Abstract
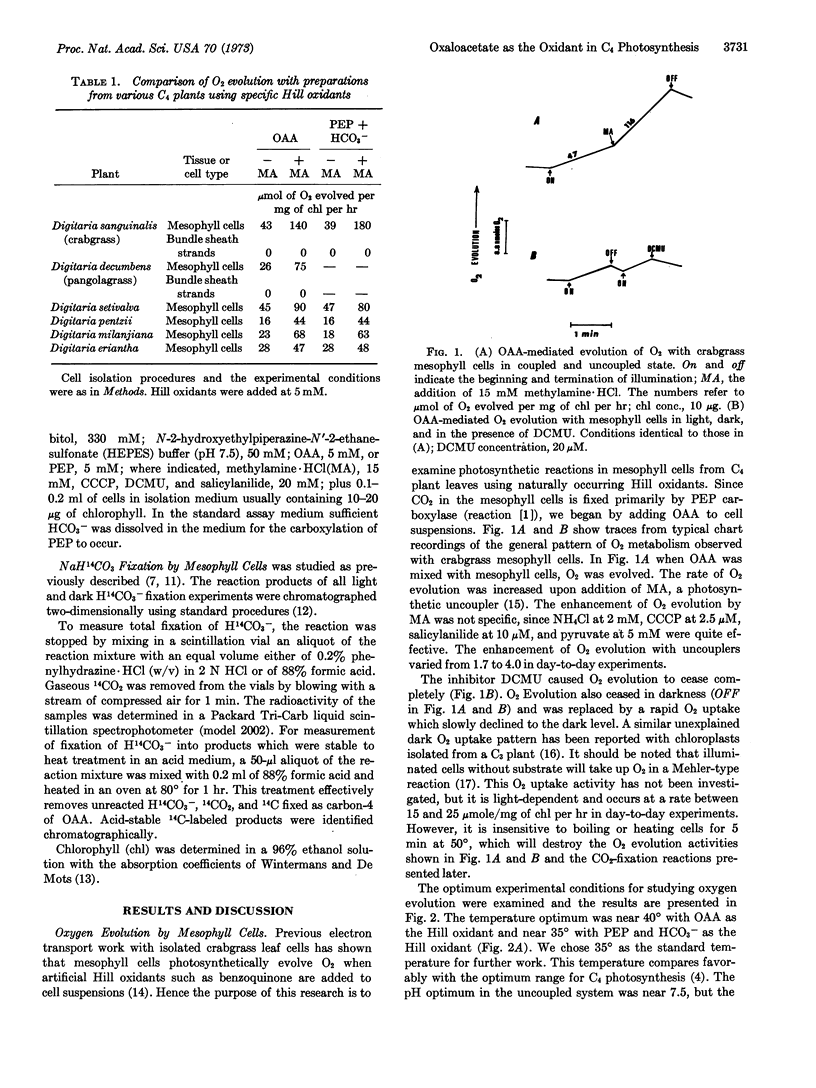
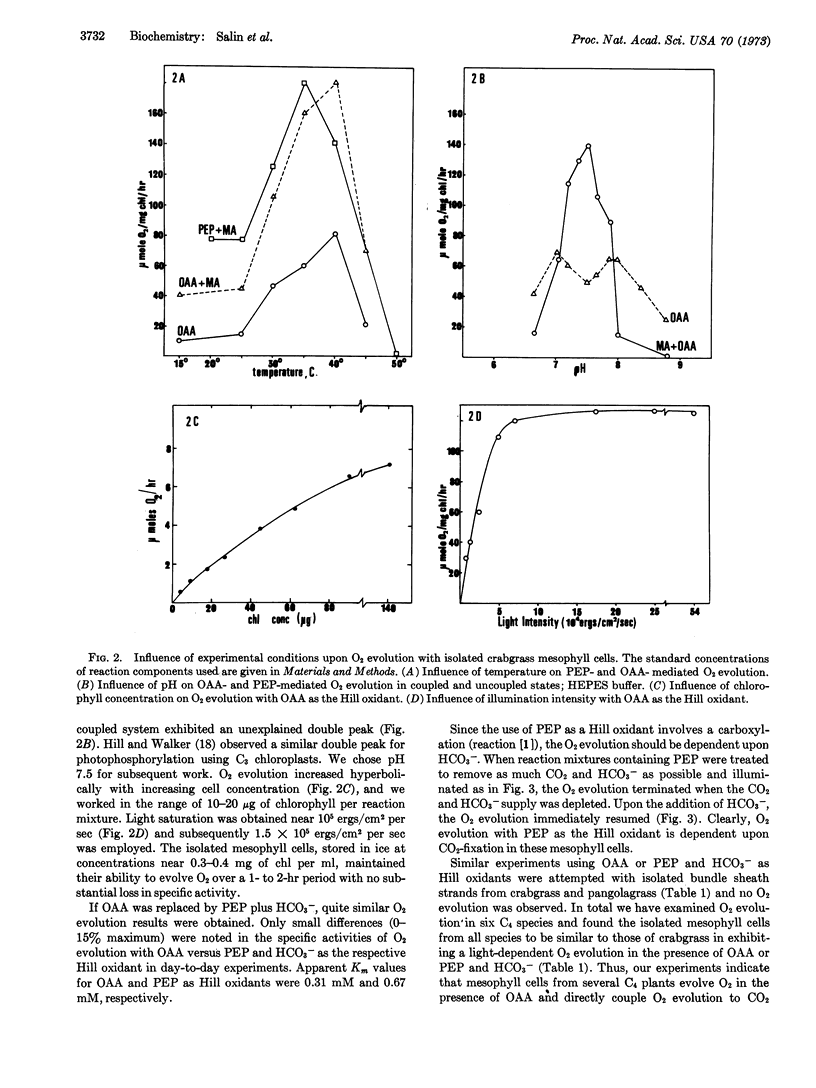
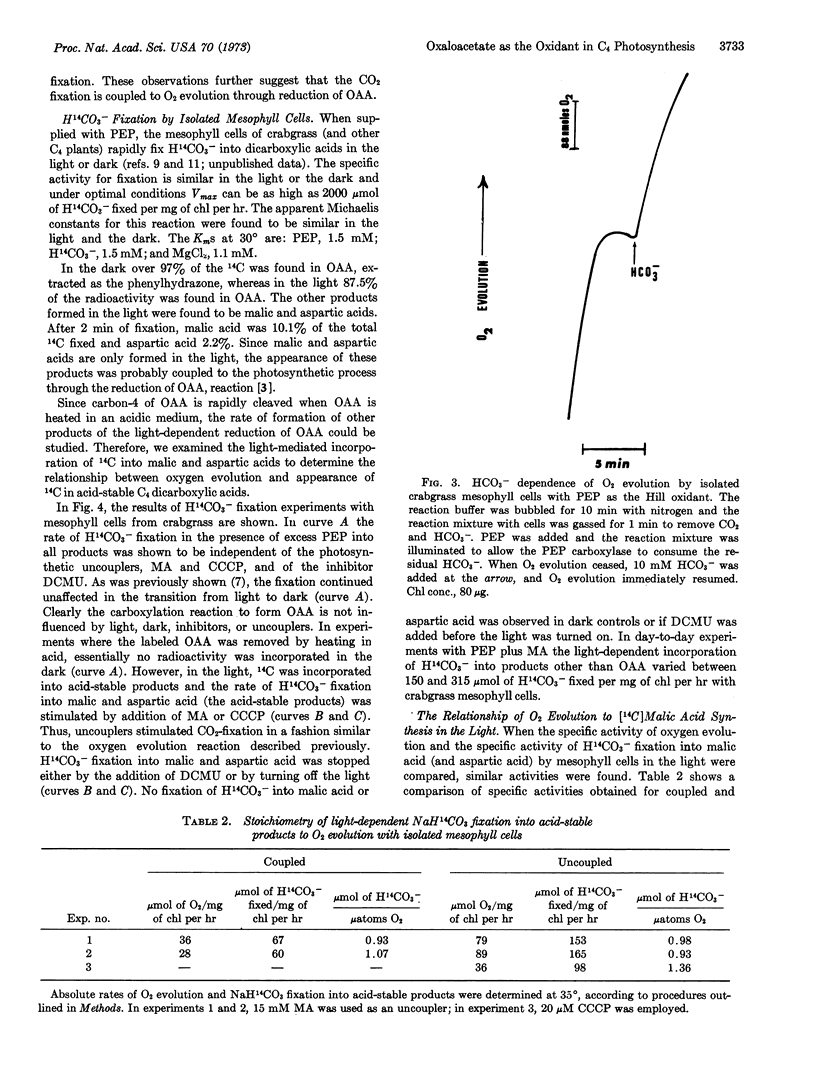
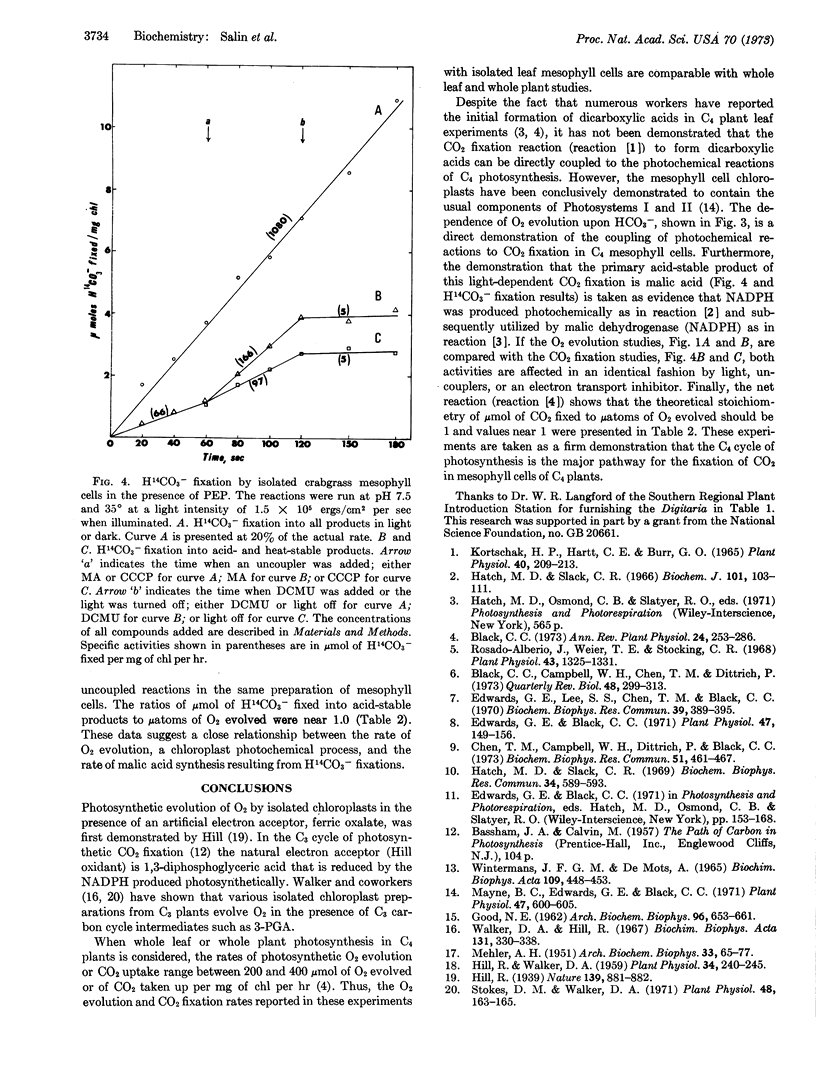
Isolated mesophyll cells from leaves of plants that use the C4 dicarboxylic acid pathway of CO2 fixation have been used to demonstrate that oxaloacetic acid reduction to malic acid is coupled to the photochemical evolution of oxygen through the presumed production of NADPH. The major acid-stable product of light-dependent CO2 fixation is shown to be malic acid. In the presence of phosphoenolpyruvate and bicarbonate the stoichiometry of CO2 fixation into acid-stable products to O2 evolution is shown to be near 1.0. Thus oxaloacetic acid acts directly as the Hill oxidant in mesophyll cell chloroplasts. The experiments are taken as a firm demonstration that the C4 dicarboxylic acid cycle of photosynthesis is the major pathway for the fixation of CO2 in mesophyll cells of plants having this pathway.
Keywords: CO2 fixation, uncoupler, photochemical reactions, electron transport, O2 evolution
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen T. M., Campbell W. H., Dittrich P., Black C. C. Distribution of carboxylation and decarboxylation enzymes in isolated mesophyll cells and bundle sheath strands of C 4 plants. Biochem Biophys Res Commun. 1973 Mar 17;51(2):461–467. doi: 10.1016/0006-291x(73)91279-5. [DOI] [PubMed] [Google Scholar]
- Edwards G. E., Black C. C. Isolation of Mesophyll Cells and Bundle Sheath Cells from Digitaria sanguinalis (L.) Scop. Leaves and a Scanning Microscopy Study of the Internal Leaf Cell Morphology. Plant Physiol. 1971 Jan;47(1):149–156. doi: 10.1104/pp.47.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G. E., Lee S. S., Chen T. M., Black C. C. Carboxylation reactions and photosynthesis of carbon compounds in isolated mesophyll and bundle sheath cells of Digitaria sanguinalis (L.) Scop. Biochem Biophys Res Commun. 1970 May 11;39(3):389–395. doi: 10.1016/0006-291x(70)90589-9. [DOI] [PubMed] [Google Scholar]
- GOOD N. E. Uncoupling of the Hill reaction from photophosphorylation by anions. Arch Biochem Biophys. 1962 Mar;96:653–661. doi: 10.1016/0003-9861(62)90352-1. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. NADP-specific malate dehydrogenase and glycerate kinase in leaves and evidence for their location in chloroplasts. Biochem Biophys Res Commun. 1969 Mar 10;34(5):589–593. doi: 10.1016/0006-291x(69)90778-5. [DOI] [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. Photosynthesis by sugar-cane leaves. A new carboxylation reaction and the pathway of sugar formation. Biochem J. 1966 Oct;101(1):103–111. doi: 10.1042/bj1010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R., Walker D. A. Pyocyanine and Phosphorylation with Chloroplasts. Plant Physiol. 1959 May;34(3):240–245. doi: 10.1104/pp.34.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortschak H. P., Hartt C. E., Burr G. O. Carbon Dioxide Fixation in Sugarcane Leaves. Plant Physiol. 1965 Mar;40(2):209–213. doi: 10.1104/pp.40.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MEHLER A. H. Studies on reactions of illuminated chloroplasts. I. Mechanism of the reduction of oxygen and other Hill reagents. Arch Biochem Biophys. 1951 Aug;33(1):65–77. doi: 10.1016/0003-9861(51)90082-3. [DOI] [PubMed] [Google Scholar]
- Mayne B. C. Spectral, Physical, and Electron Transport Activities in the Photosynthetic Apparatus of Mesophyll Cells and Bundle Sheath Cells of Digitaria sanguinalis (L.) Scop. Plant Physiol. 1971 May;47(5):600–605. doi: 10.1104/pp.47.5.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosado-Alberio J., Weier T. E., Stocking C. R. Continuity of the Chloroplast Membrane Systems in Zea mays L. Plant Physiol. 1968 Sep;43(9):1325–1331. doi: 10.1104/pp.43.9.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes D. M., Walker D. A. Phosphoglycerate as a hill oxidant in a reconstituted chloroplast system. Plant Physiol. 1971 Aug;48(2):163–165. doi: 10.1104/pp.48.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. A., Hill R. The relation of oxygen evolution to carbon assimilation with isolated chloroplasts. Biochim Biophys Acta. 1967 Mar 8;131(2):330–338. doi: 10.1016/0005-2728(67)90146-6. [DOI] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]


