Abstract
Salivary gland diseases in children are rare, apart from viral-induced diseases. Nevertheless, it is essential for the otolaryngologist to recognize these uncommon findings in children and adolescents and to diagnose and initiate the proper treatment.
The present work provides an overview of the entire spectrum of congenital and acquired diseases of the salivary glands in childhood and adolescence. The current literature was reviewed and the results discussed and summarized.
Besides congenital diseases of the salivary glands in children, the main etiologies of viral and bacterial infections, autoimmune diseases and tumors of the salivary glands were considered. In addition to the known facts, new developments in diagnostics, imaging and therapy, including sialendoscopy in obstructive diseases and chronic recurrent juvenile sialadenitis were taken into account. In addition, systemic causes of salivary gland swelling and the treatment of sialorrhoea were discussed. Although salivary gland diseases in children are usually included in the pathology of the adult, they differ in their incidence and sometimes in their symptoms. Clinical diagnostics and especially the surgical treatment are influenced by a stringent indications and a less invasive strategy. Due to the rarity of tumors of the salivary glands in children, it is recommended to treat them in a specialized center with greater surgical experience.
Altogether the knowledge of the differential diagnoses in salivary gland diseases in children is important for otolaryngologists, to indicate the proper therapeutic approach.
Keywords: salivary glands, children, inflammation, tumors, therapy
1 Embryology and anatomy of the salivary glands
The three major paired salivary glands (parotid gland, submandibular gland and sublingual gland) and the 700–1,000 minor salivary glands of the oral cavity and pharynx all come from the ectodermal germ layer. They start to appear between the sixth and tenth week of embryogenesis from cell accumulations in the embryonic foregut, with the common structural development of acini and excretory ducts. The ducts develop in parallel and have a patent lumen from the 22nd week onwards. During their growth in the surrounding mesenchymal tissue, lymphatic tissue remains within the glandular structures, especially in the case of the parotid glands. And precisely this is the reason for salivary gland involvement in certain diseases of the lymphatic system (e.g. viral infections, inflammation, and lymphomas). After birth, the glands gradually increase in size until adulthood.
The parotid gland is the largest of the salivary glands. Its 5–6 cm long excretory duct (parotid duct; Stensen’s duct) crosses the masseter muscle, curves medially and then penetrates the buccinator muscle and the buccal mucosa to open into the vestibule of the mouth opposite the second upper molar. On average, the diameter of the parotid duct is 1.4 mm at the hilum, 1.2 mm as it runs through the buccinator and 0.5 mm at the ostium [1]. The body of the gland, which consists of serous lobules, lies in the retromandibular fossa, above and posterior to the masseter muscle and the lower jaw. It is important to remember that the facial nerve in children lies much more laterally than in adults, as the mastoid cells are not yet fully developed. In young children, the nerve is even a little larger in proportion to the surrounding structures [2], [3].
The seromucous submandibular gland lies on the hyoglossal muscle between the anterior and posterior bellies of the digastric muscle. The 5–6 cm long submandibular duct passes around the mylohyoid muscle, crosses the lingual nerve and then crosses the floor of the mouth to open through the sublingual caruncle. The average diameter of the duct is 1.5 mm along its entire course from hilum to ostium, narrowing to 0.5 mm at the ostium itself [1]. The sublingual gland, a mucoserous gland, lies in the sublingual fossa, in submucosal tissue superior to the mylohyoid muscle. Its excretory duct either empties into the submandibular duct or opens separately into the oral cavity on the salivary papilla [4]. There are no estimates of duct length or diameter in childhood to be found in the literature.
2 Physiology of the salivary glands
Healthy adults produce about 1.5 litres of saliva a day. Some 20–25% comes from the parotid gland, about 70–75% from the submandibular gland and 5% from the sublingual gland. The viscosity of the saliva depends on the individual proportions secreted by the different glands [5].
Taking the total production from all of the salivary glands, 0.3 to 0.5 ml of saliva is secreted per minute at rest, while 1.5 ml/min is produced under conditions of maximum stimulation. Hidas et al. [6] studied salivary flow in children and adolescents with attention deficit hyperactivity disorder (ADHD). The rate of total salivation at rest was 1.1 ml/min in the control group and therefore similar to the recognised normal values, while the flow rate in patients with ADHD with or without medication was significantly lower. Continuous secretion is activated by a weak parasympathetic stimulus and is important in keeping the mouth moist. Central nervous system (CNS) regulation arises in the salivary nuclei of the medulla and pons and is transmitted via the autonomic nervous system. The major salivary glands are innervated by both parasympathetic and sympathetic nerves. However, unlike in other organs, they do not act antagonistically to each other. Both systems stimulate saliva secretion, albeit in different ways. Parasympathetic stimuli lead to the rapid production of a copious watery secretion that is rich in enzymes. At the same time, there is vasodilatation. In contrast, sympathetic stimulation induces the secretion of a smaller quantity of slimy viscous saliva associated with vasoconstriction. The salivary centre may be affected by external factors acting though higher CNS centres (the sight, smell or thought of food). Two reflexes are involved: a simple unconditioned reflex and an acquired conditioned reflex. The simple reflex is triggered by oral chemoreceptors and baroreceptors.
The parasympathetic component stimulates saliva secretion at the receptor level. Muscarinic receptors are important here, also in the pharmacotherapy of functional disorders. In addition, dehydration, lack of sleep and anxiety may inhibit saliva production.
Saliva consists of up to 99.5% water. Protein, electrolytes, and various bactericidal and antimicrobial factors make up just 0.5%. Roughly speaking, two characteristic secretory compartments can be distinguished: the first is the fluid serous saliva, which contains bactericidal substances such as thiocyanate, proteolytic enzymes (lysozyme) and antibodies such as IgA, as well as α-amylase to digest starches, while the second is the mucus secretion or the mucous component of saliva, which prevents the oral mucosa from drying out and makes chewing and swallowing considerably easier. It also improves the sense of taste and clarity of speech [5].
A continuous flow of saliva with this composition protects against wound infections and caries. Bacteria and food residues are washed away, so to speak. Calcium phosphate ions contribute to the remineralisation of the teeth. The bicarbonate in saliva acts as a buffer for acids from food and bacterial metabolism and this also helps to prevent tooth decay. The pH is higher with an increased salivary flow and this may possibly protect the oesophageal mucosa by buffering any acid reflux. Recent studies also credit saliva with an effect on the gland itself, at least a paracrine one (e.g. leptin) [7]. In a comparative study of the minor salivary glands, Sonneson demonstrated that even in preschool children, a high density of glands and mature innate immunity existed, which further adapts as the children grow up [8]. Studies on children’s saliva are particularly relevant to dental problems. It appears that the quality (protein content) rather than the quantity of saliva is important for caries prophylaxis. In a group of four- to six-year-olds, Bhalla et al. showed that tooth decay was inversely proportional to the proline content, but not to the total quantity of saliva produced [9]. Medicines used for oral asthma therapy, such as β2-agonists and cortisone, may reduce salivation [10]. A protein-deficient diet or chemotherapy for cancer during childhood even leads to a persistent functional impairment of the salivary glands, which may have lasting effects on the immune defences in the adult and be associated with significantly greater pathological conditions of the teeth [11], [12].
3 Clinical examination and imaging
A careful history is the first decisive step in the diagnosis of salivary gland conditions. In infants and young children it is, of course, extremely important to question the parents. As with adult patients, a relatively small number of cardinal symptoms are to be found for a large number of possible diagnoses. Most conditions affecting the salivary glands give rise to painful or painless swelling of the gland. In addition, there may be xerostomia (dry mouth), sialorrhoea (drooling) and facial palsy and, of course, corresponding generalised symptoms whenever there is an infection. The differential diagnosis to be considered in children includes infections of the salivary glands, autoimmune and systemic diseases and lymphadenopathy of various origins. Tumours and drug-induced disorders tend to be rare. Clinical examination often provides a first provisional diagnosis, for example, a ranula can easily be identified on inspection. Assessment of the saliva being secreted also indicates whether there is an acute suppurative infection (creamy yellow) or a more chronic recurrent process (flocculent secretion). Salivary stones and tumours can also usually be palpated. Imaging or laboratory tests are usually required to define the condition and sometimes confirm the diagnosis. ENT surgeons in Germany are fortunate to have ultrasound scanning available as an excellent method for assessing the three major paired salivary glands in children. As the required depth of penetration is small, frequencies of 7.5–12 MHz are suitable for scanning children to depict and anatomically classify infections, stones and tumours as well as lymph nodes. The salivary gland ducts (Stensen’s and Wharton’s ducts) can only be seen when an obstructive disease or an acute suppurative infection is present, either on initial examination or secondary to a secretory stimulus (vitamin C). Colour Doppler mode allows the internal perfusion of space-occupying lesions, the circulation in the glands themselves and non-perfused cysts to be identified. This helps to characterise lesions more precisely, especially haemangiomas and arteriovenous malformations. Conventional sialography and X-rays have no place in diagnostic investigations during childhood. In young children, sialography can anyway only be performed under sedation. Magnetic resonance imaging (MRI) and computed tomography (CT) are reserved for certain situations requiring the further workup of space-occupying lesions or processes that exceed the ultrasonographic limits of the salivary glands and extend into the para- or retropharyngeal spaces or to the base of the skull or mastoid. MRI focuses mainly on questions of soft tissue, nerves and dural infiltration, while MR sialography may be used to examine the ducts or MR angiography to demonstrate the topography of the vascular supply. On the other hand, CT scans can be used to assess bony structures and is also useful for determining the extent of large abscesses, especially in the para- and retropharyngeal spaces. Sialendoscopy, with its direct visualisation of the ducts, can fill any diagnostic gaps in obstructive salivary gland disease.
Fine needle aspiration biopsy can be used to obtain tissue for ascertaining the type of tumour and whether it is benign or malignant and material for further microbiological investigation in the case of infection. It is important to detect the pathogen by direct means or by polymerase chain reaction (PCR). Indications for fine needle aspiration are limited in children and must be considered very carefully, as the procedure usually requires general anaesthesia. Depending on the indication, an open biopsy or complete resection of a space-occupying mass can be performed for histology. The workup of an infection/inflammation includes, of course, a full or differential blood count and C-reactive protein or procalcitonin (in cases of sepsis). Antinuclear antibodies (ANAs), SS-A or SS-B (Sjögren’s syndrome antibodies to duct epithelium, formerly Ro and La) and angiotensin converting enzyme (ACE) levels may be indicated in rare childhood systemic diseases such as Sjögren’s syndrome or sarcoidosis. The determination of the salivary secretion rate is only of secondary importance in childhood.
4 Congenital salivary gland diseases
Apart from conditions that affect the saliva composition (e.g. sticky saliva in Prader-Willi syndrome) or cause an inability to swallow and lead to drooling (see below), hypoplasia or aplasia of the salivary glands is a rare occurrence and can affect just one, several or even all of the salivary glands. Aplasia of the salivary glands may occur in isolation or be associated with other malformations of the first branchial arch. These include hemifacial microsomy, Treacher Collins syndrome and other congenital malformations of the face.
A recent study from Norway looked at 21 patients with Treacher Collins syndrome [13]. In addition to ultrasound scans, it analysed salivary secretion. Almost half (48%) of the patients had abnormalities of the salivary glands. The pathology included dysplasia in six cases and aplasia in four. The salivary secretion rate was reduced in almost all the patients, although there was no correlation with subjective or ultrasound findings.
Matsuda et al. reviewed 43 cases of salivary gland aplasia appearing in the literature since the first case was reported by Gruber in 1885 [14], [15]. Nineteen more cases have been reported since 1999, of which at least five exhibited bilateral aplasia of the lower jaw and parotid gland [16]. A few of the case reports show a combination of aplasia and hypofunction of the salivary glands with other ectodermal malformations of the tear passages, skin appendages, and teeth. A familiar form seems to be inherited as an autosomal dominant with great variability of the phenotype [17]. One of the best-known conditions is the lacrimo-auriculo-dento-digital (LADD) syndrome, characterised by malformations including aplasia of the tear ducts, aplasia of the salivary glands, deafness and malformations of the ear as well as abnormalities of the teeth and limbs. Hypoplasia or aplasia of the salivary glands is often not recognised until late, since these patients, unlike those with acquired xerostomia, do not complain of their symptoms. Patients usually present with massive tooth decay associated with a dry mouth, difficulty swallowing and drinking copious amounts of fluid. Early diagnosis (on clinical examination, ultrasound, MRI and possibly scintigraphy) can result in appropriate and early prophylaxis against sequelae (teeth) and considerable relief of the symptoms.
And finally, juvenile recurrent parotitis may also be viewed as a genetic malformation of the gland [18]. Because of its topicality and new information on the treatment of this condition, it will be addressed in a separate section (see 5.2.8).
Polycystic (dysgenetic) disease of the parotid gland was described von Seifert et al. (1981) as a very rare entity that is independent of recurrent parotitis and congenital sialectasis [19]. They reported the case of a six-year-old girl who, like her father, suffered from this condition and therefore assumed an autosomal dominant inheritance. The cause is thought to be a defect in the interactions between activin, follistatin and TGF-β, leading to a developmental disorder of glandular tissue (salivary glands, pancreas and kidneys) in the mouse model [20].
Ranulas, benign tumours of the sublingual gland, may already occur before birth and large ones can cause direct respiratory problems during and after delivery. A congenital defect in the mylohyoid muscle is discussed in connection with plunging ranulas. These lesions have been observed in siblings and are more common in certain ethnic groups [21], [22], [23]. An ex utero intrapartum treatment (EXIT) procedure can be carried out electively in such a situation that could possibly even be life-threatening. During a caesarean section, the ranula is decompressed and the airways held open before the umbilical cord is cut. Only then is delivery completed [24].
Only 5% of all salivary gland tumours occur in childhood. Congenital tumours such as sialoblastomas are even less common [25], [26]. The difficulty in the histological diagnosis has led to various synonyms being used in the literature, depending on the cell type: embryoma, congenital basal cell adenoma, etc. As a rule, tumours arise in the perinatal period or can already be identified on ultrasound scans during pregnancy. The parotid and submandibular glands are most often affected. Histology shows sialoblastomas to be malignant tumours arising from the embryonic epithelial anlage and they show a wide range of differentiation. These tumours were first described in 1966 [27]. Up to 2010, a further 26 cases were reported in the literature, which have occasionally also been associated with hamartomas or hepatoblastomas [28]. T1-weighted MRI shows an isointense tumour with heterogeneous contrast enrichment. A high mitosis rate, necrosis and anaplasia on histology are considered to be unfavourable prognostic features [29]. The treatment of choice is complete resection of the tumour, although this is not always possible because of infiltration into the surrounding structures, including the base of the skull. Tumour residues usually respond well to adjuvant chemotherapy or in cases that have already metastasised, to neo-adjuvant chemotherapy [30]. As for rhabdomyosarcomas, several courses of treatment are given (e.g. ifosfamide 3 g/m2/day on days 1 and 2; vincristine 1.5 g/m2 on day 1; actinomycin D 1.5 g/m2 on day 1; doxorubicin 30 mg/m2/day on days 1 and 2) [31]. Radiotherapy at this age is associated with possible adverse effects on bone growth and not inconsiderable mutagenicity.
Local recurrence was described in 10 of the 26 cases reported, so that patients should be monitored closely. At an average follow-up of 46 months (10 days to 43 years), 18 patients (69%) were still alive. Thus the prognosis is still relatively good [28].
5 Salivary gland infection/inflammation
Inflammatory diseases are the most common conditions besides benign tumours that affect the salivary glands of children and adolescents [32]. The most important viral pathogens are the mumps virus and cytomegalovirus [33], which will be looked at in more detail in the following section. In addition, there are acute bacterial infections and chronic inflammation, as well as autoimmune diseases. It must also be mentioned that the saliva itself plays an important role in many more viral, bacterial and other diseases. The paper presented by Weidauer to the 72nd annual meeting of the German Society of Otorhinolaryngology, Head and Neck Surgery in Hamburg is of particular interest in this context [34]. According to this presentation, saliva is a reservoir of the microorganisms involved in persistent bacterial infections of the oral cavity (dental plaque), as well as of the normal oral flora with pathogenic potential, and a vector for Helicobacter pylori. In certain infections, viruses and bacteria can be demonstrated in the saliva for a long time, even without any symptoms (mumps, cytomegalovirus, human herpes viruses, HIV, rabies, hepatitis B) or are found only transiently (pertussis, diphtheria, Haemophilus influenzae, Epstein-Barr virus, adenoviruses, herpes simplex virus type 1, influenza and parainfluenza viruses, measles and rubella viruses). Organisms with oncogenic potential, such as Epstein-Barr virus, human herpes virus 8, human papilloma virus and Helicobacter pylori are of particular relevance.
5.1 Viral sialadenitis
5.1.1 Mumps
The mumps virus is an encapsulated RNA virus (15,384 nucleotides) of the paramyxovirus family, with a diameter of 200 nm. The RNA codes for six structural proteins and at least two other proteins. The capsule consists of a two-layered lipid membrane, which means that the virus is susceptible to disinfection with ether or alcohol. The virus is stable for several days at 4°C [35]. So far, twelve genotypes (A–L) of the mumps virus have been identified, with different regional distributions [36]. The disease is transmitted by droplet or contact spread. The incubation period is 15–24 days. Patients are already infectious two days before the onset of symptoms [37]. The virus can be detected in the saliva for seven days before the start of symptoms and for nine days afterwards. Virus replication usually takes place in the upper respiratory tract and in the salivary glands. Nevertheless, parotitis is not one of the first or even essential steps in the course of the infection. Replication takes place in the duct epithelium of the parotid gland and leads to oedema and a local inflammatory reaction with lymphocyte and macrophage infiltration [38].
About one-third of mumps infections are asymptomatic. The cardinal symptoms of parotitis with fever are seen in 60–70% of all infections and in 95% of symptomatic patients. The gland remains swollen for about one week. There is bilateral involvement in 90%, although the swelling may not be seen at the same time on both sides. Complications of parotitis are rare: sialectasis with further recurrent swelling has been reported [39]. The submandibular and sublingual glands may also be involved in 10% of cases. Problems with lymphatic drainage may cause bilateral cervical oedema or, in rare cases, supraglottic oedema [40]. The disease can also spread to other organs, giving rise to epididymitis in 30% and bilateral orchitis also in up to 30%. Infertility as a result of mumps infection is, however, a rare complication [41]. Oophoritis is seen in 5%, mumps meningitis in up to 10% and encephalitis in up to 1% of those infected. Typical symptoms of encephalitis are seizures, clouding of consciousness and focal neurological symptoms such as ataxia, dizziness and behavioural disorders, while EEG changes are observed in children [42]. The mortality of encephalitis may be as high as 5%. Transient high frequency hearing loss has been observed in up to 4% of cases [43]. Persistent, usually unilateral, deafness occurs in 0.005%, i.e. one in 20,000 patients [44]. Infection in the first trimester of pregnancy is particularly problematic. It is estimated that up to 27% of cases end in spontaneous abortion. Pancreatitis occurs in about 4% of people with mumps. The complications of mumps increase, above all, with age [38].
The diagnosis is made on the basis of the clinical history and symptoms, and confirmed by elevated serum levels of IgM antibody to the mumps virus (with an ELISA), although it should be noted that false negative results occur in up to 30% of cases [45]. The false negative rate can be reduced by performing the test four days after the onset of symptoms. Virus may also be demonstrated directly in certain cases, but only during the first few days of infection, or by RT-PCR. The differential diagnosis includes infection with EBV, parainfluenza viruses, adenoviruses or human herpes virus 6 [46] and, of course, all other causes of salivary gland enlargement.
As the disease is benign and usually self-limiting, treatment is purely symptomatic. The administration of immunoglobulins may be considered in isolated cases. Treatment success has been reported in presumed autoimmune sequelae such as postinfective encephalitis, Guillain-Barré syndrome and idiopathic thrombocytopenic purpura [47], [48], [49].
The incidence of mumps in Germany shows that it is still endemic despite the long-established vaccination programme (in West Germany since 1976 and throughout the whole country since 1991). Even so, thanks to the vaccine, the incidence has fallen from 100–1,000 cases per 100,000 inhabitants in the pre-vaccine era to 10.3 per 100,000 inhabitants in the period 2007–2011 [50]. However, despite the two-dose vaccine introduced in 1991, seroconversion could not be detected in 20–22% of the 0–17 age group. This may be due to the fact that some parents no longer have their children vaccinated or that secondary immunisation is suppressed because of a lack of circulating wild types. There also seems to be an increasing incidence, particularly in 20- to 29-year-olds in the Western federal states, as was seen in a large outbreak in Bavaria in 2010/11, which mainly affected people aged 15–29. Even if mumps has become less common because of the vaccine, it is still important for ENT surgeons to consider it in the differential diagnosis of salivary gland swellings.
5.1.2 Cytomegalovirus
Cytomegalovirus is a member of the herpes virus family. The infection rate in the population is 40% to 100% [51]. Up to 20% of children come into contact with the virus even before puberty. Reinfection may occur with genetically different pathogenic strains [52]. The primary infection is often unnoticed, but the virus may lie dormant in the body and cause symptoms when there is an alteration of the immune defences. The infection is transmitted by direct contact with various bodily fluids, including saliva. Serious complications may be associated with neonatal infection from the birth canal and reactivation in immunosuppressed patients or following organ transplantation. Immunocompetent patients may show a clinical picture similar to that of infectious mononucleosis with all its recognised symptoms. Hepatitis, Guillain-Barre syndrome, encephalitis, pneumonia and myocarditis are very rare. The diagnosis is confirmed by demonstrating IgM or by PCR. Treatment is purely symptomatic. It is important to consider the possibility of reactivation in immunocompromised patients. Furthermore, seronegative pregnant women should keep away from potentially infectious people because of the risk of intrauterine CMV infection of the fetus with possibly severe brain damage and visceral disease [53].
5.1.3 HIV and the salivary glands
HIV infections may also primarily involve the salivary glands, especially the parotid. The clinical picture is often one of unilateral swelling, pain and inflammatory changes in the gland, sometimes analogous to acute parotitis. On the other hand, there may just be a painless enlargement of the gland. Ultrasound scans show hypoechoic and anechoic structures, which correspond histologically to epithelial cysts and lymph nodes. In these cases, HIV testing should be added to the diagnostic tests and further cytological or histological workup considered [54]. Hobbs et al. suggested a three-stage classification in paediatric patients: persistent generalised lymphadenopathy (PGL), benign lymphoepithelial lesions (BLEL) and benign lymphoepithelial cysts (BLEC) [55]. Benign lymphoepithelial lesions in HIV are similar to those in other salivary gland diseases (sarcoidosis, Sjögren’s syndrome, chronic recurrent parotitis) and are probably the expression of an autoimmune reaction [56]. Whilst BLEL is thought to be a separate histological entity with no transformation into BLEC, the generalised lymphadenopathy is probably the precursor of BLEC. The management of benign lymphoepithelial cysts includes a watch and wait policy, repeated fine-needle aspiration (also to confirm the diagnosis) or drainage, highly active anti-retroviral therapy (HAART) [57], [58], [59], sclerotherapy (ethanol, picibanil, tetracycline, doxycycline, etc.), radiotherapy (8–24 Gy) and surgery with extracapsular dissection or total parotidectomy [60]. The choice of treatment must be determined on a case-by-case basis, especially for paediatric patients, but an initial monitoring with appropriate antiretroviral medication would appear to be possible in most cases. Radiotherapy and surgery are indicated in selected cases [55].
5.2 Bacterial sialadenitis in childhood
5.2.1 Neonatal suppurative parotitis
Neonatal suppurative parotitis has been described as a particular form of acute sialadenitis occurring immediately after birth. There is typically a warm red swelling around the affected gland. In a recent review, Ismail et al. collected 44 cases reported in the literature since 1970 [61]. The infection was unilateral in 77% and a similar percentage of the affected patients were boys. Purulent secretions were expressed from the papilla of the parotid duct in all but two patients. Only half of the patients had a raised temperature. The leucocyte count was elevated in 44%. Other laboratory tests were not particularly helpful. The organisms isolated were Staphylococcus aureus (in 61% of cases), with three patients (7%) having MRSA (methicillin resistant staphylococcus aureus) [62], other Gram-positive cocci (25%), Gram-negative rods (16%) and anaerobic bacteria (11%). Blood cultures were positive in 32%.
Possible modes of infection are bacteria ascending via the parotid duct and haematogenous spread. As the number of breast-fed children (78%) in this group was considerable higher than average, it was suggested that infants became dehydrated through inadequate breast-feeding, with ascending duct infection related to decreased salivary flow [63]. Other risk factors were high ambient temperatures, excessive sucking and feeding via a nasogastric tube [64]. In addition, the rate was considerably higher in premature babies (32%) than otherwise described. Premature birth is therefore a risk factor [65]. The majority of the patients were successfully treated with a combination of an anti-staphylococcal drug and an aminoglycoside or a third-generation cephalosporin. The duration of antibiotic therapy was between seven and 21 days, depending on the bacterial colonisation, comorbidity and spread of infection. Only 23% required surgical drainage. Most patients have an excellent prognosis with respect to morbidity and mortality; as a rule, it is very favourable. Parotid fistulas, facial nerve palsy, septicaemia, meningitis or mediastinitis occur only rarely [66].
5.2.2 Suppurative parotitis and parotid abscesses in children and adolescents
Older children and adolescents very rarely have suppurative sialadenitis. In a centre for paediatric ENT, Stong et al. treated only 17 patients with acute suppurative parotitis, and a further four patients with an acute exacerbation of juvenile recurrent parotitis, over a period of five years [67]. It must be assumed that most children with these conditions are treated by their general practitioners or in an ENT practice. The mean age of the eight boys and 13 girls was 6.5 years (6 months to 15 years). Typical symptoms were painful unilateral or bilateral swollen parotid glands and purulent secretion from the parotid duct; 62% of the patients had to be admitted to hospital with severe dehydration, leucocytosis and fever. Over half of the children admitted had serious comorbidities (cystic fibrosis, leukaemia, HIV, diabetes and infantile cerebral palsy). In two (10%) patients, an abscess developed in the gland during the course of the disease.
Treatment of acute suppurative parotitis consists of gland massage, sialagogues and antibiotic therapy to cover staphylococci and streptococci, in particular. Antibiotic resistance testing is only needed in cases of treatment failure. Ultrasound scanning is the imaging method of choice to perform after clinical examination. CT is rarely indicated, but may be used to determine the extent of an abscess. Apart from abscesses, the complications of acute parotitis include facial palsy and extensive phlegmons of the throat causing difficulty in breathing [68]. Parotidectomy is required only in rare cases [32]. Saarinen et al. from Finland reported on ten children (aged 2–16 years) who were treated within a ten-year period and calculated an incidence of 0.7 cases per million inhabitants [69]. The treatment of choice was incision and drainage of the abscess (Figure 1 (Fig. 1)). Considerations in the differential diagnosis of an abscess include infected cysts or fistulas of the first branchial arch. Even though the most common pathogens comprise a mixed flora of staphylococci, streptococci, anaerobic bacteria and Haemophilus influenzae [70], the possibility of tuberculosis and atypical mycobacterial infections should also be considered [71]. Samples should therefore be obtained before treatment and sent for microbiological tests. Salivary stones should also be ruled out.
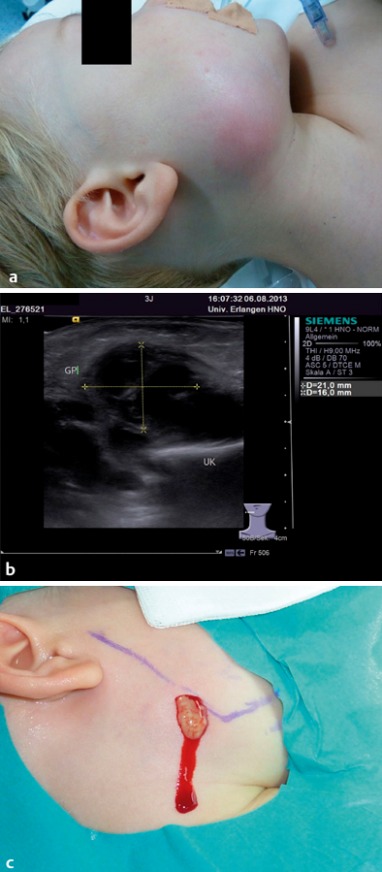
Figure 1. a) Three-year-old boy with a painful red swelling of the right cheek since 3 days. b) No improvement on antibiotics. Axial ultrasound scan shows a hypoechoic, poorly demarcated, inhomogeneous lesion, measuring 21 mm x 16 mm (+…+), in the parotid gland, consistent with the diagnosis of an abscess. c) Treatment of choice was drainage of the abscess under general anaesthetic, with irrigation and insertion of a wick. Blue line: the mandible.
5.2.3 Obstructive sialadenitis in childhood
Also in childhood are stones frequently the cause of obstruction in the excretory ducts. As a rule, idiopathic inflammatory stenosis and radioiodine-induced stenosis are found only in adults [72], [73], [74], [75], [76], [77], [78]. In our own patient population, we have also seen iatrogenic stenosis after surgical procedures on a frenulum of the tongue or ranula. One adolescent patient had traumatic papillary stenosis of the parotid duct, which is rarely seen in children and adolescents, but caused in this case by excessive bruxism. Salivary stone disease is seldom seen in children [79]. In their literature review of reports published since 1860, Reuther and Hausamen found 21 cases of submandibular gland stones in children aged between three weeks and 15 years [80]. In Japan, thirty-two cases in children below the age of ten were described up to 1994; in 29 of them, stones had formed in the submandibular gland [81].
In their study population, Zenk et al. described 39 patients below the age of 21. The youngest patients with confirmed stones in the parotid gland were a five-year-old girl and a three-year-old boy. Parotid stones are less common in adults as well [82].
As in adults, the symptoms are characteristic. There is usually recurrent swelling of the affected gland, which may be associated with pain. Sialolithiasis may also present for the first time as an acute bacterial infection of the gland. Management priorities are treating the sialadenitis and looking for the stone. The treatment of salivary stones in children today is basically not different from that in adults. As a rule, younger children require a general anaesthetic for the procedure because they are unable to cooperate. The decisive factors in selecting the therapeutic option are the site size, and mobility of the stone.
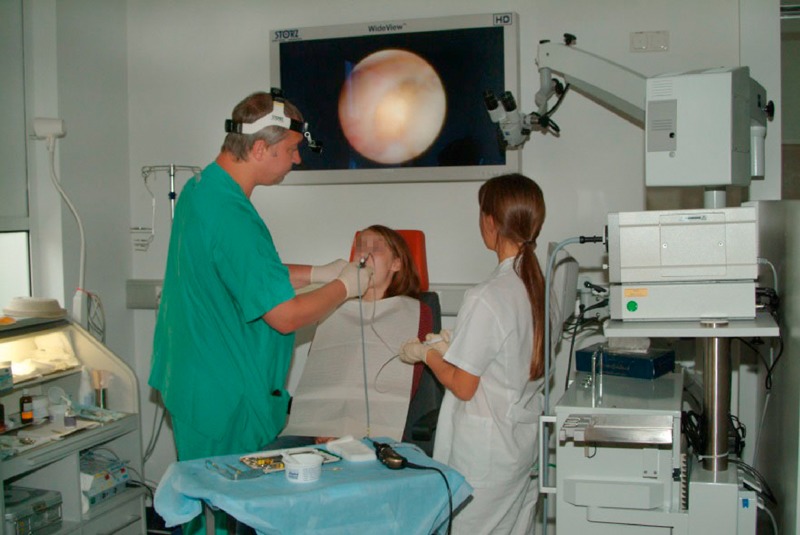
Sialendoscopy can be used initially to investigate both the parotid and submandibular ducts to determine the presence of a mobile stone, or one that can be mobilised, in the distal duct system and right up to the hilum (Figure 2 (Fig. 2)). In this case, the stone can then be extracted using a Dormia basket [83], [84].
Figure 2. Sialendoscopy performed on a 13-year-old girl to detect a stone in the hilum of the right submandibular gland (see screen) that could not be clearly demonstrated with ultrasound scanning.
Stones that have impacted anywhere up to the hilum in the submandibular gland can usually be removed transorally without problem [85] (Figure 3 (Fig. 3)). Extracorporeal shockwave lithotripsy (ESWL) is an excellent method for disintegrating or destroying impacted parotid stones, irrespective of their site and size. The stone fragments may pass spontaneously or a repeated attempt can be made to remove the stone endoscopically [86], [87], [88]. ESWL is also suitable for use in children (Figure 4 (Fig. 4)). A combined endoscopic and transcutaneous approach to stone removal can be used as an alternative [89]. Lithotripsy is also indicated for submandibular stones lying proximally. New intracorporeal methods with laser lithotripsy have been reported in a few adult study populations, but have not yet become established in routine clinical practice, partly because of possible adverse effects and partly because they are time-consuming and relatively expensive [90]. If it is not possible to relieve the symptoms with these methods, then the only remaining option is surgical excision of the gland with all its known risks [91], [92].
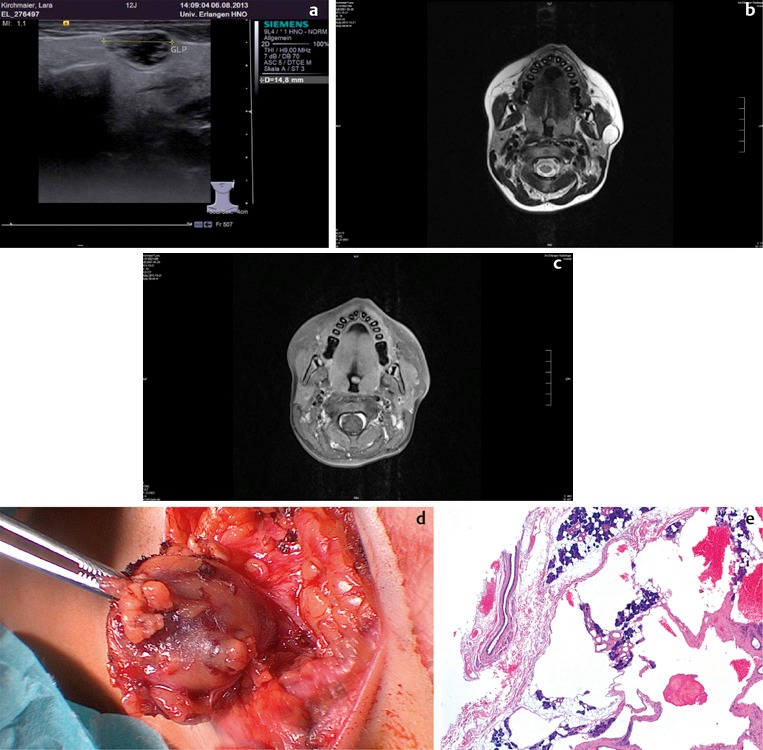
Figure 3. a) Ultrasound scan showing two hilar stones, measuring 4.4 mm and 4.7 mm, in the left submandibular gland of 14-year-old girl, before treatment. The hypoechoic changes in the parenchyma of the gland are regarded as the expression of chronic obstruction. b) Transoral stone extraction with marsupialisation of the duct under local anaesthetic in the same patient. c) Four months after the stone extraction, the patient is free of symptoms and has no new stones. The ultrasound scan shows a normal gland parenchyma (MMH: mylohyoid muscle, GSM LI: left submandibular gland). d) A neo-ostium with no signs of inflammation can be seen in the left posterior floor of the mouth.
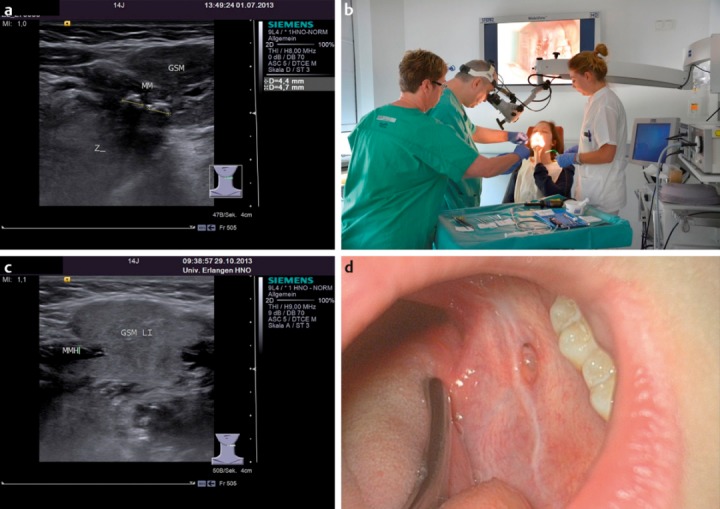
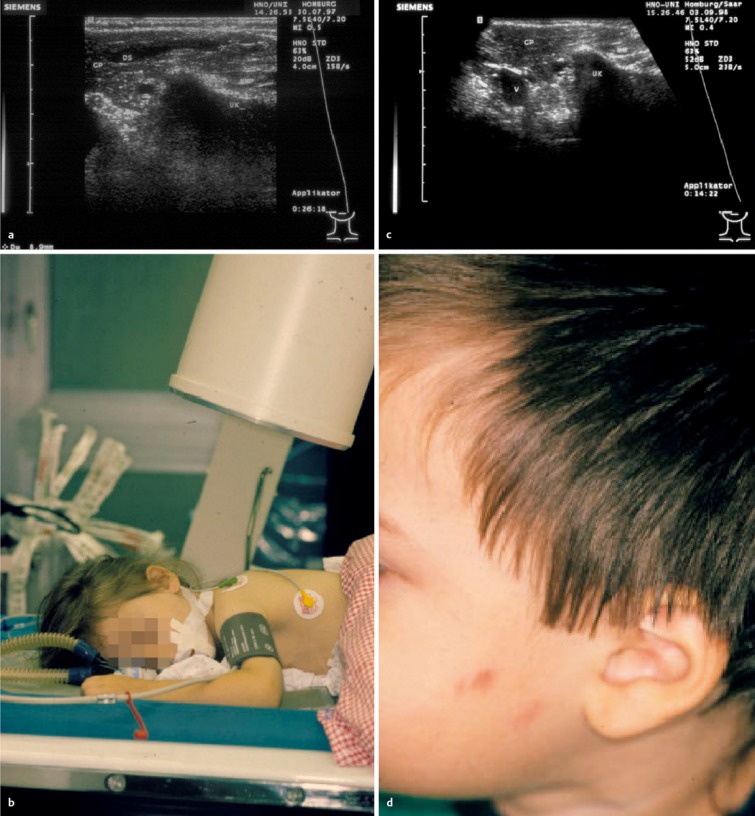
Figure 4. a) Pre-operative ultrasound scan of a 5-year-old girl with a stone measuring almost 9 mm (+…+) in the right parotid gland (DS: Stensen’s duct; GP: parotid gland; UK: lower jaw; MM: masseter muscle). b) Extracorporeal shockwave therapy under general anaesthetic. c) No stones or symptoms one year after the intervention, with an unremarkable ultrasound appearance of the right parotid gland. d) A 3-year-old boy after lithotripsy of a stone in the left parotid, showing petechial haemorrhages into the skin as a typical adverse effect of lithotripsy. These will be resorbed within a few days.
5.2.4. Pneumoparotitis
Idiopathic recurrent pneumoparotitis is a rare condition with painful swelling of the affected gland [93]. In this case, patients blow air into the excretory ducts of the parotid gland. It has been described in wind instrument players and glass blowers [94]. However, it can also be associated with diving, dental treatment or recovery from an anaesthetic (coughing fits) [95]. It may also be self-induced in adolescents with psychosocial disorders [96]. It remains unclear whether the cause involves an open parotid duct ostium or a weak buccinator muscle in combination with already dilated ducts. We ourselves can report on a 13-year-old boy who developed the recurrent parotid swelling of pneumoparotitis as a result of blowing up balloons. The diagnosis is made using ultrasonography (Figure 5 (Fig. 5)), where numerous mobile echogenic reflexes with a comet-tail artefact are apparent. The air inclusions can also be seen clearly on CT. Available treatment includes symptomatic measures such as gland massage, sialagogues, analgesics and antibiotic therapy, if necessary. The most important thing is to avoid the underlying trigger. Psychiatric treatment or behavioural therapy should also be considered for patients with self-induced disease. Surgical procedures such as duct ligation, duct repositioning into the tonsillar fossa and even excision of the gland have been described, but are indicated only in exceptional cases [97], [98].
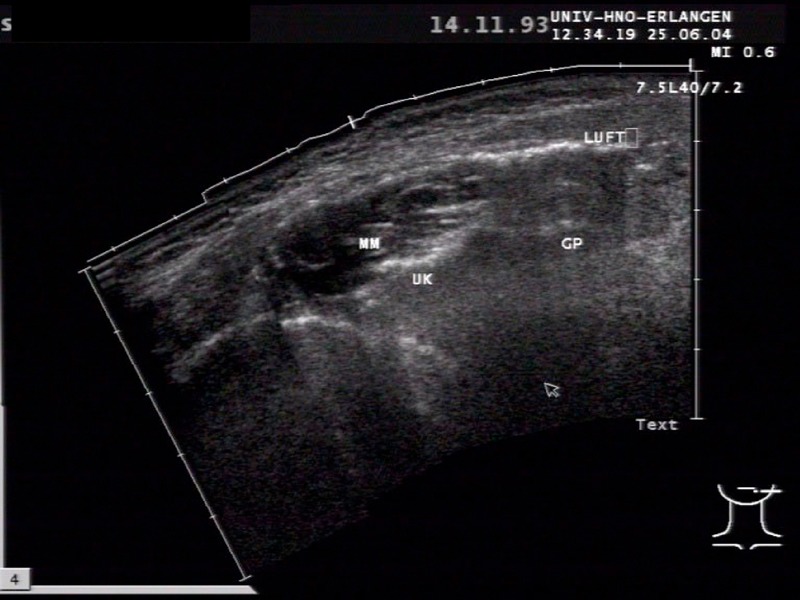
Figure 5. Sonographic imaging in an 11-year-old boy with recurrent pneumoparotitis after blowing up balloons with mobile hyperechoic reflections with acoustic shadowing (LUFT=AIR), which correspond to air inclusions in the duct system (MM: masseter muscle, UK: lower jaw, GP: parotid gland).
5.2.5. Postoperative sialadenitis
Unilateral parotid gland swelling arising immediately postoperatively (sometimes called ‘anaesthesia mumps’) usually resolves spontaneously within 48 hours [99] and can also rarely occur in children. The incidence is one patient in 1,000–3,000 surgical procedures. The underlying cause is thought to be an imbalance between secretion and obstruction [100], [101]. Dehydration and intraoperative medication, the duration of the operation and the positioning of the head are all thought to play a part [102].
5.2.6 Tuberculosis (TB) and mycobacteria other than tuberculosis (MOTT)
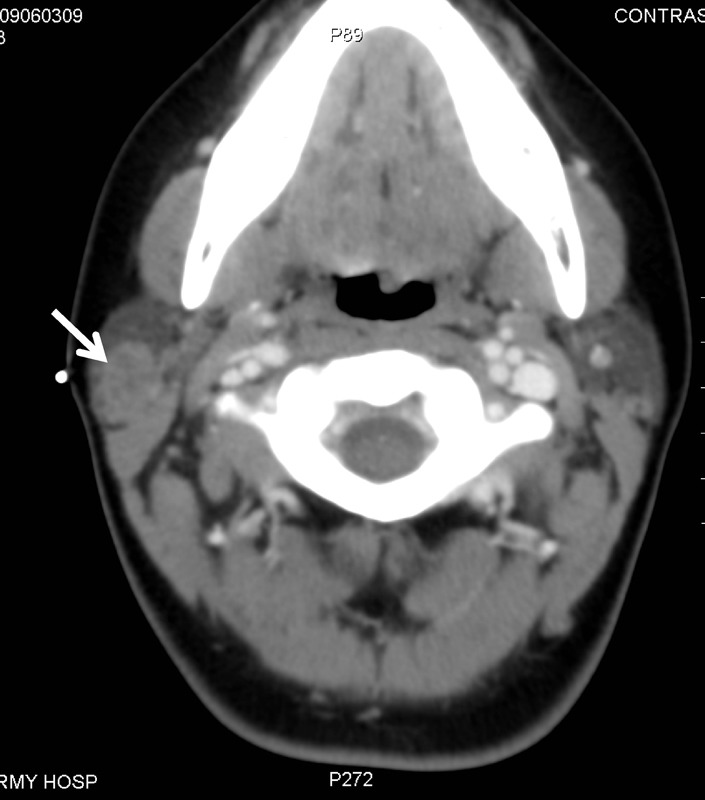
Infection with mycobacteria is particularly relevant to the parotid gland and worth mentioning once again here, since from both an anatomical and developmental point of view, this gland contains numerous lymph nodes, which correlate with disease. On the other hand, the disease may spread from the cervical lymph nodes to the salivary glands. The incidence of tuberculosis is falling in developed countries, but infections with MOTT are on the increase [103]. In children, the incidence is 7.7 per million inhabitants up to the age of 18 and as high as 23 per million inhabitants up to the age of four [104]. The children are usually otherwise healthy. Other risk groups for atypical mycobacterial infections are immunosuppressed patients [105]. The typical clinical picture is of unilateral, usually hard, enlargement of the intraglandular lymph nodes without any other signs of illness. These masses may spontaneously form fistulas and persist for months or even years. Even without any treatment, the lesions regress with time [106]. Diagnostic investigation is based on ultrasonography (Figure 6 (Fig. 6)) and the microbiological verification of the pathogenic organism (cultures, acid-fast rods seen on microscopy and PCR). The literature offers different therapeutic options: surgical excision of affected lymph nodes, pharmacotherapy or even a watch and wait policy with clinical monitoring [107]. The choice of therapy involves weighing up the extent to which the disease is affecting the patient against the possible adverse effects and complications of surgical treatment or medication.
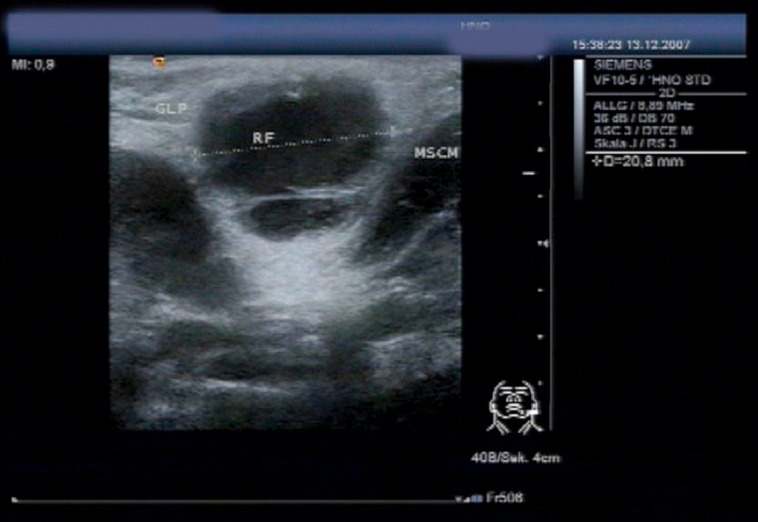
Figure 6. Ultrasound scan of an atypical mycobacterial infection in the inferior pole of the left parotid gland (RF +…+), prior to surgical excision, in a 2-year-old girl (MSCM: sternocleidomastoid muscle; GLP: parotid gland).
A prospective study showed that simple incision and drainage more often led to postoperative fistulas (91%) than complete excision (50%) [107]. In a retrospective study, disorders of wound healing were seen in 20% of patients after complete excisions and in 79% following drainage [108].
On the other hand, surgical excision has been shown to be superior to pharmacotherapy (clarithromycin and rifabutin) given for 12 weeks. The cure rates were 96% and 66%, respectively [105]. At follow-up, the aesthetic results in surgical patients were subjectively rated better [109]. Even though surgical treatment is basically superior, it can still be debated whether medication is more appropriate for lymph nodes in the parotid gland or in the region of the submandibular gland, because of the risk of facial nerve injury [106]. If a decision is made for pharmacotherapy, this should definitely take the form of a combination therapy with at least one macrolide antibiotic and rifampicin or ethambutol for a period of up to six months [110], [111].
Zeharia et al. reported on 92 patients who were merely observed [112]. After purplish discolouration, there was spontaneous rupture with a fistula for 3–8 weeks. Three-quarters of the patients had recovered completely after six months, the rest after a year at the latest. These authors did not report any complications. There are, however, no placebo-controlled trials on observation alone and patients have to put up with a fistula that persists for a relatively long time. In principle, these children also have to be regarded as infectious.
If the microbiology shows an infection with Mycobacterium tuberculosis, triple therapy with rifampicin, isoniazid and pyrazinamide for six months is indicated [113]. In these cases, it is important to check whether there is involvement of any other organs, especially of the lungs. The disease is notifiable and contact tracing should be performed to find, inform and examine all persons who have been in close contact with patients with open TB.
5.2.7 Other granulomatous and inflammatory diseases
When considering granulomatous inflammation, the differential diagnosis is important: sarcoidosis, cat-scratch disease, Kikuchi lymphadenitis and, less commonly, tularaemia and actinomycosis. In addition to demonstrating the pathogenic organism, histology is important in the diagnosis of persistent lymphadenopathy, in order to rule out benign tumours and malignant changes. Actinomycosis is due to gram-positive microaerophil bacteria, often triggered by trauma or dental treatment. The salivary glands can also be affected by the usually painless necrotising infection, which often leads to fistula formation [114]. Antibiotic therapy with Penicillin is the treatment of choice [115].
Sarcoidosis (Besnier-Boeck-Schumann’s disease) is seldom seen in childhood. Asymptomatic involvement (swelling) of the salivary glands alone does not require any treatment. The child should in any case be referred for further paediatric assessment and systemic therapy (cortisone) if necessary.
Necrotising sialometaplasia is a particular form of inflammation, which may rarely be found in children and adolescents. The World Health Organization (WHO) classifies this condition with six other lesions of the salivary glands [sialadenosis, oncocytic changes, benign lymphoepithelial lesions, salivary duct cysts (retention cyst, ranula, and dysgenetic polycystic disease), chronic sclerosing sialadenitis (Küttner’s tumour) and changes of the salivary glands with HIV infection] [116]. The disease may appear in all locations where there is salivary gland tissue and very often on the hard and soft palate. Painless ulceration, possibly caused by ischaemia following mechanical irritation, occurs in most cases and may give rise to the suspicion of malignancy [117]. In children, it may also present as a simple swelling without any necrosis [118]. Histology shows inflammation and granulation tissue, which heals spontaneously after 6–12 weeks [119].
5.2.8 Juvenile recurrent parotitis
The disease is seen clinically as an acute swelling of the affected gland, which usually affects only one side and is associated with symptoms of varying degree. The swelling appears independently of meals or season of the year. There may be reddening of the skin over the affected gland and a raised temperature or mild fever. The swelling usually persists for 24–48 hours, but may sometimes last for 1–2 weeks or rarely even months. The acute episodes (flares) appear punctuated by symptom-free intervals ranging from days to years. Hyposecretion of the gland with very viscous saliva can also be demonstrated in the intervals and, typically, flocculent secretions can be expressed at this time. Purulent secretion similar to that of acute bacterial parotitis is rare and, if seen, is found only in the acute phase.
In his review of more than 5,000 cases of chronic sialadenitis, Seifert reported chronic recurrent parotitis in 27% [120]. He distinguished between a juvenile and an adult form.
The adult form is about ten times more common than the juvenile form. It affects mainly women and is usually unilateral. Changes are seen in the distal part of the excretory duct with pronounced stenosis and dilatation.
Juvenile recurrent parotitis (JRP), which was first described in 1909 [121], occurs between the ages of 4 months and 15 years and mostly affects boys. As a rule, there is duct ectasia of the small intraparenchymal excretory ducts. Similar changes can usually also be found on the asymptomatic side. Ussmüller divided the disease into three stages on histological grounds (stage I: initial inflammatory stage; stage II: advanced inflammation with lymphatic follicles; stage III: very rare immunological end stage with complete lymphatic transformation of the gland) [122].
JRP is generally self-limiting, it goes into spontaneous remission at puberty and may even completely resolve [123]. Most patients are free of symptoms by the age of 22 [124].
The aetiology of JRP is probably multifactorial. As early as 1945, Hamilton Bailey proposed the presence of a congenital or acquired abnormal dilatation of the duct [125]. Stasis of the secretions could lead to recurrent secondary inflammation [126]. Initial inflammation is due to a reduced flow of saliva, resulting in changes to the excretory duct epithelium of the salivary gland with metaplasia, strictures, and stenosis. Metaplasia of the duct epithelium would result in a more mucous secretion. It is still not clear whether the well-documented hyposecretion, which can also be demonstrated in the contralateral clinically normal gland, is cause or effect. Other authors view the lymphocytic infiltration seen on histology to be the cause of damage to the duct walls [127]. Weakness of the surrounding connective tissue leads to the typical duct ectasia. This theory clearly explains the presence of ectasia without any signs of obstruction. The fact that changes in the ducts are also found in asymptomatic glands also supports this assumption [124], [128], [129].
In addition, genetic factors seem to contribute to the aetiology, without any specific gene or definite correlation having been found to date. Familial disease, in the sense of an autosomal dominant inheritance, has frequently been reported in the literature [18]. Wittekindt et al. published a case report relating to this, which involved identical twins with chronic recurrent parotitis in childhood [130]. Reid et al. reported a family where four members had the disease and two others had asymptomatic changes [131]. An interesting finding in JRP, as well as in Sjögren’s disease and other conditions affecting the oral mucosa, is that the levels of metalloproteinases (MMP2 and MMP9) are increased in the saliva [132], [133]. Analogous to the lymphocytic infiltration, this also ties in with the underlying cause of JRP being autoimmune disease [134], [135].
Besides the hypothesis of an allergic origin for the disease, postviral changes (e.g. mumps) and immune deficiencies (IgG3 subclass deficiency) have been suggested [136], but there is no concrete evidence for any of these.
Recent publications [137], [138] focussing intensively on the composition of saliva and on analyses of the secretion rate have concluded that the most likely pathogenesis of JRP is a congenital malformation of the excretory ducts, triggered by a bacterial infection. Albumin, IgA, lactoferrin and kallikrein in the saliva were all significantly higher than in the control group. The increase may be due to leakage in the salivary ducts even during the symptom-free intervals, which would also explain the higher concentrations of serum proteins and repeated demonstration of antibacterial substances.
Nevertheless, no acute or healed tissue damage can be seen under the light microscope. There is, therefore, no concrete evidence of active infection. On the other hand, bacteria have also been demonstrated in swabs taken between the acute episodes [124], which serve to perpetuate the disease.
The provisional diagnosis of chronic recurrent parotitis is based primarily on the history of repeated painful swelling of the gland and on the clinical examination. Even in the symptom-free interval, flocculent secretion can usually be expressed from the excretory ducts. Diagnostic imaging can then confirm the diagnosis. For a long time, sialography was the method of choice to demonstrate stenosis and strictures in adults and duct ectasia in the juvenile form. Pooling of contrast medium in the dilated interlobular portions of the ducts is consistent with sialectasis [139]. Even when the disease is clinically restricted to one side, imaging usually shows contralateral involvement. The extent of the radiologically detectable sialectasis does not correlate with the clinical symptoms [124]. However, the further development of ultrasound scanning, MRI and CT means that conventional sialography no longer has a place, at least in Germany. Apart from the radiation exposure, there is a risk of contrast medium allergy. It is also extremely difficult to perform sialography in young children, who are generally not very cooperative.
B-mode ultrasonography is a non-invasive method that is straightforward to use in children as well. It is easy for experienced ENT surgeons to recognise the typical, almost pathognomic, picture of JRP with hypoechoic and anechoic areas in the parotids, which are consistent with sialectasis and enlarged lymph nodes (Figure 7 (Fig. 7)). Ultrasound scanning is an excellent means of monitoring the condition and shows any parenchymal changes much more clearly than sialography [140]. Other possible reasons for a lymph node enlargement should, however, be considered in the differential diagnosis. A very similar picture is seen with Sjögren’s disease and sarcoidosis, but the clinical history and age of the patient are usually completely different [141].
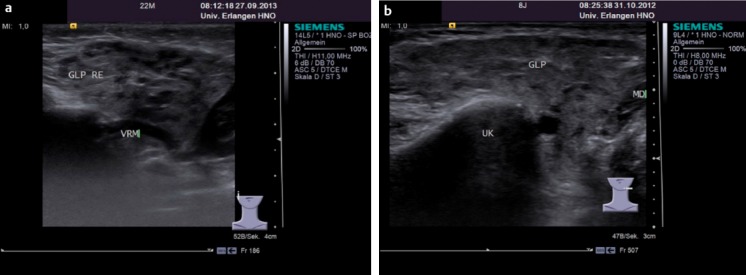
Figure 7. Typical ‘cloud-like’ appearance of the gland in the ultrasound scans of a 22-month-old girl (a) and an 8-year-old girl (b) with recurrent swelling of the right and left parotid glands, respectively (GLP: parotid gland; VRM: retromandibular vein; UK: lower jaw, MD: digastric muscle, posterior belly).
MRI and MR sialography are excellent ways of visualising salivary gland parenchyma as well as the excretory ducts. The latter can be seen without contrast enhancement in a special T2 weighting [130]. With alternative methods available, however, the time and effort required for this type of imaging in children mean that it is only worth considering for paedriatric patients in exceptional cases. CT is, in our opinion, not indicated because of the high radiation exposure and little additional benefit.
Examining affected glands with the new method of sialendoscopy shows the duct epithelium to be whitish in colour with an atrophic appearance. Blood vessels, usually clearly visible in the duct walls, are few or cannot be seen at all. The actual duct ectasia is central and therefore not accessible with the sialendoscope. This method alone is therefore not suitable for the diagnosis of JRP.
To date, there is no causal treatment for the condition. The aim of treating flares is to relieve the acute symptoms and prevent further parenchymal damage. It is expedient to give analgesics and antibiotics at this time. Amoxicillin is often used in combination with a penicillinase inhibitor, although penicillin alone, a cephalosporin or a macrolide antibiotic should suffice, since staphylococci are not usually encountered. It is debatable whether the natural course of disease is actually altered by such treatment [124], [142], [143]. Sialagogues, the local application of heat, gland massages and probing the duct may all be used additionally to promote the flow of saliva. There is no firm scientific reason to give prophylactic antibiotics to prevent acute episodes.
Radiotherapy, which was being recommended 30 years ago, is certainly obsolete these days [144]. Diamant et al. reported parotid duct ligation as a therapeutic alternative and occlusion of the duct with sodium amidotrizoate (Ethibloc®) has also been described [145], [146]. Furthermore, based on the results of animal studies, the intraductal administration of tetracycline has been recommended as a possible and effective measure [134]. However, it should be noted that tetracycline in childhood may damage the teeth and should not be given to children below the age of 14. Maier et al. irrigated the duct with aprotinin (Trasylol®), a kallikrein inhibitor, with short-term success [147]. Surgical excision of the whole gland, with all its possible complications, is seen as the last resort.
Irrigation, e.g. with normal saline or cortisone solution, has therefore also been indicated for a long time, especially in children, as in reports on sialoraphy it had been established that this diagnostic measure alone led to a clear improvement in the symptoms [72], [123], [148]. A simple instillation of normal saline or penicillin into the excretory ducts also often led to the relief of symptoms [149].
All the invasive therapeutic options have ultimately to be considered in the light of the fact that conservative measures, with a watch and wait policy, result in up to 92% of all children being free of symptoms within a follow-up period of five years [142], [143].
Based on the results of irrigation with normal saline and cortisone solution, a promising method of treating both JRP and chronic recurrent parotitis in adults has been developed in association with sialendoscopy (Figure 8 (Fig. 8)). The largest study published to date, by Shacham et al., reported on 65 children whose ducts had been irrigated and dilated with about 100 ml saline solution during sialendoscopy [150]. At the end of the procedure, the gland was irrigated with an additional 100 mg of hydrocortisone via the endoscope. One inclusion criterion for this study was at least two acute episodes within a year – so patients with little in the way of symptoms were also included. At follow-up of 6–36 months, 95% of all the patients had no symptoms after just one treatment session. Quenin et al. reported a success rate of 87% in 10 patients with 17 treated glands [151]. Out of 14 patients in Milan who underwent a single treatment session, five (36%) had recurrent symptoms within 20 months and had to be treated again [152].
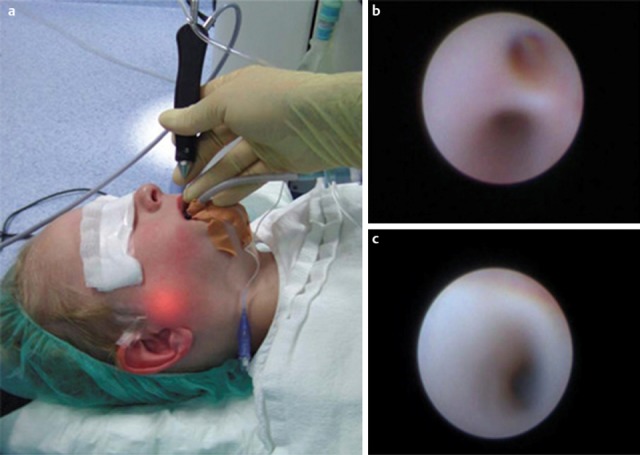
Figure 8. Endoscopy and duct irrigation with normal saline and cortisone. a) Sialendocopy of a right Stensen’s duct in a 4-year-old patient with chronic recurrent juvenile parotitis. The illuminations shows the tip of the endoscope b) Hilum and c) distal duct segment with the typical white changes of the duct wall without any vessels visible.
The first retrospective study comparing conservative management in 21 patients with JRP and sialendoscopic therapy in another 15 patients showed that there was a significant improvement in both the frequency and intensity of the gland swelling. The difference between the groups was not significant, but there was a tendency towards a more rapid relief of symptoms in the group treated with endoscopy, irrigation and cortisone [72]. Definitive results can only be expected from a prospective study.
Based on current experience and findings in the treatment of juvenile recurrent parotitis, we recommend the following treatment strategy [153]:
For mild symptoms or after a single episode: conservative symptomatic treatment, antibiotic therapy with analgesia and measures to reduce the swelling. Prophylactic antibiotics are not indicated, nor are they effective. It is worthwhile massaging the gland and giving sialagogues in the intervals.
For severe symptoms and repeated episodes of swelling: a minimally invasive procedure with sialendoscopy and duct irrigation as well as the intraductal application of prednisolone in normal physiological saline solution (100 mg/100 ml), instilling 30–40 ml in each gland. Children require a general anaesthetic.
Complete parotidectomy: as the last resort, for persistent symptoms after failure of the previously mentioned measures or with the rare stage III disease (immunological end stage with complete lymphatic transformation).
5.2.9. Juvenile Sjögren’s syndrome
Sjögren’s syndrome is an autoimmune disease characterised by progressive lymphocytic and plasmacytic infiltration, mainly of the salivary glands and lacrimal glands. According to Vitali et al. [154], there are six criteria for the diagnosis of Sjögren’s syndrome: ocular symptoms, oral symptoms, objective ocular signs (Schirmer’s test), histologically detectable lymphocytic infiltration (>1 focus of 50 lymphocytes/4 mm2), objectively verifiable findings in the salivary glands (<1.5 ml saliva/15 min, positive imaging) and autoantibodies. Sjögren’s syndrome is confirmed when at least four of these are present together with at least positive histology or serology.
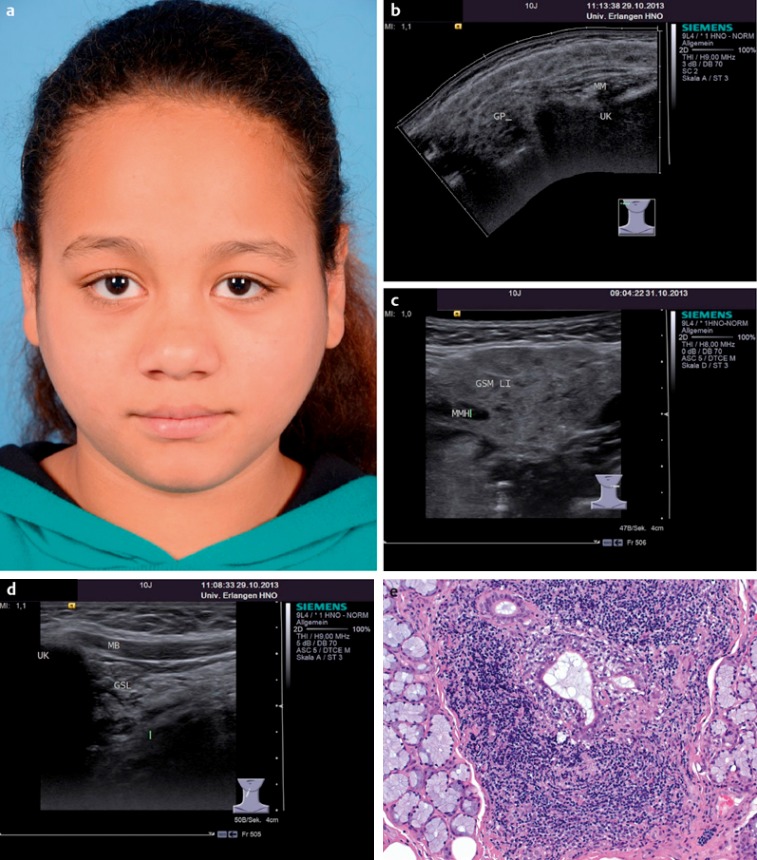
Sjögren’s syndrome occurs considerably more often in women than men (9:1); the onset is usually between the ages of 40 and 50. Juvenile Sjögren’s syndrome is rare or underdiagnosed. Children and adolescents often have predominant recurrent swelling of the parotid gland at the onset as well as during the course of disease, whilst ocular and oral symptoms are less common. Systemic extraglandular manifestations (vasculitis, cryoglobulinaemia, autoimmune hepatitis, alveolitis, neuropathy and B-cell lymphomas) are also seen less often during the course of disease [155], [156]. Sjögren’s syndrome may occasionally give rise to life-threatening situations, such as hypocalcaemic paralysis due to renal tubular acidosis. In addition, it may involve the central nervous system and liver [157], [158]. A total of 81 patients aged between four and 16 (mean 10 years of age) were reported in the literature from 2000 to 2010 [159]. Swelling of the parotid gland was found in 65%, xerostomia in 22% and ocular symptoms in 33% of the cases. Rheumatoid factor was positive in 54%, SS-A (ro) in 67%, SS-B (la) in 62% and antinuclear antibody in 72%. Nikitakis et al. gave higher figures for xerostomia (64%) and ocular symptoms (72%) in 95 patients studied in the period 1995–2002 [160]. Here, too, the cardinal symptom was enlargement of the parotid gland (74%), which showed the typical changes seen on ultrasound (Figure 9 (Fig. 9)).
Figure 9. a) Sjögren’s syndrome in a 10-year-old girl with only the symptoms of non-specific bilateral parotid swellings. Ultrasonography showed typical hypoechoic cloud-like changes in all three major salivary glands. b) Left parotid gland (GP: parotid gland; MM: masseter muscle, UK: lower jaw). c) Left submandibular gland (GSM LI: left submandibular gland; MMH: mylohyoid muscle). d) Left sublingual gland (GSL: sublingual gland; UK: lower jaw; MB: floor of the mouth). In this case, the biopsy to confirm the diagnosis was taken from the sublingual gland. e) Dense lymphocytic infiltration of the sublingual gland parenchyma with the formation of lymphoepithelial lesions (reproduced with the kind permission of Prof. Agaimy, Department of Pathology, and Erlangen, Germany).
As the ocular and oral symptoms are not as pronounced in children as in adult patients, the usual criteria have a lower sensitivity to the presence of juvenile Sjögren’s syndrome. This disease should always be considered, however, when there is recurrent swelling of the salivary glands without any other relevant symptoms [156]. Recurrent conjunctivitis also suggests Sjögren’s syndrome, as do elevated amylase levels, renal tubular acidosis, leucocytopenia, ANAs, a positive rheumatoid factor and hypergammaglobulinaemia. Taking a cut-off of >1 focus/4 mm2, the histological findings of the minor labial salivary glands are often negative. The sensitivity is greatly increased by using a score of >0 focus/4 mm2 [161]. As in adults, the biopsy of the parotid gland is of considerably more diagnostic use in children. McGuirt et al. reported on six patients: biopsy of the minor salivary glands was negative in four of them, while parotid biopsies performed in all patients were positive [162]. The treatment of juvenile Sjögren’s syndrome is largely symptomatic. As in adults, the aims are to replace the function of the affected glands (sialagogues, artificial saliva and tear substitutes) and reduce the sequelae (dental care, etc.) [163]. The management of juvenile Sjögren’s syndrome should be discussed in detail with a paediatric or general medical rheumatologist. To date, no studies on systemic therapy of juvenile Sjögren’s syndrome are to be found in the literature [161], so that recourse has to be made to the experience gained in treating adults. This is based on the administration of corticosteroids, with cyclosporin A given in severe cases [164]. Immunosuppressant therapy may control the symptoms of the disease and reduce the lymphocytic infiltration and fibrosis of the salivary glands. Studies have shown a partial success of the anti-B-cell antibody rituximab in adults, but, even though the circulating B cells are clearly suppressed, antibody-producing B cells remain in the glands. Rituximab is currently recommended for systemic involvement [165], [166]. Gene therapy approaches and stem cell transplantation are still in the animal study stage [167], [168].
6 Salivary gland tumours in children and adolescents
6.1. Ranula
Histologically speaking and according to the WHO classifiaction, ranulas and salivary gland retention cysts belong to the group of tumour-like lesions [116]. As mentioned previously, these lesions may already be present at birth. Ranulas are extravasation mucoceles, arising from either the ruptured and then blocked main excretory duct of the sublingual gland, or the numerous subsidiary ducts or the acini [169]. In so-called plunging ranulas, the accumulation of secretions extends into the soft tissues of the neck. Pre-existing gaps in the floor of the mouth or directly in the mylohyoid muscle predispose to these lesions [21], [22], [170]. Ranulas can usually be diagnosed on visual inspection and confirmed by an ultrasound scan. Conservative methods, including sclerosis (OK-432) and injection with botulinum toxin A, have been described with mixed results [171], [172], [173], [174]. Complete excision of the sublingual gland is the surgical procedure with the best results for recurrence rate, but it also carries the highest risk of complications [175]. In a retrospective study on 13 children carried out by our research group, we also found that removal of the sublingual gland was associated with the lowest rate of recurrence (<2%) [176]. On the other hand, extensive marsupialisation of a ranula is a viable alternative. The success rate is well over 85% and the gland can still be removed later if there is a recurrence.
6.2 Haemangiomas and vascular malformations
Haemangiomas are the most common vascular tumours in childhood. Following a growth phase, the vascular epithelium often undergoes spontaneous involution that may last for months or even years. Haemangiomas account for almost 60% of tumours in children, of which 80% occur in the parotid gland (Figure 10 (Fig. 10)) and 18% in the submandibular region, while 2% are associated with the minor salivary glands [114]. Vascular malformations are pathologically anomalous structures arising from an abnormal development and morphogenesis of arterial, venous and/or lymphatic vessels. Section 2 of this book takes a closer look at the classification, diagnostic investigation and treatment of haemangiomas and vascular malformations.
Figure 10. a) Unclear findings on ultrasonography of the left parotid gland of a 12-year-old girl. MRI showed a space-occupying lesion with high signal intensity in the T2 weighted scan and (b) a hypointense, inhomogeneous structure taking up contrast medium in an enhanced T1 weighted scan (c). The diagnosis of an atypical pleomorphic adenoma seemed most likely. After extracapsular dissection of the tumour (d), histology showed a cavernous haemangioma with cystic, partially thrombosed vascular clefts with residual acini in between them (e: reproduced with the kind permission of Prof. Agaimy, Department of Pathology, Erlangen, Germany).
6.3 Epithelial salivary gland tumours
On the whole, salivary glands tumours are rare in children and adolescents. Seifert and co-workers estimated the incidence to be 4% in patients up to the age of 20 [177]. They reported 80 epithelial salivary gland tumours in the register, corresponding to 2.5%. Of the 53 benign tumours (66%), 52 were pleomorphic adenomas. The 27 malignant tumours (34%) consisted of twelve mucoepidermoid carcinomas, five acinus cell carcinomas and ten showing other histology. Three of them, however, were adenoid cystic carcinomas, making this type of cancer the third most common. Cunningham et al. analysed malignant head and neck tumours in children over a period of 20 years [178]. Malignant lymphomas were present in 60% of the 241 children with cancer. Salivary gland carcinomas were very rare, accounting for only 2.5% overall. The Surveillance, Epidemiology and End Results (SEER) database also records rhabdomyosarcomas of the salivary glands in 8% of 113 cases [179]. Recent studies from China, with 119 patients, confirm the distribution of the different histological findings. Tumours were benign in 73% of the patients (n=87), with pleomorphic adenomas of the parotid gland, submandibular gland and minor salivary glands in all but three patients, [180]. Most tumours (77%) arose after the age of ten years [181]. An older review article [2] shows that the majority (85%) of tumours arise in the parotid gland with 48% of them being benign; 11.7% of tumours are found in the submandibular gland, with benign lesions accounting for two-thirds or more [179], [182]. A further 3.2% arise in the sublingual gland, all of which are benign. Conversely, the incidence of malignant lesions in adults increases from the parotid gland to the sublingual [183]. Lymphomas occur primarily in the parotid gland, representing some 2%-5% of all salivary gland neoplasms. About one-third of these lymphomas are mucosa-associated lymphoid tissue (MALT) lymphomas, another third are follicular lymphomas and the remaining third are diffuse large cell B lymphomas [184].
However, when children present with a firm solid swelling of the salivary gland, a higher suspicion of malignancy is needed than in adults. Local pain and especially facial palsy are further indications of possible malignancy. In addition to the clinical examination, ultrasonography, MRI and possibly CT are required to determine the extent of the lesion and any local metastasis. The following section takes a closer look at the most common benign and malignant tumours.
6.3.1 Benign epithelial tumours
A retrospective analysis of the Hamburg tumour register with a total of 549 surgical specimens from children showed adenomas in 47 cases (12%) [185]. 71% of these were pleomorphic adenomas, less commonly a Warthin’s tumour, oncocytoma, basal cell adenoma, inverted ductal papilloma and other rare benign tumours. The pleomorphic adenomas most commonly seen usually occur between the ages of 11 and 18. They are found in the parotid gland, the submandibular gland and also in the minor salivary glands [186]. Treatment of these benign tumours consists of complete surgical resection. Tumours in the superficial part of the parotid gland can be removed with a superficial parotidectomy (lateral lobectomy), whilst tumours in the deep lobe require complete parotidectomy [187]. There is no information on how often a superficial parotidectomy or a extracapsular dissection is performed at this age, although, on the basis of the currently available data, such a procedure is, in principle, also considered possible, depending on the site of the tumour [188]. Surgery must aim for a complete resection of the tumour, without any residual disease, whilst preserving the facial nerve [183]. Submandibular gland resection is indicated for tumours in the submandibular gland, with complete local resection for the small salivary glands. As in adults, recurrences may occur if any tumour has been left behind and carcinomas may develop in longstanding pleomorphic adenomas.
6.3.2. Malignant epithelial tumours of the salivary glands
As mentioned previously, malignant epithelial salivary gland tumours are rare, mostly presenting around the age of 14 [189]. Girls seem to be affected more often than boys. The site tends to be the parotid gland more often than the submandibular gland, the sublingual gland and the minor salivary glands [186]. In principle, all tumours of the recognised WHO classification can, just as in adults, occur histologically. Low-grade or intermediate-grade mucoepidermoid carcinomas are the malignant tumours most frequently described in childhood [190], followed by acinus cell carcinomas (Figure 11 (Fig. 11)) and adenoid cystic carcinomas. These three types of cancer account for 80–90% of malignant salivary gland tumours in children and adolescents. Adenocarcinomas, basal cell carcinomas and squamous cell carcinomas of the salivary glands are less common.
Figure 11. A 12-year-old boy with a mobile, slowly growing space-occupying lesion in the right parotid gland (arrow). After parotidectomy, histology showed an acinus cell carcinoma (image kindly supplied by Prof. M.B. Gillespie, University of South Carolina, Charleston, USA).
The treatment of choice is surgical removal of the tumour with a superficial or total parotidectomy, combined if necessary with neck dissection [114]. As for adenomas, total parotidectomy is preferred for tumours in the deep lobe [191]. But it is well known that the extent of the resection impacts the recurrence rate. A study from the Mayo Clinic found the recurrence rate of mucoepidermoid carcinomas to be 48% after simple enucleation, 31% after lateral parotidectomy and 0% after total parotidectomy [192]. In view of these results, it can certainly be argued that total parotidectomy should be performed for all parotid gland malignancies in childhood.
When the facial nerve has been infiltrated by tumour, it must also be resected to obtain an R0 resection and appropriate rehabilitation measures carried out [193]. Removal of the gland, with or without neck dissection, is indicated for cancer of the submandibular or sublingual gland. Malignancy of the minor salivary glands requires complete resection (clinically extending into healthy tissue). Neck dissection in children with salivary gland cancer can be considered in the same way as for adults. Such a dissection is indicated whenever abnormal lymph nodes are picked up on clinical examination or imaging. Elective neck dissection should be carried out whenever there are undifferentiated or large tumours (T3–4) and in certain individual cases [32], [194]. Given the 36% incidence of cervical node metastasis (N+) [191], with predominantly poorly differentiated tumours, it is our opinion that neck dissection can be considered as a basic elective procedure.
Postoperative radiotherapy for children is, of course, indicated in selected cases only (R1 resection, perineural invasion, advanced tumour stage, and poorly differentiated tumour) [195]. Nevertheless, it must be remembered that radiotherapy in children may affect growth of the skull bones and even cause osteoradionecrosis, trismus, dental problems, visual disorders and, in particular, radiation-induced second malignancies [191]. The indication for chemotherapy is found mainly in palliative situations or following recurrence, especially when surgery and radiotherapy are no longer possible [196].
Kupferman et al. carefully analysed the risk factors and the prognosis of childhood epithelial salivary gland cancer in a large population of 61 patients over a long follow-up period (median: 114 months) [191]. They found that 72% of the cases had T1 or T2 tumours and only 7% were classed as T4. Metastasis to the cervical lymph nodes (N+) was present in 36% of the cases. The five- and ten-year overall survival rates were 93% and 84%, respectively, with figures of 85% and 80% for disease-free survival. Seven patients died, five of them from the primary cancer. Univariate analysis of their data showed that patients aged more than 14 years, of non-Caucasian descent, with undifferentiated tumours, and with perineural invasion had a significantly worse disease-specific survival rate. The age, histology, ethnic origin and positive cervical lymph nodes were statistically related to poorer overall survival. Mucoepidermoid carcinomas have a relatively favourable prognosis, even when they arise as second malignancies (e.g. following treatment of lymphoma) with a 5-year survival rate of almost 94% or even better [197], [198]. A similarly good outcome, with a disease-specific survival of 88.4%, is also achieved with cancer of the minor salivary glands, if small, well-differentiated tumours are completely resected [199]. On the other hand, the survival rate falls with the corresponding risk factors, e.g. to 30–50% with undifferentiated tumours. Just like adult patients with such cancer, these children have to be followed up long-term over 10–20 years [200].
7 Systemic causes of salivary gland swelling
Systemic diseases, whose pathogenesis is not primarily related to salivary gland disease, may also cause swelling of the salivary glands in children. Today, many of these diseases can be treated successfully, so that changes in the salivary glands may not be seen so often. These diseases include hormone disorders such as hypothyroidism, diabetes mellitus and disorders of the pituitary gland [201]. The submandibular gland in particular may be enlarged in cystic fibrosis [202]. Nutritional disorders (obesity, malnutrition, hyperlipoproteinemia and vitamin deficiencies) may cause salivary gland enlargement. Bulimia or anorexia must also be considered, particularly in adolescents with bilateral parotid swellings. The actual pathological mechanism for the swelling is not known. Ultrasound scans show homogeneously enlarged glands similar to those seen with sialadenosis [203]. Medications responsible for drug-induced enlargement of salivary glands are not often prescribed for children.
8 Sialorrhoea
Sialorrhoea (excessive salivation or drooling) impacts strongly on the mental state of the patient and is therefore a medical condition that needs to be clarified and treated. Sialorrhoea carries a social stigma, due to the impossibility of holding the saliva in the mouth and is usually found in association with an underlying neurological disease. In the long term, drooling leads to a considerable reduction in the quality of life of the affected children and their families.
The indication for treatment depends on the quantity of saliva produced and the individual situation of the child or adolescent. Patients with mild sialorrhoea, but of normal intelligence and perhaps with a slight speech impediment, feel socially incompetent and rejected. At the other end of the scale are children with physical and learning disabilities, who have profuse sialorrhoea. They wear 10–15 bibs a day to catch the saliva, need frequent changes of clothes, and often need to be looked after in care facilities. These patients dribble all the time, and over everything. Consequently, they are often socially isolated and receive less loving care and attention.
The complexity of sialorrhoea and the many possibilities for treatment make interdisciplinary management essential. A team consisting of an ENT surgeon, dentist and orthodontist as well as a maxillofacial surgeon, neurologist or paediatric neurologist and speech-language therapist should agree on the best possible treatment. Together, they must consider whether spontaneous improvement is likely and, if not, they must decide which therapeutic modality to use, in particular, whether surgery is required [204], [205].
Increased salivation, in the sense of sialorrhoea, is physiological up to the age of four years. Excessive salivation in children over the age of four, who are awake, must, however, be considered pathological.
The most common reason for drooling is a neurological disorder, with an incidence of 10% to 30% [206]. Peripheral disorders of the trigeminal or facial nerves can also lead to drooling. With neurological disorders, difficulty in swallowing is a much more prominent feature than the hypersecretion of saliva. A lack of lip closure is a prime feature [207]. Centrally regulated sialorrhoea may be caused by raised intraluminal oesophageal pressure, but is far more often due to interruption of the first (oral) phase of swallowing. Uncoordinated movements of the tongue impede the transport of saliva from the mouth to the oropharynx. Sialorrhoea has been studied particularly well in children with Down’s syndrome. General muscular hypotonia and relative macroglossia with a wide mouth opening have a deciding aetiological role [205].
Drug-induced hypersalivation, especially due to neuroleptic agents, can be distinguished from sialorrhoea of neurological origin. Alpha-adrenergic and muscarinic receptors in particular are involved in the pathophysiology of neuroleptic-induced salivation [208].
Idiopathic hypersalivation is another form of sialorrhoea of unknown origin. A study by Johnson et al. showed that children who are developing normally may also show delay in saliva control. This problem is usually self-limiting without any treatment, as the child continues to develop [209].
Besides the obvious causes, important cofactors impacting on sialorrhoea include the current emotional status, ability to concentrate, disorders of occlusion, tooth damage and posture. Dental or gingival damage naturally increases salivation, whilst inclining the head forward obviously encourages saliva to flow out of the mouth under the force of gravity.
In addition, patients with difficulty in nasal breathing and wide-open mouths tend to salivate more. Treatment with anticonvulsants also promotes hypersalivation [205].
Gastro-oesophageal reflux may be a problem in children and it has been suggested that reflux may induce hyperstimulation of the salivary glands with increased saliva production [204]. Even though an effect on drooling has not yet been confirmed for anti-reflux therapy, such treatment has led to considerable improvement in a few cases [210].
A rough estimate of the severity of the sialorrhoea is the minimum required to assess and compare different therapeutic approaches and outcomes. The problem with sialorrhoea is that it undergoes extreme temporal variation. Blasco et al. divided the various methods for quantifying the degree of drooling into three categories [210]:
Basic clinical estimation (e.g. the number of bibs or T-shirts required)
Drooling severity scales and frequency based on systematically timed observations [211].
Volumetric measurement of saliva for absolute quantification of the saliva flow using special external or intraoral collection devices [212], [213]
However, none of these different methods can replace the long-term observations of the parents or carers. The best semiquantitative measure is the number of bibs or T-shirts required during the course of a ‘typical day’, a ‘bad day’ or a ‘good day’.
Interestingly, numerous surgical procedures have been reported in the literature of English-speaking countries, while conservative treatment is preferred in German and European sources. The problems of drooling also seem to be perceived differently in different societies.
Before beginning any specific treatment of sialorrhoea, the potentially exacerbating factors first need to be corrected. Dealing with tooth decay, resecting hypertrophic tonsils or correcting malocclusion often leads to a distinct improvement in the symptoms. The treatment of sialorrhoea always requires interdisciplinary cooperation between dentist, ENT surgeon, orthodontist, maxillofacial surgeon and neurologist or paediatric neurologist.
8.1 Conservative therapeutic approaches
Behavioural modification and biofeedback therapy
These therapeutic methods aim to trigger swallowing in response to an auditory signal [214]. In a study on EMG biofeedback, a significant reduction in salivary flow with a simultaneous slightly increased rate of swallowing could be achieved. The success of the method depends on improved coordination during the oral phase of swallowing, but it is very time consuming. Nevertheless, Rapp reported a sustained benefit in children with a mental age of between 18 months and 8 years [215].
Oral-motor therapy and orofacial regulation therapy
In 1978, McCracken reported on the treatment of three cases using a sensorimotor technique. Vibration was applied to the masseter as well as to the anterior belly of the digastric muscle and the lip area for two to three minutes [216]. Harris and Dignam also found this therapeutic technique to be successful [213]. However, the available reports on this method are ultimately concerned with small patient groups only. In Germany and South America, the literature focuses on the Castillo-Morales concept of orofacial regulation therapy [217], [218], [219], [220]. This method essentially involves the application of acrylic plates in the region of the palate and oral vestibule of the upper and lower lips, which function as active components stimulating the intra- and circumoral muscle, in combination with oral and facial physiotherapy. This treatment method can be started in infants as young as six weeks old. Its effects have been monitored particularly well in several studies on patients with Down’s syndrome. Here, improvement was seen in the symptoms of hypotonia with the resulting optimisation of swallowing and expression [218]. Furthermore, it reduced tongue protrusion with improved lip closure. Similar observations have also been made in children with central motor injury. An improvement in the spontaneous position of the tongue, coordination of tongue movements, eating, speech development,and drooling was seen in almost half of the cases (n=71) [218], [220].
In summary, it can be said that although orofacial therapy at least reduces drooling, it can rarely return salivation to normal [205]. Treatment should always start before the age of four, otherwise the palatal stimulating plate is not tolerated and there is no guarantee of success. It is not known whether the stimulating plate is better used alone or with the additional physiotherapy [204].
Speech therapy
Even though experimental attempts have been made to reduce drooling with speech therapy, it seems to be too time-consuming and is only successful with continuous application [216].
8.2. Medication for sialorrhoea
The basis of many pharmacotherapeutic approaches to drooling is an anticholinergic effect. One of the oldest and best-known active substances is scopolamine, a cholinergic muscarinic receptor-antagonist, which causes a significant reduction in salivation, even in comparison with atropine [221]. Giving medication to children and adolescents has to be considered very carefully and is, at most, indicated in exceptional cases for a short time only. The best-known method of administration is the Scopoderm patch (Scopoderm TTS®), which can be affixed directly to the skin behind the ear, after the area has been cleansed with 70% alcohol. The patch can be left in situ for up to four days after which time it is replaced with a new patch. The onset of action is about 15 minutes after applying the patch [222]. Very good results have been achieved with Scopoderm patches in adults. Adverse effects, such as local allergic reactions, acute psychotic and confusional states, dry mouth, constipation, urinary retention, reduced sweating and flushing, may occur. Hypertension and glaucoma are relative contraindications [205].
An important recent addition is the use of botulinum toxin A to inhibit salivary gland secretion. Botulinum toxin inhibits the reuptake of the neurotransmitter acetylcholine from the synaptic cleft, thereby blocking signal transmission and reducing saliva production; this, in turn, leads to a considerable reduction of drooling [223], [224], [225], [226], [227], [228], [229]. However, it must be remembered that treating hypersalivation with botulinum toxin is currently off-label use, i.e. using an approved medicinal product for an indication not included in its marketing authorisation. In terms of prescribing freedom, however, the medicinal product may also be used for a non-approved indication when this is justified and medically necessary. On the basis of existing studies, its use in the salivary glands falls into this category. According to Ellies et al., the best way is to inject the toxin, under ultrasound guidance, into both parotid glands (21 units of Botox® each side) and both submandibular glands (10 units of Botox® each side). Improvement can already be seen three days after the injection [224], [225], [226]. Bothwell et al. used botulinum toxin A in a population of children with sialorrhoea associated with various neurological diseases. They injected 5 units of botulinum toxin into the parotid gland. All patients had a reduced incidence of hypersalivation after four weeks. Eight of the nine patients also showed a reduction in the objectively measured salivary flow; 55% of the parents were of the opinion that the treatment was successful. The reported adverse reactions included difficulty chewing, dry mouth, and transient weakness of jaw closing – the last probably due to inaccurate injection into the masseter muscle [223]. The possibility of transient facial paresis should also be mentioned, although this has not been described in the literature with regard to the use of botulinum toxin. The effects of botulinum toxin A last 8–16 weeks and then the injections have to be repeated. Compared with anticholinergic medication (see above) the success rate is about the same.
8.3 Surgical treatment of sialorrhoea
Despite conservative methods as well as the use of medication and even acupuncture, surgical procedures may be indicated for drooling because of adverse drug reactions or treatment failure [230], [231], [232].
These operations essentially aim to reduce saliva production, change the outflow of saliva or involve a combination of the two. Transtympanic neurectomy of the tympanic nerve [233] often leads to recurrence and especially to a loss of taste sensation or possibly even to xerostomia [234]. For this reason, it is nowadays no longer performed. Distortion or even loss of the sense of taste is particularly serious for children and those with mental impairment, as oral sensations are often the only pleasures in their lives.
Reduction in saliva production can obviously be achieved by resecting the gland. Here, the simplest solution for pronounced drooling is certainly to remove both submandibular glands (Figure 12 (Fig. 12)).
Figure 12. Boy with persistent drooling despite four sessions of botox therapy in both parotid and submandibular glands and rerouting the submandibular ducts into the tonsillar fossa (a). Clear improvement was seen only after bilateral submandibular gland resection (b).
Simple ligation of the parotid and/or submandibular ducts have been described [235]. Instead of using ligatures, one research group occluded the parotid duct using Nd:YAG laser impulses, resulting in stenosis. With this procedure, they achieved symptomatic improvement in more than 90% of the 48 patients [236]. However, a manipulation of the parotid duct on its own is somewhat disputed in the literature, as the parotid gland produces only a small proportion of the saliva at rest, even though it is of greater importance when secretion is stimulated [205]. Other surgical techniques consist of rerouting the excretory ducts. For this, the parotid ducts and possibly also the submandibular ducts are dissected out from their usual anatomical positions and reimplanted in the tonsillar fossa. In 1967, Wilkie was the first to propose the procedure, which was subsequently named after him. The parotid ducts were implanted in the tonsillar fossae, in order to trigger a direct swallowing reflex on salivation [237]. Wilkie and Brody modified the procedure in 1977, by removing the submandibular glands during the same operation. Tonsillectomy is also carried out as part of the procedure, in order to prevent stenosis and recurrent infection of the excretory ducts or salivary glands [238], [239]. Even the rerouting of the parotid duct with autologous venous interposition grafts has been tried as a variation of Wilkie’s procedure [240]. Rerouting of the submandibular duct into the tonsillar fossa has also been described, a procedure that is associated with the risk of a ranula developing [241], [242], [243].
One obvious disadvantage of these operations is that the patient has to be admitted to hospital. In addition, there is a risk of a branchial cyst developing, as well as severe caries [243].
Rerouting the excretory duct into the tonsillar fossa is contraindicated in patients being aspirated. Figures from 67% to more than 90% have been reported for the success rates of the surgical procedures.
9 Parotidectomy in children: indications and complications
Overall, parotidectomy is very rarely indicated in children. In their analysis of 22 parotidectomies (six total), Xie and Kubba [244] demonstrated the different indications very well. Congenital abnormalities, atypical mycobacterial infections and tumours were each responsible for about one third of the patients requiring surgery. This is very different from the reasons seen in adults [32], [245]. Typical complications, such as transient or persistent facial palsy (Figure 13 (Fig. 13)) and Frey’s syndrome, occur about as frequently as in adult patients. We recommend the use of facial nerve monitoring and nerve stimulation during surgery in children. It is important to remember that the facial nerve may lie much more laterally in children than in adults, as the mastoid cells are not yet fully developed. In proportion to the surrounding structures, the nerve is even slightly larger in young children [2], [246]. There are no contraindications to this procedure. Transient facial weakness has been reported in 36% and persistent palsy in 11% of lateral or total parotidectomies [191]. Other authors give figures of 23% to 50% for transient paresis and complete recovery with time. The incidence of short-term facial nerve weakness is higher with total than with superficial parotidectomy (25% vs 16%) [32]. A mild Frey’s syndrome that did not require treatment was found in 19% of children following lateral parotidectomy [183], [244]. Hypertrophic scarring requiring treatment (14%) seems to be more common in children than in adults. Treatment is usually intralesional injection of steroids. One study found the recurrence rate of pleomorphic adenomas in children to be 20% [2]. The authors attributed this to the smaller anatomical structures, which make capsular rupture and tumour seeding more likely. In contrast, Malone and Baker [247] found no recurrence in their patients after up to 20 years’ follow-up.
Figure 13. Permanent facial palsy (House-Brackman VI) after resection of a fistula in the right parotid gland, in a one-year-old patient operated on elsewhere. Revision surgery was performed with nerve interposition grafting.

In principle, surgeons must be aware of the special features of parotidectomy in childhood that have been mentioned and discuss them in detail with the parents as well as with the children, if they are older. The surgeon should already have extensive experience in parotid gland surgery.
Notes
Competing interests
The authors declare that they have no competing interests.
References
- 1.Zenk J, Zikarsky B, Hosemann WG, Iro H. Die Durchmesser des Stenon- und Wharton-Ganges. Bedeutung für Diagnostik und Therapie. [The diameter of the Stenon and Wharton ducts. Significance for diagnosis and therapy]. HNO. 1998 Dec;46(12):980–985. doi: 10.1007/s001060050345. (Ger). Available from: http://dx.doi.org/10.1007/s001060050345. [DOI] [PubMed] [Google Scholar]
- 2.Shikhani AH, Johns ME. Tumors of the major salivary glands in children. Head Neck Surg. 1988 Mar-Apr;10(4):257–263. doi: 10.1002/j.1930-2398.1988.tb00009.x. [DOI] [PubMed] [Google Scholar]
- 3.Swilley S, Strickland DK, Davila R, Levstik M, Ribeiro R, Hudson MM. Hepatitis C infection during treatment for childhood cancer: pitfalls in diagnosis and management. Med Pediatr Oncol. 2002 Jul;39(1):58–59. doi: 10.1002/mpo.10051. Available from: http://dx.doi.org/10.1002/mpo.10051. [DOI] [PubMed] [Google Scholar]
- 4.Seifert G, Miehlke A, Haubrich J, Chilla R. Speicheldrüsenkrankheiten. Pathologie, Klinik, Therapie, Fazialischirurgie. Stuttgart: Thieme; 1984. pp. 91–97. [Google Scholar]
- 5.Holmes S. Xerostomia: aetiology and management in cancer patients. Support Care Cancer. 1998 Jul;6(4):348–355. doi: 10.1007/s005200050176. Available from: http://dx.doi.org/10.1007/s005200050176. [DOI] [PubMed] [Google Scholar]
- 6.Hidas A, Noy AF, Birman N, Shapira J, Matot I, Steinberg D, Moskovitz M. Oral health status, salivary flow rate and salivary quality in children, adolescents and young adults with ADHD. Arch Oral Biol. 2011 Oct;56(10):1137–1141. doi: 10.1016/j.archoralbio.2011.03.018. Available from: http://dx.doi.org/10.1016/j.archoralbio.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Bohlender J, Rauh M, Zenk J, Gröschl M. Differential distribution and expression of leptin and the functional leptin receptor in major salivary glands of humans. J Endocrinol. 2003 Aug;178(2):217–223. doi: 10.1677/joe.0.1780217. Available from: http://dx.doi.org/10.1677/joe.0.1780217. [DOI] [PubMed] [Google Scholar]
- 8.Sonesson M. On minor salivary gland secretion in children, adolescents and adults. Swed Dent J Suppl. 2011;(215):9–64. [PubMed] [Google Scholar]
- 9.Bhalla S, Tandon S, Satyamoorthy K. Salivary proteins and early childhood caries: A gel electrophoretic analysis. Contemp Clin Dent. 2010 Jan;1(1):17–22. doi: 10.4103/0976-237X.62515. Available from: http://dx.doi.org/10.4103/0976-237X.62515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sag C, Ozden FO, Acikgoz G, Anlar FY. The effects of combination treatment with a long-acting beta2-agonist and a corticosteroid on salivary flow rate, secretory immunoglobulin A, and oral health in children and adolescents with moderate asthma: a 1-month, single-blind clinical study. Clin Ther. 2007 Oct;29(10):2236–2242. doi: 10.1016/j.clinthera.2007.10.014. Available from: http://dx.doi.org/10.1016/j.clinthera.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 11.Psoter WJ, Spielman AL, Gebrian B, St Jean R, Katz RV. Effect of childhood malnutrition on salivary flow and pH. Arch Oral Biol. 2008 Mar;53(3):231–237. doi: 10.1016/j.archoralbio.2007.09.007. Available from: http://dx.doi.org/10.1016/j.archoralbio.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avsar A, Elli M, Darka O, Pinarli G. Long-term effects of chemotherapy on caries formation, dental development, and salivary factors in childhood cancer survivors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007 Dec;104(6):781–789. doi: 10.1016/j.tripleo.2007.02.029. Available from: http://dx.doi.org/10.1016/j.tripleo.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 13.Østerhus IN, Skogedal N, Akre H, Johnsen UL, Nordgarden H, Åsten P. Salivary gland pathology as a new finding in Treacher Collins syndrome. Am J Med Genet A. 2012 Jun;158A(6):1320–1325. doi: 10.1002/ajmg.a.35331. Available from: http://dx.doi.org/10.1002/ajmg.a.35331. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda C, Matsui Y, Ohno K, Michi K. Salivary gland aplasia with cleft lip and palate: a case report and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999 May;87(5):594–599. doi: 10.1016/S1079-2104(99)70140-X. Available from: http://dx.doi.org/10.1016/S1079-2104(99)70140-X. [DOI] [PubMed] [Google Scholar]
- 15.Gruber W. Congenitaler Mangel beider Glandulae submaxillares bei einem wohlgebildeten, erwachsenen Subjecte. Arch Pathol Anat Physiol Klin Med. 1885;102(1):9–11. doi: 10.1007/BF01932760. Available from: http://dx.doi.org/10.1007/BF01932760. [DOI] [Google Scholar]
- 16.Taji SS, Savage N, Holcombe T, Khan F, Seow WK. Congenital aplasia of the major salivary glands: literature review and case report. Pediatr Dent. 2011 Mar-Apr;33(2):113–118. [PubMed] [Google Scholar]
- 17.Ferreira AP, Gomez RS, Castro WH, Calixto NS, Silva RA, Aguiar MJ. Congenital absence of lacrimal puncta and salivary glands: report of a Brazilian family and review. Am J Med Genet. 2000 Sep 4;94(1):32–34. doi: 10.1002/1096-8628(20000904)94:1<32::aid-ajmg7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 18.Wiedemann HR. Salivary gland disorders and heredity. Am J Med Genet. 1997 Jan 20;68(2):222–224. doi: 10.1002/(sici)1096-8628(19970120)68:2<222::aid-ajmg20>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 19.Seifert G, Thomsen S, Donath K. Bilateral dysgenetic polycystic parotid glands. Morphological analysis and differential diagnosis of a rare disease of the salivary glands. Virchows Arch A Pathol Anat Histol. 1981;390(3):273–288. doi: 10.1007/BF00496559. Available from: http://dx.doi.org/10.1007/BF00496559. [DOI] [PubMed] [Google Scholar]
- 20.Ritvos O, Tuuri T, Erämaa M, Sainio K, Hildén K, Saxén L, Gilbert SF. Activin disrupts epithelial branching morphogenesis in developing glandular organs of the mouse. Mech Dev. 1995 Apr;50(2-3):229–245. doi: 10.1016/0925-4773(94)00342-K. Available from: http://dx.doi.org/10.1016/0925-4773(94)00342-K. [DOI] [PubMed] [Google Scholar]
- 21.Morton RP, Ahmad Z, Jain P. Plunging ranula: congenital or acquired? Otolaryngol Head Neck Surg. 2010 Jan;142(1):104–107. doi: 10.1016/j.otohns.2009.10.014. Available from: http://dx.doi.org/10.1016/j.otohns.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Mahadevan M, Vasan N. Management of pediatric plunging ranula. Int J Pediatr Otorhinolaryngol. 2006 Jun;70(6):1049–1054. doi: 10.1016/j.ijporl.2005.10.022. Available from: http://dx.doi.org/10.1016/j.ijporl.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 23.Hopp E, Mortensen B, Kolbenstvedt A. Mylohyoid herniation of the sublingual gland diagnosed by magnetic resonance imaging. Dentomaxillofac Radiol. 2004 Sep;33(5):351–353. doi: 10.1259/dmfr/31454077. Available from: http://dx.doi.org/10.1259/dmfr/31454077. [DOI] [PubMed] [Google Scholar]
- 24.Chan DF, Lee CH, Fung TY, Chan DL, Abdullah V, Ng PC. Ex utero intrapartum treatment (EXIT) for congenital giant ranula. Acta Paediatr. 2006 Oct;95(10):1303–1305. doi: 10.1080/08035250600580545. Available from: http://dx.doi.org/10.1080/08035250600580545. [DOI] [PubMed] [Google Scholar]
- 25.Harris M, Blewitt RW, Davies VJ, Steward WP. High-grade non-Hodgkin’s lymphoma complicating polypoid nodular lymphoid hyperplasia and multiple lymphomatous polyposis of the intestine. Histopathology. 1989 Oct;15(4):339–350. doi: 10.1111/j.1365-2559.1989.tb01586.x. Available from: http://dx.doi.org/10.1111/j.1365-2559.1989.tb01586.x. [DOI] [PubMed] [Google Scholar]
- 26.Taylor GP. Congenital epithelial tumor of the parotid-sialoblastoma. Pediatr Pathol. 1988;8(4):447–452. doi: 10.3109/15513818809041583. Available from: http://dx.doi.org/10.3109/15513818809041583. [DOI] [PubMed] [Google Scholar]
- 27.Vawter GF, Tefft M. Congenital tumors of the parotid gland. Arch Pathol. 1966 Sep;82(3):242–245. [PubMed] [Google Scholar]
- 28.Prigent M, Teissier N, Peuchmaur M, El Maleh-Berges M, Philippe-Chomette P, Cardin P, Orbach D. Sialoblastoma of salivary glands in children: chemotherapy should be discussed as an alternative to mutilating surgery. Int J Pediatr Otorhinolaryngol. 2010 Aug;74(8):942–945. doi: 10.1016/j.ijporl.2010.01.026. Available from: http://dx.doi.org/10.1016/j.ijporl.2010.01.026. [DOI] [PubMed] [Google Scholar]
- 29.Williams SB, Ellis GL, Warnock GR. Sialoblastoma: a clinicopathologic and immunohistochemical study of 7 cases. Ann Diagn Pathol. 2006 Dec;10(6):320–326. doi: 10.1016/j.anndiagpath.2006.02.008. Available from: http://dx.doi.org/10.1016/j.anndiagpath.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Scott JX, Krishnan S, Bourne AJ, Williams MP, Agzarian M, Revesz T. Treatment of metastatic sialoblastoma with chemotherapy and surgery. Pediatr Blood Cancer. 2008 Jan;50(1):134–137. doi: 10.1002/pbc.20788. Available from: http://dx.doi.org/10.1002/pbc.20788. [DOI] [PubMed] [Google Scholar]
- 31.Bergeron C, Thiesse P, Rey A, Orbach D, Boutard P, Thomas C, Schmitt C, Scopinaro MJ, Bernard F, Stevens M, Oberlin O. Revisiting the role of doxorubicin in the treatment of rhabdomyosarcoma: an up-front window study in newly diagnosed children with high-risk metastatic disease. Eur J Cancer. 2008 Feb;44(3):427–431. doi: 10.1016/j.ejca.2007.12.007. Available from: http://dx.doi.org/10.1016/j.ejca.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Orvidas LJ, Kasperbauer JL, Lewis JE, Olsen KD, Lesnick TG. Pediatric parotid masses. Arch Otolaryngol Head Neck Surg. 2000 Feb;126(2):177–184. doi: 10.1001/archotol.126.2.177. Available from: http://dx.doi.org/10.1001/archotol.126.2.177. [DOI] [PubMed] [Google Scholar]
- 33.Ellies M, Laskawi R. Diseases of the salivary glands in infants and adolescents. Head Face Med. 2010;6:1. doi: 10.1186/1746-160X-6-1. Available from: http://dx.doi.org/10.1186/1746-160X-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weidauer H. AIDS in der Hals-Nasen-Ohrenheilkunde. [AIDS in otorhinolaryngology]. Laryngorhinootologie. 2000 Nov;79(11):662–663. doi: 10.1055/s-2000-8277. (Ger). Available from: http://dx.doi.org/10.1055/s-2000-8277. [DOI] [PubMed] [Google Scholar]
- 35.Webster RG, Granoff A, Rima BK, editors. Encyclopedia of Virology. Vol 2. New York: Academic Press; 1994. Mumps virus; pp. 876–883. [Google Scholar]
- 36.Mühlemann K. The molecular epidemiology of mumps virus. Infect Genet Evol. 2004 Sep;4(3):215–219. doi: 10.1016/j.meegid.2004.02.003. Available from: http://dx.doi.org/10.1016/j.meegid.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Richardson M, Elliman D, Maguire H, Simpson J, Nicoll A. Evidence base of incubation periods, periods of infectiousness and exclusion policies for the control of communicable diseases in schools and preschools. Pediatr Infect Dis J. 2001 Apr;20(4):380–391. doi: 10.1097/00006454-200104000-00004. Available from: http://dx.doi.org/10.1097/00006454-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 38.Hviid A, Rubin S, Mühlemann K. Mumps. Lancet. 2008 Mar 15;371(9616):932–944. doi: 10.1016/S0140-6736(08)60419-5. Available from: http://dx.doi.org/10.1016/S0140-6736(08)60419-5. [DOI] [PubMed] [Google Scholar]
- 39.Travis LW, Hecht DW. Acute and chronic inflammatory diseases of the salivary glands: diagnosis and management. Otolaryngol Clin North Am. 1977 Jun;10(2):329–338. [PubMed] [Google Scholar]
- 40.Ishida M, Fushiki H, Morijiri M, Maruyama M, Motoshima H, Asai M, Watanabe Y. Mumps virus infection in adults: three cases of supraglottic edema. Laryngoscope. 2006 Dec;116(12):2221–2223. doi: 10.1097/01.mlg.0000246189.38777.2c. Available from: http://dx.doi.org/10.1097/01.mlg.0000246189.38777.2c. [DOI] [PubMed] [Google Scholar]
- 41.Bertschat FL, Alexander M. Infertilität nach Mumpsorchitis. [Infertility after mumps orchitis (author's transl)]. MMW Munch Med Wochenschr. 1981 Apr;123(15):606–608. (Ger). [PubMed] [Google Scholar]
- 42.Koskiniemi M, Donner M, Pettay O. Clinical appearance and outcome in mumps encephalitis in children. Acta Paediatr Scand. 1983 Jul;72(4):603–609. doi: 10.1111/j.1651-2227.1983.tb09778.x. Available from: http://dx.doi.org/10.1111/j.1651-2227.1983.tb09778.x. [DOI] [PubMed] [Google Scholar]
- 43.Vuori M, Lahikainen EA, Peltonen T. Perceptive deafness in connectionwith mumps. A study of 298 servicemen suffering from mumps. Acta Otolaryngol. 1962 Sep;55:231–236. doi: 10.3109/00016486209127357. Available from: http://dx.doi.org/10.3109/00016486209127357. [DOI] [PubMed] [Google Scholar]
- 44.Everberg G. Deafness following mumps. Acta Otolaryngol. 1957 Nov-Dec;48(5-6):397–403. doi: 10.3109/00016485709126900. Available from: http://dx.doi.org/10.3109/00016485709126900. [DOI] [PubMed] [Google Scholar]
- 45.Cusi MG, Bianchi S, Valassina M, Santini L, Arnetoli M, Valensin PE. Rapid detection and typing of circulating mumps virus by reverse transcription/polymerase chain reaction. Res Virol. 1996 Jul-Aug;147(4):227–232. doi: 10.1016/0923-2516(96)89653-1. Available from: http://dx.doi.org/10.1016/0923-2516(96)89653-1. [DOI] [PubMed] [Google Scholar]
- 46.Davidkin I, Jokinen S, Paananen A, Leinikki P, Peltola H. Etiology of mumps-like illnesses in children and adolescents vaccinated for measles, mumps, and rubella. J Infect Dis. 2005 Mar;191(5):719–723. doi: 10.1086/427338. Available from: http://dx.doi.org/10.1086/427338. [DOI] [PubMed] [Google Scholar]
- 47.Buyukavci M, Yildirim ZK, Tan H. High-dose IVIG therapy in a child with idiopathic thrombocytopenic purpura associated with mumps. J Pediatr Hematol Oncol. 2005 Jan;27(1):56–57. doi: 10.1097/01.mph.0000151804.79843.c2. Available from: http://dx.doi.org/10.1097/01.mph.0000151804.79843.c2. [DOI] [PubMed] [Google Scholar]
- 48.Bajaj NP, Rose P, Clifford-Jones R, Hughes PJ. Acute transverse myelitis and Guillain-Barré overlap syndrome with serological evidence for mumps viraemia. Acta Neurol Scand. 2001 Oct;104(4):239–242. doi: 10.1034/j.1600-0404.2001.00340.x. Available from: http://dx.doi.org/10.1034/j.1600-0404.2001.00340.x. [DOI] [PubMed] [Google Scholar]
- 49.Sonmez FM, Odemis E, Ahmetoglu A, Ayvaz A. Brainstem encephalitis and acute disseminated encephalomyelitis following mumps. Pediatr Neurol. 2004 Feb;30(2):132–134. doi: 10.1016/j.pediatrneurol.2003.09.004. Available from: http://dx.doi.org/10.1016/j.pediatrneurol.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 50.Takla A, Wichmann O, Klinc C, Hautmann W, Rieck T, Koch J. Mumps epidemiology in Germany 2007-11. Euro Surveill. 2013;18(33):20557. doi: 10.2807/1560-7917.es2013.18.33.20557. Available from: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20557. [DOI] [PubMed] [Google Scholar]
- 51.de Jong MD, Galasso GJ, Gazzard B, Griffiths PD, Jabs DA, Kern ER, Spector SA. Summary of the II International Symposium on Cytomegalovirus. Antiviral Res. 1998 Oct;39(3):141–162. doi: 10.1016/S0166-3542(98)00044-8. Available from: http://dx.doi.org/10.1016/S0166-3542(98)00044-8. [DOI] [PubMed] [Google Scholar]
- 52.Bale JF, Jr, Petheram SJ, Souza IE, Murph JR. Cytomegalovirus reinfection in young children. J Pediatr. 1996 Mar;128(3):347–352. doi: 10.1016/S0022-3476(96)70279-2. Available from: http://dx.doi.org/10.1016/S0022-3476(96)70279-2. [DOI] [PubMed] [Google Scholar]
- 53.Ussmüller J. Virale Speicheldrüsenentzündungen. [Viral sialadenitis]. HNO. 2010 Mar;58(3):225–228. doi: 10.1007/s00106-009-2077-y. (Ger). Available from: http://dx.doi.org/10.1007/s00106-009-2077-y. [DOI] [PubMed] [Google Scholar]
- 54.Mayer M, Haddad J., Jr Human immunodeficiency virus infection presenting with lymphoepithelial cysts in a six-year-old child. Ann Otol Rhinol Laryngol. 1996 Mar;105(3):242–244. doi: 10.1177/000348949610500312. [DOI] [PubMed] [Google Scholar]
- 55.Hobbs CG, Groves E, Davenport V, Bailey M, Williams NA, Birchall MA, Heyderman RS. Tonsil T cell immunity to human papillomavirus in the absence of detectable virus in healthy adults. Laryngoscope. 2008 Mar;118(3):459–463. doi: 10.1097/MLG.0b013e31815aedb3. Available from: http://dx.doi.org/10.1097/MLG.0b013e31815aedb3. [DOI] [PubMed] [Google Scholar]
- 56.Del Bono V, Pretolesi F, Pontali E, Martinoli C, Bassetti M, Mazzarello G, Chiaramondia M, Derchi LE, Bassetti D. Possible malignant transformation of benign lymphoepithelial parotid lesions in human immunodeficiency virus-infected patients: report of three cases. Clin Infect Dis. 2000 Jun;30(6):947–949. doi: 10.1086/313813. Available from: http://dx.doi.org/10.1086/313813. [DOI] [PubMed] [Google Scholar]
- 57.Morales-Aguirre JJ, Patiño-Niño JA, Mendoza-Azpiri M, Villalobos-Acosta CP, Gómez-Barreto D, de la Torre C, Cashat-Cruz M. Parotid cysts in children infected with human immunodeficiency virus: report of 4 cases. Arch Otolaryngol Head Neck Surg. 2005 Apr;131(4):353–355. doi: 10.1001/archotol.131.4.353. Available from: http://dx.doi.org/10.1001/archotol.131.4.353. [DOI] [PubMed] [Google Scholar]
- 58.Syebele K, Bütow KW, Webber L, Manda SO. Quantification of HIV-1 viral load in the fluid of ranulas in HIV-positive patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011 Jun;111(6):715–719. doi: 10.1016/j.tripleo.2011.01.038. Available from: http://dx.doi.org/10.1016/j.tripleo.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 59.Syebele K, Bütow KW. Comparative study of the effect of antiretroviral therapy on benign lymphoepithelial cyst of parotid glands and ranulas in HIV-positive patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011 Feb;111(2):205–210. doi: 10.1016/j.tripleo.2010.09.067. Available from: http://dx.doi.org/10.1016/j.tripleo.2010.09.067. [DOI] [PubMed] [Google Scholar]
- 60.Steehler MK, Steehler MW, Davison SP. Benign lymphoepithelial cysts of the parotid: long-term surgical results. HIV AIDS (Auckl) 2012;4:81–86. doi: 10.2147/HIV.S27755. Available from: http://dx.doi.org/10.2147/HIV.S27755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ismail EA, Seoudi TM, Al-Amir M, Al-Esnawy AA. Neonatal suppurative parotitis over the last 4 decades: report of three new cases and review. Pediatr Int. 2013 Feb;55(1):60–64. doi: 10.1111/j.1442-200X.2012.03738.x. Available from: http://dx.doi.org/10.1111/j.1442-200X.2012.03738.x. [DOI] [PubMed] [Google Scholar]
- 62.Managoli S, Chaturvedi P. Suppurative parotitis in a neonate. Indian Pediatr. 2002 Apr;39(4):407–408. [PubMed] [Google Scholar]
- 63.Moritz ML, Ayus JC. Hospital-induced hyponatremia. J Pediatr. 2005 Aug;147(2):273–274. doi: 10.1016/j.jpeds.2005.01.052. Available from: http://dx.doi.org/10.1016/j.jpeds.2005.01.052. [DOI] [PubMed] [Google Scholar]
- 64.Chevalier J, Jadcherla SR. Parotid swelling in a premature neonate. Am J Perinatol. 2002 Nov;19(8):435–438. doi: 10.1055/s-2002-36842. Available from: http://dx.doi.org/10.1055/s-2002-36842. [DOI] [PubMed] [Google Scholar]
- 65.Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, Rubens C, Menon R, Van Look PF. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010 Jan;88(1):31–38. doi: 10.2471/BLT.08.062554. Available from: http://dx.doi.org/10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leake D, Leake R. Neonatal suppurative parotitis. Pediatrics. 1970 Aug;46(2):202–207. [PubMed] [Google Scholar]
- 67.Stong BC, Sipp JA, Sobol SE. Pediatric parotitis: a 5-year review at a tertiary care pediatric institution. Int J Pediatr Otorhinolaryngol. 2006 Mar;70(3):541–544. doi: 10.1016/j.ijporl.2005.08.001. Available from: http://dx.doi.org/10.1016/j.ijporl.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 68.Raad II, Sabbagh MF, Caranasos GJ. Acute bacterial sialadenitis: a study of 29 cases and review. Rev Infect Dis. 1990 Jul-Aug;12(4):591–601. doi: 10.1093/clinids/12.4.591. Available from: http://dx.doi.org/10.1093/clinids/12.4.591. [DOI] [PubMed] [Google Scholar]
- 69.Saarinen RT, Kolho KL, Pitkäranta A. Cases presenting as parotid abscesses in children. Int J Pediatr Otorhinolaryngol. 2007 Jun;71(6):897–901. doi: 10.1016/j.ijporl.2007.02.011. Available from: http://dx.doi.org/10.1016/j.ijporl.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 70.Giglio MS, Landaeta M, Pinto ME. Microbiology of recurrent parotitis. Pediatr Infect Dis J. 1997 Apr;16(4):386–390. doi: 10.1097/00006454-199704000-00010. Available from: http://dx.doi.org/10.1097/00006454-199704000-00010. [DOI] [PubMed] [Google Scholar]
- 71.Chatterjee A, Varman M, Quinlan TW. Parotid abscess caused by Mycobacterium tuberculosis. Pediatr Infect Dis J. 2001 Sep;20(9):912–914. doi: 10.1097/00006454-200109000-00019. Available from: http://dx.doi.org/10.1097/00006454-200109000-00019. [DOI] [PubMed] [Google Scholar]
- 72.Schneider H, Koch M, Künzel J, Gillespie MB, Grundtner P, Iro H, Zenk J. Juvenile recurrent parotitis: a retrospective comparison of sialendoscopy versus conservative therapy. Laryngoscope. 2014 Feb;124(2):451–455. doi: 10.1002/lary.24291. Available from: http://dx.doi.org/10.1002/lary.24291. [DOI] [PubMed] [Google Scholar]
- 73.Koch M, Iro H, Klintworth N, Psychogios G, Zenk J. Results of minimally invasive gland-preserving treatment in different types of parotid duct stenosis. Arch Otolaryngol Head Neck Surg. 2012 Sep;138(9):804–810. doi: 10.1001/archoto.2012.1618. Available from: http://dx.doi.org/10.1001/archoto.2012.1618. [DOI] [PubMed] [Google Scholar]
- 74.Koch M, Iro H, Künzel J, Psychogios G, Bozzato A, Zenk J. Diagnosis and gland-preserving minimally invasive therapy for Wharton's duct stenoses. Laryngoscope. 2012 Mar;122(3):552–558. doi: 10.1002/lary.22452. Available from: http://dx.doi.org/10.1002/lary.22452. [DOI] [PubMed] [Google Scholar]
- 75.Koch M, Iro H, Zenk J. Stenosen und andere nicht steinbedingte Obstruktionen der Speicheldrüsenausführungsgänge. Moderne Therapiekonzepte. [Stenosis and other non-sialolithiasis-related obstructions of the major salivary gland ducts. Modern treatment concepts]. HNO. 2010 Mar;58(3):218–224. doi: 10.1007/s00106-009-2076-z. (Ger). Available from: http://dx.doi.org/10.1007/s00106-009-2076-z. [DOI] [PubMed] [Google Scholar]
- 76.Koch M, Zenk J, Iro H. Algorithms for treatment of salivary gland obstructions. Otolaryngol Clin North Am. 2009 Dec;42(6):1173–92, Table of Contents. doi: 10.1016/j.otc.2009.08.002. Available from: http://dx.doi.org/10.1016/j.otc.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 77.Koch M, Iro H, Zenk J. Sialendoscopy-based diagnosis and classification of parotid duct stenoses. Laryngoscope. 2009 Sep;119(9):1696–1703. doi: 10.1002/lary.20522. Available from: http://dx.doi.org/10.1002/lary.20522. [DOI] [PubMed] [Google Scholar]
- 78.Koch M, Constantinidis J, Dimmler A, Strauss C, Iro H. Langzeit-Erfahrungen in der Therapie des Asthesioneuroblastoms. [Long-term experiences in the therapy of esthesioneuroblastoma]. Laryngorhinootologie. 2006 Oct;85(10):723–730. doi: 10.1055/s-2006-925298. (Ger). Available from: http://dx.doi.org/10.1055/s-2006-925298. [DOI] [PubMed] [Google Scholar]
- 79.Bodner L, Azaz B. Submandibular sialolithiasis in children. J Oral Maxillofac Surg. 1982 Sep;40(9):55l–554. doi: 10.1016/0278-2391(82)90281-6. Available from: http://dx.doi.org/10.1016/0278-2391(82)90281-6. [DOI] [PubMed] [Google Scholar]
- 80.Reuther J, Hausamen JE. Sialolithiasis der Glandula submandibularis im Kindesalte. [Submaxillary salivary calculus in children (author's transl)]. Klin Padiatr. 1976 May;188(3):285–288. (Ger). [PubMed] [Google Scholar]
- 81.Horie N, Shimoyama T, Tanebayashi Y, Ide F. Parotid sialolithiasis in a child. J Clin Pediatr Dent. 1995;20(1):61–62. [PubMed] [Google Scholar]
- 82.Zenk J, Constantinidis J, Kydles S, Hornung J, Iro H. Klinische und diagnostische Befunde bei der Sialolithiasis. [Clinical and diagnostic findings of sialolithiasis]. HNO. 1999 Nov;47(11):963–969. doi: 10.1007/s001060050476. (Ger). Available from: http://dx.doi.org/10.1007/s001060050476. [DOI] [PubMed] [Google Scholar]
- 83.Zenk J, Koch M, Klintworth N, König B, Konz K, Gillespie MB, Iro H. Sialendoscopy in the diagnosis and treatment of sialolithiasis: a study on more than 1000 patients. Otolaryngol Head Neck Surg. 2012 Nov;147(5):858–863. doi: 10.1177/0194599812452837. Available from: http://dx.doi.org/10.1177/0194599812452837. [DOI] [PubMed] [Google Scholar]
- 84.Hackett AM, Baranano CF, Reed M, Duvvuri U, Smith RJ, Mehta D. Sialoendoscopy for the treatment of pediatric salivary gland disorders. Arch Otolaryngol Head Neck Surg. 2012 Oct;138(10):912–915. doi: 10.1001/2013.jamaoto.244. Available from: http://dx.doi.org/10.1001/2013.jamaoto.244. [DOI] [PubMed] [Google Scholar]
- 85.Zenk J, Gottwald F, Bozzato A, Iro H. Speichelsteine der Glandula submandibularis. Steinentfernung mit Organerhalt. [Submandibular sialoliths. Stone removal with organ preservation]. HNO. 2005 Mar;53(3):243–249. doi: 10.1007/s00106-004-1110-4. (Ger). Available from: http://dx.doi.org/10.1007/s00106-004-1110-4. [DOI] [PubMed] [Google Scholar]
- 86.Ottaviani F, Marchisio P, Arisi E, Capaccio P. Extracorporeal shockwave lithotripsy for salivary calculi in pediatric patients. Acta Otolaryngol. 2001 Oct;121(7):873–876. doi: 10.1080/00016480152602366. Available from: http://dx.doi.org/10.1080/00016480152602366. [DOI] [PubMed] [Google Scholar]
- 87.Zenk J, Iro H. Die Sialolithiasis und deren Behandlung. Laryngorhinootologie. 2001;80(1):115–136. [Google Scholar]
- 88.Zenk J, Koch M, Mantsopoulos K, Klintworth N, Schapher M, Iro H. Der Stellenwert der extrakorporalen Stosswellenlithotripsie bei der Therapie der Sialolithiasis. [The significance of extracorporeal shock wave lithotripsy in sialolithiasis therapy]. HNO. 2013 Apr;61(4):306–311. doi: 10.1007/s00106-013-2677-4. (Ger). Available from: http://dx.doi.org/10.1007/s00106-013-2677-4. [DOI] [PubMed] [Google Scholar]
- 89.Koch M, Bozzato A, Iro H, Zenk J. Combined endoscopic and transcutaneous approach for parotid gland sialolithiasis: indications, technique, and results. Otolaryngol Head Neck Surg. 2010 Jan;142(1):98–103. doi: 10.1016/j.otohns.2009.10.022. Available from: http://dx.doi.org/10.1016/j.otohns.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 90.Zenk J, Koch M, Iro H. Extracorporeal and intracorporeal lithotripsy of salivary gland stones: basic investigations. Otolaryngol Clin North Am. 2009 Dec;42(6):1115–37, Table of Contents. doi: 10.1016/j.otc.2009.08.005. Available from: http://dx.doi.org/10.1016/j.otc.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 91.Witt RL, Iro H, Koch M, McGurk M, Nahlieli O, Zenk J. Minimally invasive options for salivary calculi. Laryngoscope. 2012 Jun;122(6):1306–1311. doi: 10.1002/lary.23272. Available from: http://dx.doi.org/10.1002/lary.23272. [DOI] [PubMed] [Google Scholar]
- 92.Iro H, Zenk J, Koch M. Moderne Konzepte zur Diagnostik und Therapie der Sialolithiasis. [Modern concepts for the diagnosis and therapy of sialolithiasis]. HNO. 2010 Mar;58(3):211–217. doi: 10.1007/s00106-009-2075-0. (Ger). Available from: http://dx.doi.org/10.1007/s00106-009-2075-0. [DOI] [PubMed] [Google Scholar]
- 93.McCormick ME, Bawa G, Shah RK. Idiopathic recurrent pneumoparotitis. Am J Otolaryngol. 2013 Mar-Apr;34(2):180–182. doi: 10.1016/j.amjoto.2012.11.005. Available from: http://dx.doi.org/10.1016/j.amjoto.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 94.Saunders HF. Wind parotitis. N Engl J Med. 1973 Sep 27;289(13):698. doi: 10.1056/nejm197309272891322. [DOI] [PubMed] [Google Scholar]
- 95.Piette E, Walker RT. Pneumoparotid during dental treatment. Oral Surg Oral Med Oral Pathol. 1991 Oct;72(4):415–417. doi: 10.1016/0030-4220(91)90550-V. Available from: http://dx.doi.org/10.1016/0030-4220(91)90550-V. [DOI] [PubMed] [Google Scholar]
- 96.Markowitz-Spence L, Brodsky L, Seidell G, Stanievich JF. Self-induced pneumoparotitis in an adolescent. Report of a case and review of the literature. Int J Pediatr Otorhinolaryngol. 1987 Dec;14(2-3):113–121. doi: 10.1016/0165-5876(87)90021-8. [DOI] [PubMed] [Google Scholar]
- 97.Chilla R, Meyfarth HO, Arglebe C. Uber die operative Behandlung der chronischen Ohrspeicheldrüsenentzündung. [Surgical treatment of chronic parotitis (author’s transl)]. Arch Otorhinolaryngol. 1982;234(1):53–63. doi: 10.1007/BF00453538. (Ger). Available from: http://dx.doi.org/10.1007/BF00453538. [DOI] [PubMed] [Google Scholar]
- 98.Han S, Isaacson G. Recurrent pneumoparotid: cause and treatment. Otolaryngol Head Neck Surg. 2004 Nov;131(5):758–761. doi: 10.1016/j.otohns.2004.04.035. Available from: http://dx.doi.org/10.1016/j.otohns.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 99.Rowell J, Lynn AM, Filardi TZ, Celix J, Ojemann JG. Acute unilateral enlargement of the parotid gland immediately post craniotomy in a pediatric patient: a case report. Childs Nerv Syst. 2010 Sep;26(9):1239–1242. doi: 10.1007/s00381-010-1186-y. Available from: http://dx.doi.org/10.1007/s00381-010-1186-y. [DOI] [PubMed] [Google Scholar]
- 100.Attas M, Sabawala PB, Keats AS. Acute transient sialadenopathy during induction of anesthesia. Anesthesiology. 1968 Sep-Oct;29(5):1050–1052. doi: 10.1097/00000542-196809000-00036. Available from: http://dx.doi.org/10.1097/00000542-196809000-00036. [DOI] [PubMed] [Google Scholar]
- 101.Bonchek LI. Salivary gland enlargement during induction of anesthesia. JAMA. 1969 Sep;209(11):1716–1718. doi: 10.1001/jama.1969.03160240072025. Available from: http://dx.doi.org/10.1001/jama.1969.03160240072025. [DOI] [PubMed] [Google Scholar]
- 102.Berker M, Sahin A, Aypar U, Ozgen T. Acute parotitis following sitting position neurosurgical procedures: review of five cases. J Neurosurg Anesthesiol. 2004 Jan;16(1):29–31. doi: 10.1097/00008506-200401000-00007. Available from: http://dx.doi.org/10.1097/00008506-200401000-00007. [DOI] [PubMed] [Google Scholar]
- 103.Tremblay V, Ayad T, Lapointe A, Giguàre C, Quintal MC, Arcand P, Abela A. Nontuberculous mycobacterial cervicofacial adenitis in children: epidemiologic study. J Otolaryngol Head Neck Surg. 2008 Oct;37(5):616–622. [PubMed] [Google Scholar]
- 104.Haverkamp MH, Arend SM, Lindeboom JA, Hartwig NG, van Dissel JT. Nontuberculous mycobacterial infection in children: a 2-year prospective surveillance study in the Netherlands. Clin Infect Dis. 2004 Aug;39(4):450–456. doi: 10.1086/422319. Available from: http://dx.doi.org/10.1086/422319. [DOI] [PubMed] [Google Scholar]
- 105.Lindeboom JA, Kuijper EJ, Bruijnesteijn van Coppenraet ES, Lindeboom R, Prins JM. Surgical excision versus antibiotic treatment for nontuberculous mycobacterial cervicofacial lymphadenitis in children: a multicenter, randomized, controlled trial. Clin Infect Dis. 2007 Apr;44(8):1057–1064. doi: 10.1086/512675. Available from: http://dx.doi.org/10.1086/512675. [DOI] [PubMed] [Google Scholar]
- 106.Harris RL, Modayil P, Adam J, Sharland M, Heath P, Planche T, Daya H. Cervicofacial nontuberculous mycobacterium lymphadenitis in children: is surgery always necessary? Int J Pediatr Otorhinolaryngol. 2009 Sep;73(9):1297–1301. doi: 10.1016/j.ijporl.2009.06.006. Available from: http://dx.doi.org/10.1016/j.ijporl.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 107.Iversen RH, Illum P. Cervicofacial nontuberculous mycobacterial lymphadenitis in children. Dan Med J. 2012 Jan;59(1):A4349. [PubMed] [Google Scholar]
- 108.Flint D, Mahadevan M, Barber C, Grayson D, Small R. Cervical lymphadenitis due to non-tuberculous mycobacteria: surgical treatment and review. Int J Pediatr Otorhinolaryngol. 2000 Jul 14;53(3):187–194. doi: 10.1016/S0165-5876(00)82006-6. Available from: http://dx.doi.org/10.1016/S0165-5876(00)82006-6. [DOI] [PubMed] [Google Scholar]
- 109.Lindeboom JA, Lindeboom R, Bruijnesteijn van Coppenraet ES, Kuijper EJ, Tuk J, Prins JM. Esthetic outcome of surgical excision versus antibiotic therapy for nontuberculous mycobacterial cervicofacial lymphadenitis in children. Pediatr Infect Dis J. 2009 Nov;28(11):1028–1030. doi: 10.1097/INF.0b013e3181aa6411. Available from: http://dx.doi.org/10.1097/INF.0b013e3181aa6411. [DOI] [PubMed] [Google Scholar]
- 110.Griffith DE. Therapy of nontuberculous mycobacterial disease. Curr Opin Infect Dis. 2007 Apr;20(2):198–203. doi: 10.1097/QCO.0b013e328055d9a2. Available from: http://dx.doi.org/10.1097/QCO.0b013e328055d9a2. [DOI] [PubMed] [Google Scholar]
- 111.Haase G, Kentrup H, Skopnik H, Springer B, Böttger EC. Mycobacterium lentiflavum: an etiologic agent of cervical lymphadenitis. Clin Infect Dis. 1997 Nov;25(5):1245–1246. doi: 10.1086/516958. Available from: http://dx.doi.org/10.1086/516958. [DOI] [PubMed] [Google Scholar]
- 112.Zeharia A, Eidlitz-Markus T, Haimi-Cohen Y, Samra Z, Kaufman L, Amir J. Management of nontuberculous mycobacteria-induced cervical lymphadenitis with observation alone. Pediatr Infect Dis J. 2008 Oct;27(10):920–922. doi: 10.1097/INF.0b013e3181734fa3. Available from: http://dx.doi.org/10.1097/INF.0b013e3181734fa3. [DOI] [PubMed] [Google Scholar]
- 113.Jawahar MS, Rajaram K, Sivasubramanian S, Paramasivan CN, Chandrasekar K, Kamaludeen MN, Thirithuvathas AJ, Ananthalakshmi V, Prabhakar R. Treatment of lymph node tuberculosis – a randomized clinical trial of two 6-month regimens. Trop Med Int Health. 2005 Nov;10(11):1090–1098. doi: 10.1111/j.1365-3156.2005.01493.x. Available from: http://dx.doi.org/10.1111/j.1365-3156.2005.01493.x. [DOI] [PubMed] [Google Scholar]
- 114.Mehta D, Willging JP. Pediatric salivary gland lesions. Semin Pediatr Surg. 2006 May;15(2):76–84. doi: 10.1053/j.sempedsurg.2006.02.004. Available from: http://dx.doi.org/10.1053/j.sempedsurg.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 115.Rice DH. Chronic inflammatory disorders of the salivary glands. Otolaryngol Clin North Am. 1999 Oct;32(5):813–818. doi: 10.1016/S0030-6665(05)70174-2. Available from: http://dx.doi.org/10.1016/S0030-6665(05)70174-2. [DOI] [PubMed] [Google Scholar]
- 116.Seifert G. Tumour-like lesions of the salivary glands. The new WHO classification. Pathol Res Pract. 1992 Oct;188(7):836–846. [PubMed] [Google Scholar]
- 117.Fowler CB, Brannon RB. Subacute necrotizing sialadenitis: report of 7 cases and a review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000 May;89(5):600–609. doi: 10.1067/moe.2000.105943. Available from: http://dx.doi.org/10.1067/moe.2000.105943. [DOI] [PubMed] [Google Scholar]
- 118.Ylikontiola L, Siponen M, Salo T, Sándor GK. Sialometaplasia of the soft palate in a 2-year-old girl. J Mich Dent Assoc. 2010 Apr;92(4):38–40. [PubMed] [Google Scholar]
- 119.Brannon RB, Fowler CB, Hartman KS. Necrotizing sialometaplasia. A clinicopathologic study of sixty-nine cases and review of the literature. Oral Surg Oral Med Oral Pathol. 1991 Sep;72(3):317–325. doi: 10.1016/0030-4220(91)90225-2. Available from: http://dx.doi.org/10.1016/0030-4220(91)90225-2. [DOI] [PubMed] [Google Scholar]
- 120.Seifert G. Aetiological and histological classification of sialadenitis. Pathologica. 1997 Feb;89(1):7–17. [PubMed] [Google Scholar]
- 121.von Reuss A. Über chronische Erkrankungen der Parotis im Kindesalter. Jahrb Kinderheilk. 1909;70:161–173. [Google Scholar]
- 122.Ussmüller J, Donath K. Klinische, histopathologische und immunhistochemische Untersuchungen zur chronischen sialektatischen Parotitis im Kindes- und Jugendalter. [Clinical, histopathologic and immunohistochemical studies of chronic sialectatic parotitis in childhood and adolescence]. Klin Padiatr. 1999 May-Jun;211(3):165–171. doi: 10.1055/s-2008-1043780. (Ger). Available from: http://dx.doi.org/10.1055/s-2008-1043780. [DOI] [PubMed] [Google Scholar]
- 123.Galili D, Marmary Y. Juvenile recurrent parotitis: clinicoradiologic follow-up study and the beneficial effect of sialography. Oral Surg Oral Med Oral Pathol. 1986 Jun;61(6):550–556. doi: 10.1016/0030-4220(86)90091-5. Available from: http://dx.doi.org/10.1016/0030-4220(86)90091-5. [DOI] [PubMed] [Google Scholar]
- 124.Ericson S, Zetterlund B, Ohman J. Recurrent parotitis and sialectasis in childhood. Clinical, radiologic, immunologic, bacteriologic, and histologic study. Ann Otol Rhinol Laryngol. 1991 Jul;100(7):527–535. doi: 10.1177/000348949110000702. [DOI] [PubMed] [Google Scholar]
- 125.Bailey H. Local infiltration of procaine hydrochloride solution combined with intravenous administration of pentothal sodium. J Int Coll Surg. 1945 Sep-Oct;8:427–430. [PubMed] [Google Scholar]
- 126.Maynard JD. Recurrent parotid enlargement. Br J Surg. 1965 Oct;52(10):784–789. doi: 10.1002/bjs.1800521021. Available from: http://dx.doi.org/10.1002/bjs.1800521021. [DOI] [PubMed] [Google Scholar]
- 127.Hemenway WG. Chronic punctate parotitis. Laryngoscope. 1971 Apr;81(4):485–509. doi: 10.1288/00005537-197104000-00001. Available from: http://dx.doi.org/10.1288/00005537-197104000-00001. [DOI] [PubMed] [Google Scholar]
- 128.Zou ZJ, Wang SL, Zhu JR, Yu SF, Ma DQ, Wu YT. Recurrent parotitis in children. A report of 102 cases. Chin Med J (Engl) 1990 Jul;103(7):576–582. [PubMed] [Google Scholar]
- 129.Konno A, Ito E. A study on the pathogenesis of recurrent parotitis in childhood. Ann Otol Rhinol Laryngol Suppl. 1979 Nov-Dec;88(6 Pt 4 Suppl 63):1–20. doi: 10.1177/00034894790886s301. [DOI] [PubMed] [Google Scholar]
- 130.Wittekindt C, Jungehülsing M, Fischbach R, Landwehr P. Chronisch rezidivierende Parotitis des Kindesalters bei eineiigen Zwillingen. Magnetresonanzsialographie. [Chronic recurrent parotitis in childhood in monozygotic twins. Magnetic resonance sialography]. HNO. 2000 Mar;48(3):221–225. doi: 10.1007/s001060050036. (Ger). Available from: http://dx.doi.org/10.1007/s001060050036. [DOI] [PubMed] [Google Scholar]
- 131.Reid E, Douglas F, Crow Y, Hollman A, Gibson J. Autosomal dominant juvenile recurrent parotitis. J Med Genet. 1998 May;35(5):417–419. doi: 10.1136/jmg.35.5.417. Available from: http://dx.doi.org/10.1136/jmg.35.5.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Morales-Bozo I, Landaeta M, Urzúa-Orellana B, Retamales P. Association between the occurrence of matrix metalloproteinases 2 and 9 in parotid saliva with the degree of parotid gland damage in juvenile recurrent parotitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008 Sep;106(3):377–383. doi: 10.1016/j.tripleo.2008.02.006. Available from: http://dx.doi.org/10.1016/j.tripleo.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 133.Wu AJ, Lafrenie RM, Park C, Apinhasmit W, Chen ZJ, Birkedal-Hansen H, Yamada KM, Stetler-Stevenson WG, Baum BJ. Modulation of MMP-2 (gelatinase A) and MMP-9 (gelatinase B) by interferon-gamma in a human salivary gland cell line. J Cell Physiol. 1997 May;171(2):117–124. doi: 10.1002/(SICI)1097-4652(199705)171:2<117::AID-JCP1>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 134.Bowling DM, Ferry G, Rauch SD, Goodman ML. Intraductal tetracycline therapy for the treatment of chronic recurrent parotitis. Ear Nose Throat J. 1994 Apr;73(4):262–274. [PubMed] [Google Scholar]
- 135.Andrade RE, Hagen KA, Manivel JC. Distribution and immunophenotype of the inflammatory cell population in the benign lymphoepithelial lesion (Mikulicz’s disease) Hum Pathol. 1988 Aug;19(8):932–941. doi: 10.1016/S0046-8177(88)80009-1. Available from: http://dx.doi.org/10.1016/S0046-8177(88)80009-1. [DOI] [PubMed] [Google Scholar]
- 136.Marsman WA, Sukhai RN. Recurrent parotitis and isolated IgG3 subclass deficiency. Eur J Pediatr. 1999 Aug;158(8):684. doi: 10.1007/s004310051177. Available from: http://dx.doi.org/10.1007/s004310051177. [DOI] [PubMed] [Google Scholar]
- 137.Ericson S, Sjöbäck I. Salivary factors in children with recurrent parotitis. Part 2: Protein, albumin, amylase, IgA, lactoferrin lysozyme and kallikrein concentrations. Swed Dent J. 1996;20(5):199–207. [PubMed] [Google Scholar]
- 138.Ericson S, Sjöbäck I. Salivary factors in children with recurrent parotitis. Part 1: Salivary flow rate, buffering capacity and inorganic components. Swed Dent J. 1996;20(4):121–132. [PubMed] [Google Scholar]
- 139.Mandel L, Witek EL. Chronic parotitis: diagnosis and treatment. J Am Dent Assoc. 2001 Dec;132(12):1707–1711. doi: 10.14219/jada.archive.2001.0125. Available from: http://dx.doi.org/10.14219/jada.archive.2001.0125. [DOI] [PubMed] [Google Scholar]
- 140.Shimizu M, Ussmüller J, Donath K, Yoshiura K, Ban S, Kanda S, Ozeki S, Shinohara M. Sonographic analysis of recurrent parotitis in children: a comparative study with sialographic findings. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998 Nov;86(5):606–615. doi: 10.1016/S1079-2104(98)90355-9. Available from: http://dx.doi.org/10.1016/S1079-2104(98)90355-9. [DOI] [PubMed] [Google Scholar]
- 141.Iro H, Uttenweiler V, Zenk J. Kopf-Hals-Sonographie. Eine Anleitung zur praxisbezogenen Ultraschalluntersuchung. Berlin: Springer; 2000. Available from: http://dx.doi.org/10.1007/978-3-642-57012-4. [DOI] [Google Scholar]
- 142.Watkin GT, Hobsley M. Natural history of patients with recurrent parotitis and punctate sialectasis. Br J Surg. 1986 Sep;73(9):745–748. doi: 10.1002/bjs.1800730922. Available from: http://dx.doi.org/10.1002/bjs.1800730922. [DOI] [PubMed] [Google Scholar]
- 143.Geterud A, Lindvall AM, Nylén O. Follow-up study of recurrent parotitis in children. Ann Otol Rhinol Laryngol. 1988 Jul-Aug;97(4 Pt 1):341–346. doi: 10.1177/000348948809700403. [DOI] [PubMed] [Google Scholar]
- 144.Glasenapp GB, Schmidt W, Kessler L, Otto HJ. Zur Behandlung der chronisch rekurrierenden Parotitis mit Röntgenbestrahlung unter szintigraphischer Kontrolle. [Treatment of chronic recurrent parotitis by roentgen irradiation under scintigraphic control]. Z Laryngol Rhinol Otol. 1970 Aug;49(8):520–525. (Ger). [PubMed] [Google Scholar]
- 145.Diamant H, Enfors B. Treatment of chronic recurrent parotitis. Laryngoscope. 1965 Jan;75:153–160. doi: 10.1288/00005537-196501000-00017. Available from: http://dx.doi.org/10.1288/00005537-196501000-00017. [DOI] [PubMed] [Google Scholar]
- 146.Rettinger G, Stolte M, Bäumler C. Ausschaltung von Speicheldrüsen durch temporäre Okklusion des Gangsystems mit einer Aminosäurenlösung. Tierexperimentelle Studie zu einem neuen Therapieverfahren. [Elimination of major salivary glands by temporary medicamentous occlusion of the excretory ducts (author's transl)]. HNO. 1981 Sep;29(9):294–299. (Ger). [PubMed] [Google Scholar]
- 147.Maier H, Adler D, Lenarz T, Müller-Esterl W. New concepts in the treatment of chronic recurrent parotitis. Arch Otorhinolaryngol. 1985;242(3):321–328. doi: 10.1007/BF00453557. Available from: http://dx.doi.org/10.1007/BF00453557. [DOI] [PubMed] [Google Scholar]
- 148.Rubaltelli L, Sponga T, Candiani F, Pittarello F, Andretta M. Infantile recurrent sialectatic parotitis: the role of sonography and sialography in diagnosis and follow-up. Br J Radiol. 1987 Dec;60(720):1211–1214. doi: 10.1259/0007-1285-60-720-1211. Available from: http://dx.doi.org/10.1259/0007-1285-60-720-1211. [DOI] [PubMed] [Google Scholar]
- 149.Antoniades D, Harrison JD, Epivatianos A, Papanayotou P. Treatment of chronic sialadenitis by intraductal penicillin or saline. J Oral Maxillofac Surg. 2004 Apr;62(4):431–434. doi: 10.1016/j.joms.2003.07.007. Available from: http://dx.doi.org/10.1016/j.joms.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 150.Shacham R, Droma EB, London D, Bar T, Nahlieli O. Long-term experience with endoscopic diagnosis and treatment of juvenile recurrent parotitis. J Oral Maxillofac Surg. 2009 Jan;67(1):162–167. doi: 10.1016/j.joms.2008.09.027. Available from: http://dx.doi.org/10.1016/j.joms.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 151.Quenin S, Plouin-Gaudon I, Marchal F, Froehlich P, Disant F, Faure F. Juvenile recurrent parotitis: sialendoscopic approach. Arch Otolaryngol Head Neck Surg. 2008 Jul;134(7):715–719. doi: 10.1001/archotol.134.7.715. Available from: http://dx.doi.org/10.1001/archotol.134.7.715. [DOI] [PubMed] [Google Scholar]
- 152.Capaccio P, Sigismund PE, Luca N, Marchisio P, Pignataro L. Modern management of juvenile recurrent parotitis. J Laryngol Otol. 2012 Dec;126(12):1254–1260. doi: 10.1017/S0022215112002319. Available from: http://dx.doi.org/10.1017/S0022215112002319. [DOI] [PubMed] [Google Scholar]
- 153.Zenk J, Koch M, Klintworth N, Iro H. Die chronisch rezidivierende Parotitis. [Chronic recurrent parotitis]. HNO. 2010 Mar;58(3):237–243. doi: 10.1007/s00106-009-2079-9. (Ger). Available from: http://dx.doi.org/10.1007/s00106-009-2079-9. [DOI] [PubMed] [Google Scholar]
- 154.Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, Pillemer SR, Talal N, Weisman MH European Study Group on Classification Criteria for Sjögren’s Syndrome. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002 Jun;61(6):554–558. doi: 10.1136/ard.61.6.554. Available from: http://dx.doi.org/10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cimaz R, Casadei A, Rose C, Bartunkova J, Sediva A, Falcini F, Picco P, Taglietti M, Zulian F, Ten Cate R, Sztajnbok FR, Voulgari PV, Drosos AA. Primary Sjögren syndrome in the paediatric age: a multicentre survey. Eur J Pediatr. 2003 Oct;162(10):661–665. doi: 10.1007/s00431-003-1277-9. Available from: http://dx.doi.org/10.1007/s00431-003-1277-9. [DOI] [PubMed] [Google Scholar]
- 156.Bartunková J, Sedivá A, Vencovský J, Tesar V. Primary Sjögren’s syndrome in children and adolescents: proposal for diagnostic criteria. Clin Exp Rheumatol. 1999 May-Jun;17(3):381–386. [PubMed] [Google Scholar]
- 157.Houghton K, Malleson P, Cabral D, Petty R, Tucker L. Primary Sjögren’s syndrome in children and adolescents: are proposed diagnostic criteria applicable? J Rheumatol. 2005 Nov;32(11):2225–2232. [PubMed] [Google Scholar]
- 158.Houghton KM, Cabral DA, Petty RE, Tucker LB. Primary Sjögren's syndrome in dizygotic adolescent twins: one case with lymphocytic interstitial pneumonia. J Rheumatol. 2005 Aug;32(8):1603–1606. [PubMed] [Google Scholar]
- 159.de Souza TR, Silva IH, Carvalho AT, Gomes VB, Duarte AP, Leão JC, Gueiros LA. Juvenile Sjögren syndrome: distinctive age, unique findings. Pediatr Dent. 2012 Sep-Oct;34(5):427–430. [PubMed] [Google Scholar]
- 160.Nikitakis NG, Rivera H, Lariccia C, Papadimitriou JC, Sauk JJ. Primary Sjögren syndrome in childhood: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003 Jul;96(1):42–47. doi: 10.1016/S1079-2104(03)00159-8. Available from: http://dx.doi.org/10.1016/S1079-2104(03)00159-8. [DOI] [PubMed] [Google Scholar]
- 161.Lieberman SM. Childhood Sjögren syndrome: insights from adults and animal models. Curr Opin Rheumatol. 2013 Sep;25(5):651–657. doi: 10.1097/BOR.0b013e328363ed23. Available from: http://dx.doi.org/10.1097/BOR.0b013e328363ed23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.McGuirt WF, Jr, Whang C, Moreland W. The role of parotid biopsy in the diagnosis of pediatric Sjögren syndrome. Arch Otolaryngol Head Neck Surg. 2002 Nov;128(11):1279–1281. doi: 10.1001/archotol.128.11.1279. Available from: http://dx.doi.org/10.1001/archotol.128.11.1279. [DOI] [PubMed] [Google Scholar]
- 163.Ramos-Casals M, Tzioufas AG, Stone JH, Sisó A, Bosch X. Treatment of primary Sjögren syndrome: a systematic review. JAMA. 2010 Jul;304(4):452–460. doi: 10.1001/jama.2010.1014. Available from: http://dx.doi.org/10.1001/jama.2010.1014. [DOI] [PubMed] [Google Scholar]
- 164.Skalova S, Minxova L, Slezak R. Hypokalaemic Paralysis Revealing Sjogren’s Syndrome in a 16-Year Old Girl. Ghana Med J. 2008 Sep;42(3):124–128. [PMC free article] [PubMed] [Google Scholar]
- 165.Vitali C, Bootsma H, Bowman SJ, Dorner T, Gottenberg JE, Mariette X, Ramos-Casals M, Ravaud P, Seror R, Theander E, Tzioufas AG. Classification criteria for Sjogren’s syndrome: we actually need to definitively resolve the long debate on the issue. Ann Rheum Dis. 2013 Apr;72(4):476–478. doi: 10.1136/annrheumdis-2012-202565. Available from: http://dx.doi.org/10.1136/annrheumdis-2012-202565. [DOI] [PubMed] [Google Scholar]
- 166.Gottenberg JE, Cinquetti G, Larroche C, Combe B, Hachulla E, Meyer O, Pertuiset E, Kaplanski G, Chiche L, Berthelot JM, Gombert B, Goupille P, Marcelli C, Feuillet S, Leone J, Sibilia J, Zarnitsky C, Carli P, Rist S, Gaudin P, Salliot C, Piperno M, Deplas A, Breban M, Lequerre T, Richette P, Ghiringhelli C, Hamidou M, Ravaud P, Mariette X Club Rhumatismes et Inflammations and the French Society of Rheumatology. Efficacy of rituximab in systemic manifestations of primary Sjogren’s syndrome: results in 78 patients of the AutoImmune and Rituximab registry. Ann Rheum Dis. 2013 Jun;72(6):1026–1031. doi: 10.1136/annrheumdis-2012-202293. Available from: http://dx.doi.org/10.1136/annrheumdis-2012-202293. [DOI] [PubMed] [Google Scholar]
- 167.Baum BJ, Alevizos I, Zheng C, Cotrim AP, Liu S, McCullagh L, Goldsmith CM, Burbelo PD, Citrin DE, Mitchell JB, Nottingham LK, Rudy SF, Van Waes C, Whatley MA, Brahim JS, Chiorini JA, Danielides S, Turner RJ, Patronas NJ, Chen CC, Nikolov NP, Illei GG. Early responses to adenoviral-mediated transfer of the aquaporin-1 cDNA for radiation-induced salivary hypofunction. Proc Natl Acad Sci USA. 2012 Nov;109(47):19403–19407. doi: 10.1073/pnas.1210662109. Available from: http://dx.doi.org/10.1073/pnas.1210662109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xu J, Wang D, Liu D, Fan Z, Zhang H, Liu O, Ding G, Gao R, Zhang C, Ding Y, Bromberg JS, Chen W, Sun L, Wang S. Allogeneic mesenchymal stem cell treatment alleviates experimental and clinical Sjögren syndrome. Blood. 2012 Oct;120(15):3142–3151. doi: 10.1182/blood-2011-11-391144. Available from: http://dx.doi.org/10.1182/blood-2011-11-391144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Harrison JD. Modern management and pathophysiology of ranula: literature review. Head Neck. 2010 Oct;32(10):1310–1320. doi: 10.1002/hed.21326. Available from: http://dx.doi.org/10.1002/hed.21326. [DOI] [PubMed] [Google Scholar]
- 170.Zhi K, Wen Y, Ren W, Zhang Y. Management of infant ranula. Int J Pediatr Otorhinolaryngol. 2008 Jun;72(6):823–826. doi: 10.1016/j.ijporl.2008.02.012. Available from: http://dx.doi.org/10.1016/j.ijporl.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 171.Chow TL, Chan SW, Lam SH. Ranula successfully treated by botulinum toxin type A: report of 3 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008 Jan;105(1):41–42. doi: 10.1016/j.tripleo.2007.04.007. Available from: http://dx.doi.org/10.1016/j.tripleo.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 172.Niccoli-Filho W, Morosolli AR. Surgical treatment of ranula with carbon dioxide laser radiation. Lasers Med Sci. 2004;19(1):12–14. doi: 10.1007/s10103-004-0293-y. Available from: http://dx.doi.org/10.1007/s10103-004-0293-y. [DOI] [PubMed] [Google Scholar]
- 173.McGurk M. Management of the ranula. J Oral Maxillofac Surg. 2007 Jan;65(1):115–116. doi: 10.1016/j.joms.2006.05.033. Available from: http://dx.doi.org/10.1016/j.joms.2006.05.033. [DOI] [PubMed] [Google Scholar]
- 174.Ohta N, Fukase S, Suzuki Y, Ishida A, Aoyagi M. Treatments of various otolaryngological cystic diseases by OK-4321: its indications and limitations. Laryngoscope. 2010 Nov;120(11):2193–2196. doi: 10.1002/lary.21141. Available from: http://dx.doi.org/10.1002/lary.21141. [DOI] [PubMed] [Google Scholar]
- 175.Zhao YF, Jia Y, Chen XM, Zhang WF. Clinical review of 580 ranulas. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004 Sep;98(3):281–287. doi: 10.1016/S1079210404000800. Available from: http://dx.doi.org/10.1016/S1079210404000800. [DOI] [PubMed] [Google Scholar]
- 176.Sigismund PE, Bozzato A, Schumann M, Koch M, Iro H, Zenk J. Management of ranula: 9 years' clinical experience in pediatric and adult patients. J Oral Maxillofac Surg. 2013 Mar;71(3):538–544. doi: 10.1016/j.joms.2012.07.042. Available from: http://dx.doi.org/10.1016/j.joms.2012.07.042. [DOI] [PubMed] [Google Scholar]
- 177.Seifert G, Okabe H, Caselitz J. Epithelial salivary gland tumors in children and adolescents. Analysis of 80 cases (Salivary Gland Register 1965-1984) ORL J Otorhinolaryngol Relat Spec. 1986;48(3):137–149. doi: 10.1159/000275859. [DOI] [PubMed] [Google Scholar]
- 178.Cunningham MJ, Myers EN, Bluestone CD. Malignant tumors of the head and neck in children: a twenty-year review. Int J Pediatr Otorhinolaryngol. 1987 Oct;13(3):279–292. doi: 10.1016/0165-5876(87)90109-1. Available from: http://dx.doi.org/10.1016/0165-5876(87)90109-1. [DOI] [PubMed] [Google Scholar]
- 179.Shapiro NL, Bhattacharyya N. Clinical characteristics and survival for major salivary gland malignancies in children. Otolaryngol Head Neck Surg. 2006 Apr;134(4):631–634. doi: 10.1016/j.otohns.2005.11.018. Available from: http://dx.doi.org/10.1016/j.otohns.2005.11.018. [DOI] [PubMed] [Google Scholar]
- 180.Deng R, Huang X, Hao J, Ding J, Hu Q. Salivary gland neoplasms in children. J Craniofac Surg. 2013 Mar;24(2):511–513. doi: 10.1097/SCS.0b013e3182801866. Available from: http://dx.doi.org/10.1097/SCS.0b013e3182801866. [DOI] [PubMed] [Google Scholar]
- 181.Zeng D, Yu G, Zhang P, Feng Z. Production of chitosan used for flocculant in medium scale. Huan Jing Ke Xue. 2002 Jan;23(1):62–65. [PubMed] [Google Scholar]
- 182.Hockstein NG, Samadi DS, Gendron K, Carpentieri D, Wetmore RF. Pediatric submandibular triangle masses: a fifteen-year experience. Head Neck. 2004 Aug;26(8):675–680. doi: 10.1002/hed.20019. Available from: http://dx.doi.org/10.1002/hed.20019. [DOI] [PubMed] [Google Scholar]
- 183.Bradley P, McClelland L, Mehta D. Paediatric salivary gland epithelial neoplasms. ORL J Otorhinolaryngol Relat Spec. 2007;69(3):137–145. doi: 10.1159/000099222. Available from: http://dx.doi.org/10.1159/000099222. [DOI] [PubMed] [Google Scholar]
- 184.Weber AL, Rahemtullah A, Ferry JA. Hodgkin and non-Hodgkin lymphoma of the head and neck: clinical, pathologic, and imaging evaluation. Neuroimaging Clin N Am. 2003 Aug;13(3):371–392. doi: 10.1016/S1052-5149(03)00039-X. Available from: http://dx.doi.org/10.1016/S1052-5149(03)00039-X. [DOI] [PubMed] [Google Scholar]
- 185.Muenscher A, Diegel T, Jaehne M, Ussmüller J, Koops S, Sanchez-Hanke M. Benign and malignant salivary gland diseases in children A retrospective study of 549 cases from the Salivary Gland Registry, Hamburg. Auris Nasus Larynx. 2009 Jun;36(3):326–331. doi: 10.1016/j.anl.2008.07.006. Available from: http://dx.doi.org/10.1016/j.anl.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 186.da Cruz Perez DE, Pires FR, Alves FA, Almeida OP, Kowalski LP. Salivary gland tumors in children and adolescents: a clinicopathologic and immunohistochemical study of fifty-three cases. Int J Pediatr Otorhinolaryngol. 2004 Jul;68(7):895–902. doi: 10.1016/j.ijporl.2004.02.004. Available from: http://dx.doi.org/10.1016/j.ijporl.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 187.Lee DH, Yoon TM, Lee JK, Lim SC. Clinical features of pediatric parotid tumors: 10-year experience of a single institute. Acta Otolaryngol. 2013 Nov;133(11):1213–1218. doi: 10.3109/00016489.2013.822554. Available from: http://dx.doi.org/10.3109/00016489.2013.822554. [DOI] [PubMed] [Google Scholar]
- 188.Iro H, Zenk J, Koch M, Klintworth N. Follow-up of parotid pleomorphic adenomas treated by extracapsular dissection. Head Neck. 2013 Jun;35(6):788–793. doi: 10.1002/hed.23032. Available from: http://dx.doi.org/10.1002/hed.23032. [DOI] [PubMed] [Google Scholar]
- 189.Aro K, Leivo I, Grénman R, Mäkitie AA. Paediatric salivary gland cancer in Finland. Int J Pediatr Otorhinolaryngol. 2012 Sep;76(9):1304–1307. doi: 10.1016/j.ijporl.2012.05.024. Available from: http://dx.doi.org/10.1016/j.ijporl.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 190.Ellies M, Schaffranietz F, Arglebe C, Laskawi R. Tumors of the salivary glands in childhood and adolescence. J Oral Maxillofac Surg. 2006 Jul;64(7):1049–1058. doi: 10.1016/j.joms.2006.03.006. Available from: http://dx.doi.org/10.1016/j.joms.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 191.Kupferman ME, de la Garza GO, Santillan AA, Williams MD, Varghese BT, Huh W, Roberts D, Weber RS. Outcomes of pediatric patients with malignancies of the major salivary glands. Ann Surg Oncol. 2010 Dec;17(12):3301–3307. doi: 10.1245/s10434-010-1165-2. Available from: http://dx.doi.org/10.1245/s10434-010-1165-2. [DOI] [PubMed] [Google Scholar]
- 192.Orvidas LJ, Kasperbauer JL, Lewis JE, Olsen KD, Lesnick TG. Pediatric parotid masses. Arch Otolaryngol Head Neck Surg. 2000 Feb;126(2):177–184. doi: 10.1001/archotol.126.2.177. Available from: http://dx.doi.org/10.1001/archotol.126.2.177. [DOI] [PubMed] [Google Scholar]
- 193.Callender DL, Frankenthaler RA, Luna MA, Lee SS, Goepfert H. Salivary gland neoplasms in children. Arch Otolaryngol Head Neck Surg. 1992 May;118(5):472–476. doi: 10.1001/archotol.1992.01880050018003. Available from: http://dx.doi.org/10.1001/archotol.1992.01880050018003. [DOI] [PubMed] [Google Scholar]
- 194.Ethunandan M, Ethunandan A, Macpherson D, Conroy B, Pratt C. Parotid neoplasms in children: experience of diagnosis and management in a district general hospital. Int J Oral Maxillofac Surg. 2003 Aug;32(4):373–377. doi: 10.1054/ijom.2002.0381. Available from: http://dx.doi.org/10.1054/ijom.2002.0381. [DOI] [PubMed] [Google Scholar]
- 195.Sultan I, Rodriguez-Galindo C, Al-Sharabati S, Guzzo M, Casanova M, Ferrari A. Salivary gland carcinomas in children and adolescents: a population-based study, with comparison to adult cases. Head Neck. 2011 Oct;33(10):1476–1481. doi: 10.1002/hed.21629. Available from: http://dx.doi.org/10.1002/hed.21629. [DOI] [PubMed] [Google Scholar]
- 196.Ryan JT, El-Naggar AK, Huh W, Hanna EY, Weber RS, Kupferman ME. Primacy of surgery in the management of mucoepidermoid carcinoma in children. Head Neck. 2011 Dec;33(12):1769–1773. doi: 10.1002/hed.21675. Available from: http://dx.doi.org/10.1002/hed.21675. [DOI] [PubMed] [Google Scholar]
- 197.Védrine PO, Coffinet L, Temam S, Montagne K, Lapeyre M, Oberlin O, Orbach D, Simon C, Sommelet D. Mucoepidermoid carcinoma of salivary glands in the pediatric age group: 18 clinical cases, including 11 second malignant neoplasms. Head Neck. 2006 Sep;28(9):827–833. doi: 10.1002/hed.20429. Available from: http://dx.doi.org/10.1002/hed.20429. [DOI] [PubMed] [Google Scholar]
- 198.Rahbar R, Grimmer JF, Vargas SO, Robson CD, Mack JW, Perez-Atayde AR, Marcus KJ, Grier HE, Healy GB, McGill TJ. Mucoepidermoid carcinoma of the parotid gland in children: A 10-year experience. Arch Otolaryngol Head Neck Surg. 2006 Apr;132(4):375–380. doi: 10.1001/archotol.132.4.375. Available from: http://dx.doi.org/10.1001/archotol.132.4.375. [DOI] [PubMed] [Google Scholar]
- 199.Galer C, Santillan AA, Chelius D, Huh W, El-Naggar A, Hanna E, Weber RS, Kupferman ME. Minor salivary gland malignancies in the pediatric population. Head Neck. 2012 Nov;34(11):1648–1651. doi: 10.1002/hed.21980. Available from: http://dx.doi.org/10.1002/hed.21980. [DOI] [PubMed] [Google Scholar]
- 200.Levine SB, Potsic WP. Acinic cell carcinoma of the parotid gland in children. Int J Pediatr Otorhinolaryngol. 1986 Sep;11(3):281–286. doi: 10.1016/S0165-5876(86)80040-4. Available from: http://dx.doi.org/10.1016/S0165-5876(86)80040-4. [DOI] [PubMed] [Google Scholar]
- 201.Strome M. Non-neoplastic salivary gland diseases in children. Otolaryngol Clin North Am. 1977 Jun;10(2):391–398. [PubMed] [Google Scholar]
- 202.White AK. Salivary gland disease in infancy and childhood: non-malignant lesions. J Otolaryngol. 1992 Dec;21(6):422–428. [PubMed] [Google Scholar]
- 203.Bozzato A, Burger P, Zenk J, Uter W, Iro H. Salivary gland biometry in female patients with eating disorders. Eur Arch Otorhinolaryngol. 2008 Sep;265(9):1095–1102. doi: 10.1007/s00405-008-0598-8. Available from: http://dx.doi.org/10.1007/s00405-008-0598-8. [DOI] [PubMed] [Google Scholar]
- 204.Lloyd Faulconbridge RV, Tranter RM, Moffat V, Green E. Review of management of drooling problems in neurologically impaired children: a review of methods and results over 6 years at Chailey Heritage Clinical Services. Clin Otolaryngol Allied Sci. 2001 Apr;26(2):76–81. doi: 10.1046/j.1365-2273.2001.00434.x. Available from: http://dx.doi.org/10.1046/j.1365-2273.2001.00434.x. [DOI] [PubMed] [Google Scholar]
- 205.Nunn JH. Drooling: review of the literature and proposals for management. J Oral Rehabil. 2000 Sep;27(9):735–743. doi: 10.1046/j.1365-2842.2000.00575.x. Available from: http://dx.doi.org/10.1046/j.1365-2842.2000.00575.x. [DOI] [PubMed] [Google Scholar]
- 206.Hillel AD, Miller R. Bulbar amyotrophic lateral sclerosis: patterns of progression and clinical management. Head Neck. 1989 Jan-Feb;11(1):51–59. doi: 10.1002/hed.2880110110. Available from: http://dx.doi.org/10.1002/hed.2880110110. [DOI] [PubMed] [Google Scholar]
- 207.Lespargot A, Langevin MF, Muller S, Guillemont S. Swallowing disturbances associated with drooling in cerebral-palsied children. Dev Med Child Neurol. 1993 Apr;35(4):298–304. doi: 10.1111/j.1469-8749.1993.tb11641.x. Available from: http://dx.doi.org/10.1111/j.1469-8749.1993.tb11641.x. [DOI] [PubMed] [Google Scholar]
- 208.Fischer RB. Was tun bei neuroleptikainduzierter Hypersalivation. [What to do in neuroleptic-induced sialorrhea]. Psychiatr Prax. 2001 Jul;28(5):249–250. (Ger). [PubMed] [Google Scholar]
- 209.Johnson H, King J, Reddihough DS. Children with sialorrhoea in the absence of neurological abnormalities. Child Care Health Dev. 2001 Nov;27(6):591–602. doi: 10.1046/j.1365-2214.2001.00233.x. Available from: http://dx.doi.org/10.1046/j.1365-2214.2001.00233.x. [DOI] [PubMed] [Google Scholar]
- 210.Blasco PA. Management of drooling: 10 years after the Consortium on Drooling, 1990. Dev Med Child Neurol. 2002 Nov;44(11):778–781. doi: 10.1111/j.1469-8749.2002.tb00286.x. Available from: http://dx.doi.org/10.1111/j.1469-8749.2002.tb00286.x. [DOI] [PubMed] [Google Scholar]
- 211.Allaire J. American Academy for Cerebral Palsy and Developmental Medicine AACPDM Annual Meeting; Toronto; 2000. [Google Scholar]
- 212.Brown C. American Academy for Cerebral Palsy and Developmental Medicine AACPDM Annual Meeting; Toronto; 2000. [Google Scholar]
- 213.Harris MM, Dignam PF. A non-surgical method of reducing drooling in cerebral-palsied children. Dev Med Child Neurol. 1980 Jun;22(3):293–299. doi: 10.1111/j.1469-8749.1980.tb03708.x. Available from: http://dx.doi.org/10.1111/j.1469-8749.1980.tb03708.x. [DOI] [PubMed] [Google Scholar]
- 214.Koheil R, Sochaniwskyj AE, Bablich K, Kenny DJ, Milner M. Biofeedback techniques and behaviour modification in the conservative remediation of drooling by children with cerebral palsy. Dev Med Child Neurol. 1987 Feb;29(1):19–26. doi: 10.1111/j.1469-8749.1987.tb02103.x. Available from: http://dx.doi.org/10.1111/j.1469-8749.1987.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 215.Rapp D. Drool control: long-term follow-up. Dev Med Child Neurol. 1980 Aug;22(4):448–453. doi: 10.1111/j.1469-8749.1980.tb04349.x. Available from: http://dx.doi.org/10.1111/j.1469-8749.1980.tb04349.x. [DOI] [PubMed] [Google Scholar]
- 216.McCracken A. Drool control and tongue thrust therapy for the mentally retarded. Am J Occup Ther. 1978 Feb;32(2):79–85. [PubMed] [Google Scholar]
- 217.Fischer-Brandies H, Avalle C, Limbrock GJ. Therapy of orofacial dysfunctions in cerebral palsy according to Castillo-Morales: first results of a new treatment concept. Eur J Orthod. 1987 May;9(2):139–143. doi: 10.1093/ejo/9.2.139. Available from: http://dx.doi.org/10.1093/ejo/9.2.139. [DOI] [PubMed] [Google Scholar]
- 218.Limbrock GJ, Hoyer H, Scheying H. Drooling, chewing and swallowing dysfunctions in children with cerebral palsy: treatment according to Castillo-Morales. ASDC J Dent Child. 1990 Nov-Dec;57(6):445–451. [PubMed] [Google Scholar]
- 219.Hoyer H, Limbrock GJ. Orofacial regulation therapy in children with Down syndrome, using the methods and appliances of Castillo-Morales. ASDC J Dent Child. 1990 Nov-Dec;57(6):442–444. [PubMed] [Google Scholar]
- 220.Limbrock GJ, Hoyer H, Scheying H. Regulation therapy by Castillo-Morales in children with Down syndrome: primary and secondary orofacial pathology. ASDC J Dent Child. 1990 Nov-Dec;57(6):437–441. [PubMed] [Google Scholar]
- 221.Talmi YP, Finkelstein Y, Zohar Y, Laurian N. Reduction of salivary flow with Scopoderm TTS. Ann Otol Rhinol Laryngol. 1988 Mar-Apr;97(2 Pt 1):128–130. doi: 10.1177/000348948809700206. [DOI] [PubMed] [Google Scholar]
- 222.Dettman CE. Suppression of salivation in wind-instrument players with scopolamine. N Engl J Med. 1984 May;310(21):1396. doi: 10.1056/NEJM198405243102122. Available from: http://dx.doi.org/10.1056/NEJM198405243102122. [DOI] [PubMed] [Google Scholar]
- 223.Bothwell JE, Clarke K, Dooley JM, Gordon KE, Anderson R, Wood EP, Camfield CS, Camfield PR. Botulinum toxin A as a treatment for excessive drooling in children. Pediatr Neurol. 2002 Jul;27(1):18–22. doi: 10.1016/S0887-8994(02)00381-8. Available from: http://dx.doi.org/10.1016/S0887-8994(02)00381-8. [DOI] [PubMed] [Google Scholar]
- 224.Ellies M, Rohrbach-Volland S, Arglebe C, Wilken B, Laskawi R, Hanefeld F. Successful management of drooling with botulinum toxin A in neurologically disabled children. Neuropediatrics. 2002 Dec;33(6):327–330. doi: 10.1055/s-2002-37084. Available from: http://dx.doi.org/10.1055/s-2002-37084. [DOI] [PubMed] [Google Scholar]
- 225.Ellies M, Laskawi R, Rohrbach-Volland S, Arglebe C, Beuche W. Botulinum toxin to reduce saliva flow: selected indications for ultrasound-guided toxin application into salivary glands. Laryngoscope. 2002 Jan;112(1):82–86. doi: 10.1097/00005537-200201000-00015. Available from: http://dx.doi.org/10.1097/00005537-200201000-00015. [DOI] [PubMed] [Google Scholar]
- 226.Ellies M, Laskawi R, Rohrbach-Volland S, Rödel R, Beuche W. Sekretionshemmung exokriner Drüsen des Kopf-Hals-Bereiches durch Applikation von Botulinum Toxin A. Therapie seltener Krankheitsbilder. [Blocking secretion of exocrine glands in the head-neck area by administration of botulinum toxin A. Therapy of a rare disease picture]. HNO. 2001 Oct;49(10):807–813. doi: 10.1007/s001060170028. (Ger). Available from: http://dx.doi.org/10.1007/s001060170028. [DOI] [PubMed] [Google Scholar]
- 227.Giess R, Naumann M, Werner E, Riemann R, Beck M, Puls I, Reiners C, Toyka KV. Injections of botulinum toxin A into the salivary glands improve sialorrhoea in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatr. 2000 Jul;69(1):121–123. doi: 10.1136/jnnp.69.1.121. Available from: http://dx.doi.org/10.1136/jnnp.69.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Jongerius PH, van Tiel P, van Limbeek J, Gabreëls FJ, Rotteveel JJ. A systematic review for evidence of efficacy of anticholinergic drugs to treat drooling. Arch Dis Child. 2003 Oct;88(10):911–914. doi: 10.1136/adc.88.10.911. Available from: http://dx.doi.org/10.1136/adc.88.10.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Jongerius PH, Joosten F, Hoogen FJ, Gabreels FJ, Rotteveel JJ. The treatment of drooling by ultrasound-guided intraglandular injections of botulinum toxin type A into the salivary glands. Laryngoscope. 2003 Jan;113(1):107–111. doi: 10.1097/00005537-200301000-00020. Available from: http://dx.doi.org/10.1097/00005537-200301000-00020. [DOI] [PubMed] [Google Scholar]
- 230.Ekedahl C, Mansson I, Sandberg N. Swallowing disorders and drooling. JFORL J Fr Otorhinolaryngol Audiophonol Chir Maxillofac. 1974 Oct;23(8):727–731. [PubMed] [Google Scholar]
- 231.Ekedahl C, Månsson I, Sandberg N. Swallowing dysfunction in the brain-damaged with drooling. Acta Otolaryngol. 1974 Jul-Aug;78(1-2):141–149. doi: 10.3109/00016487409126339. Available from: http://dx.doi.org/10.3109/00016487409126339. [DOI] [PubMed] [Google Scholar]
- 232.Ekedahl C. Surgical treatment of drooling. Acta Otolaryngol. 1974 Mar;77(3):215–220. doi: 10.3109/00016487409124619. Available from: http://dx.doi.org/10.3109/00016487409124619. [DOI] [PubMed] [Google Scholar]
- 233.Ross JA. The function of the tympanic plexus as related to Frey's syndrome. Laryngoscope. 1970 Dec;80(12):1816–1833. doi: 10.1002/lary.5540801204. Available from: http://dx.doi.org/10.1002/lary.5540801204. [DOI] [PubMed] [Google Scholar]
- 234.Michel RG, Johnson KA, Patterson CN. Parasympathetic nerve section for control of sialorrhea. Arch Otolaryngol. 1977 Feb;103(2):94–97. doi: 10.1001/archotol.1977.00780190074008. Available from: http://dx.doi.org/10.1001/archotol.1977.00780190074008. [DOI] [PubMed] [Google Scholar]
- 235.Dundas DF, Peterson RA. Surgical treatment of drooling by bilateral parotid duct ligation and submandibular gland resection. Plast Reconstr Surg. 1979 Jul;64(1):47–51. doi: 10.1097/00006534-197907000-00009. Available from: http://dx.doi.org/10.1097/00006534-197907000-00009. [DOI] [PubMed] [Google Scholar]
- 236.Chang CJ, May-Kuen Wong A. Intraductal laser photocoagulation of the bilateral parotid ducts for reduction of drooling in patients with cerebral palsy. Plast Reconstr Surg. 2001 Apr;107(4):907–913. doi: 10.1097/00006534-200104010-00001. Available from: http://dx.doi.org/10.1097/00006534-200104010-00001. [DOI] [PubMed] [Google Scholar]
- 237.Wilkie TF. The problem of drooling in cerebral palsy: a surgical approach. Can J Surg. 1967 Jan;10(1):60–67. [PubMed] [Google Scholar]
- 238.Massengill R., Jr A follow-up investigation of patients who have had parotid duct transplantation surgery to control drooling. Ann Plast Surg. 1979 Mar;2(3):205–208. doi: 10.1097/00000637-197903000-00005. Available from: http://dx.doi.org/10.1097/00000637-197903000-00005. [DOI] [PubMed] [Google Scholar]
- 239.Wilkie TF, Brody GS. The surgical treatment of drooling. A ten-year review. Plast Reconstr Surg. 1977 Jun;59(6):791–797. doi: 10.1097/00006534-197706000-00001. Available from: http://dx.doi.org/10.1097/00006534-197706000-00001. [DOI] [PubMed] [Google Scholar]
- 240.Ozgenel GY, Ozcan M. Bilateral parotid-duct diversion using autologous vein grafts for the management of chronic drooling in cerebral palsy. Br J Plast Surg. 2002 Sep;55(6):490–493. doi: 10.1054/bjps.2002.3884. Available from: http://dx.doi.org/10.1054/bjps.2002.3884. [DOI] [PubMed] [Google Scholar]
- 241.Crysdale WS. Management options for the drooling patient. Ear Nose Throat J. 1989 Nov;68(11):820, 825–826, 829. [PubMed] [Google Scholar]
- 242.Crysdale WS, White A. Submandibular duct relocation for drooling: a 10-year experience with 194 patients. Otolaryngol Head Neck Surg. 1989 Jul;101(1):87–92. doi: 10.1177/019459988910100114. [DOI] [PubMed] [Google Scholar]
- 243.Crysdale WS, Raveh E, McCann C, Roske L, Kotler A. Management of drooling in individuals with neurodisability: a surgical experience. Dev Med Child Neurol. 2001 Jun;43(6):379–383. doi: 10.1017/S0012162201000718. Available from: http://dx.doi.org/10.1017/S0012162201000718. [DOI] [PubMed] [Google Scholar]
- 244.Xie CM, Kubba H. Parotidectomy in children: indications and complications. J Laryngol Otol. 2010 Dec;124(12):1289–1293. doi: 10.1017/S0022215110001209. Available from: http://dx.doi.org/10.1017/S0022215110001209. [DOI] [PubMed] [Google Scholar]
- 245.Wright GL, Smith RJ, Katz CD, Atkins JH., Jr Benign parotid diseases of childhood. Laryngoscope. 1985 Aug;95(8):915–920. doi: 10.1288/00005537-198508000-00006. Available from: http://dx.doi.org/10.1288/00005537-198508000-00006. [DOI] [PubMed] [Google Scholar]
- 246.Ribeiro Kde C, Kowalski LP, Saba LM, de Camargo B. Epithelial salivary glands neoplasms in children and adolescents: a forty-four-year experience. Med Pediatr Oncol. 2002 Dec;39(6):594–600. doi: 10.1002/mpo.10168. Available from: http://dx.doi.org/10.1002/mpo.10168. [DOI] [PubMed] [Google Scholar]
- 247.Malone B, Baker SR. Benign pleomorphic adenomas in children. Ann Otol Rhinol Laryngol. 1984 May-Jun;93(3 Pt 1):210–214. doi: 10.1177/000348948409300304. [DOI] [PubMed] [Google Scholar]


