Clinical Vignette
A 9 year old boy with a history of chronic atopic dermatitis with recurrent skin infections presented with extensive eczematous dermatitis that started behind his knees at the age of 4 years and steadily worsened to include the popliteal and antecubital fossae, arms and abdomen. He scratched the lesions, especially at night, with the result that his sleep was disturbed. Despite the use of chronic moisturizing therapy and topical corticosteroids, he has seasonal flares of eczema during the winter months. When he was 6 years old, he also had experienced bronchial asthma with persistent cough. He is not allergic to any food. Regarding family history of other atopic conditions, his father has asthma and his younger sister has allergic rhinitis and cow’s milk allergy. He was referred to an allergist for further evaluation and management.
Physical examination showed injected conjunctivae and presence of Dennie-Morgan lines. There was a ‘cobblestone’ appearance of his posterior pharynx. Lungs were clear bilaterally with good aeration. Cardiac and abdominal examinations were unremarkable. Skin examination demonstrated scattered scaly eczematous patches along the flexural areas of his upper and lower extremities. Impetigo “crust like” lesions with serum oozing were found on the left elbow.
Laboratory testing to an environmental allergen panel was performed, including common indoor and outdoor allergens, as skin testing could not be performed due to eczematous lesion on arms. His total serum IgE level was markedly elevated at 4300 IU/ml with highly positive ImmunoCAP specific IgE levels to dust mite, mouse, and cockroach, along with multiple tree and grass pollens. Dust samples vacuumed from his home and sent to a commercial laboratory showed very high levels of house dust mite.
Appropriate allergen avoidance and integrative pest management (IPM) strategies were discussed with the patient’s family. As he was symptomatic despite traditional emollient and topical corticosteroid therapy, he was started on cyclosporine and topical tacrolimus and the possibility of allergen immunotherapy was discussed with his family.
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by pruritic skin lesions, disrupted skin barrier function, dysregulation of the immune system, and allergic reactions to food and environmental allergens. It generally presents in early childhood. Scratching at the area of pruritis leads to redness, cracking, scaling and potential superinfection of the skin. Most AD patients have personal or family history of atopy (allergic rhinitis, allergic conjunctivitis, AD or asthma). The differential diagnosis of AD is listed in Table 1.
Table 1.
| Diagnosis | Description |
|---|---|
| Seborrheic dermatitis | Red, shiny, relatively well-demarcated eruptions typically involving the diaper area. The lower abdomen and armpits may also be involved, and scalp scaling may be present. |
| Discoid (nummular) eczema | Circular “cracked” areas of erythema 1 to 5 cm in diameter are present initially on limbs, often with secondary infection |
| Irritant contact dermatitis | Cumulative damage to the skin barrier from irritants such as soaps and detergents is present. The clinical appearance can be identical to that of atopic dermatitis, but location at sites of maximal exposure. |
| Allergic contact dermatitis | hypersensitivity reaction exists after sensitization to specific substances (e.g., nickel in jewelry, the rubber in gloves) |
| Frictional lichenoid dermatitis | Shiny papules occur at elbows, knees, and backs of hands, probably related to friction. The diagnosis may be common, and may be more so in patients with atopic dermatitis. |
| Scabies | Infestation may produce nonspecific eczematous changes on the entire body. Burrows and pustules on palms, soles, genitalia, and between fingers help to establish diagnosis |
| Cutaneous T-cell lymphoma (Mycosis Fungoides) | Adult with history of eczema; diagnosis by biopsy |
| Zinc deficiency; acrodermatitis enteropathica | Infant with eczema that does not respond to steroid; Periorificail rash; Necrotic areas around nose |
| Netherton syndrome | Dermatitis, scaly skin, with short, spiky, brittle hair or “bamboo hair” |
| Immune deficiencies Wiskott-Aldrich syndrome Immunodysregulation, polyendocrinopathy, enteropathy, X linked (IPEX syndrome) Hyper IgE syndrome Severe combined immunodeficiency (SCID) |
Dermatitis, recurrent infection, and low platelet Dermatitis, intractable diarrhea, diabetes, and hypothyroid Chronic eczema with impetigo Dermatitis, failure to thrive, diarrhea, and life threatening infections |
Data from the National Survey of Children’s Health in the United States demonstrates a wide range of prevalence from 8.7% to 18.1% in various locales with the higher prevalence reported in the east coast states.1 In Europe, 10% to 20% of children and teenagers are affected by AD.2 Approximately 50% of patients develop this disease during first year of life and 30% between the ages of 1–5 years.3
Pathogenesis
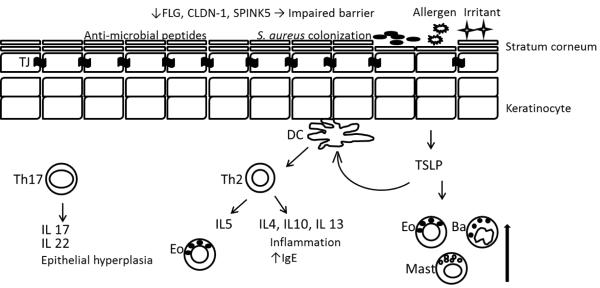
Atopic dermatitis is a condition that requires interplay from several factors to explain its pathogenesis. Defects in epidermal barriers, dysregulation of various types of immune responses, genetic polymorphisms and environmental factors have been implicated in the pathogenesis of the disease. 4, 5 Figure 1 provides a schematic of the immunologic pathology associated with atopic dermatitis that is explained in the following paragraphs.
Figure 1.
Defective epidermal barrier in the pathogenesis of AD. A reduction in FLG, CLDN-1, SPINK5, and other injuries lead to increase permeation of allergen and increase trans-epidermal water loss. Decreased antimicrobial peptides, like beta-defensins and cathelicidins result in bacterial colonization. Activated DCs by TSLP from keratinocytes and by antigens stimulate proliferation of Th2 cells. Th2 cells secrete inflammatory cytokines that worsen AD severity. Mast cells, basophils, and eosinophils are induced by TSLP. Th17 cells secrete IL17 and IL22 that can cause epithelial hyperplasia. FLG: filaggrin, CLDN-1: claudin 1, SPINK5: Kazal-type serine protease inhibitor, TJ: tight junction, DC: dendritic cell, TSLP: Thymic stromal lymphopoietin, Eo: eosinophil, Ba: basophil, Mast: mast cell.
Defective epidermal barrier
The epidermis has 4 layers. The outermost layer, called the stratum corneum, serves as a barrier to reduce water evaporation and penetration of exogenous allergens and microbes. The stratum corneum is composed of cells that have keratin proteins and structural components, such as ceramides, filaggrin and lipids. Filaggrin protein (FLG) plays a crucial role in maintaining the structure of the epidermis by aggregating keratin filaments to form a cytoskeleton in epidermal cells. FLG is released from keratohyalin F granules as an inactive form and is converted to an active form by proteolysis and dephosphorylation. Studies have found that mutations in FLG gene, specifically R501X and 2282del4, can induce a reduction of natural moisturizing factors including sodium pyrroloidone carboxylic acid, urocanic acid, and lipoprotein components especially ceramides.6, 7 A meta-analysis of these nonsense mutations has confirmed that these mutations represent the most compelling genetic risk for AD.6 The alteration of the epidermal structure due to this protein mutation leads to trans-epidermal water loss and evaporation, resulting in dry skin and itching. Apart from FLG, a reduction of SPINK5 gene expression, which encodes Kazal-type 5 serine protease inhibitor, can increase cleavage of intercellular attachments in the stratum corneum and can compromise barrier function.8 Additionally, De Benedetto et al. demonstrated that reduced expression of epidermal claudin-1, a transmembrane protein component of tight junctions (TJs), can cause an impairment in TJs which leads to skin barrier dysfunction in patients with AD.9
Dysregulation of cutaneous immune response
Thymic stromal lymphopoietin (TSLP) plays an important role in AD. TSLP expression in keratinocytes is induced by mechanical injury as well as stimulation of Toll-like receptors 2, 5 and 6. TSLP activates dendritic cells. This leads to proliferation of CD4 T cells which then differentiate into T-Helper 2 cells. Next, inflammatory cytokines such as IL4, IL5 and IL13 are produced and released. Furthermore, TSLP also stimulates mast cells, basophils and eosinophils which play a crucial role in cutaneous inflammation.8 It has been shown that scratching can induce TSLP expression and aggravate the course of AD resulting in a vicious cycle among itching, scratching, TSLP expression and Th2 upregulation.10 Furthermore, Th2 cells produce IL-31, which provokes pruritus. Th17 cells, which can produce IL17 and IL22, are also involved in AD pathogenesis.11 Th17 cells are normally found in acutely inflamed skin lesions. An increase in the number of Th17 cells correlates with the severity of AD. In a mouse model, FLG deficiency leads to Th17 dominated skin inflammation.12 Additionally, IL22 induces epidermal hyperplasia that may lead to epidermal acanthosis in the chronic stages of AD.12
Treatment
Education of patients and families is one of the most effective treatments for AD. Information about avoidance of irritants and allergens is important in preventing AD exacerbations. In addition, written eczema action plans may be beneficial by enabling eczema self-management by reminding patients and caregivers of maintenance regimens and additional therapies to incorporate during flares.13
In order to assess the severity and extent of AD, several parameters have been used such as The Eczema Area and Severity Index (EASI) , Rajka and Lengeland: Grading of severity of atopic dermatitis, and The scoring of atopic dermatitis (SCORAD).14 SCORAD is one of parameters widely used to assess disease severity and determine whether the treatment is effective. There are 3 major components in SCORAD: A) percentage of affected surface area, B) intensity of eczema at lesions, on a scale of 0 to 3, composed of erythema, edema, excoriations, oozing, lichenification, and skin dryness, and C) functional impact evaluated by visual scale (0-10) composed of pruritus and sleep disturbance. The SCORAD score is calculated by using the formula A/5 + 7B/2 + C. SCORAD scores <20, 20-40, and >40 suggest mild, moderate, and severe AD, respectively. 14
An updated practice parameter in 2012 discusses a step-wise approach based on severity, including allergen/irritant avoidance, skin barrier repair, and use of anti-inflammatory and antimicrobial agents.15 However, difficult to control AD can still be a therapeutic challenge. There are a variety of proven AD therapies (Tables 2 & 3). Topical steroids and calcineurin inhibitors are preferred in the treatment of severe AD. In addition, systemic anti-inflammatory treatment, allergen specific immunotherapy and phototherapy are options for management of refractory AD cases. In rare cases, hospitalization might be needed to temporarily reduce exposure to environmental triggers while initiating intensive patient education, further diagnostic testing, and administration of antibiotics among other aggressive treatments. Outpatient therapy may also include the use of bleach baths, vitamin D supplementation, immunomodulatory and biologic therapies.
Table 2.
| Treatment | Action | Common adverse effect |
|---|---|---|
| Emollient, moisturizers | Moisturize dry skin and help repair the defective skin barrier | No significant side effect |
| Avoidance of triggers | Prevent known allergic reactions | - |
| Topical corticosteroids | Anti-inflammatory response | Straie and atrophy of the skin, rosacea, suppression of the hypothalamic- pituitary-adrenal axis |
| Topical calcineurin inhibitors | Anti-inflammatory response, proactive treatment (tacrolismus) | Burning sensation of the skin, facial flushing, itching |
| Vitamin D | Improve cathelicidin production | Hypercalcemia,nausea, vomiting |
| Antihistamines | Sedating effect aid in sleeping, decrease night-time scratching and skin excoriation |
Drowsiness, dizziness |
| Bleach Bath | Decrease microbial load on AD skin, esp. S. aureus | No significant side effect |
| Topical/Systemic antibiotic | Treat cutaneous bacterial, fungal, or viral infection | Depend on each drug's adverse effect |
| Immunomodulation agents | ||
| Systemic corticosteroids | Anti-inflammatory response | Abdominal discomfort, increase risk of infection, suppression of the hypothalamic-pituitary-adrenal axis |
| Azathioprine | Inhibit purine biosynthesis with anti-inflammatory activity | Myelosupression, elevated liver enzyme |
| Cyclosporin A | Suppress inflammatory cytokine gene transcription in T cells | Abdominal discomfort, hypertrichosis, paresthesias,hypertension, hyperbilirubinemia, renal impairment |
| Mycophenolate | Inhibit purine biosynthesis with immunosuppressive activity | Gastrointestinal symtomps (nausea, diarrhea), leukopenia, thrombopenia |
| Methotrexate | Inhibit purine and pyrimidine systhesis | Nausea, elevated liver enzyme level |
| Alitretinoin | Bind to retinoid and rexinoid receptors with anti-inflammatory activity | Headache, elevated TSH and serum lipid level |
| IFNgamma | Downregulate Th2 cell function | Influenza-like symptoms |
| Phototherapy | ||
| Narrow-band UVB (peak: 331-313 nm) | Anti-inflammatory response through inhibition of langerhans cells and Alteration of cytokine production. Antimicrobial effects by reducing the colonization of S. aureus. |
Short term: skin erythema, pain, pruritus , pigmentation Long term: premature skin aging and cutaneous malignanacy |
| Broadband UVB (280-320 nm) | ||
| UVA1 (340-400 nm) | ||
| Allergen Immunotherapy | Induce apoptosis/anergy of T cells and induces immune-regulatory responses and immune deviation towards Th1 |
Transient increase in serum IgE levels, transient eczema flares, increase risk for anaphylaxis reaction, or transient exacerbation of underlying atopic disease |
Table 3.
Potency rating of topical corticosteroids38
| Potency (Group) | Medication | Dosage Vehicle |
|---|---|---|
| Ultra high (I) | Augmented betamethasone dipropionate 0.05% |
G, O |
| Clobetasol propionate 0.05% | L, Sh, F, C, G, O |
|
| Diflorasone diacetate 0.05% | O | |
| Fluocinonide 0.1% | C | |
| Flurandrenolide 4 mcg per m2 | T | |
| Halobetasol propionate 0.05% | C, O | |
|
| ||
| High (II) | Amcinonide 0.1% | O |
| Augmented betamethasone dipropionate 0.05% |
L, C | |
| Betamethasone dipropionate 0.05% | O | |
| Desoximetasone | C, G, O | |
| Diflorasone diacetate 0.05% | C | |
| Fluocinonide 0.05% | C, G, O | |
| Halcinonide 0.1% | C, O, So | |
|
| ||
| Medium to high (III) | Amcinonide 0.1% | C |
| Betamethasone dipropionate 0.05% | C | |
| Fluticasone propionate 0.005% | O | |
| Triamcinolone acetonide 0.5% | C, O | |
|
| ||
| Medium (IV and V) | Betamethasone valerate | C, L, F |
| Desoximetasone 0.05% | C | |
| Fluocinolone acetonide 0.025% | C, O | |
| Fluticasone propionate 0.05% | C | |
| Hydrocortisone butyrate 0.1% | O | |
| Hydrocortisone probutate 0.1% | C | |
| Hydrocortisone valerate 0.2% | C, O | |
| Mometasone furoate 0.1% | C, L, O | |
| Triamcinolone acetonide 0.025% | C, L, O | |
| Triamcinolone acetonide 0.1% | C, L,O | |
|
| ||
| Low (VI) | Alclometasone dipropionate 0.05% | C, O |
| Desonide 0.05% | G, C, O, L, F | |
| Fluocinolone 0.01% | C | |
| Hydrocortisone butyrate 0.1% | C | |
|
| ||
| Least potent (VII) | Hydrocortisone 1%, 2.5% | C, L, O |
* C = cream; F = foam; G = gel; L = lotion; O = ointment; Sh = shampoo; So = solution; T = tape
Bleach baths
The current recommendation for the concentration of bleach in the bath is 0.005%. To reach this concentration, 1/4 cup of bleach is added to a half-filled 40-gallon bath. Huang et al,16 reported that with twice-weekly bathing, eczema severity and the affected body surface area significantly decreased. Two recent controlled trial studies have found no significant between treatment and placebo groups.17, 18 Dry skin, burning sensation were reported as side-effects of using bleach bath.17 However, these dilute bleach baths have been recommended as a maintenance therapy to suppressing S. aureus overgrowth.15
Vitamin D
Normally, antimicrobial peptides prevent infection by this microbe, however, their protective roles are inefficient as a result of deficient beta-defensin and cathelicidin expression in patients with AD.19 Studies have demonstrated the beneficial effects of vitamin D on the innate immune response in patients with AD. Peroni et al, evaluated the severity of disease in 37 children and found that levels of 25(OH)D were significantly higher in patients with mild AD compared to those with moderate and severe AD.20 Furthermore, patients with AD who took an oral dose of 4000 IU of vitamin D daily for 3 weeks had a significant increase in cathelicidin expression,21 suggesting that supplementation with oral vitamin D could improve the innate antimicrobial protection in patients with AD.
Systemic immunomodulatory agents
In severe AD, systemic steroids are usually effective, but should not be used for a long period of time because of side effects. Therefore, systemic immunomodulatory treatments should be considered instead. Consideration should be given to the specific potential side effects attributable to the specific agent when selecting a treatment and monitoring plan.15, 22 For example, cyclosporine A is an effective drug but has a narrow therapeutic index. Patients receiving cyclosporine have to be monitored closely for alterations in blood pressure and renal function. Azathioprine has a slow onset of action and myeolosuppression is a major potential adverse effect. However, screening for thiopurine methyltransferase activity before initiating treatment may identify those at high risk. Mycophenolate mofetil has been used to treat AD in both adults and children and has a more favorable safety profile. Methotrexate has been shown to be effective in adult patients with AD but pediatric clinical trial data is lacking. Liver toxicity and teratogenicity are its main adverse effects. IFN gamma is known to antagonize the Th2 immune response and has the ability to decrease blood eosinophilia. Flu-like symptoms are common adverse effects and limit the use of this treatment clinically.
Phototherapy
Phototherapy is another useful treatment of chronic and recalcitrant AD. Narrow-band UVB (peak: 331-313 nm), broadband UVB (280-320 nm), and UVA1 (340-400 nm) are commonly used. Clayton et al, reported that of 60 children with severe AD (age range 4-16 year) who receiving narrow-band UVB treatment, 40% had complete clearance and 46% had moderate to good improvement.23 Adverse effects include skin erythema, skin pain and pruritus. Cutaneous malignancy and premature skin aging are potential long-term adverse effects.
Allergen specific immunotherapy
Allergen specific immunotherapy (SIT) can be an effective treatment for AD associated with allergen sensitivity. SIT can be administered subcutaneously (SCIT) or sublingually (SLIT). Both SLIT and SCIT have shown promising results in reducing topical steroid use and improving SCORAD scores in patients with AD.24 The safety and efficacy of sublingual immunotherapy using aqueous preparations for subcutaneous administration has not been established by the US FDA.25 Patients with a positive skin test and corresponding history of AD exacerbations are good candidates for SIT.26 Recently, a meta-analysis by Jung Min Bae provides the evidence for the efficacy of SIT for the treatment of AD. This study found that SIT had significant positive effect on AD with odds ratio (OR) 5.35 (95% confidence interval (CI) 1.61-17.77). Moreover, patients with severe AD showed significant improvement with SIT (odds ratio 6.42, 95%CI 1.31-7.48).27 Additionally, A multi-centre, randomized dose–response, double-blind trial by Werfel et al28 investigated SIT in 89 adults with chronic AD and sensitized to house dust mite. The result showed that subcutaneous immunotherapy with dust mite allergen extract administered weekly for 1 year was able to improve eczema in sensitized patients and reduce the use of steroids. Adverse effects included transient increase in serum IgE levels, transient eczema flares, increased risk for anaphylaxis, and transient exacerbation of underlying atopic disease.
Biologic therapy
With greater understanding of the immunopathogenesis of AD, biologic therapies present a promising therapeutic option. Omalizumab, an exogenous monoclonal anti-IgE antibody, has shown efficacy in the treatment of severe asthma. To date, it has not been observed to have significant clinical benefit in most patients with AD.29 Anti-TSLP is of great interest and antagonists of TSLP are under investigation for patients with AD or asthma30 and currently in Phase I clinical trials (clinicaltrials.gov). Treatments targeting IL31 are in Phase I clinical trials, as well. Rituximab, a monoclonal anti-CD20 antibody, was demonstrated by Simon et al.31 to improve skin symptoms in patients with severe AD when treated with 2 intravenous infusions of 1000 mg administered 2 weeks apart. However, Sediva et al,32 found that treatment with 500 mg of rituximab administered intravenously twice over a 2-week interval to patients with severe AD resulted in transient improvement followed by deterioration. Additionally, the use of rituximab could lead to depletion of CD20+cells31, affecting immunoglobulin production, and increase the risk of infection. Recently, a randomized, double-blind study led by Beck et al, showed efficacy of dupilumab, a monoclonal antibody to the alpha subunit of the interleukin-4 receptor, in improving symptoms of moderate-to-severe atopic dermatitis with few adverse events or infections.33 Although there is no approved biologic therapy for AD, these and other immune-targeted molecules show promise for future therapeutic agents. Further studies will be needed to fully understand the efficacy and safety of biologic therapy.
Conclusion
Atopic Dermatitis is an inflammatory skin condition which may have a chronic or intermittent course. Many patients are effectively treated by using emollients in combination with topical corticosteroids. However, severe AD may require the use of immunomodulating systemic or topical therapy, as in the case presented, or advanced therapies such as phototherapy and SIT. A variety of novel biologic agents that target pathogenic immune molecules, such as anti-CD20, TSLP, and IL31 are currently being investigated. Advanced understanding in the pathogenesis underlying this disease, briefly depicted in Figure 1, is essential for developing new targets for effective treatments to improve the quality of life for patients with AD.
Acknowledgments
This article was supported by K24 AI 106822, U10HL098102 (PI: Phipatanakul) and K23AI106945 (PI: Gaffin) from the National Institutes of Health, Research Scholarship from Ramathibodi Hospital, Mahidol University, Bangkok, Thailand (Kanchongkittiphon),
This work was also conducted with the support from Harvard Catalyst. The Harvard Clinical and Translational Science Center (NIH Award # UL1 TR001102 and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, the National Center for Research Resources, or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Financial Interests Declaration: All authors declare that they have no competing financial interests.
References
- 1.Shaw TE, Currie GP, Koudelka CW, Simpson EL. Eczema prevalence in the United States: data from the 2003 National Survey of Children's Health. The Journal of investigative dermatology. 2011;131(1):67–73. doi: 10.1038/jid.2010.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters AS, Kellberger J, Vogelberg C, et al. Prediction of the incidence, recurrence, and persistence of atopic dermatitis in adolescence: a prospective cohort study. The Journal of allergy and clinical immunology. 2010;126(3):590–5. doi: 10.1016/j.jaci.2010.06.020. e1-3. [DOI] [PubMed] [Google Scholar]
- 3.Leung DYM, Eichenfield LF, Boguniewicz M. In: Atopic dermatitis (atopic eczema) 7th Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, Wolff K, editors. McGraw-Hill; New York: 2008. [Google Scholar]
- 4.Sicherer SH, Leung DY. Advances in allergic skin disease, anaphylaxis, and hypersensitivity reactions to foods, drugs, and insects in 2013. The Journal of allergy and clinical immunology. 2014;133(2):324–34. doi: 10.1016/j.jaci.2013.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Sprecher E, Leung DY. Atopic dermatitis: scratching through the complexity of barrier dysfunction. The Journal of allergy and clinical immunology. 2013;132(5):1130–1. doi: 10.1016/j.jaci.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez E, Baurecht H, Herberich E, et al. Meta-analysis of filaggrin polymorphisms in eczema and asthma: robust risk factors in atopic disease. The Journal of allergy and clinical immunology. 2009;123(6):1361–70. doi: 10.1016/j.jaci.2009.03.036. e7. [DOI] [PubMed] [Google Scholar]
- 7.Gao PS, Rafaels NM, Hand T, et al. Filaggrin mutations that confer risk of atopic dermatitis confer greater risk for eczema herpeticum. The Journal of allergy and clinical immunology. 2009;124(3):507–13. doi: 10.1016/j.jaci.2009.07.034. 13 e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziegler SF. Thymic stromal lymphopoietin and allergic disease. The Journal of allergy and clinical immunology. 2012;130(4):845–52. doi: 10.1016/j.jaci.2012.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Benedetto A, Rafaels NM, McGirt LY, et al. Tight junction defects in patients with atopic dermatitis. The Journal of allergy and clinical immunology. 2011;127(3):773–86. doi: 10.1016/j.jaci.2010.10.018. e1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simon D, Kernland Lang K. Atopic dermatitis: from new pathogenic insights toward a barrier-restoring and anti-inflammatory therapy. Current opinion in pediatrics. 2011;23(6):647–52. doi: 10.1097/MOP.0b013e32834cad0a. [DOI] [PubMed] [Google Scholar]
- 11.Cornelissen C, Marquardt Y, Czaja K, et al. IL-31 regulates differentiation and filaggrin expression in human organotypic skin models. The Journal of allergy and clinical immunology. 2012;129(2):426–33. doi: 10.1016/j.jaci.2011.10.042. 33 e1-8. [DOI] [PubMed] [Google Scholar]
- 12.Nograles KE, Zaba LC, Shemer A, et al. IL-22-producing "T22" T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells. The Journal of allergy and clinical immunology. 2009;123(6):1244–52. doi: 10.1016/j.jaci.2009.03.041. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rork JF, Sheehan WJ, Gaffin JM, et al. Parental response to written eczema action plans in children with eczema. Archives of dermatology. 2012;148(3):391–2. doi: 10.1001/archdermatol.2011.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology. 1993;186(1):23–31. doi: 10.1159/000247298. Severity scoring of atopic dermatitis: the SCORAD index. Basel, Switzerland. [DOI] [PubMed] [Google Scholar]
- 15.Schneider L, Tilles S, Lio P, et al. Atopic dermatitis: a practice parameter update 2012. The Journal of allergy and clinical immunology. 2013;131(2):295–9. doi: 10.1016/j.jaci.2012.12.672. e1-27. [DOI] [PubMed] [Google Scholar]
- 16.Huang JT, Abrams M, Tlougan B, Rademaker A, Paller AS. Treatment of Staphylococcus aureus colonization in atopic dermatitis decreases disease severity. Pediatrics. 2009;123(5):e808–14. doi: 10.1542/peds.2008-2217. [DOI] [PubMed] [Google Scholar]
- 17.Wong SM, Ng TG, Baba R. Efficacy and safety of sodium hypochlorite (bleach) baths in patients with moderate to severe atopic dermatitis in Malaysia. The Journal of dermatology. 2013;40(11):874–80. doi: 10.1111/1346-8138.12265. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan SL, Forbes A, Hammerman WA, et al. Randomized trial of "bleach baths" plus routine hygienic measures vs. routine hygienic measures alone for prevention of recurrent infections. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014;58(5):679–82. doi: 10.1093/cid/cit764. [DOI] [PubMed] [Google Scholar]
- 19.Nizet V, Ohtake T, Lauth X, et al. Innate antimicrobial peptide protects the skin from invasive bacterial infection. Nature. 2001;414(6862):454–7. doi: 10.1038/35106587. [DOI] [PubMed] [Google Scholar]
- 20.Peroni DG, Piacentini GL, Cametti E, Chinellato I, Boner AL. Correlation between serum 25-hydroxyvitamin D levels and severity of atopic dermatitis in children. The British journal of dermatology. 2011;164(5):1078–82. doi: 10.1111/j.1365-2133.2010.10147.x. [DOI] [PubMed] [Google Scholar]
- 21.Mutgi K, Koo J. Update on the role of systemic vitamin D in atopic dermatitis. Pediatric dermatology. 2013;30(3):303–7. doi: 10.1111/j.1525-1470.2012.01850.x. [DOI] [PubMed] [Google Scholar]
- 22.Ring J, Alomar A, Bieber T, et al. Guidelines for treatment of atopic eczema (atopic dermatitis) part I. Journal of the European Academy of Dermatology and Venereology : JEADV. 2012;26(8):1045–60. doi: 10.1111/j.1468-3083.2012.04635.x. [DOI] [PubMed] [Google Scholar]
- 23.Clayton TH, Clark SM, Turner D, Goulden V. The treatment of severe atopic dermatitis in childhood with narrowband ultraviolet B phototherapy. Clinical and experimental dermatology. 2007;32(1):28–33. doi: 10.1111/j.1365-2230.2006.02292.x. [DOI] [PubMed] [Google Scholar]
- 24.Compalati E, Rogkakou A, Passalacqua G, Canonica GW. Evidences of efficacy of allergen immunotherapy in atopic dermatitis: an updated review. Current opinion in allergy and clinical immunology. 2012;12(4):427–33. doi: 10.1097/ACI.0b013e328354e540. [DOI] [PubMed] [Google Scholar]
- 25.Cox L. Sublingual immunotherapy for aeroallergens: status in the United States. Allergy and asthma proceedings : the official journal of regional and state allergy societies. 2014;35(1):34–42. doi: 10.2500/aap.2014.35.3708. [DOI] [PubMed] [Google Scholar]
- 26.Cox L, Nelson H, Lockey R, et al. Allergen immunotherapy: a practice parameter third update. The Journal of allergy and clinical immunology. 2011;127(1 Suppl):S1–55. doi: 10.1016/j.jaci.2010.09.034. [DOI] [PubMed] [Google Scholar]
- 27.Bae JM, Choi YY, Park CO, Chung KY, Lee KH. Efficacy of allergen-specific immunotherapy for atopic dermatitis: a systematic review and meta-analysis of randomized controlled trials. The Journal of allergy and clinical immunology. 2013;132(1):110–7. doi: 10.1016/j.jaci.2013.02.044. [DOI] [PubMed] [Google Scholar]
- 28.Werfel T, Breuer K, Rueff F, et al. Usefulness of specific immunotherapy in patients with atopic dermatitis and allergic sensitization to house dust mites: a multi-centre, randomized, dose-response study. Allergy. 2006;61(2):202–5. doi: 10.1111/j.1398-9995.2006.00974.x. [DOI] [PubMed] [Google Scholar]
- 29.Heil PM, Maurer D, Klein B, Hultsch T, Stingl G. Omalizumab therapy in atopic dermatitis: depletion of IgE does not improve the clinical course - a randomized, placebo-controlled and double blind pilot study. Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology : JDDG. 2010;8(12):990–8. doi: 10.1111/j.1610-0387.2010.07497.x. [DOI] [PubMed] [Google Scholar]
- 30.Gauvreau GM, O'Byrne PM, Boulet LP, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. The New England journal of medicine. 2014;370(22):2102–10. doi: 10.1056/NEJMoa1402895. [DOI] [PubMed] [Google Scholar]
- 31.Simon D, Hosli S, Kostylina G, Yawalkar N, Simon HU. Anti-CD20 (rituximab) treatment improves atopic eczema. The Journal of allergy and clinical immunology. 2008;121(1):122–8. doi: 10.1016/j.jaci.2007.11.016. [DOI] [PubMed] [Google Scholar]
- 32.Sediva A, Kayserova J, Vernerova E, et al. Anti-CD20 (rituximab) treatment for atopic eczema. The Journal of allergy and clinical immunology. 2008;121(6):1515–6. doi: 10.1016/j.jaci.2008.03.007. author reply 6-7. [DOI] [PubMed] [Google Scholar]
- 33.Beck LA, Thaci D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. The New England journal of medicine. 2014;371(2):130–9. doi: 10.1056/NEJMoa1314768. [DOI] [PubMed] [Google Scholar]
- 34.Williams HC. Clinical practice. Atopic dermatitis. The New England journal of medicine. 2005;352(22):2314–24. doi: 10.1056/NEJMcp042803. [DOI] [PubMed] [Google Scholar]
- 35.Calabria C, Commins S, Gerrish P, et al. Hypersensitivity Disorders: American College of Allergy. Asthma & Immunology. 2010 [Google Scholar]
- 36.Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: Section 2. Management and treatment of atopic dermatitis with topical therapies. Journal of the American Academy of Dermatology. 2014;71(1):116–32. doi: 10.1016/j.jaad.2014.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: Section 3. Management and treatment with phototherapy and systemic agents. Journal of the American Academy of Dermatology. 2014 doi: 10.1016/j.jaad.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ference JD, Last AR. Choosing topical corticosteroids. American family physician. 2009;79(2):135–40. [PubMed] [Google Scholar]