Abstract
Traumatic brain injury (TBI) is deemed the “signature injury” of recent military conflicts in Afghanistan and Iraq, largely because of increased blast exposure. Injuries to the brain can often be misdiagnosed, leading to further complications in the future. Therefore, the use of protein biomarkers for the screening and diagnosis of TBI is urgently needed. In the present study, we have investigated the plasma levels of soluble cellular prion protein (PrPC) as a novel biomarker for the diagnosis of primary blast-induced TBI (bTBI). We hypothesize that the primary blast wave can disrupt the brain and dislodge extracellular localized PrPC, leading to a rise in concentration within the systemic circulation. Adult male Sprague–Dawley rats were exposed to single pulse shockwave overpressures of varying intensities (15-30 psi or 103.4–206.8 kPa] using an advanced blast simulator. Blood plasma was collected 24 h after insult, and PrPC concentration was determined with a modified commercial enzyme-linked immunosorbent assay (ELISA) specific for PrPC. We provide the first report that mean PrPC concentration in primary blast exposed rats (3.97 ng/mL±0.13 SE) is significantly increased compared with controls (2.46 ng/mL±0.14 SE; two tailed test p<0.0001). Furthermore, we report a mild positive rank correlation between PrPC concentration and increasing blast intensity (psi) reflecting a plateaued response at higher pressure magnitudes, which may have implications for all military service members exposed to blast events. In conclusion, it appears that plasma levels of PrPC may be a novel biomarker for the detection of primary bTBI.
Key words: : blast exposure, blood plasma, brain injury, ELISA, PrPC
Introduction
Traumatic brain injury (TBI) is the leading cause of mortality and disability in individuals under<45 years of age in North America, and is estimated to occur in 600/100,000 people.1–3 Most cases of TBI are a result of a significant impact or penetrating injury to the head leading to the disruption of normal brain functions.4 The leading causes of TBI include vehicle accidents, falls, assaults, and sports-related injuries. However, over the past decade, the scope of concern over TBI has expanded to include injuries sustained during military operations and urban terrorist activities.5 Since the beginning of the global war on terror, it is estimated that 15–20% of returning service members have sustained a TBI, representing>300,000 cases for the period between 2001 and 2014.6,7 Therefore, TBI is described as the signature injury of these conflicts and has been associated with a spectrum of post-deployment health issues, including decline in long-term motor, psychosocial, and cognitive ability; sleep disturbance; psychiatric disorders; post-concussive syndrome; substance abuse; and chronic traumatic encephalopathy.8–12 Of all military-related TBI incidences, blast exposure is by far the leading cause, accounting for ∼47% of TBI cases in Afghanistan and 64% of those in Iraq, thereupon establishing blast-induced TBI (bTBI) as its own category distinct from the spectrum of non-blast TBI.13,14
The rising incidence of bTBI in the military over the past decade has been largely the result of the expanded use of various explosive munitions such as grenades, improvised explosive devices (IEDs), and land mines.9,15 Moreover, improvements in medical treatment and protective equipment have increased the survival rate, concomitant with increased TBI reporting in those sustaining injuries previously considered fatal.16,17 Despite these advancements, bTBI remains a large concern, as the effects of blast exposure, particularly chronic exposure, to the human brain are not clearly understood because of their complex and heterogeneous nature.18 During an explosive detonation, the instantaneous conversion of a solid material into gas produces a supersonic overpressure wave, termed the primary blast wave.10 Additional byproducts of this reaction can include extreme heat, toxic gases, electromagnetic pulses, and winds generated by the abrupt air pressure changes produced in the blast wave.10,13,19–21 Considering that the manifestations of bTBI may be a consequence of certain or even all such blast-induced forces, there is no clinical standard for assessing bTBI other than using conventional TBI diagnostic guidelines. Of the various patterns of bTBI, those caused by the primary blast wave itself are the least recognized and understood.22 Therefore, considerable effort has been directed toward the development of reliable screening procedures to help determine the degree of head trauma suffered by military service personnel.
To date, various clinical assessment guidelines and advanced neuroimaging techniques remain the standard tools for determining bTBI; however, there is no guarantee of proper diagnosis and timely management, because of the inconsistent indications of bTBI. A promising approach to circumvent these uncertainties has focused on the detection of protein biomarkers symptomatic of bTBI. Typical markers examined for bTBI may include such proteins as S100 calcium binding protein B (S100B), glial fibrillary acidic protein (GFAP), neuron specific enolase (NSE), cleaved tau (C-tau), and various others.23–27 However, to our knowledge, there are no reports indicating cellular prion protein (PrPC) as a potential biomarker of bTBI. The present study seeks to address this question as to whether assessment of plasma PrPC may also be indicative of bTBI.
PrPC is a ubiquitous glycoprotein distributed throughout many cell types and tissues in mammals, with preponderance within the central nervous system (CNS).28–30 PrPC is 208–209 amino acids long, and is almost entirely located extracellularly on dynamic lipid raft domains tethered by a glycophosphatidylinositol (GPI) anchor.30,31 Efforts to elucidate the physiological role of PrPC in the CNS have determined its involvement in various functions, including cellular adhesion, cell signaling, ion homeostasis, and neuroprotection.32–44 Because of PrPC's extracellular nature, it is possible that during blast exposure, shearing forces from the primary blast wave traversing across the brain may cause the tenuously bound PrPC to dislodge and collect within the systemic circulation. We addressed this hypothesis by subjecting adult male Sprague–Dawley (S-D) rats to controlled single pulse shockwaves closely simulating free field blast and collecting blood plasma afterwards for quantification of PrPC.45 In this study, we established 1) plasma PrPC as a potential biomarker for primary bTBI and 2) a positive correlation between plasma PrPC and blast wave intensity exposure.
Methods
Advanced blast simulator (ABS)
A custom‐built ABS (∼30.5cm in diameter and 5.79 m in length) located at Defence Research and Development Canada (DRDC) Suffield was used for producing simulated blast waves.45 The ABS consisted of a “driver” section filled with high-pressure gas, and a low-pressure test section, separated by a frangible cellulose acetate diaphragm. Closely controlled pressurization of the driver causes rupture of the diaphragm, releasing high-pressure gas into the test section, and generating a shockwave down the length of the test section. The inclusion of a custom-designed divergent driver and an end wave eliminator in this ABS system enables the highly reproducible generation of single pulse shockwaves.45 Compressed helium and varying thicknesses of cellulose acetate sheets were employed to obtain the desired target pressure. A complete description of the development and operation of the ABS is described in a manuscript nearing completion, to be submitted to the open literature by Sawyer and colleagues.
Animal exposure to simulated blast
In conducting this research, the authors adhered to the Guide to the Care and Use of Experimental Animals and The Ethics of Animal Experimentation, published by the Canadian Council on Animal Care. Adult male S-D rats were acquired from Charles River Laboratories (St. Constant, Quebec, Canada) and acclimated for at least 1 week prior to exposure. The animals were kept on a 12 h light/dark cycle and fed ad libitum. On the day of use, the animals (∼350–400 g) were anaesthetized with 3% isoflurane in oxygen for 3 min in a closed induction chamber. Anesthesia was maintained using a face mask, and the animal was placed into a restraint consisting of a clear plastic cylindrical sleeve, with the neck encircled in a closely fitting plastic collar with the head protruding from the end. The hindquarters were supported using an end cap fitted with a piston. To the left of the head, a mesh netting was secured between two pins placed vertically in line with the side of, and above and below the head. The head was placed against this vertical netting, and then held in place using additional netting around the head. This was locked into place using Velcro on the side of the head opposite the direction of shockwave propagation. After a minimum of 8 min of anesthetic exposure, the cylindrical restraint containing the animal was set into the wall of the ABS, such that only the head protruded into the test section. Test groups consisted of sham control, and head-only, side-on exposures of single pulse shockwave overpressures of 15, 20, 25, and 30 psi or 103.4, 137.9, 172.4, or 206.8 kPa.
After exposure, the animals were immediately removed from the shock tube and animal restraint, and were closely observed for at least 30 min post-exposure, or until no signs of stress were evident. The animals were returned to the dedicated animal holding facility where they had been observed on a daily basis prior to testing. At 24 h, the animals were anaesthetized and euthanized by decapitation prior to blood sample collection.
Sample collection
Following anesthesia, whole trunk blood samples were collected from both control (n=19) and blast (n=33) group rats following decapitation into potassium ethylene diamine tetraacetic acid (K2EDTA) coated blood collection tubes. Samples were centrifuged for 10 min at 2000g, and the separated plasma supernatant was collected. To avoid excessive freeze–thaw cycles, blood plasma aliquots were made and stored at −20°C for short-term use, and the rest were stored at −80°C.
Plasma PrPC ELISA
For sensitive quantification of full-length soluble PrPC, we employed an ELISA technique using a commercially available assay kit (Spi Bio A05201, Paris, France). The kit is typically used for qualitative screening in animal products; therefore, we modified the manufacturer's protocol to allow sensitive and accurate quantification. Pure full-length recombinant PrPC (Prionatis, α-Rec Mouse PrP-RPA0101S, Zurich, Switzerland) was used for producing serial dilutions (0.625–20 ng/mL) in order to establish the calibration curve for quantifying samples. All samples and PrPC protein standards were diluted in the manufacturer's provided dilution buffer solution (1 M phosphate, 1% bovine serum albumin [BSA], 4 M NaCl, 10 mM EDTA, and 0.1% sodium azide). Remaining solutions and reagents provided by the manufacturer were reconstituted and prepared according to the suggested protocol.
Briefly, overall protein concentration of individual samples was first determined in triplicate using the Bio-Rad DC protein assay (Sigma-Aldrich, bovine albumin, A-9647, Oakville, ON). Samples and standards were loaded in equal volume in triplicate in the kit's 96 microwell plate strips. Diluted samples were loaded such that each well contained overall protein amounts of ∼ 75–100 μg. The plate is then incubated overnight at 4°C with shaking to allow adequate antigen binding to well-embedded monoclonal antibodies, specific to the 144–153 amino acid sequence. After rigorous washing (4M phosphate, pH 7.4), the wells were incubated with an acetylcholinesterase- (AChE) Fab’ conjugated antibody solution for 2 h at room temperature (RT) with shaking, thus completing a double-antibody sandwich. After another cycle of rigorous washing, Ellman's reagent was added in equal volume to each well, and incubated in the dark for 30 min at RT with shaking. Any immobilized AChE-conjugated antibody bound to PrPC therefore reacted with Ellman's reagent to produce a colorimetric reaction in solution proportional to the concentration of PrPC, which was read using a microplate reader at 405 nm (Molecular Devices, LLC., SpectraMax M5, Sunnyvale CA). Raw absorbance values were interpolated along the standard calibration curve and converted into PrPC concentration values.
PrPC Western blotting
Western blotting was conducted as previously described.46 Briefly, diluted plasma samples were separated with sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE), transferred onto a polyvinylidene difluoride (PVDF) membrane, and probed with an anti-PrPC primary antibody (Santa Cruz, goat IgG anti-PrP C-20 pAb, 1:500, sc-7693). For sequential reprobing of the same blots, the membranes were stripped and subjected to immunoblotting with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) primary antibody (AbCam, mouse IgG2b mAb, 1:2000, ab9484). Blots were developed using enhanced chemiluminescence detection (Amersham) and exposed to x-ray film. Band intensities were quantified using National Institutes of Health (NIH) ImageJ software and normalized to the quantity of GAPDH in each sample lane. Blots were developed in triplicate, and a representative image is provided (see Fig. 1).
FIG. 1.
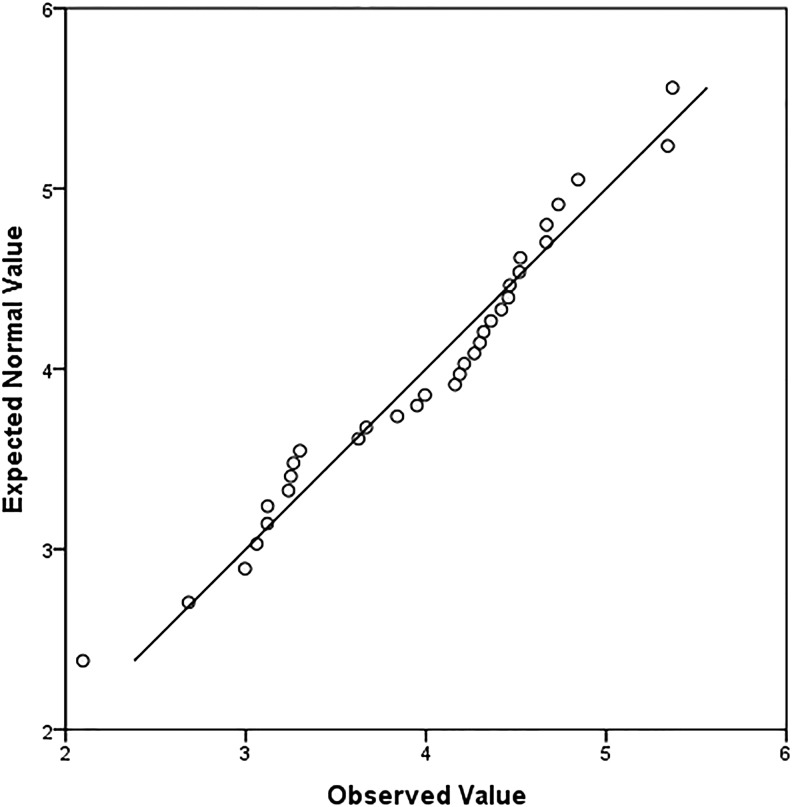
Quantile-quantile Q-Q plot of blast soluble cellular prion protein (PrPC) distribution. Most of the blast group PrPC concentration results depart from the normal distribution reference line (y=x). Therefore, data are not normally distributed and are considered nonparametric.
Statistical analysis
Statistical analysis for all data was performed using the IBM SPSS 21 Statistical Package. Nonparametric data were appropriately analyzed using the Kruskall–Wallis test for comparison of mean rank values of control and the different blast intensity groups. Post-hoc Mann–Whitney U test with a Bonferroni correction for 95% confidence interval (CI) was used for determining statistical significance between mean rank values of control and individual blast group PrPC concentration. The Jonckheere trend test was used to determine a significant relationship between blast intensity and PrPC concentration. Kendall's tau-b test determined the nature and degree of association for said relationship. Receiver operating characteristic (ROC) analysis was performed for determining accuracy of classifier performance. The measure of general predictiveness of classifiers was determined by area under the ROC curve (AUC). Two-graph ROC (TG-ROC) analysis was used for determining the cutoff value, as described by Greiner and coworkers, between control and blast exposure groups, and positive and negative predictive values (PPV and NPV) were subsequently calculated.47 For all tests, statistical significance was determined when p≤0.05.
Results
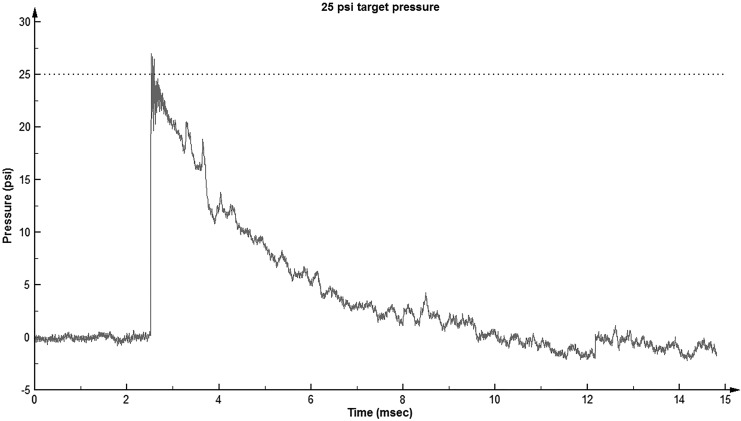
The use of the ABS system, in concert with the head restraint configuration employed in this work, has been shown to minimize concussive and whiplash forces and produce a “clean” primary single pulse shockwave insult (manuscript in preparation by Sawyer et al.). Figure 2 shows a representative example of the single pulse shock wave produced with a 25 psi target pressure. The overpressures obtained for the four test groups were: 15±0.2, 20±0.8, 25±0.3, and 30±0.9 psi (mean±SD). Immediately after exposure, the animals showed no obvious signs of injury and revived from the anesthetic with no visible differences compared with sham controls with respect to time to revival and time to mobility. No signs of distress or injury were noted either immediately after regaining consciousness after exposure, or the following day.
FIG. 2.
Primary blast profile: Representative single pulse wave form generated in the advanced blast simulator (ABS) at the test location at a target overpressure of 25 psi. The pressure perturbations at ∼ 3.6–4.0 msec are caused by the placement of the head within the shock flow.
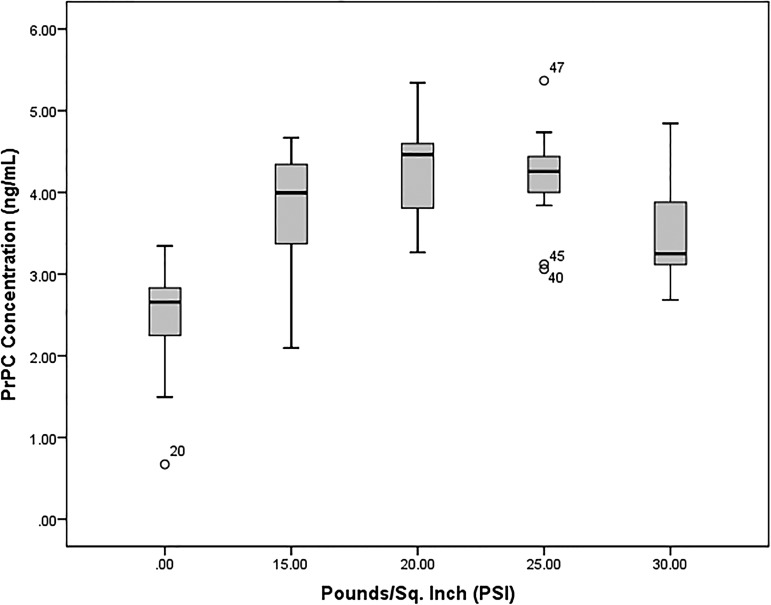
Quantitative analysis of blood plasma PrPC from both control (n=19, 0 psi) and blast (n=33, 15–30 psi) groups was performed using a modified commercial ELISA kit specific for PrPC; for results summary see Table 1. Graphic representation of PrPC concentration results is provided in a box-and-whisker plot (see Fig. 3) showing that the majority of blast group values lie above the control group median value (2.66 ng/mL), indicating that blast group concentrations are distinct from control results. A quantile-quantile (Q-Q) plot of blast group results distribution reveals that most data points deviate from the normal distribution line (y=x), and, therefore, these data are considered nonparametric (see Fig. 1). As such, the Kruskall–Wallis test for nonparametrics was appropriately used for determining differences in PrPC concentration mean rank values of sham controls and individual blast intensity groups. There was a statistically significant difference (χ2=31.62, p<0.0001) between sham control (mean rank=11.84, n=19), 15 psi (mean rank=31.14, n=7), 20 psi (mean rank=40.00, n=7), 25 psi (mean rank=37.58, n=12), and 30 psi (mean rank=29.14, n=7) blast exposure groups. Post-hoc Mann–Whitney U test with a Bonferroni correction for multiple comparisons with an adjusted level of significance (α=0.0125) determined statistical difference of PrPC concentration mean rank between sham controls and 15 psi (10.89 vs. 20.57, U=17, p=0.004), 20 psi (10.05 vs. 22.86, U=1, p=0.0001), 25 psi (10.16 vs. 25.25, U=3, p<0.0001), and 30 psi (10.74 vs. 21.00, U=14, p=0.002) blast exposure groups.
Table 1.
Plasma PrPC ELISA Results Summary
| PrPC Concentration (ng/mL) | ||||||
|---|---|---|---|---|---|---|
| Group | Target pressure (psi) | Actual pressure (psi) | n | Mean±SE | Median | Range |
| Sham control | 0 | 0 | 19 | 2.46±0.14 | 2.66 | 0.67–3.35 |
| Blast | 15 | 15±0.2 | 7 | 3.74±0.34 | 3.99 | 2.10–4.67 |
| 20 | 20±0.8 | 7 | 4.27±0.26 | 4.47 | 3.27–5.34 | |
| 25 | 25±0.3 | 12 | 4.18±0.18 | 4.26 | 3.06–5.37 | |
| 30 | 30±0.9 | 7 | 3.54±0.30 | 3.25 | 2.68–4.84 | |
| 15-30 | 33 | 3.97±0.13 | 4.19 | 2.10–5.37 | ||
Blood plasma from control (n=19, 0 psi) and blast (n=33, 15–30 psi) group rats were assayed using a modified commercial PrPC ELISA kit for quantification. Individual results not provided.
PrPC, soluble cellular prion protein; ELISA, enzyme-linked immunosorbent assay.
FIG. 3.
Box-and-whisker plot of soluble cellular prion protein (PrPC) concentrations. Box plot comparison of control (0 psi, n=19) and blast (15 psi, n=7; 20 psi, n=7; 25 psi, n=12; 30 psi, n=7) groups illustrate that the majority of blast group PrPC concentrations (interquartile range Q1-Q3) lie above the median (Q2) of the control group. Data points 20, 40, 45, and 47 are considered outliers from group distribution.
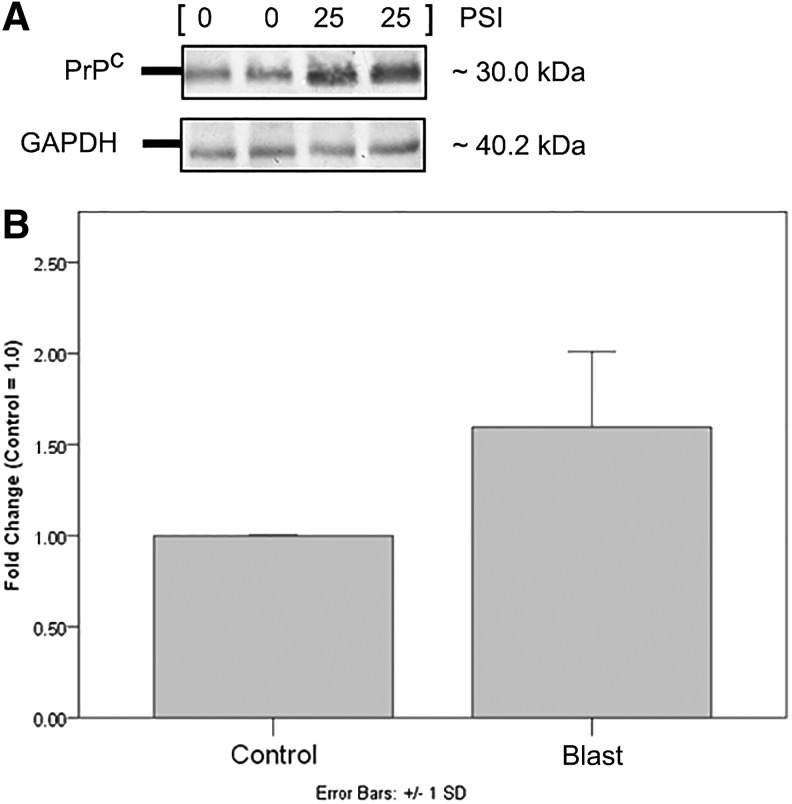
Quantified differences between blast and control group PrPC concentration is demonstrated with Western blotting (see Fig. 4). Densitometric analysis using NIH ImageJ software calculated PrPC band intensity in relation to GAPDH loading control in blast group plasma determined a 1.60±0.41 fold increase (n=4, two tailed test p<0.001) when compared with controls. To determine a significant relationship between increasing blast pressure intensity (psi) and plasma PrPC content, Jonckheere trend test was used, which showed an ordered relationship between blast intensity and PrPC concentration (J-T=773.00, p<0.0001). Additionally, Kendall's tau-b test determined the correlation coefficient at 0.446 (p<0.0001), reflecting a positive trend association between increasing blast intensity groups and their respective median PrPC concentrations.
FIG. 4.
Western blot of soluble cellular prion protein (PrPC) (A) Results are semiquantitative, and are for the purpose of simple visualization of increased PrPC in blast group plasma compared with control. (B) Numerical (fold) change bar graph represents a mean fold increase of 1.60±0.41 compared with control values, given an arbitrary value of 1.0 (n=4, two tailed test p<0.05).
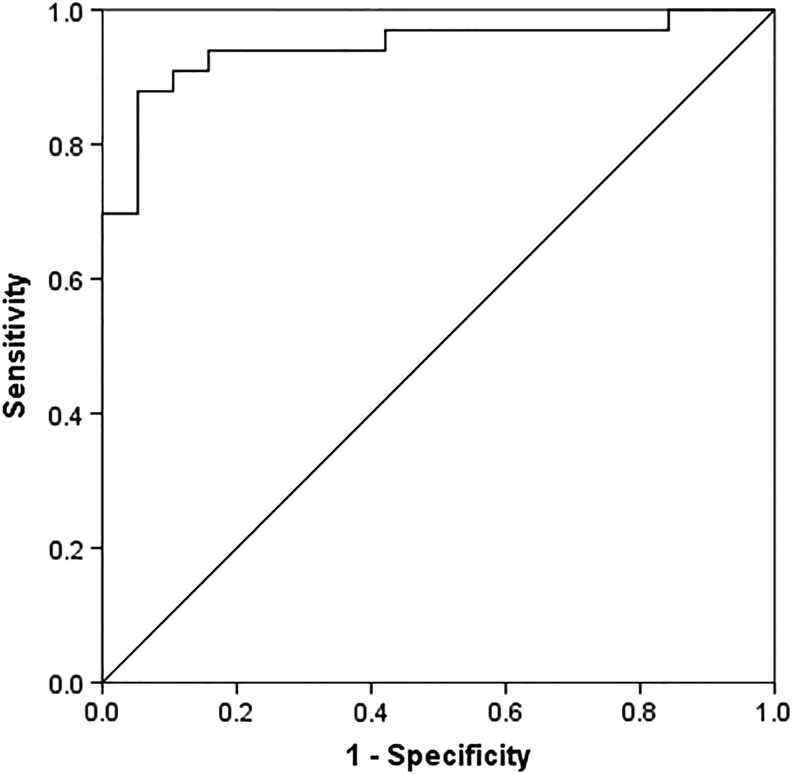
ROC analysis was performed for determining accuracy of our ELISA test based on the predictability of control and blast group classifiers (see Fig. 5). ROC analysis allows comparison of PrPC sensitivity against the inverse specificity over a range of thresholds for evaluating overall test accuracy. The AUC was determined at 0.944±0.032 SE (95% CI, 0.881–1.000, p<0.0001) indicating ELISA test results to be highly accurate for distinguishing between control and blast groups. As there is presently no standard reference database available for rat plasma PrPC, we performed TG-ROC analysis using values obtained to determine the minimum cutoff value defining blast exposure. We chose a conservative cutoff of 2.78 ng/mL, which yielded 79.1% sensitivity and specificity, 81.6% PPV, and 85.7% NPV.
FIG. 5.
Receiver operating characteristic (ROC) analysis of plasma soluble cellular prion protein (PrPC) for test of blast exposure. Control (n=19) versus blast (n=33) area under the curve (AUC) is 0.944±0.032 S.E. (95% CI, 0.881–1.000, p<0.0001).
Discussion
Increased blast exposure during the recent military conflicts in Afghanistan and Iraq has not surprisingly been concomitant with increased reports of TBI among service members.6,16,17 TBI is typically brought about by direct impact or acceleration forces to the head, leading to collision between the brain and skull, as well as shearing strain on brain tissue and vasculature.3,48 Proper diagnosis of TBI caused by blast is especially difficult, given the potential absence of physical symptoms or presence of nonspecific ones, thus confounding the recognition of mild indications such as sleep disturbance, fatigue, headaches, and loss of concentration that are often overlooked and underreported by service members.9,49 A possible approach toward addressing this issue is in screening individuals for protein biomarkers specific for bTBI. Various proteins have been investigated, but none has been conclusively established as having clinically practical screening qualities.23,24 For example, the S100B protein is frequently used as a biomarker for TBI, and has been thoroughly investigated because of its strong NPV; however, its value for predicting TBI outcome is questionable, because of its high correlation with bone fractures without TBI, extracranial injury, and even melanoma.50–53 Another protein, GFAP, has shown correlation with TBI outcome, but has been inconsistent in discerning between those with TBI and uninjured victims.54 As such, investigation toward establishing both a highly predictive and reliable protein biomarker continues. Therefore, in collaboration with the DRDC, Suffield Research Center, we sought to establish the use of a novel protein biomarker, PrPC, within the blood plasma of rats exposed to simulated primary blast. This is the shockwave component of a blast, and is distinct from the other blast components that may cause injury, such as penetrating fragments (secondary), blast wind effects (tertiary), and noxious gases, heat, or dust (quaternary). Because of technical difficulties, the experimental replication of clean primary blast conditions has traditionally been problematic. However, recent developments in these laboratories have enabled the consistent replication of single pulse shockwaves with minimal concussive and whiplash forces (manuscript in preparation by Sawyer et al.), which is highly reminiscent of a free field blast.45
We subjected adult male S-D rats to single pulse shockwaves of varying intensities, localized only to the head, in order to determine whether there was an appreciable rise in plasma PrPC concentration, which we quantified using a modified commercial ELISA kit. We hypothesized that the blast-induced shearing forces as described by Schardin may cause the predominantly extracellular, GPI-anchored PrPC to be dislodged from its neuronal lipid raft location.55 Previous reports have shown increased plasma PrPC concentration following stroke, and in patients with various neurodegenerative diseases.56,57 Additionally, there is a growing body of evidence reporting neurodegenerative changes post-TBI, which may possibly allude to an association with elevated plasma PrPC levels.58–63
In our current study, we have identified the rise in plasma levels of PrPC as a novel biomarker for detection of primary bTBI; and based on current literature search, this is the first report of such an association. Statistical analysis determined that mean PrPC concentration in simulated primary blast exposed rats was significantly greater than in controls. Moreover, we also determined a mild positive correlation between plasma PrPC levels and increasing blast intensity (psi). Results showed dramatic increase of plasma PrPC in the 15 and 20 psi blast group, with levels plateauing at higher intensities. The reason or mechanism for this effect is not yet known, and will be the subject of further investigation, specifically at blast exposure of lower intensity (<15 psi), to better discern any functional relationship. In this regard, this initial finding suggests that subjects exposed to lower blast intensity elicit a similar plasma PrPC profile to those at higher magnitudes. These findings are in agreement with our recent immunohistochemical staining for neurofilament phosphorylation (unpublished data).
The translation of this finding to humans may mean that military service members exposed to primary blast waves only, including those at lower intensities, experience a similar effect to those exposed to waves at higher intensity, but may not receive medical attention because of lack of apparent injuries. Immunoblotting additionally confirmed, albeit semiquantitatively, that there is an apparent increase in plasma PrPC content after blast exposure compared with controls, which is consistent with quantitative ELISA results obtained. We determined the PrPC concentration cutoff value for blast exposure conservatively at 2.78 ng/mL (79.1% sensitivity and specificity, 81.6% PPV, and 85.7% NPV). It is noteworthy that there is currently no known standard reference database for normal rat plasma PrPC concentrations; therefore, the cutoff value determined is based on the normal concentration values that we have established.
In summary, our findings support our working hypothesis that a primary blast force of sufficient intensity passing through brain tissue may dislodge the loosely attached PrPC from its extracellular domain, which subsequently accumulates within the systemic circulation.
The neuropathology of bTBI is not entirely clear, but reports have noted, among other symptoms, brain edema, cerebral pseudoaneurysms, intracerebral hemorrhaging, microlesions, cell death, and axonal injury as a result of blast exposure.5,64–66 Such evidence establishes the basis that blast exposure can cause damage to brain tissue and vasculature. Furthermore, recent studies have shown that patients with cerebrovascular disease or vascular endothelial damage had higher levels of plasma PrPC than control values.64–66 We cannot at this time discern whether our observation of increased plasma PrPC following primary blast exposure is exclusively of neural origin or if it also arises from the surrounding cerebrovasculature, which may also be subjected to primary blast-induced damage. Furthermore, PrPC has been reported to be upregulated following focal cerebral ischemia; therefore, it is possible that the rise in plasma PrPC content may be partially attributed to by damaged ischemic regions in the brain as a result of blast exposure.67 Because the extent of blast-induced damage in the brain is unclear, it is possible that PrPC mRNA and protein expression may also be affected.
It is certain, however, that the rise in PrPC concentration is yet another part of the unique pathology complex associated with primary bTBI. In relation to primary bTBI, the neuroprotective function of PrPC may be of interest, as studies have noted its involvement in the context of hypoxia, epilepsy, oxidative stress, neurotoxicity, ischemic injury, and even in limiting brain damage in an animal model of TBI.67–76 Further studies are needed to test our findings in the acute period following primary bTBI to definitively acknowledge it as a reliable biomarker, as well as to investigate the potential contributions of the other, non-primary aspects of bTBI (i.e., penetrating bodies, blunt trauma) to PrPC release, and its subsequent diagnostic utility.
Acknowledgments
This research is supported by DRDC Contract W7702-145664. The authors thank the following individuals: Drs. Venkat Gopalakrishnan and John Mikler for their useful critical reviewing of our manuscript; Stephen Bjarnason at the DRDC for his managerial support for this project; Tracy Weiss, Peggy Nelson, Grant Hennes, and Julia Barnes for their excellent technical help during the blast exposure and blood collection processes; and Bing Yan and Dr. June Lim for their valuable assistance and consultation regarding statistical analysis.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Rao V., and Lyketsos C. (2000). Neuropsychiatric sequelae of traumatic brain injury. Psychosomatics 41, 95–103 [DOI] [PubMed] [Google Scholar]
- 2.Cassidy J.D., L.J., Carrol L.J., Peloso P.M., Borg J., von Holst H., Holm L., Kraus J., and Coronado V.G. (2004). Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. S43, 28–60 [DOI] [PubMed] [Google Scholar]
- 3.Rosenfeld J.V., McFarlane A.C., Bragge P., Armonda R.A., Grimes J.B., and Ling G.S. (2013). Blast-related traumatic brain injury. Lancet Neurol. 12, 882–893 [DOI] [PubMed] [Google Scholar]
- 4.Menon D.K., Schwab K., Wright D.W., and Mass A.I. (2010). Position statement: definition of traumatic brain injury. Arch. Phys. Med. Rehabil. 91, 1637–1640 [DOI] [PubMed] [Google Scholar]
- 5.Ling G., Bandak F., Armonda R., Grant G., and Ecklund J. (2009). Explosive blast neurotrauma. J. Neurotrauma 26, 815–825 [DOI] [PubMed] [Google Scholar]
- 6.Hoge C.W., McGurk D., Thomas J.L., Cox A.L., Engel C.C., and Castro C.A. (2008). Mild traumatic brain injury in U.S. soldiers returning from Iraq. N. Engl. J. Med. 358, 453–456 [DOI] [PubMed] [Google Scholar]
- 7.Defense and Veterans Brain Injury Center (2014). DoD Worldwide Numbers for TBI. Available at: http://www.dvbic.org/dod-worldwide-numbers-tbi Accessed May1, 2013
- 8.Hall R.C., and Chapman M.J. (2005). Definition, diagnosis, and forensic implications of postconcussive syndrome. Psychosomatics 46, 195–202 [DOI] [PubMed] [Google Scholar]
- 9.Tanielian T., and Jaycox L.H. (2008). Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences and Services to Assist Recovery. Rand Corp, MG 720-CCF: Santa Monica, CA [Google Scholar]
- 10.Wolf S.J., Bebarta V.S., Bonnett C.J., Pons P.T., and Cantrill S.V. (2009). Blast injuries. Lancet 374, 405–415 [DOI] [PubMed] [Google Scholar]
- 11.Goldstein L.E., Fisher A.M., Tagge C.A., Zhang X., et al. (2012) Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci. Transl. Med. 4, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrie E.C., Cross D.J., Yarnykh V.L., Richards T., Martin N.M., Pagulayan K., Hoff D., Hart k., Mayer C., Tarabochia M., Raskind M.A., Minoshima S., and Peskind E.R. (2014). Neuroimaging, behavioral, and psychological sequelae of repetitive combined blast/impact mild traumatic brain injury in Iraq and Afghanistan war veterans. J. Neurotrauma 31, 425–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elder G.A., Mitsis E.M., Ahlers S.T., and Cristian A. (2010). Blast-induced mild traumatic brain injury. Psychiatr. Clin. N. Am. 33, 757–781 [DOI] [PubMed] [Google Scholar]
- 14.Wojcik B.E., Stein C.R., Bagg K., Humphrey R.J., and Orosco J. (2010) Traumatic Brain Injury Hospitalization of U.S. Army Soldiers Deployed to Afghanistan and Iraq. Am. J. Prev. Med. 38, S108–S116 [DOI] [PubMed] [Google Scholar]
- 15.Ramasamy A., Hill A.M., Hepper A.E., Bull A.M., and Clasper J.C. (2009). Blast mines: physics, injury mechanisms and vehicle protection. J. R. Army Med. Corps 155, 258–264 [DOI] [PubMed] [Google Scholar]
- 16.Warden D. (2006). Military TBI during the Iraq and Afghanistan wars. J. Head Trauma Rehabil. 21, 398–402 [DOI] [PubMed] [Google Scholar]
- 17.Okie S. (2005) Traumatic brain injury in the war zone. N. Engl. J. Med. 352, 2043–2047 [DOI] [PubMed] [Google Scholar]
- 18.Graner J., Oakes T.R., French L.M., and Riedy G. (2013). Functional MRI in the investigation of blast-related traumatic brain injury. Front. Neurol. 4, 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Champion H.R., Holcomb J.B., and Young L.A. (2009). Injuries from explosions: physics, biophysics, pathology, and required research focus. J. Trauma 66, 1468–1477 [DOI] [PubMed] [Google Scholar]
- 20.Hicks R.R., Fertig S.J., Desrocher R.E., Koroshetz W.J., and Pancrazio J.J. (2010). Neurological effects of blast injury. J. Trauma 68, 1257–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakagawa A., Manley G.T., Gean A.D., Ohtani K., Armonda R., Tsukamoto A., Yamamoto H., Takayama K., and Tominaga T. (2011). Mechanisms of primary blast-induced traumatic brain injury: insights from shock-wave research. J. Neurotrauma 28, 1101–1119 [DOI] [PubMed] [Google Scholar]
- 22.MacDonald C.L., Johnson A.M., Cooper D., Nelson E.C., and Brody D.L. (2011). Detection of blast-related traumatic brain injury in U.S. military personnel. N. Engl. J. Med. 364, 2091–2100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agoston D.V., Gyorgy A., Eidelman O., and Pollard H.B. (2009). Proteomic biomarkers for blast neurotrauma: targeting cerebral edema, inflammation, and neuronal death cascades. J. Neurotrauma 26, 901–911 [DOI] [PubMed] [Google Scholar]
- 24.Agoston D.V., and Elsayed M. (2012). Serum-based protein biomarkers in blast-induced traumatic brain injury spectrum disorder. Front. Neurol. 3, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kobeissy F., Mondello S., Tumer N., Toklu H.Z., Whidden M.A., Kirichenko N., Zhang Z., Prima V., Yassin W., Anagli J., Chandra N., Svetlov S., and Wang K.K.W. (2013). Assessing neuro-systemic and behavioral components in the pathophysiology of blast-related brain injury. Front. Neurol. 4, 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kochanek P.M., Dixon E.D., Shellington D.K., Shin S.S., and Jenkins L.W. (2013). Screening of biochemical and molecular mechanisms of secondary injury and repair in the brain after experimental blast-induced traumatic brain injury in rats. J. Neurotrauma 30, 920–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arun P., Abu-Taleb R., Oguntayo S., Tanaka M., Wang Y., Valiyaveettil M., Long J.B., Zhang Y., and Nambiar M.P. (2013). Distinct patterns of expression of traumatic brain injury biomarkers after blast exposure: role of compromised cell membrane integrity. Neurosci. Lett. 552, 87–91 [DOI] [PubMed] [Google Scholar]
- 28.Bendheim P.E., Brown H.R., Rudelli R.D., et al. (1992). Nearly ubiquitous tissue distribution of the scrapie agent precursor protein. Neurology 42, 149–156 [DOI] [PubMed] [Google Scholar]
- 29.Moser M., Colello R.J., Pott U., and Oesch B. (1995). Developmental expression of the prion protein gene in glial cells. Neuron 14, 509–517 [DOI] [PubMed] [Google Scholar]
- 30.Aguzzi A., and Polymenidou M. (2004) Mammalian prion biology: one century of evolving concepts. Cell 116, 313–327 [DOI] [PubMed] [Google Scholar]
- 31.Sales N., Rodolfo K., Hassig R., et al. (1998). Cellular prion protein localization in rodent and primate brain. Eur. J. Neurosci. 10, 2464–2471 [DOI] [PubMed] [Google Scholar]
- 32.Martins V.R., and Brentani R.R. (2002). The biology of the cellular prion protein. Neurochem. Int. 41, 353–355 [DOI] [PubMed] [Google Scholar]
- 33.Collinge J., Whittington M.A., Sidle K.C.L., et al. (1994). Prion protein is necessary for normal synaptic function. Nature 370, 295–297 [DOI] [PubMed] [Google Scholar]
- 34.Didonna A. (2012). Prion protein and its role in signal transduction. Cell. Mol. Biol. Lett. 18, 209–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown D.R., Qin K., Herms J.W., Madlung A., Manson J., Strome R., Fraser P.E., Kruck T., von Bohlen A., Schulz–Schaeffer W., Giese A., Westaway D., and Kretzschmar H. (1997). The cellular prion protein binds copper in vivo. Nature 390, 684–687 [DOI] [PubMed] [Google Scholar]
- 36.Watt N.T., Taylor D.R., Kerrigan T.L., et al. (2012). Prion protein facilitates uptake of zinc into neuronal cells. Nat. Commun. 3, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh A., Kong Q., Luo X., Petersen R.B., Meyerson H., and Singh N. (2009) Prion protein (PrP) knock-out mice show altered iron metabolism: a functional role for PrP in iron uptake and transport. PLoS One 4, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ford M.J., Burton L.J., Li H., Graham C.H., Frobert Y., Grassi J., Hall S.M., and Morris R.J. (2002). A marked disparity between the expression of prion protein and its message by neurones of the CNS. Neuroscience 111, 533–551 [DOI] [PubMed] [Google Scholar]
- 39.Biasini E., Turnbaugh J.A., Unterberger U., and Harris D.A. (2012). Prion protein at the crossroads of physiology and disease. Trends Neurosci. 35, 92–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shmerling D., Hegyi I., Fischer M., et al. (1998). Expression of amino-terminally truncated PrP in the mouse leading to ataxia and specific cerebellar lesions. Cell 93, 203–214 [DOI] [PubMed] [Google Scholar]
- 41.Kuwahara C, Takeuchi AM, Nishimura T, Haraguchi K, et al. (1999). Prions prevent neuronal cell-line death. Nature 400, 255–256 [DOI] [PubMed] [Google Scholar]
- 42.Coulpier M., Messiaen S., Boucreaux D., and Elliot M. (2006). Axotomy-induced motoneuron death is delayed in mice overexpressing PrPc. Neuroscience 141, 1827–1834 [DOI] [PubMed] [Google Scholar]
- 43.Mitteregger G., Vosko M., Krebs B., et al. (2007). The role of the octarepeat region in neuroprotective function of the cellular prion protein. Brain Pathol. 17, 174–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carnini A., Casha S., Yong V.W., Hurlbert R.J., and Braun J.E.A. (2010). Reduction of PrPC in human cerebrospinal fluid after spinal cord injury. Prion 4, 80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ritzel D.V., Parks S.A., Roseveare J., Rude G., and Sawyer T.W. (2011). Experimental Blast Simulation for Injury Studies. NATO HFM; 207: Halifax [Google Scholar]
- 46.Taghibiglou C., Lu J., Mackenzie I.R., Wang Y.T., and Cashman N.R. (2011). Sterol regulatory element binding protein-1 (SREBP1) activation in motor neurons in excitotoxicity and amylotrophic lateral sclerosis (ALS): Indip, a potential therapeutic peptide. Biochem. Biophys. Res. Commun. 413, 159–163 [DOI] [PubMed] [Google Scholar]
- 47.Greiner M., Sohr D., and Gobel P. (1995) A modified ROC analysis for the selection of cut-off values and the definition of intermediate results of serodiagnostic tests. J. Immunol. Methods 185, 123–132 [DOI] [PubMed] [Google Scholar]
- 48.Barkhoudarian G., Hovda D.A., and Giza C.C. (2011). The molecular pathophysiology of concussive brain injury. Clin. Sports Med. 30, 33–48 [DOI] [PubMed] [Google Scholar]
- 49.DeKosky S.T., Ikonomovic M.D., and Gandy S. (2010). Traumatic brain injury—football, warfare, and long-term effects. N. Engl. J. Med. 14, 1293–1296 [DOI] [PubMed] [Google Scholar]
- 50.Unden J., Bellner J., Alling C., Ingebrigtsen T., and Rommer B. (2005). Raised serum S100B levels after acute bone fractures without cerebral injury. J. Trauma 58, 59–61 [DOI] [PubMed] [Google Scholar]
- 51.Savola O., Pyhtinen J., Leino T.K., Siitonen S., Niemela O., and Hillbom M. (2004). Effects of head and extracranial injuries on serum protein S100B levels in trauma patients. J. Trauma 56, 1229–1234 [DOI] [PubMed] [Google Scholar]
- 52.Anderson R.E., Hansson L.O., Nilsson O., Dijlai–Merzoug R., and Settergren G. (2001). High serum S100B levels for trauma patients without head injuries. Neurosurgery 48, 1255–1258 [DOI] [PubMed] [Google Scholar]
- 53.Harpio R., and Einarsson R. (2004). S100 proteins as cancer biomarkers with focus on S100B in malignant melanoma. Clin. Biochem. 37, 512–528 [DOI] [PubMed] [Google Scholar]
- 54.Metting Z., Wilczak N., Rodiger L.A., Schaaf J.M., and van der Naalt J. (2012). GFAP and S100B in the acute phase of mild traumatic brain injury. Neurology 78, 1428–1433 [DOI] [PubMed] [Google Scholar]
- 55.Schardin H. (1950). The Physical Principles of the Effects of a Detonation. German Aviation Medicine, World War II. Department of the US Air Force, Office of the Surgeon General: Washington DC [Google Scholar]
- 56.Mitsios N., Saka M., Krupinski J., Pennucci R., Sanfeliu C., Miguel T.M., Gaffney J., Kumar P., Kumar S., Sullivan M., and Slevin M. (2007). Cellular prion protein is increased in the plasma and peri-infarcted brain tissue after acute stroke. J. Neurosci. Res. 85, 602–611 [DOI] [PubMed] [Google Scholar]
- 57.Volkel D., Zimmermann K., Zerr I., Bodemer M., Linder T., Turecek P.L., Poser S., and Schwarz H.P. (2001). Immunochemical determination of cellular prion protein in plasma from healthy subjects and patients with sporadic CJD or other neurologic diseases. Transfusion 41, 441–448 [DOI] [PubMed] [Google Scholar]
- 58.Smith D.H., Uryu K., Saatman K.E., Trojanowski J.Q., and McIntosh T.K. (2003). Protein accumulation in traumatic brain injury. Neuromolecular Med. 4, 59–72 [DOI] [PubMed] [Google Scholar]
- 59.Uryu K., Giasson B.I., Longhi L., Martinez D., Murray I., Conte V., Nakamura M., Saatman K., Talbot K., Horiguchi T., McIntosh T., Lee V.M.Y., and Trojanowski J.Q. (2003). Age-dependent synuclein pathology following traumatic brain injury in mice. Exp. Neurol. 184, 214–224 [DOI] [PubMed] [Google Scholar]
- 60.Sidaros A., Skimminge A., Liptrot M.G., Sidaros K., Engberg A.W. Herning M., Paulson O.B., Jernigan T.L., and Rostrup E. (2009). Long-term global and regional brain volume changes following severe traumatic brain injury: A longitudinal study with clinical correlates. NeuroImage 44, 1–8 [DOI] [PubMed] [Google Scholar]
- 61.Stern R.A., Riley D.O., Daneshvar D.H., Nowinski C.J., Cantu R.C., and McKee A.C. (2011). Long-term consequences of repetitive brain trauma: chronic traumatic encephalopathy. Am. Acad of Phys. Med. Rehabil. 3, S460–S467 [DOI] [PubMed] [Google Scholar]
- 62.Small G.W., Kepe V., Siddarth P., Ercoli L.M., Merrill D.A., Donoghue N., Bookheimer S.Y., Martinez J., Omalu B., Bailes J., and Barrio J.R. (2013). PET scanning of brain TAU in retired national football league players: preliminary findings. Am. J. Geriatr. Psychol. 2, 138–144 [DOI] [PubMed] [Google Scholar]
- 63.Johnson V.E., Stewart J.E., Begbie F.D., Trojanowski J.Q., Smith D.H., and Stewart W. (2013). Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 136, 28–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krupinski J., Turu M.M., Lugue A., Badimon L., and Slevin M. (2008). Increased PrPc expression correlates with endoglin (CD105) positive microvessels in advanced carotid lesions. Acta. Neuropathol. 116, 537–545 [DOI] [PubMed] [Google Scholar]
- 65.Starke R., Drummond O., MacGregor I., Biggerstaff J., Gale R., Camilleri R., Mackie I., Machin S., and Harrison P. (2002). The expression of prion protein by endothelial cells: a source of the plasma form of prion protein? Br. J. Haematol. 119, 863–873 [DOI] [PubMed] [Google Scholar]
- 66.Simak J., Holada K., D'Agnillo F., Janota J., and Vostal J.G. (2002). Cellular prion protein is expressed on endothelial cells and is released during apoptosis on membrane microparticles found in human plasma. Transfusion 42, 334–342 [DOI] [PubMed] [Google Scholar]
- 67.Weise J., Crome O., Sandau R., Schulz–Schaeffer W., Bahr M., and Zerr I. (2004). Upregulation of cellular prion protein (PrPC) after focal cerebral ischaemia and influence of lesion severity. Neurosci. Lett. 372, 146–150 [DOI] [PubMed] [Google Scholar]
- 68.McLennan N.F., Brennan P.M., McNeil A., Davies I., et al. (2004). Prion protein accumulation and neuroprotection in hypoxic brain damage. Am. J. Pathol. 165, 227–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Walz R., Amaral O.B., Rockenbach I.C., et al. (1999). Increased sensitivity to seizures in mice lacking cellular prion protein. Epilepsia 40, 1679–1682 [DOI] [PubMed] [Google Scholar]
- 70.Milhavet O., McMahon H.E.M., Rachidi W., Nishida N., Katamine S., Mange A., Arlotto M., Casanova D., Riondel J., Favier A., and Lehmann S. (2000) Prion infection impairs the cellular response to oxidative stress. Proc. Natl. Acad. Sci. U.S.A. 97, 13,937–13,942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rangel A., Burgaya F., Gavin R., Soriano E., Aguzzi A., and del Rio J.A. (2007). Enhanced susceptibility of Prnp-deficient mice to kainite-induced seizures, neuronal apoptosis, and death: Role of AMPA/kainite receptors. J. Neurosci. Res. 85, 2741–2755 [DOI] [PubMed] [Google Scholar]
- 72.You H., Tsutsui S., Hameed S., et al. (2012). Aβ neurotoxicity depends on interactions between copper ions, prion protein, and N-methyl-D-aspartate receptors. Proc. Natl. Acad. Sci. U.S.A. 109, 1737–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weise J., Sandau R., Schwarting S., et al. (2006). Deletion of cellular prion protein results in reduced Akt activation, enhanced post-ischemic caspase-3 activation, and exacerbation of ischemic brain injury. Stroke 37, 1296–1300 [DOI] [PubMed] [Google Scholar]
- 74.Shyu W.C., Lin S.Z., Chiang M.F., Ding D.C., Li K.W., Chen S.F., Yang H.I., and Li H. (2005). Overexpression of PrPC by adenovirus-mediated gene targeting reduces ischemic injury in a stroke rat model. J. Neurosci. 25, 8967–8977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spudich A., Frigg R., Kilic E., et al. (2005) Aggravation of ischemic brain injury by prion protein deficiency: role of ERK-1/-2 and STAT-1. Neurobiol. Dis. 20, 442–449 [DOI] [PubMed] [Google Scholar]
- 76.Hoshino S., Inoue K., Yokoyama T., Kobayashi S., Asakura T., Teramoto A., and Itohara S. (2003). Prions prevent brain damage after experimental brain injury: a preliminary report. Acta Neurochir. S86, 297–299 [DOI] [PubMed] [Google Scholar]