Abstract
Torres-Peralta, Rafael, José Losa-Reyna, Miriam González-Izal, Ismael Perez-Suarez, Jaime Calle-Herrero, Mikel Izquierdo, and José A.L. Calbet. Muscle activation during exercise in severe acute hypoxia: Role of absolute and relative intensity. High Alt Med Biol 15:472–482, 2014.—The aim of this study was to determine the influence of severe acute hypoxia on muscle activation during whole body dynamic exercise. Eleven young men performed four incremental cycle ergometer tests to exhaustion breathing normoxic (FIo2=0.21, two tests) or hypoxic gas (FIo2=0.108, two tests). Surface electromyography (EMG) activities of rectus femoris (RF), vastus medialis (VL), vastus lateralis (VL), and biceps femoris (BF) were recorded. The two normoxic and the two hypoxic tests were averaged to reduce EMG variability. Peak Vo2 was 34% lower in hypoxia than in normoxia (p<0.05). The EMG root mean square (RMS) increased with exercise intensity in all muscles (p<0.05), with greater effect in hypoxia than in normoxia in the RF and VM (p<0.05), and a similar trend in VL (p=0.10). At the same relative intensity, the RMS was greater in normoxia than in hypoxia in RF, VL, and BF (p<0.05), with a similar trend in VM (p=0.08). Median frequency increased with exercise intensity (p<0.05), and was higher in hypoxia than in normoxia in VL (p<0.05). Muscle contraction burst duration increased with exercise intensity in VM and VL (p<0.05), without clear effects of FIo2. No significant FIo2 effects on frequency domain indices were observed when compared at the same relative intensity. In conclusion, muscle activation during whole body exercise increases almost linearly with exercise intensity, following a muscle-specific pattern, which is adjusted depending on the FIo2 and the relative intensity of exercise. Both VL and VM are increasingly involved in power output generation with the increase of intensity and the reduction in FIo2.
Key Words: : electromyogram; exercise; fatigue; human; hypoxia, median frequency; root mean square
Introduction
Muscle activation, as represented by the amplitude of surface electromyogram (EMG) increases during incremental exercise to exhaustion (Taylor and Bronks, 1996; Osawa et al., 2011). Greater EMG amplitude may originate from the combination of progressive recruitment of additional motor units and increases in the firing rate to raise muscle contraction intensity with the progression of power output, as shown using different contraction modes (Gottlieb and Agarwal 1971; Ericson 1986; Weir et al., 1992; Gonzalez-Izal et al., 2012). Muscle activation is also increased during repeated static (Viitasalo and Komi, 1977; Hausswirth et al., 2000) and dynamic (Sarre and Lepers, 2005) submaximal muscle contractions at a given absolute exercise intensity, mostly through additional motor unit recruitment as fatigue develops (Bigland-Ritchie et al., 1986; Fulco et al., 1996). The latter may be accompanied by increasing mean power frequency (MF) during low-intensity prolonged isometric contractions (10%–20% of maximal voluntary contraction (MVC)) or decreasing MF at slightly higher intensities (30%–40% of MVC) (Arendt-Nielsen et al., 1989).
A clear decrement of median power frequency (MPF) is observed during repeated high-intensity dynamic muscle contractions when power output is also declining due to fatigue (Tesch et al., 1990; Izquierdo et al., 2011). However, MPF increases (vastus lateralis) or remains at the same level (vastus medialis) during 5 sec knee extension isometric contractions going from 10% to 90% of the MVC interspaced with 2 min recovery periods (Pincivero et al., 2001). During repeated dynamic muscle contractions at high intensities, a decline in MPF reflects muscle fatigue (Amann et al., 2006), particularly if power output is declining (Tesch et al. 1990; Izquierdo et al., 2011), while an elevated MPF (or MF) may be indicative of fatigue during prolonged exercise at a fixed power output when the intensity of exercise is low or moderate (Sarre and Lepers, 2005). Nevertheless, a reduction in MPF has been also reported during prolonged exercise to exhaustion (Hausswirth et al., 2000).
At a given absolute intensity, dynamic exercise is perceived as harder during exercise in severe hypoxia (i.e., FIo2 <0.115), and the relative intensity of exercise is higher due to the lower Vo2max (Calbet et al., 2003). Consequently, the amount of muscle mass used and EMG amplitude is expected to be higher during exercise at the same absolute intensity in hypoxia, since the relative intensity is higher in hypoxia. The increase of exercise intensity, particularly above the lactate threshold, causes progressive muscle recruitment as shown using magnetic resonance (Endo et al., 2007). However, severe hypoxia has been shown to reduce central motor output (Millet et al., 2012) and, hence, muscle activation due to lower brain oxygenation in hypoxia than in normoxia (Goodall et al., 2012). So far experimental data are contradictory, with some studies reporting no effect of severe hypoxia on EMG amplitude during dynamic ( Taylor and Bronks, 1996; Donnelly and Green, 2013) or static (Millet et al., 2012) muscle contractions, and other reporting increased activity (Fulco et al., 1996). Moreover, the relationship between central motor output, voluntary activation, and EMG parameters is quite uncertain (Verges et al., 2012).
Part of the discrepancies between studies could be due to the different muscles and/or different muscle type of actions (i.e., isometric vs. dynamic), evaluated in each study, since it has been recently shown that muscle activation patterns during incremental exercise in normoxia show marked intra and between-muscle heterogeneity (Hug et al., 2004), as reflected by the tissue water spin-spin transverse relaxation time (T2) from 1H magnetic resonance imaging combined with local measures of exercise 31P chemical shift imaging (Cannon et al., 2013). It remains unknown if EMG amplitude or MPF are affected by changes in the relative intensity of exercise due to differences in oxygenation during dynamic muscle actions.
Therefore, the aim of this study was to determine the influence of severe acute hypoxia on thigh muscle activation, assessed with surface EMG, during dynamic exercise. We hypothesized that muscle activation would be higher during exercise in acute hypoxia with a muscle-specific pattern. Since severe hypoxia may reduce central motor output (Millet et al., 2012), we hypothesized also that at the same relative intensity muscle activation would be lower in severe hypoxia. To reduce EMG variability, two incremental exercise tests in normoxia were averaged and compared with the averages of two incremental exercise tests in severe hypoxia.
Methods
Subjects
Eleven physically active and healthy men [mean±SD: 21.2±2 years old, 71.7±9 kg body weight, 173.6±8 cm height, 16±5% body fat, 52.4±5 mL.kg−1.min−1 maximal oxygen consumption (Vo2max)] volunteered to participate in this project. Prior, to the experiment, all procedures and any potential risks were explained to each subject, and an informed consent document was signed. This study was approved by the ethics committee of the University of Las Palmas de Gran Canaria, and all experiments were performed in accordance with the Declaration of Helsinki.
General procedures
On the first visit to the laboratory, the body composition was determined by dual-energy x-ray absorptiometry (Hologic QDR-1500, Hologic Corp., software version 7.10, Waltham, MA) as described elsewhere (Calbet et al., 1998). Thereafter, subjects reported to the laboratory to become familiar with maximal exercise tests in normoxia and normobaric hypoxia (Altitrainer 200, SMTEC, Switzerland) on separate days. An average of 10 days later, subjects reported to the laboratory on 2 different test days, at least 1 week apart. In each test day, two sets of incremental cycle ergometer (Lode Excalibur Sport 925900, Groningen, The Netherlands) exercise tests to exhaustion, interspaced by a 90 min rest period, one in normoxia (inspired oxygen pressure, PIo2=143±1 mmHg) and another one in acute hypoxia PIo2=74±1 mmHg, were carried out in random order. In hypoxia, subjects were connected to the Altitrainer and after 2 min resting recordings were started. In both conditions, resting values were recorded during 2 min prior to the start of exercise. Thus, subjects were exposed to hypoxia 4 min before the start of the test in hypoxia. After the resting period, the load was set to 60W (hypoxia) or 80W (normoxia), and after 2 min the intensity was increased by 20–30W (hypoxia) or 30–40 W (normoxia) every 2 min until exhaustion, to have incremental exercise tests not too different in terms of duration between normoxia and hypoxia. Subjects were requested to keep a pedaling rate of 80 rpm. Exhaustion was defined as the inability to maintain a pedaling rate above 50 rpm despite strong verbal encouragement during 5 seconds. Oxygen uptake was measured with a metabolic cart (Vmax N29; Sensormedics, California, USA), calibrated prior to each test according to the manufacturer instructions. Respiratory variables were analyzed breath-by-breath and averaged every 20 sec for the assessment of Vo2peak and every minute for submaximal loads. The value recorded during the last minute of each submaximal load was used in the analyses.
Electromyography
Electrical muscle activation was monitored by means of surface electromyography (EMG). EMG signals were continuously recorded from four muscles of the left lower limb: rectus femoris (RF), vastus medialis (VM), vastus lateralis (VL), and biceps femoris (BF). Prior to the application of the EMG electrodes the skin surface was carefully shaved and wiped with alcohol to reduce skin impedance. Bipolar single differential electrodes were placed longitudinally on the muscles following the SENIAM recommendations (Merletti and Hermens, 2000) and taped to the skin to minimize movement artifacts. The reference electrode was placed on the skin over the acromion. The position of the electrodes was marked on the skin with indelible ink, and these references were used for precise electrode placement on repeated experiments. The EMG signals were acquired using a 16-channel recording system (Myomonitor IV, Delsys Inc., Boston, MA) at a sampling rate of 1000 Hz using rectangular-shaped (19.8 mm wide and 35 mm long) bipolar surface electrodes with 1 x 10 mm 99.9% Ag conductors, and an inter-conductor distance of 10 mm (DE-2.3 Delsys Inc.) and filtered with a high pass filter of 20 Hz and low pass filter of 450 Hz. The system has an input impedance of>1015Ω // 0.2pF, a common mode rejection ratio of>80 dB, signal-to-noise ratio<1.2 μV, and a pre-amplifier gain 1000 V/V±1%. Each pedal revolution was detected by using an electrogoniometer (Goniometer Biosignal Sensor S700 Joint Angle Shape Sensor; Delsys Inc.) fixed on the left knee and sampled at 500 Hz. EMG; joint movement were simultaneously recorded by a portable device (Myomonitor IV, Delsys Inc.) and wireless transmitted to a computer (EMGWorks Wireless application and EMGWorks Acquisition 3.7.1.3; Delsys, Inc.).
EMG recordings were later analyzed using a custom-made application (Matlab R2012b, MathWorks, Natick, MA). The EMG signals were full wave rectified and RMS calculated using a 25 ms rolling window. Burst onset and offset detection was determined using as a reference 20% of the maximal RMS activity of each burst (Baum and Li, 2003; Hug and Dorel, 2009), rather than using a mean threshold value from 15 consecutive bursts (Ozgunen et al., 2010). This approach yielded the same result as direct simple visual discrimination, with 100% detection of all bursts in the four muscles. The RMS recorded during the last min of a 2 min 80 W load (in normoxia) was used to normalize the rest of the RMS data. In addition, we calculated a total activity index per minute (TAI) defined as TAI=RMS x burst duration (ms) x pedaling rate (rpm), which is similar to the integrated EMG signal, but computing separately each burst and excluding the baseline EMG between burst. The TAI recorded during the last min of a 2 min 80 W load (in normoxia) was used to normalize the rest of the TAI values.
Mean (MF) and median (MPF) power spectrum frequencies were calculated using Fast Fourier Transform (FFT) (Solomonow et al., 1990). All variables were reported as the mean values of the pedal strokes recorded during the last minute of each load, or the fraction completed in the case of the last load.
Methodological considerations
Disagreements between previous studies could have been caused by the intrinsic variability of EMG recordings (Taylor and Bronks, 1995; Hug et al., 2004). For example, integrated EMG (iEMG) increases with increasing angular velocity during concentric contractions (Westing et al., 1991; Amiridis et al., 1996). Several normalization procedures have been used to reduce EMG variability. Normalization is achieved by comparing the root mean square (RMS) signal recorded during a given experimental condition to a reference RMS signal recorded during standardized reproducible conditions. This approach allows the comparison of RMS across muscles, time, and subjects (Albertus-Kajee et al., 2010). The most applied normalization method is achieved by dividing the RMS recorded during dynamic or static contractions by that obtained during a maximal voluntary contraction (MVC) under static conditions (isometric contraction) (Marsh and Martin, 1995; Hug and Dorel, 2009). This method of normalization is appropriate for static conditions, especially if performed at muscle length and joints angles close to those used in the reference contraction. However, this approach is less specific and less reproducible when the RMS obtained during an MVC is used to normalize dynamic contractions. An alternative procedure is to use RMS obtained during a reference dynamic condition as the normalizing value (Westing et al., 1991; Taylor and Bronks, 1995; Amiridis et al., 1996). Variability could be also reduced averaging some experiments performed under similar conditions, as usually done in O2 kinetics studies (Jones et al., 2012). However, this latter approach has not been applied in EMG research.
Statistical analysis
A Students t-test was used to determine if there was a test order effect between the two tests performed in similar conditions. Since there were no significant test order effects, or differences between the tests performed in the same conditions, the two normoxic exercise tests were averaged and the two hypoxic tests, as well. Thus, only one set of data was left to represent each condition (normoxia and hypoxia). Exercise tests were compared using a two-way ANOVA for repeated measures followed by pairwise comparisons with the Student's t-test adjusted for multiple comparisons with the Bonferroni-Holm correction. The impact of pedaling rate on burst duration was assessed with ANCOVA for repeated measures using pedaling rate as a covariate. P≤0.05 was considered significant. Analysis was performed using a commercially available software package (SPSS version 15.0, SPSS, Inc., Chicago, IL). Data are reported as means±standard deviation (SD), unless otherwise stated.
Results
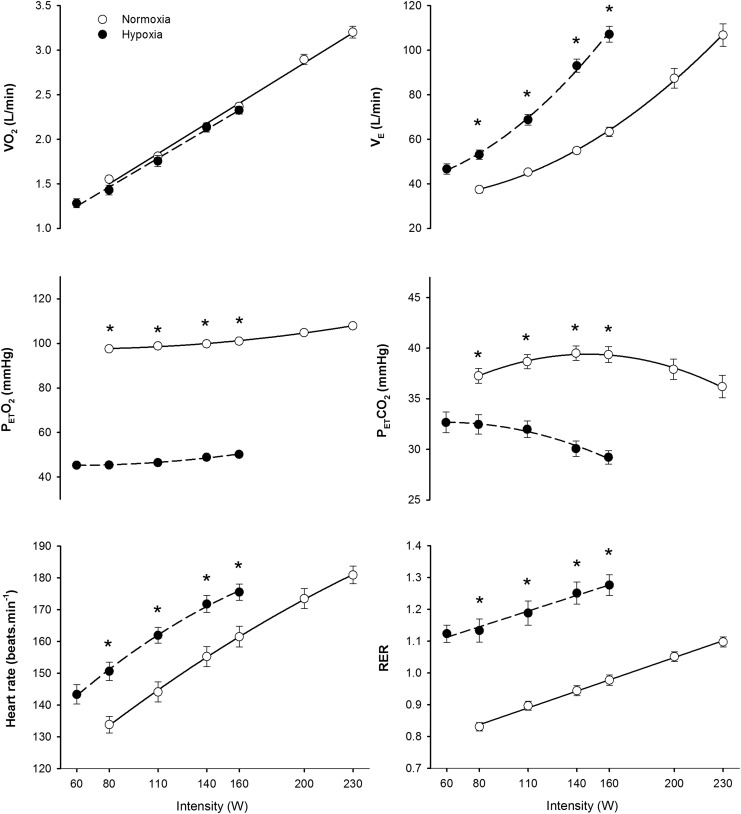
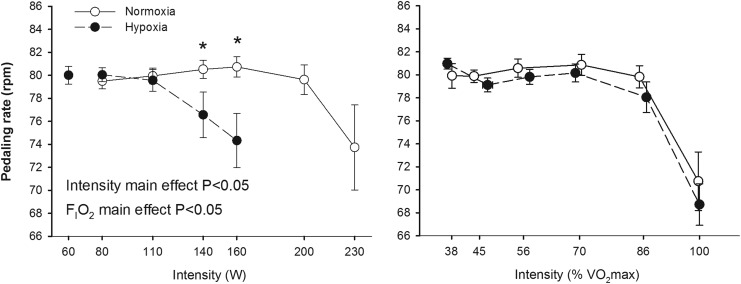
Similar results were obtained in the two test performed in hypoxia (184±23 and 182±23 W, respectively, p=0.72) and normoxia (284±30 and 278±34 W, respectively, p=0.34). However, the tests in normoxia were slightly longer than in hypoxia (850±109 and 747±84 sec, p<0.05). No significant differences were observed in the Vo2/power relationship between normoxia and hypoxia; however, peak Vo2 was 34% lower in hypoxia than in normoxia (p<0.05). At the same absolute load, pulmonary ventilation (VE), heart rate and RER were higher in hypoxia than in normoxia (all, p<0.05), whilst PETO2 and PETCO2 were lower in hypoxia (p<0.05) (Fig. 1). Pedaling rate was maintained around 80 rpm up to 86% of Vo2max; then it declined attaining a value close to 70 rpm in the last min of exercise in both conditions (Fig. 2). At high submaximal exercise intensities, pedaling rate was lower in hypoxia than in normoxia (p<0.05), while it was not affected by FIo2 at the same relative intensity (Fig. 2).
FIG. 1.
Ergospirometric variables during incremental exercise to exhaustion in normoxia (FIo2=0.21, PIo2=141 mmHg) and hypoxia (FIo2=0.108, PIo2=74 mmHg), each point represents the mean and the error bars the standard error of the mean (n=11), only the points for which n=11 are depicted. *P<0.05 normoxia vs. hypoxia, same point.
FIG. 2.
Pedaling rate during incremental exercise to exhaustion in normoxia (FIo2=0.21, PIo2=141 mmHg) and hypoxia (FIo2=0.108, PIo2=74 mmHg), each point represents the mean and the error bars the standard error of the mean (n=11). *P<0.05 normoxia vs. hypoxia, same point. There was a significant main effect of relative intensity on pedaling rate (p<0.05).
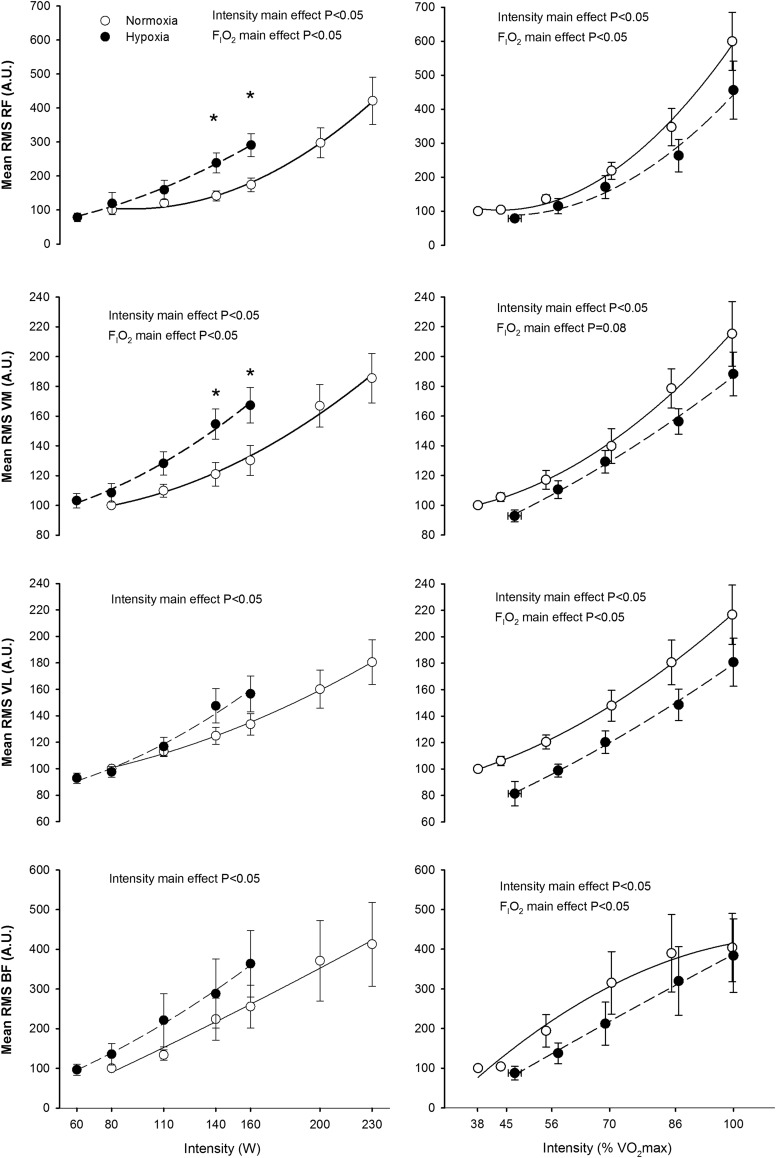
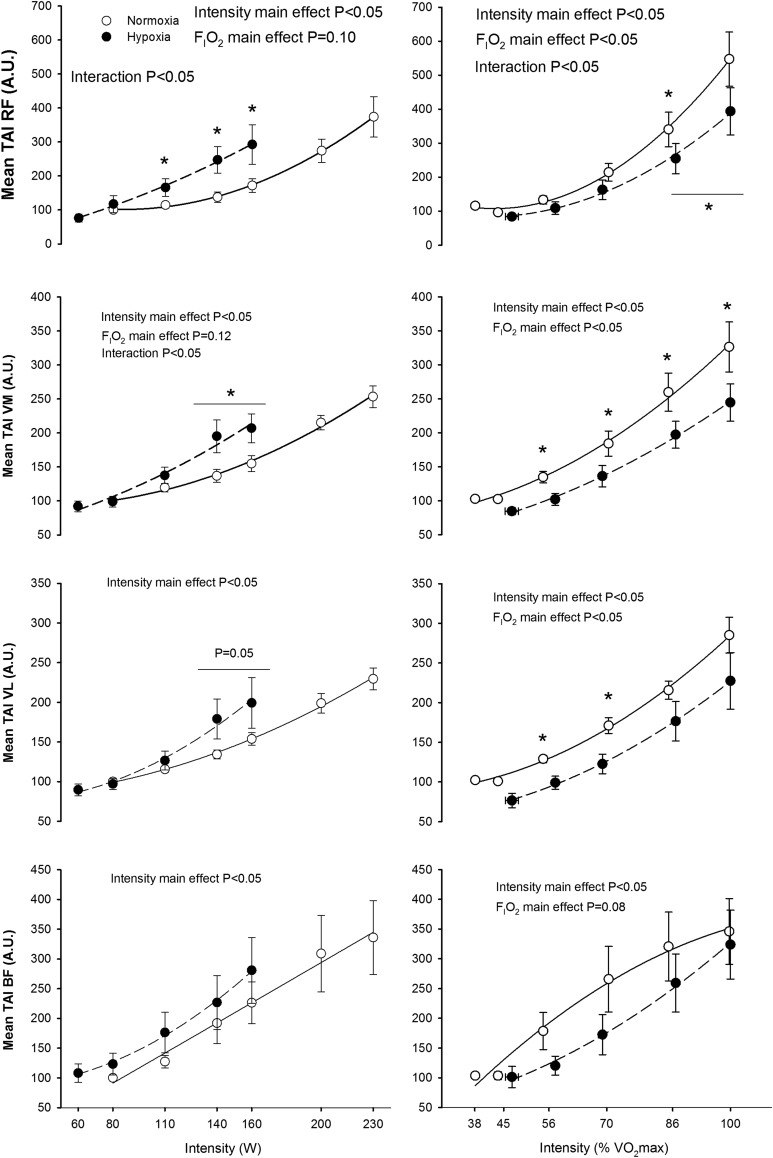
The RMS and TAIm increased with exercise intensity in all the examined muscles; this effect being more accentuated in the RF than in the other muscles (p<0.05) (Figs. 3 and 4). The rate of increase of RMS and TAIm with absolute exercise intensity was greater in hypoxia than in normoxia in the RF and VM (interaction intensity x FIo2 p<0.05), while a similar trend was seen in VL for RMS (p=0.10). No significant FIo2 effects were seen in RMS and TAI responses of the BF. However, at the same relative intensity the RMS was higher in normoxia than in hypoxia in RF, VL and BF (both p<0.05), while a similar trend was seen in VM (p=0.08). Similar results were obtained in TAI [i.e., greater values in normoxia than in hypoxia in RF, VM, and VL (the three, p<0.05), but not in BF (p=0.08)] (Fig. 4).
FIG. 3.
Root mean square (RMS) during incremental exercise to exhaustion in normoxia (FIo2=0.21, PIo2=141 mmHg) and hypoxia (FIo2=0.108, PIo2=74 mmHg), each point represent the mean and the error bars the standard error of the mean (o=11). *P<0.05 normoxia vs. hypoxia, same point.
FIG. 4.
Total activation index per minute (TAI) during incremental exercise to exhaustion in normoxia (FIo2=0.21, PIo2=141 mmHg) and hypoxia (FIo2=0.108, PIo2=74 mmHg), each point represents the mean and the error bars the standard error of the mean (n=11). *P<0.05 normoxia vs. hypoxia, same point. The horizontal line indicates that the mean of the last two relative loads was compared between conditions using a t-test.
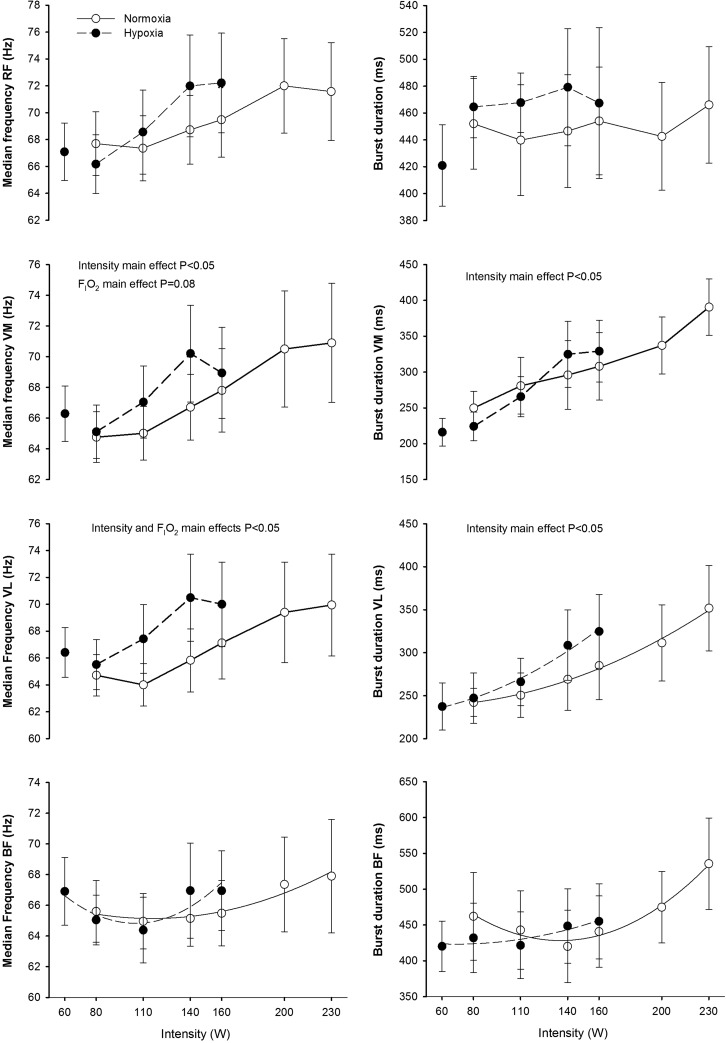
At the same absolute intensity, median frequency in VL was higher in hypoxia, while no significant effects of FIo2 on median frequency were observed in the other muscles (Fig. 5). A significant increase of median frequency with exercise intensity was observed in VM and VL, while no significant influence of exercise intensity on RF and BF median frequencies were observed. The duration of the burst increased with exercise intensity only in VM and VL (p<0.05), while no significant FIo2 effects on burst duration were observed in any muscle (Fig. 5). This effect remained after accounting for small changes in pedaling rate. Mean frequency results (data not shown) were essentially similar to MPF. No significant effects of FIo2 on frequency domain indices were observed when comparisons were performed at the same relative intensity.
FIG. 5.
Median power frequency and burst duration during incremental exercise to exhaustion in normoxia (FIo2=0.21, PIo2=141 mmHg) and hypoxia (FIo2=0.108, PIo2=74 mmHg), each point represents the mean and the error bars the standard error of the mean (n=11). Post hoc pair-wise comparisons at the same time points between conditions yielded nonstatistically significant differences.
Discussion
Although the exercise protocols were slightly different, this study shows that exercise muscle activation increases almost linearly with exercise intensity, and is modulated by the inspired O2 fraction. We have also shown that FIo2 influences muscle activation with a muscle-specific pattern. During cycling at the same absolute intensity, greater muscle activation in severe hypoxia is only clearly seen in RF and VM, although a trend to higher levels of activation was also detected in VL and BF. In contrast, the muscle activation is lower in hypoxia than in normoxia when compared at the same relative intensity. Median and mean frequencies remained at the same level or increased slightly with exercise intensity, following also a muscle specific pattern modulated by FIo2. Finally, we have also shown that the duration of the burst increases (37%–55%) with exercise intensity mostly in VL and VM. On the basis of increases in both RMS and burst duration, it can be inferred that the contribution of VL and VM to the overall mechanical impulse increases with exercise intensity similarly in normoxia and hypoxia.
This muscle specificity may originate from changes in the neural activation pattern caused by the effect of FIo2 on central nervous system oxygenation (Amann et al., 2007; Millet et al., 2012), afferent modulation of corticospinal motor drive (Gerdle and Fugl-Meyer, 1992), and differences in muscle metabolic response to hypoxia (Parolin et al., 2000), muscle fiber type composition and muscle vascularization (Moritani et al., 1992). For example, more active motoneurones have an increased metabolic demand, which under circumstances of limited O2 supply to the brain, as during exercise in severe acute hypoxia, may lead to increased glycolysis, brain release of lactate, and alteration of neuronal metabolism and function (Rasmussen et al., 2010; Overgaard et al., 2012). Experiments using intrathecal fentanyl in humans during cycling have shown that opioid-mediated muscle afferents inhibit central motor drive (Amann et al., 2009; Gagnon et al., 2012); thus changes in afferent discharge with fatigue or oxygenation are expected to alter the pattern of muscle activation. Although a comparative analysis of the metabolic responses of the different portions of the quadriceps muscle in man has not been performed, rodent studies have shown that the metabolic response to hypoxia are region specific, depending on the predominant muscle fiber type and the degree of capillarization (Wust et al., 2009).
In agreement with our results, increased quadriceps muscle iEMG was observed during dynamic knee extension exercise at 21 W in hypobaric hypoxia (barometric pressure: 464 mmHg) compared to normoxic exercise (Fulco et al., 1996). Likewise, increased mean VL iEMG during cycling in hypoxia at FIo2 of 0.10 (Amann et al., 2007) and 0.116 (Taylor et al., 1997) was observed, compared to normoxia, after one minute of exercise at the same absolute intensity. Nevertheless, VL iEMG was not significantly increased when the FIo2 was 0.15 (Amann et al., 2007), indicating that the increase in muscle activation at a given absolute exercise intensity depends on the magnitude of hypoxia during whole body exercise. In partial agreement with our results, Peltonen et al. (1997) reported reduced iEMG (sum of gastrocnemius, VL, RF, BF, gluteus maximus, erector spinae, and biceps brachii muscles) during a 2500 m rowing test in mild hypoxia (FIo2=0.158) compared to normoxia without significant effects on mean power frequency. No muscle specific analysis was reported by Peltonen et al. (1997).
In contrast with our results, Taylor and Bronks (1996) observed similar iEMG responses in RF, VM, and VL at the same absolute intensities in normoxia and moderate hypoxia (FIo2=0.135) during cycling. It should be noticed that, although in Taylor and Bronks (1996) the differences were not statistically significant, the mean iEMG values were higher in hypoxia than in normoxia. Given the intrinsic variability of the EMG, the results of Taylor and Bronks (1996) could just be due to a type II error caused by the combination of smaller effect of a milder level of hypoxia with the intrinsic variability of EMG. Goodall et al. (2010) measured RMS during submaximal fatiguing isometric leg extension contractions and reported no significant differences in VL between severe hypoxia (FIo2=0.10) and normoxia. Likewise, Donnelly and Green (2013) reported no effect during graded exercise of severe hypoxia (FIo2=0.105) on RMS in the triceps surae muscles, with the exception of gastrocnemius medialis, which reached relatively higher RMS values in hypoxia. Millet et al. (2012) reported similar biceps brachii RMS responses during submaximal contractions in normoxia and severe hypoxia (FIo2=0.09). Thus, it seems that when the active muscle mass is small (arm flexion, leg extension, and in some instances knee extension exercise), the impact of hypoxia on muscle activation may be absent or is lower than observed during exercise with a large muscle mass, such as cycling.
The increased EMG amplitude during submaximal exercise at a given absolute intensity in hypoxia may reflect increased motor unit recruitment to compensate for fatigue of active muscle units (Moritani et al., 1992). In fact, using wire electrodes, Moritani et al. (1992) showed both increases in amplitude and firing frequency of individual motor units with fatigue. Motor unit recruitment strategies can be indirectly assessed by determining the MPF of the power spectral analysis of the EMG (Solomonow et al., 1990; Sbriccoli et al., 2003). The fact that the pattern of muscle activation was altered by hypoxia is clearly demonstrated by the reduced pedaling rate at 140 and 160 W in hypoxia (Fig. 2). The influence of pedaling cadence on EMG activity is controversial, but in general it seems that EMG activity increases with pedaling rate with a muscle-specific pattern. EMG activity has been reported to increase with cadence in VL (Marsh and Martin, 1995; Bieuzen et al., 2007), VM (Neptune et al., 1997), BF (Neptune et al., 1997), RF (Marsh and Martin, 1995; Sarre et al., 2003), and medial gastrocnemius (Neptune et al., 1997), whereas no changes in EMG with cadence has been also reported for VM (Sarre et al., 2003), VL (Sarre et al., 2003), RF (Neptune et al., 1997; Bieuzen et al., 2007), and BF (Marsh and Martin, 1995; Bieuzen et al., 2007). At a given absolute exercise intensity, the relative intensity increases with cadences above 60 rpm (Chavarren and Calbet, 1999), implying that part of the increase in EMG amplitude with cadence is likely due to the increase in relative intensity. Despite the slightly lower cadence during exercise in hypoxia at 140 and 160 W, EMG activity was higher in hypoxia than in normoxia. Furthermore, pedaling rate declined similarly in normoxia and hypoxia at exercise intensities above 86% of Vo2max, implying that the relative intensity rather than the small differences in pedaling rate was the main factor dictating the motor activation strategy.
Supraspinal fatigue has been defined as an exercise-induced decline in force caused by suboptimal output from the motor cortex (Gandevia, 2001). Reduced brain oxygenation may cause central fatigue during exercise, particularly in severe acute hypoxia (Goodall et al., 2010; Millet et al., 2012) leading to the corticospinal inhibition of motor drive. Interestingly, hypoxia has, if any, a small effect on muscle metabolism and exercise capacity when the muscle mass recruited is small (Roach et al., 1999; Calbet et al., 2009) or the impairment in exercise capacity is only observed in severe hypoxia (Goodall et al., 2010). Moreover, during exercise with a small muscle mass in severe acute hypoxia, pulmonary gas exchange is less perturbed and consequently, brain oxygenation is less altered than during exercise with a large muscle mass (Amann and Calbet, 2008; Calbet and Lundby, 2009; Calbet et al., 2009). The energy charge of the cell is less reduced during submaximal cycling at the same relative intensity in hypoxia (FIo2=0.115; 72% of Vo2max) than in normoxia (73% of Vo2max) (Wadley et al., 2006), implying a similar or milder alteration of muscle metabolism in hypoxia. The reduction in muscle activation at the same relative intensity in hypoxia compared to normoxia, observed in the present investigation, could originate from both differences in muscle metabolism and changes in corticospinal drive. On the other hand, the potential effects due to differences in pedaling rate can be ruled out, since pedaling rates were similar between the two conditions when compared at the same relative intensities. It should be taken also into consideration that, in severe acute hypoxia, the absolute intensity is much lower than in normoxia, when exercising at the same relative intensity.
In summary, muscle activation during whole body exercise increases almost linearly with exercise intensity, following a muscle-specific pattern, which is modulated depending on FIo2 and the relative intensity of exercise. In general, at a given absolute intensity, muscle activation is higher in hypoxia than in normoxia. Conversely, at a given relative intensity muscle activation is reduced in severe acute hypoxia. Median and mean frequencies remain at the same level or increase slightly with exercise intensity, following also a muscle specific pattern modulated by FIo2. Since both the duration of VL and VM bursts and RMS increase with exercise intensity, it can be inferred that these two muscles are increasingly involved in power output generation as the exercise intensity is elevated, an effect that is accentuated in hypoxia.
Acknowledgments
Special thanks are given to José Navarro de Tuero for his excellent technical assistance. Thanks are also expressed to Lorena Rodríguez-García and Jesus Gustavo Ponce González who helped occasionally during the execution of the experiments.
Author Disclosure Statement
The authors have no conflict of interest to disclose. This study was supported by a grant from the Ministerio de Educación y Ciencia of Spain (DEP2009-11638 and FEDER).
References
- Albertus-Kajee Y, Tucker R, Derman W, and Lambert M. (2010). Alternative methods of normalising EMG during cycling. J Electromyogr Kinesiol 20:1036–1043 [DOI] [PubMed] [Google Scholar]
- Amann M, and Calbet JA. (2008). Convective oxygen transport and fatigue. J Appl Physiol 104:861–870 [DOI] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Pegelow DF, and Dempsey JA. (2009). Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol 587:271–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Romer LM, Pegelow DF, Jacques AJ, Hess CJ, and Dempsey JA. (2006). Effects of arterial oxygen content on peripheral locomotor muscle fatigue. J Appl Physiol 101:119–127 [DOI] [PubMed] [Google Scholar]
- Amann M, Romer LM, Subudhi AW, Pegelow DF, and Dempsey JA. (2007). Severity of arterial hypoxaemia affects the relative contributions of peripheral muscle fatigue to exercise performance in healthy humans. J Physiol 581:389–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiridis IG, Martin A, Morlon B, Martin L, Cometti G, Pousson M, and van Hoecke J. (1996). Co-activation and tension-regulating phenomena during isokinetic knee extension in sedentary and highly skilled humans. Eur J Appl Physiol Occup Physiol 73:149–156 [DOI] [PubMed] [Google Scholar]
- Arendt-Nielsen L, Mills KR, and Forster A. (1989). Changes in muscle fiber conduction velocity, mean power frequency, and mean EMG voltage during prolonged submaximal contractions. Muscle Nerve 12:493–497 [DOI] [PubMed] [Google Scholar]
- Baum BS, and Li L. (2003). Lower extremity muscle activities during cycling are influenced by load and frequency. J Electromyogr Kinesiol 13:181–190 [DOI] [PubMed] [Google Scholar]
- Bieuzen F, Lepers R, Vercruyssen F, Hausswirth C, and Brisswalter J. (2007). Muscle activation during cycling at different cadences: effect of maximal strength capacity. J Electromyogr Kinesiol 17:731–0738 [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Cafarelli E, and Vollestad NK. (1986). Fatigue of submaximal static contractions. Acta Physiol Scand Suppl 556:137–148 [PubMed] [Google Scholar]
- Calbet JA, Boushel R, Radegran G, Sondergaard H, Wagner PD, and Saltin B. (2003). Determinants of maximal oxygen uptake in severe acute hypoxia. Am J Physiol Regul Integr Comp Physiol 284:R291–R303 [DOI] [PubMed] [Google Scholar]
- Calbet JA, and Lundby C. (2009). Air to muscle O2 delivery during exercise at altitude. High Alt Med Biol 10:123–134 [DOI] [PubMed] [Google Scholar]
- Calbet JA, Moysi JS, Dorado C, and Rodriguez LP. (1998). Bone mineral content and density in professional tennis players. Calcif Tissue Int 62:491–496 [DOI] [PubMed] [Google Scholar]
- Calbet JA, Radegran G, Boushel R, and Saltin B. (2009). On the mechanisms that limit oxygen uptake during exercise in acute and chronic hypoxia: Role of muscle mass. J Physiol 587:477–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon DT, Howe FA, Whipp BJ, et al. (2013). Muscle metabolism and activation heterogeneity by combined 31P chemical shift and T2 imaging, and pulmonary O2 uptake during incremental knee-extensor exercise. J Appl Physiol (1985) 115:839–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavarren J, and Calbet JA. (1999). Cycling efficiency and pedalling frequency in road cyclists. Eur J Appl Physiol 80:555–563 [DOI] [PubMed] [Google Scholar]
- Donnelly J, and Green S. (2013). Effect of hypoxia on the dynamic response of hyperaemia in the contracting human calf muscle. Exp Physiol 98:81–93 [DOI] [PubMed] [Google Scholar]
- Endo MY, Kobayakawa M, Kinugasa R, Kuno S, Akima H, Rossiter HB, Miura A, and Fukuba Y. (2007). Thigh muscle activation distribution and pulmonary VO2 kinetics during moderate, heavy, and very heavy intensity cycling exercise in humans. Am J Physiol Regul Integr Comp Physiol 293:R812–820 [DOI] [PubMed] [Google Scholar]
- Ericson M. (1986). On the biomechanics of cycling. A study of joint and muscle load during exercise on the bicycle ergometer. Scand J Rehabil Med Suppl 16,1–43 [PubMed] [Google Scholar]
- Fulco CS, Lewis SF, Frykman PN, et al. (1996). Muscle fatigue and exhaustion during dynamic leg exercise in normoxia and hypobaric hypoxia. J Appl Physiol 81:1891–1900 [DOI] [PubMed] [Google Scholar]
- Gagnon P, Bussieres JS, Ribeiro F, et al. (2012). Influences of spinal anesthesia on exercise tolerance in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 186:606–615 [DOI] [PubMed] [Google Scholar]
- Gandevia SC. (2001). Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81:1725–1789 [DOI] [PubMed] [Google Scholar]
- Gerdle B, and Fugl-Meyer AR. (1992). Is the mean power frequency shift of the EMG a selective indicator of fatigue of the fast twitch motor units? Acta Physiologica Scandinavica 145:129–138 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Izal M, Malanda A, Gorostiaga E, and Izquierdo M. (2012). Electromyographic models to assess muscle fatigue. J Electromyogr Kinesiol 22:501–512 [DOI] [PubMed] [Google Scholar]
- Goodall S, Gonzalez-Alonso J, Ali L, Ross EZ, and Romer LM. (2012). Supraspinal fatigue after normoxic and hypoxic exercise in humans. J Physiol 590:2767–2782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodall S, Ross EZ, and Romer LM. (2010). Effect of graded hypoxia on supraspinal contributions to fatigue with unilateral knee-extensor contractions. J Appl Physiol 109:1842–1851 [DOI] [PubMed] [Google Scholar]
- Gottlieb GL, and Agarwal GC. (1971). Dynamic relationship between isometric muscle tension and the electromyogram in man. J Appl Physiol 30:345–351 [DOI] [PubMed] [Google Scholar]
- Hausswirth C, Brisswalter J, Vallier JM, Smith D, and Lepers R. (2000). Evolution of electromyographic signal, running economy, and perceived exertion during different prolonged exercises. Int J Sports Med 21:429–436 [DOI] [PubMed] [Google Scholar]
- Hug F, Bendahan D, Le Fur Y, Cozzone PJ, and Grelot L. (2004). Heterogeneity of muscle recruitment pattern during pedaling in professional road cyclists: A magnetic resonance imaging and electromyography study. Eur J Appl Physiol 92:334–342 [DOI] [PubMed] [Google Scholar]
- Hug F, and Dorel S. (2009). Electromyographic analysis of pedaling: A review. J Electromyogr Kinesiol 19:182–198 [DOI] [PubMed] [Google Scholar]
- Izquierdo M, Gonzalez-Izal M, Navarro-Amezqueta I, et al. (2011). Effects of strength training on muscle fatigue mapping from surface EMG and blood metabolites. Med Sci Sports Exerc 43:303–311 [DOI] [PubMed] [Google Scholar]
- Jones AM, Krustrup P, Wilkerson DP, Berger NJ, Calbet JA, and Bangsbo J. (2012). Influence of exercise intensity on skeletal muscle blood flow, O2 extraction and O2 uptake on-kinetics. J Physiol 590:4363–4376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AP, and Martin PE. (1995). The relationship between cadence and lower extremity EMG in cyclists and noncyclists. Med Sci Sports Exerc 27:217–225 [PubMed] [Google Scholar]
- Merletti R, and Hermens H. (2000). Introduction to the special issue on the SENIAM European Concerted Action. J Electromyogr Kinesiol 10:283–236 [DOI] [PubMed] [Google Scholar]
- Millet GY, Muthalib M, Jubeau M, Laursen PB, and Nosaka K. (2012). Severe hypoxia affects exercise performance independently of afferent feedback and peripheral fatigue. J Appl Physiol 112:1335–1344 [DOI] [PubMed] [Google Scholar]
- Moritani T, Sherman WM, Shibata M, Matsumoto T, and Shinohara M. (1992). Oxygen availability and motor unit activity in humans. Eur J Appl Physiol Occup Physiol 64:552–556 [DOI] [PubMed] [Google Scholar]
- Neptune RR, Kautz SA, and Hull ML. (1997). The effect of pedaling rate on coordination in cycling. J Biomech 30:1051–1058 [DOI] [PubMed] [Google Scholar]
- Osawa T, Kime R, Hamaoka T, Katsumura T, and Yamamoto M. (2011). Attenuation of muscle deoxygenation precedes EMG threshold in normoxia and hypoxia. Med Sci Sports Exerc 43:1406–1413 [DOI] [PubMed] [Google Scholar]
- Overgaard M, Rasmussen P, Bohm AM, et al. (2012). Hypoxia and exercise provoke both lactate release and lactate oxidation by the human brain. FASEB J 26:3012–3020 [DOI] [PubMed] [Google Scholar]
- Ozgunen KT, Celik U, and Kurdak SS. (2010). Determination of an optimal threshold value for muscle activity eetection in EMG analysis. J Sports Sci Med 9:620–628 [PMC free article] [PubMed] [Google Scholar]
- Parolin ML, Spriet LL, Hultman E, Hollidge-Horvat MG, Jones NL, and Heigenhauser GJ. (2000). Regulation of glycogen phosphorylase and PDH during exercise in human skeletal muscle during hypoxia. Am J Physiol Endocrinol Metab 278:E522–534 [DOI] [PubMed] [Google Scholar]
- Peltonen JE, Rusko HK, Rantamaki J, Sweins K, Niittymaki S, and Viitasalo JT. (1997). Effects of oxygen fraction in inspired air on force production and electromyogram activity during ergometer rowing. Eur J Appl Physiol Occup Physiol 76:495–503 [DOI] [PubMed] [Google Scholar]
- Pincivero DM, Campy RM, Salfetnikov Y, Bright A, and Coelho AJ. (2001). Influence of contraction intensity, muscle, and gender on median frequency of the quadriceps femoris. J Appl Physiol (1985) 90:804–810 [DOI] [PubMed] [Google Scholar]
- Rasmussen P, Nielsen J, Overgaard M, Krogh-Madsen R, Gjedde A, Secher NH, and Petersen NC. (2010). Reduced muscle activation during exercise related to brain oxygenation and metabolism in humans. J Physiol 588:1985–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach RC, Koskolou MD, Calbet JA, and Saltin B. (1999). Arterial O2 content and tension in regulation of cardiac output and leg blood flow during exercise in humans. Am J Physiol 276:H438–445 [DOI] [PubMed] [Google Scholar]
- Sarre G, and Lepers R. (2005). Neuromuscular function during prolonged pedalling exercise at different cadences. Acta Physiol Scand 185:321–328 [DOI] [PubMed] [Google Scholar]
- Sarre G, Lepers R, Maffiuletti N, Millet G, and Martin A. (2003). Influence of cycling cadence on neuromuscular activity of the knee extensors in humans. Eur J Appl Physiol 88:476–479 [DOI] [PubMed] [Google Scholar]
- Sbriccoli P, Bazzucchi I, Rosponi A, Bernardi M, De Vito G, and Felici F. (2003). Amplitude and spectral characteristics of biceps brachii sEMG depend upon speed of isometric force generation. J Electromyogr Kinesiol 13:139–147 [DOI] [PubMed] [Google Scholar]
- Solomonow M, Baten C, Smit J, Baratta R, Hermens H, D'Ambrosia R, and Shoji H. (1990). Electromyogram power spectra frequencies associated with motor unit recruitment strategies. J Appl Physiol (1985) 68:1177–1185 [DOI] [PubMed] [Google Scholar]
- Taylor AD, and Bronks R. (1995). Reproducibility and validity of the quadriceps muscle integrated electromyogram threshold during incremental cycle ergometry. Eur J Appl Physiol Occup Physiol 70:252–257 [DOI] [PubMed] [Google Scholar]
- Taylor AD, and Bronks R. (1996). Effect of acute normobaric hypoxia on quadriceps integrated electromyogram and blood metabolites during incremental exercise to exhaustion. Eur J Appl Physiol Occup Physiol 73:121–129 [DOI] [PubMed] [Google Scholar]
- Taylor AD, Bronks R, Smith P, and Humphries B. (1997). Myoelectric evidence of peripheral muscle fatigue during exercise in severe hypoxia: Some references to m.vastus lateralis myosin heavy chain composition. Eur J Appl Physiol Occup Physiol 75:151–159 [DOI] [PubMed] [Google Scholar]
- Tesch PA, Dudley GA, Duvoisin MR, Hather BM, and Harris RT. (1990). Force and EMG signal patterns during repeated bouts of concentric or eccentric muscle actions. Acta Physiol Scand 138:263–271 [DOI] [PubMed] [Google Scholar]
- Verges S, Rupp T, Jubeau M, Wuyam B, Esteve F, Levy P, Perrey S, and Millet GY. (2012). Cerebral perturbations during exercise in hypoxia. Am J Physiol Regul Integr Comp Physiol 302:R903–916 [DOI] [PubMed] [Google Scholar]
- Viitasalo JH, and Komi PV. (1977). Signal characteristics of EMG during fatigue. Eur J Appl Physiol Occup Physiol 37:111–121 [DOI] [PubMed] [Google Scholar]
- Wadley GD, Lee-Young RS, Canny BJ, et al. (2006). Effect of exercise intensity and hypoxia on skeletal muscle AMPK signaling and substrate metabolism in humans. Am J Physiol Endocrinol Metab 290:E694–702 [DOI] [PubMed] [Google Scholar]
- Weir JP, Wagner LL, and Housh TJ. (1992). Linearity and reliability of the IEMG v torque relationship for the forearm flexors and leg extensors. Am J Phys Med Rehabil 71:283–287 [DOI] [PubMed] [Google Scholar]
- Westing SH, Cresswell AG, and Thorstensson A. (1991). Muscle activation during maximal voluntary eccentric and concentric knee extension. Eur J Appl Physiol Occup Physiol 62:104–108 [DOI] [PubMed] [Google Scholar]
- Wust RC, Jaspers RT, van Heijst AF, et al. (2009). Region-specific adaptations in determinants of rat skeletal muscle oxygenation to chronic hypoxia. Am J Physiol Heart Circ Physiol 297:H364–374 [DOI] [PubMed] [Google Scholar]