Abstract
The pathology of spinal cord injury (SCI) makes it appropriate for cell-based therapies. Treatments using neural stem cells (NSCs) in animal models of SCI have shown positive outcomes, although uncertainty remains regarding the optimal cell source. Pluripotent cell sources such as embryonic stem cells (ESCs) provide a limitless supply of therapeutic cells. NSCs derived using embryoid bodies (EB) from ESCs have shown tumorigenic potential. Clonal neurosphere generation is an alternative method to generate safer and more clinically relevant NSCs without the use of an EB stage for use in cell-based therapies. We generated clonally derived definitive NSCs (dNSCs) from ESC. These cells were transplanted into a mouse thoracic SCI model. Embryonic stem cell-derived definitive neural stem cell (ES-dNSC)-transplanted mice were compared with controls using behavioral measures and histopathological analysis of tissue. In addition, the role of remyelination in injury recovery was investigated using transmission electron microscopy. The SCI group that received ES-dNSC transplantation showed significant improvements in locomotor function compared with controls in open field and gait analysis. The cell treatment group had a significant enhancement of spared neural tissue. Immunohistological assessments showed that dNSCs differentiated primarily to oligodendrocytes. These cells were shown to express myelin basic protein, associate with axons, and support nodal architecture as well as display proper compact, multilayer myelination in electron microscopic analysis. This study provides strong evidence that dNSCs clonally derived from pluripotent cells using the default pathway of neuralization improve motor function after SCI and enhance sparing of neural tissue, while remaining safe and clinically relevant.
Introduction
The Christopher and Dana Reeve Foundation reports spinal cord injury (SCI) as the leading cause of paralysis in North America, constituting 24% of this population. Despite progress in the acute medical and surgical care of SCI [1], a significant portion of SCI patients suffer from severe neurological disability, leading to substantial physical, emotional, social, and economic burden. It has been estimated that the average lifetime cost for a 25-year-old suffering from an SCI with tetraplegia is roughly US$3 million [2]. There are currently no effective clinical treatments to restore neurological function after an SCI, and this remains an area of continued research [3].
One area of intense research focus has been cell-based repair strategies for the injured spinal cord. SCI is a complex injury that stems from an initial mechanical insult followed by a series of molecular and cellular events that form the secondary injury. A hallmark of this secondary injury is demyelination that leads to an interruption of signal transmission and contributes to axonal degeneration [4]. Many cell types have emerged as possible candidates for SCI therapy, each with unique advantages, disadvantages, and challenges in clinical translation (reviewed by Tetzlaff et al. [5]). Neural stem cells (NSCs) are a well-studied cell type that have shown promise in SCI. NSCs can be derived from areas in adult brain tissue, areas lining the central spinal cord [5,6], or from embryonic stem cells (ESCs) within the blastocyst [7–11]. NSCs derived from adult tissue expand in vitro [12,13] and respond to in vivo cues when transplanted into the spinal cord to form neural lineage cells that promote functional recovery in SCI [14,15]. Unfortunately, NSCs derived from adult tissue reside primarily in the periventricular sub-ependymal layer of the brain and the risks associated with harvesting this cell type may preclude their clinical translation [16]. In light of this, ESCs have emerged as a very attractive source of transplantable NSCs.
Studies using ESC-derived NSCs transplants have shown that these cells confer functional and structural benefits in animal SCI models [11,17–22]. Despite the promise of NSC-ESCs for cell-based repair in SCI, grave safety concerns are associated with their teratoma and tumor-forming potential [23,24]. The most common techniques of deriving NSCs from ESCs involve embryoid body (EB) formation [9]. These approaches have shown some functional success, but an EB intermediate in the generation of NSCs carries a serious risk of non-neural lineage ESCs from the EB persisting after transplant [25]. Non-neural lineage cells—particularly those expressing endoderm lineage genes and pluripotency markers—carry a distinct potential for tumorigenesis [23,25,26]. Tumors and teratomas have been observed in in vivo transplants of NSCs derived from ESCs using the EB formation step [26,27], indicating a clear need for an alternative approach to generate safe NSCs from ESCs.
We used the default pathway of neural induction [25,28,29] to generate a clinically relevant clonally derived NSC population that is derived from ESCs without the need for EB formation (described extensively by Rowland et al. [25]). The default pathway of neuralization from pluripotent ESCs to transplantable NSCs involves the formation of leukemia inhibitory factor (LIF)-dependent neurospheres [referred to as primitive neural stem cells (pNSCs)]. Subsequently, primitive neurospheres are passaged in the absence of LIF in serum-free media (SFM) containing fibroblast growth factor (FGF), yielding a definitive NSC (dNSC). These dNSCs are committed to a neural lineage, lose pluripotency markers, and have a substantially decreased tumorigenic potential [25,28,29].
In this study, we investigated the transplantation of dNSCs that are clonally selected from ESCs, without EB formation, in a mouse injury model. We hypothesized that transplanted dNSCs would survive and differentiate into neural-lineage cells, neurons, oligodendrocytes, and astrocytes. We further hypothesized that these transplanted cells would provide support to the injured spinal cord and facilitate axonal remyelination, thus leading to a functional benefit to the injured mouse, measurable by a mouse behavioral assessment novel to cell transplantation—the CatWalk-assisted gait analysis [30]—and by a validated tool, the Basso Mouse Scale (BMS) [20,21,31,32]. This study is the first to use dNSCs clonally derived from ESCs, without EB formation in a SCI model. It is also the first to use a graded clip-compression injury in a mouse model of cell transplantation. This study provides evidence supporting the efficacy of dNSCs clonally selected from ESCs in functional recovery after SCI, and lends support to the concept that this treatment is a safe alternative to NSCs derived from ESCs by EB formation.
Materials and Methods
Animals used
All experimental protocols were approved by the Animal Care Committee at the Toronto Western Research Institute in accordance with the policies of the Guide to the Care and Use of Experimental Animals as per the Canadian Council of Animal Care. Female, adult wild-type C57BL/6 mice (15 to 20 g; Jackson Laboratories) aged 8 to 10 weeks were used for injury, cell transplantation, behavioral analysis, histology, immunohistochemistry, and electron microscopy.
Expansion of ESCs in culture
R1 murine ESCs were used in all experiments [33]. These cells were initially generated from 129 strain mice and constitutively express green fluorescent protein (GFP). The ESCs were cultured using standard serum and feeder layer conditions [25,34]. In brief, the ESCs were plated on NUNC-coated dishes on a mitomycin C inactivated mouse embryonic fibroblast (MEF) feeder layer in media containing Dulbecco's Modified Eagle Medium, ESC qualified fetal bovine serum, L-glutamine, penicillin/streptomycin, nonessential amino acids, and β-Mercaptoethanol+LIF (1,000 U/mL; Millipore). For passage, the confluent cells were incubated with 0.05% Trypsin–ethylenediaminetetra-acetic acid (EDTA) for disassociation and plated at a 1:5 ratio.
Generation of primary and definitive neurospheres
The generation of primitive and definitive neurospheres followed the protocol previously described [25,28,34]. ESCs were dissociated using TrypLE Select (Invitrogen) and spun at 1,500 rpm for 5 min. Cells were re-suspended in ES media and plated on a NUNC coated dish so that MEFs were able to attach. The supernatant was collected, spun down, and rinsed twice with SFM. Primary ESC-derived pNSC were seeded in low attachment flasks at a cell density of 10 cell/μL in SFM containing LIF (1,000 U/mL). Primary primitive neurospheres were present after 7 days, on which single neurospheres could be dissociated using TrypLE Select and in SFM media+LIF (Millipore), FGF2 (10 mg/100 mL; Sigma), and Heparin (2 mg/100 mL; Sigma) to yield secondary primitive neurospheres and so on. To generate dNSCs after pNSC, the cells were seeded at 10 cells/μL in SFM without LIF, containing only FGF2 (Sigma) and Heparin (Sigma). B27 supplement (Gibco) was added to increase cell survival. The dNSCs can be continually passaged in the SFM+FGF, Heparin, and B27.
Spinal cord injury
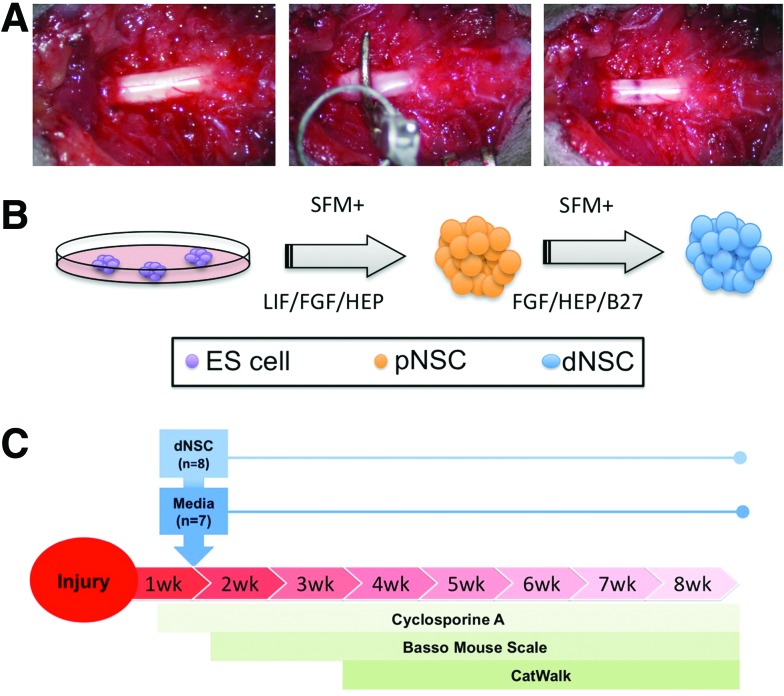
Spinal cord injuries in mice were performed similarly to previously described techniques [35]. Mice were anesthetized using a mixture of isoflurane, nitrous oxide, and oxygen. Under aseptic conditions, A T5 to T7 laminectomy was performed exposing the spinal cord. The spinal cord was compressed ventrally and dorsally at T6 using a modified aneurysm clip (FEJOTA™ mouse clip, University Health Network, Canada), described in previously reported studies [36,37]. The clip was released with a closing force of 6.5 g and the clip remained closed on the cord for 30 s (Fig. 1A bottom-left), resulting in a moderate compression injury (Fig. 1B bottom-right). To prevent excessive scar formation and adhesion of surrounding tissues to the dura, absorbable surgical foam (Surgifoam; Ethicon) was applied directly dorsal to the exposed dura of the spinal cord. Sham injured mice received all of the steps of surgery described earlier, including the laminectomy, but not the passage of the dissecting hook or the clip-compression injury. Saline was administered subcutaneously to replace lost blood volume, and buprenorphine (0.05 mg/kg) was administered subcutaneously to alleviate postoperative pain. During recovery from anesthesia, mice were placed in sterile cages under a heating lamp and eventually housed in a temperature-controlled room at 27°C for the 8 weeks of experimentation. Food and water were provided ad libitum, and mice received antibiotic in their drinking water for 3 days after surgery (Clavamox drops; Pfizer Animal Health). Buprenorphine was administered subcutaneously twice daily for 2 days after surgery. Bladders were manually voided twice daily until bladder function returned.
FIG. 1.
Schematic of spinal cord injury (SCI) and cell intervention. (A) All injured mice received a central incision and blunt dissection to expose the dorsal spinal column. A laminectomy is performed at T5–7 exposing the spinal cord (left). A modified aneurysm clip was closed on the spinal cord at T6 with a closing force of 6.5 g for 30 s (center), resulting in a moderate contusion/compression injury (right). (B) Definitive neural stem cells (dNSCs) were generated from pluripotent embryonic stem (ES) cells grown on a feeder cell layer that were passaged as single cells to serum-free media (SFM) containing LIF to form floating neurospheres of primitive neural stem cells (pNSCs) and subsequently passaged in SFM with FGF to yield clonally expanded free-floating neurospheres of dNSCs. (C) Timeline for experimental groups. Mice received SCI at t=0; dNSC transplants or media only injections at t=1 week; sacrificed at t=8 weeks. FGF, fibroblast growth factor; LIF, leukemia inhibitory factor. Color images available online at www.liebertpub.com/scd
dNSC transplantation
One week after injury, mice were randomized to receive either dNSC injections or media alone. Cell transplantation was performed similarly to previously described techniques [25]. Before surgery, mice were immunosuppressed for 48 h with cyclosporine A (20 mg/kg) in their drinking water. Cyclosporine A treatment was continued after transplant for the remaining 7 weeks of experimentation. Although the transplantation was nonxenographic, immunosuppression was required due to variability between strains. At the time of transplantation, mice were prepared for surgery as described earlier and anesthesia was induced under the same conditions. The injured spinal cord was re-exposed and a cell injection at four sites was mapped bilaterally adjacent to the midline dorsal vein at 1 mm rostral and caudal to the site of clip injury. Cells were injected using a pulled glass micropipette attached to a 5 μL Hamilton syringe at a depth of 0.5 mm into the spinal cord. Cells were prepared for transplantation by dissociation and re-suspension at a concentration of 50,000 per microliter in SFM. The injection volume was 1 μL per site, and cells were injected at a rate of 0.5 μL/min by a computer-controlled syringe pump (World Precision Instruments).
Assessment of motor functional recovery
Functional recovery in both ESC-transplanted mice and media-injected controls was assessed using two behavioral assessment tools that are validated for use in mice—the BMS [31] and CatWalk-assisted gait analysis [30]. Neuropathic pain was also evaluated using von Frey filaments for mechanical allodynia and tail flick latency for thermal allodynia.
Treatment-blinded researchers performed all behavioral data acquisition and analysis. The BMS is a global measure of hindlimb function in mice. It is scored on a scale from 0 to 9 (0 denotes complete lack of hindlimb movement, 9 denotes normal hindlimb movement, coordination, and stability). Mice were placed in an open field free of tactile stimulation and observed by two raters. The BMS score was evaluated once per week, on the same day and at the same time for 8 weeks for experimentation.
The CatWalk assessment measures many specific aspects of mouse limb movement, including swing speed, stride length, and hindlimb intensity. Catwalk analysis was performed from week 3 post-transplant (when a majority of mice had regained some hindlimb function) until the end of experimentation at week 8. Only the mice able to traverse the platform were used in the gait parameter analysis.
All injured mice were tested for mechanical and thermal allodynia weekly, starting at 6 weeks post-SCI using established methods [38]. In brief, the mice were allowed to acclimate to the testing environment for 30 min before testing. Mechanical allodynia was assessed using a 4 g von Frey filament (Semmes-Weinstein monofilaments; Stoelting). The mouse enclosure had a metal mesh floor that allows for application of the filament to the plantar surface of the hind paws. Ten trials were carried out during the testing week with a minimum of 15 min between each trial. Paw withdrawal after stimulus was counted as a response. Thermal allodynia was assessed by tail flick latency time in response to a light beam using a Tail flick test analgesia Meter Apparatus (Model 336T6; IITC Life Science). The latency of the mouse to remove its tail from the heat was recorded.
Histology and lesional tissue quantification
Eight weeks after injury, mice were overdosed with isoflurane, and transcardially perfused with ice-cold phosphate-buffered solution (PBS) followed by 4% paraformaldehyde (PFA) in PBS (pH 7.4). Spinal cords were collected and fixed. The spinal cords were then cryoprotected using sucrose. A 1.2 cm length of spinal cord centered at the injury epicenter was then embedded in Embedding Matrix (M1 Embedding Matrix; Thermo Scientific). Serial cryosections of 35 μm thickness were taken and mounted on glass slides. To assess total cord area, gray matter area, white matter area, and lesion tissue area, sections were stained using standard hematoxylin/eosin (H&E) and Luxol fast blue (LFB) [39]. H&E and LFB stained sections were analyzed using the Cavalieri Probe in a Stereo Investigator (Stereo Investigator 64-bit software; MBF Bioscience). Tissue sections were analyzed for lesional tissue (dense fibrous tissue, inconsistent matrix), gray matter (stained red due to eosin), and white matter (stained blue due to LFB). The injury epicenter was defined as the section with the largest cross-sectional area of lesional tissue. Experimenters were blinded to the treatment group of the tissue sections.
Immunohistochemistry
Cross-sectional spinal cord tissue was incubated with phosphate-buffered saline containing 1% bovine serum albumin, 5% normal goat serum, 5% nonfat milk powder, and 0.25% Triton X-100 for 1 h to block nonspecific binding. The slides were incubated overnight at 4°C with a primary antibody (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/scd) diluted in the same blocking solution. The slides were incubated for 1 h with an appropriate secondary antibody (Invitrogen). Finally, slides were mounted with the Vectashield mounting medium with 4-6-diamidino-2-phenylindole (DAPI; Vector Labs) for nuclear counterstaining. Total GFP+ cell survival was estimated using Stereo Investigator optical fractionator software on a Nikon Eclipse E800 microscope. For fate analysis of exogenous cells, nonoverlapping high magnification confocal fields were imaged. ImageJ software was used to count all GFP+ and lineage marker positive cells in each field. The rostro-caudal sampling was even distributed along the engrafted tissue and included the injury epicenter. These data were expressed as a proportion of total GFP+ cells imaged.
Electron microscopy
Fixed spinal cords were rinsed in 1× PBS and embedded in 2% agarose for vibratome sectioning at a thickness of 50 μm. Immunoperoxidase staining with photo-oxidation of GFP was performed following adapted methods from Grabenbauer et al. [40]. In brief, endogenous peroxidases were quenched using a 0.1% H2O2, blocked in 5% goat serum, 1% BSA, and 0.03% Triton X for 2 h, and incubated overnight at 4C in primary antibody (1:100, Rb anti-GFP, AB3080; Chemicon). HRP-rabbit secondary was applied for 2 h at room temperature followed by postfixation in 0.1% glutaraldeyde. Incubation with 0.05% DAB with 0.1% H2O2 was performed under a light microscope to visualize the DAB reaction product as well as using a X-Cite 120Q series fluorescence lamp (Luman Dynamics) with appropriate settings for GFP excitations to bleach sample. Tissue sections were stored in PBS before transmission preparation of tissue.
Tissue is embedded in Epon-Araldite epoxy resin following a modified technique by Schurch et al. [41] In brief, the tissue samples are removed, placed in a drop of fixative, cut into small (3 mm) cubes or sectioned, and then fixed for 2 h with Karnovsky's style fixative in Sorensen's phosphate buffer at room temperature. The fixative is removed and replaced with fresh fixative, and the samples are stored at 4°C. Dehydration is performed using a graded series of ethanol/distilled water: 30%, 50%, 70%, and 95% twice each for 10 min followed by three changes of 100% ethanol for 15 min each, all at room temperature. The samples are washed with a transitional solvent, propylene oxide, twice for 15 min each. The samples are infiltrated with Epon Araldite (E/A) resin using a graded series of E/A and propylene oxide (PO) mixture. The samples are then placed into polyethylene BEEM capsules, or flat embedded with appropriate labels. Fresh E/A resin is added to the molds, and all of the samples are placed in an oven. Polymerization occurs for 48 h at 60°C. After complete polymerization, the solid resin blocks containing the samples are sectioned on a Reichert Ultracut E microtome at 70 to 80 nm thickness and collected on 300 mesh copper grids. Flat embedded samples should be cut out and glued onto stubs for sectioning. The sections are counter-stained using saturated uranyl acetate for 15–20 min, rinsed in distilled water, followed by Reynold's lead citrate for 15–20 min, and rinsed again in distilled water. The sections are examined and photographed in a Hitachi H7000 transmission electron microscope at an accelerating voltage of 75 kV.
Experimental design and data analysis
All animals were randomized into injury groups as well as to treatment. Behavioral and histological analysis was performed by two independent and blinded researchers. Data are presented as mean±standard error of the mean Catwalk data were analyzed using a one-way analysis of variance (ANOVA). The BMS was analyzed using a two-way ANOVA with repeated measures. Histological data were analyzed between all groups using two-way ANOVA with repeated measures. For post-hoc comparison, Fishers least-significant difference analysis was used. The significance level of all analyses was set at P<0.05.
Results
dNSC transplant leads to functional recovery in injured mice
dNSCs were generated from clonally selected ESCs using the default pathway of neuralization previously described by our lab [25]. In addition to their previously published in vitro characterization, the capacity of the embryonic stem cell-derived definitive neural stem cell (ES-dNSCs) to express neurotropic gene markers was evaluated using a reverse transcriptase polymer chain reaction (Supplementary Fig. S1).
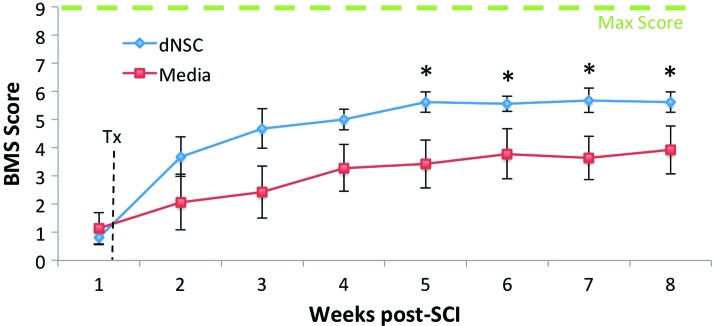
18 mice were injured as described earlier, with 15 mice (83.33%) surviving all 8 weeks of experimentation. Mice were transplanted with dNSCs (n=8) or with SFM only (n=7) at 7 days from injury (Fig. 1). In open field locomotion testing using the BMS, mice transplanted with dNSCs performed significantly better than control mice beginning at 28 days postinjury and this significant difference was maintained to the 8 week experimental endpoint (Fig. 2). Sham injured animals did not display a motor deficit and received a maximum BMS of nine for all weeks postsurgery.
FIG. 2.
dNSC Transplant results in improved locomotor function in open field test after SCI. dNSC transplant results in improvements on the BMS. dNSC-treated mice (blue line) and media only (red line) have significant differences in performance from week 5 onward. Data represent mean±SEM, * indicates P<0.05 two-way ANOVA with LSD. Sham, n=3; dNSC, n=8; media, n=7. ANOVA, analysis of variance; BMS, Basso Mouse Scale; LSD, least significant difference; SEM, standard error of the mean. Color images available online at www.liebertpub.com/scd
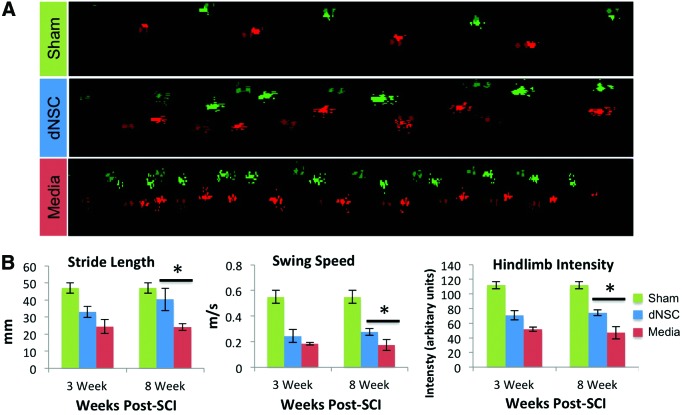
CatWalk-assisted gait analysis was performed from week 3 until the end of experimentation at week 8—weeks 3, and 8 mice were analyzed across treatment groups. When representative CatWalk runs are visualized for each group, mouse footstep patterns are noticeably different between dNSC-treated and media-treated control mice (Fig. 3A). For gait to be analyzed, the animal needs to be able to achieve plantar stepping. At week 3, all mice receiving dNSC treatment were able to complete the CatWalk, while only four of seven media control mice were able to complete it. By 8 weeks post-SCI, all animals had recovered to the extent that they were able to complete the CatWalk. At week 3, no significant differences were noted for any of the measures between treatment groups described earlier, although a trend toward an improvement with dNSC treatment was observed (Fig. 3B). Eight weeks post-SCI, dNSC-treated mice showed significant improvements in hindlimb step intensity (P=0.02); hindlimb swing speed (P=0.05); and hindlimb stride length (P=0.05) compared with the control injured mice (Fig. 3B). The Catwalk-assisted gait analysis also allows for an objective measure of coordinated stepping complementing the BMS data. Eighty-eight percent of injury mice receiving dNSC transplants executed at least three coordinated stepping patterns during the gait analysis, while only 57% of media-treated animals achieved this level of coordination (Data not shown).
FIG. 3.
dNSC transplantation after SCI improves various gait parameters. (A) Representative footprint analysis at 8 weeks from dNSC and media-transplanted mice as well as sham injured. (B) Hindlimb comparisons on CatWalk-assisted gait analysis at 3 weeks (21 days) and 8 weeks (56 days) between dNSC (blue) and media-only (red) transplanted mice on stride length (left), swing speed (center), and step intensity (right). There was a significant difference in these gait variables at 8 week post-SCI. Data represent mean±SEM; * indicates P<0.05 one-way ANOVA with LSD post-hoc; Sham, n=3; dNSC, n=8; media, n=7. Color images available online at www.liebertpub.com/scd
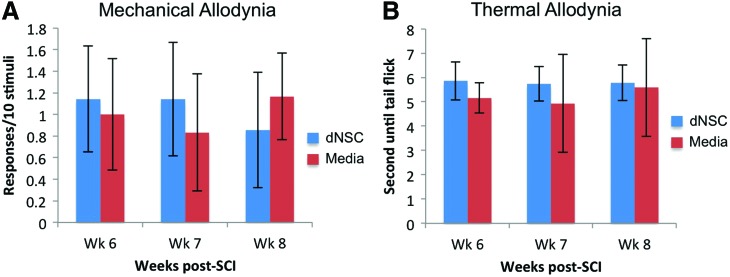
Intraspinal cellular transplantation after neurological insult has been associated with the exacerbation of neuropathic pain [42]. To address this, both mechanical and thermal allodynia were evaluated once per week for the final 3 weeks of the experimental protocol. There was no difference in responses to Von Frey filament application between SCI groups at any time point (Fig. 4A). In addition, the latency of tail removal from thermal stimuli was similar among both groups at all time points (Fig. 4B). This indicates that the transplantation of exogenous cells does not increase neuropathic pain.
FIG. 4.
The transplantation of dNSCs does not increase neuropathic pain. (A) The number of responses per 10 applications of a 4.0 g von frey filament to the plantar surface of the hind paws of injured mice that received dNSC or media transplantation were recorded. There were no significant differences observed in number responses. (B) The latency time for the mouse to remove its tail from a focal heat source was recorded for each experimental group. There were no significant differences observed in tail flick times. Data represent mean±SEM; dNSC, n=8; media, n=7. Color images available online at www.liebertpub.com/scd
dNSC transplant results in greater neural tissue sparing at the lesion after SCI
To assess histological differences between the dNSC transplant group and media-only injections, mouse spinal cords were sectioned at a thickness of 35 μm, sections were spaced 140 μm apart and spanned 2,380 μm rostral and caudal to the injury epicenter. H&E staining with LFB was applied to the sections, and Cavalieri probing in a Stereo Investigator was used to calculate cross-sectional areas of total cord, white matter, gray matter, and lesional tissue for each sequential tissue section. Representative H&E and LFB stained sections are shown for each group at the injury epicenter and at 1,120 μm caudal and rostral to the epicenter (Fig. 5A). Significant histology differences are seen between dNSC transplanted and media control. Both groups have dense networks of lesion tissue and clusters of small basophilic cells at the injury epicenter, but dNSC spinal cords appear to maintain a round, uniform contour that is closer in shape to sham injury cords. In contrast, growth media-treated cords appear to be compressed, misshapen and lack a smooth, uniform contour. In addition, dNSC-treated cords appear to have increased white matter sparing at the outer rim of the cord, while media-treated cords have loose connective tissue deposits extending to the edge of the cord. Histological changes between dNSC-treated and media-treated cords persist at 1,120 μm rostral and caudal to the site of injury—most notable is the overall compression of growth media-treated cords and the persistence of disorganized connective tissue within the gray matter of media-treated cords. It is important to note that there was no evidence of teratoma or tumor formation in any mice receiving the dNSC treatment. dNSC-transplanted mice had significantly more total spinal tissue than media-treated control mice when comparing the spinal cord volume between the two groups (P=0.044), and at many sections rostral and caudal to the injury epicenter when comparing total section area at individual points between dNSC and media treatment groups (Fig. 5B). dNSC-transplanted mice also had significantly more gray matter preservation than media-treated mice when comparing the gray matter volume between groups (P=0.011), and at many sections rostral and caudal to the epicenter when comparing individual gray matter area between dNSC and media-injected treatment groups (Fig. 5C). No significant differences in white matter preservation were seen between dNSC and media-injected treatment groups when examining white volume between groups; however, a trend toward increased white matter preservation was noted (P=0.0596) (Fig. 5D). In addition, dNSC-treated mice had significantly greater white matter area than media-treated mice at several individual tissue sections caudal and rostral to the injury epicenter. No significant differences were seen between dNSC-treated mice and media-injected treatment groups with regard to lesion area between groups along the entire cord (P=0.932) or at individual section points (Fig. 5E), suggesting that lesional tissue may be mostly influenced by primary injury mechanisms.
FIG. 5.
dNSC transplant results in increased tissue sparing after SCI. (A) Representative histological images with H&E and LFB stains are shown for each group at the injury epicenter and 1,120 μm rostral and caudal to the injury epicenter. Scale bar represents 500 μm (B, C) Histological analysis of spinal cord sections from dNSC transplantation, media, and sham injured mice revealed significant differences between dNSC and growth media groups in total cord and gray matter volume across the entire cord and at several individual section points. (D) A trend but no significant differences were seen between dNSC and growth media groups in white matter volume, although significant differences were found at certain individual section points caudal to the injury epicenter. (E) No significant differences were seen between dNSC and media groups in lesion volume or at individual section points. Data represent mean±SEM, * indicates P<0.05 two-way ANOVA with LSD post-hoc for area analysis and one-way ANOVA with LSD post-hoc for volume analysis; Sham, n=3; dNSC, n=6; growth media, n=6. LFB, Luxol fast blue. Color images available online at www.liebertpub.com/scd
Integration and survival of ES-dNSC in the spinal cord at 8 weeks after SCI
The extent of survival and integration of the transplanted cells was evaluated by examining the GFP label constitutively expressed by the ES-dNSC in vivo. Although auto-fluorescence can be a concern when imaging damaged tissue, the GFP+ expressing exogenous cells were clearly distinguishable from the background and minimal auto-fluorescence was present (Supplementary Fig. S2). The distribution shows that more cells are present in and around the epicenter of injury (Fig. 6A left), compared with a cross-section caudal and rostral to the region. The pattern of integration appears more random toward the epicenter, while the extra-lesional tissue shows integration of the GFP+ cells in the area associated with white matter tracts (Fig. 6A right). In addition, the GFP+ areas were observed throughout the injury and extended as far as ∼1,400 μm caudal and rostral to the injury epicenter (Fig. 6B). Using a Stereo Investigator optical fractionator to calculate the total number of GFP+ cells, we found that 36.2%±14.2% of the total number of cells were transplanted, suggesting robust survival. This does not, however, preclude the possibility of cell proliferation after transplantation in the spinal cord.
FIG. 6.
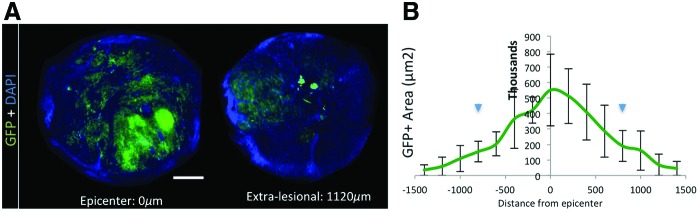
Integration and distribution of exogenous GFP+ dNSC at 8 weeks post-SCI. (A) Representative cross-sectional images displaying GFP+ cell integration at the epicenter of injury (left) and extra-lesional (1,120 μm from epicenter, right). The pattern of integration is more random toward the epicenter, while the extra-lesional tissue has an integration of the GFP+ cells in area associated with white matter tracks. (B) The GFP+ area measured across the graft length shows that more cells are present in and around the epicenter of injury. Scale bar represents 750 μm. Data represent mean±SEM, n=3, Arrowheads denote inject sites. GFP, green fluorescent protein. Color images available online at www.liebertpub.com/scd
Transplanted ES-dNSC differentiate to neural cells in the injured spinal cord
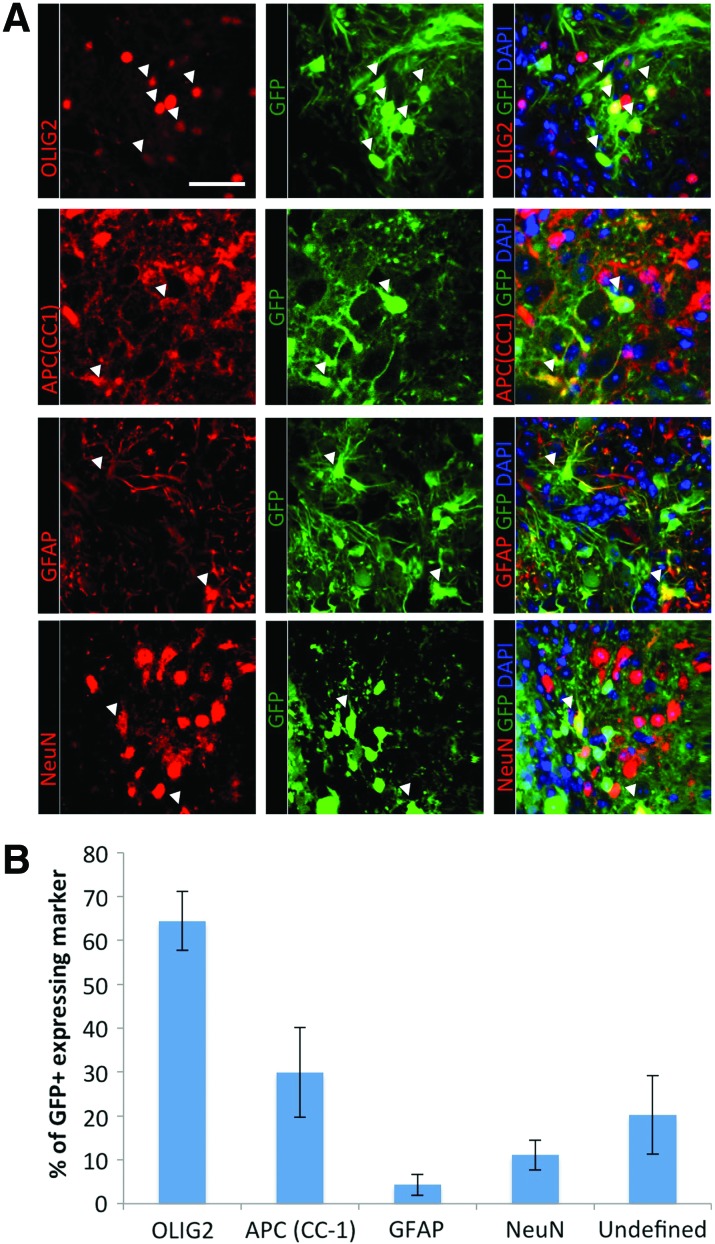
The differentiated fate of the transplanted cells was determined by immunohistochemistry for neural specific markers. Oligodendrocytes were labeled using the pan-oligodendrocyte marker OLIG-2, which is expressed by oligodendrocytes at all stages of their differentiation, and APC(CC1), which labels only mature oligodendrocytes. Astrocytes were labeled with GFAP, while NeuN was used to identify neurons. Representative images of immunohistochemical stained tissue show that all three neural cell types are present in the exogenous GFP+ cell population (Fig. 7A). The distribution pattern of differentiated cells shows that oligodendrocytes are primarily produced with 64.4%±6.7% of GFP+ cells expressing OLIG-2 and 29.9%±10.2% of GFP+ cells expressing APC-CC1. Neurons and astrocytes represent 11.1%±3.4% and 4.3%±2.4% of the GFP+ cells, respectively. Although the majority of the exogenous cells expressed a neural cell marker, there was a population of cells that remained undefined (20.2%±8.9%) (Fig. 7B). Nestin was examined as a marker of undifferentiated dNSCs; however, it was not expressed in any of the cell grafts (Supplementary Fig. S3). Of note, absence of labeling for the Schwann cell marker, p75, and the peripheral myelin marker, p0 on the exogenous GFP+ cells, was used to confirm an exclusively central nervous system nature of the restricted dNSCs and any resultant myelination (Supplementary Fig. S4).
FIG. 7.
Transplanted dNSCs survive in vivo and primarily differentiate to oligodendrocytes. (A) Immunofluorescence images of neural markers (Olig2; oligodendrocytes and precursors. APC(CC1); mature oligodendrocytes. GFAP; astrocytes, NeuN; neurons). (B) Cell counting for immunofluorescent-positive cells shows that the differentiation pattern for ES-dNSC predominantly differentiated in oligodendrocytes lineages, although all neural cell types are present. Scale bar represents 50 μm. Data represent mean±SEM, n=3. ES-dNSC, embryonic stem cell-derived definitive neural stem cell. Color images available online at www.liebertpub.com/scd
Transplanted ES-dNSC-derived oligodendrocytes associate with host axons and contribute to remyelination
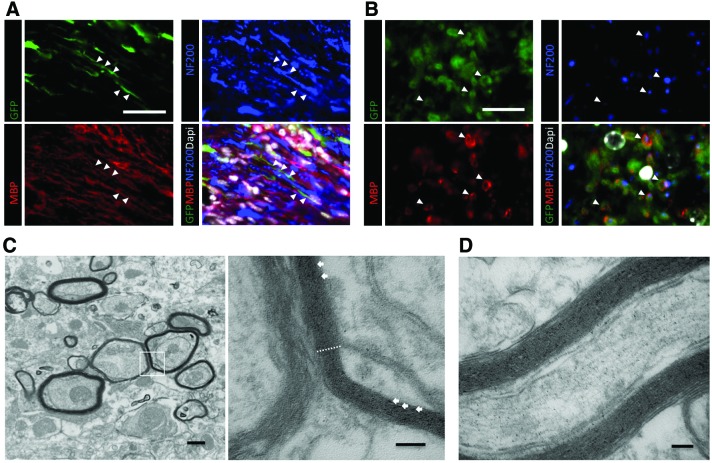
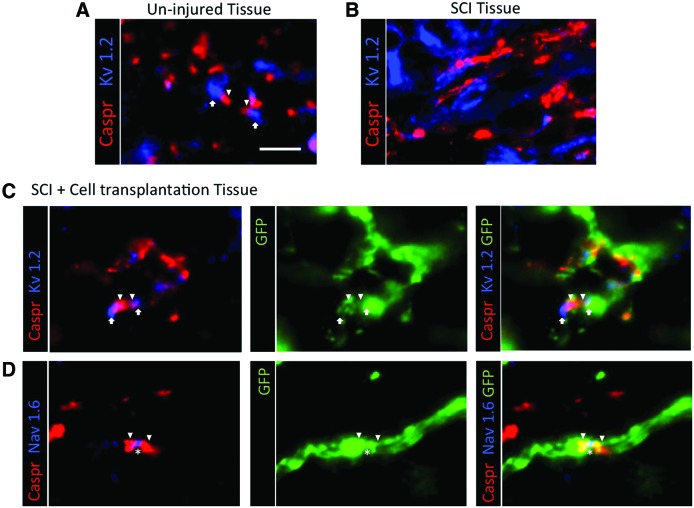
To assess whether oligodendrocytes derived from dNSC-transplanted cells contribute to myelin formation in injured spinal cords, dNSC-transplanted mice were sectioned and stained for MBP, NF200, GFP, and DAPI. In injured spinal cords, dNSC-derived oligodendrocytes closely associated with host axons. These oligodendrocytes co-localized with axon-ensheathing myelin, indicating the dNSC-derived oligodendrocytes are capable of remyelinating host axons in the injured spinal cord (Fig. 8A, B). The myelinating capacity of dNSC-derived oligodendrocytes was further evaluated using transmission electron microscopy. Multi-layer exogenous myelination is observed in the injured spinal cord (Fig. 8C, D). Moreover, normal nodal architecture and ion channel distribution (Fig. 9A) is disrupted after demyelination in SCI as demonstrated by a lack of distinct localization of nodal elements (Fig. 9B). Importantly, GFP+ ES-dNSCs transplanted into the injured spinal cord were associated with normal nodal architecture as evidenced by the appropriate localization of nodal (NaV1.6), juxtaparanodal (Caspr), and paranodal markers (Kv1.2) (Fig. 9C, D).
FIG. 8.
Transplanted dNSCs associate with host axons and contribute to remyelination. Immunofluorescence images from longitudinal (A) and transverse (B) sections show GFP+ ES-dNSC expressing MBP that associates with NF200+ axons. Scale bars represents 50 and 25 μm. Transmission electron microscopy of spinal tissue with GFP photo-oxidized and labeled with DAB shows exogenous, multilayer myelination of host axons in transverse (C) and longitudinal (D) sections. The dotted lines shows an area of ∼100 nm thick compacted myelin consisting of >20 lamellae. Arrows denote peroxidase reaction product. Scale bars represent 500 nm (C-left), 100 nm (C-right), and 100 nm (D). Color images available online at www.liebertpub.com/scd
FIG. 9.
Exogenous ES-dNSCs contribute to proper nodal architecture after SCI. (A) CASPR and Kv1.2 are shown to assemble paranodal and juxaparanodal areas, respectively, in health spinal tissue. (B) Disruption in nodal organization as a result of SCI is characterized by the lack of distinct localization of these nodal elements. (C, D) Exogenous GFP+ cells are shown to associate with proper distribution of these nodal architecture proteins as shown by co-labeling with CASPR and Kv1.2 as well as CASPR with nodal element Nav1.6. Arrowhead denotes paranodal CASPR, arrows denote juxaparanodal Kv1.2, and asterisk denotes nodal Nav1.6. Scale bar represents 15 μm. Color images available online at www.liebertpub.com/scd
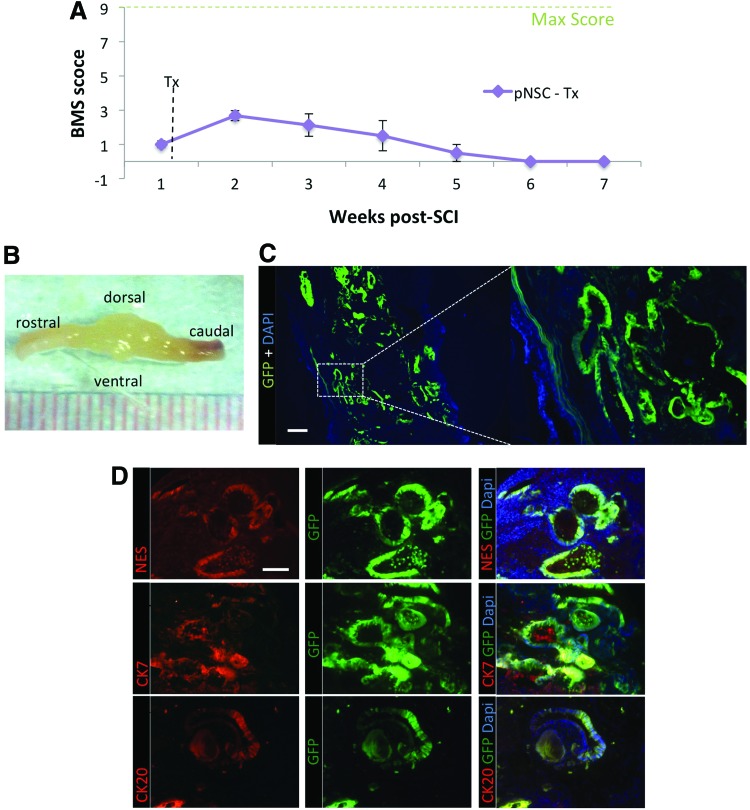
Primitive NSC transplantation results in teratoma formation
The default pathway of neuralization from pluripotent ESCs to transplantable NSCs involves the formation of LIF-dependent neurospheres or pNSCs as an intermediate stage. These pNSCs are incompletely committed to a neural lineage, possess many pluripotency markers, and may be tumorigenic (for many of the same reasons that NSCs cultured via EB formation are) [29]. Since pNSCs are a step in the default pathway of neuralization, we sought to characterize their tumorigenic potential when transplanted in the mouse spinal cord, as well as to reiterate the importance of clonal expansion of dNSC before in vivo transplantation. As described earlier, mice were transplanted at 1 week after injury. However, rather than transplant with ES-dNSC, the mice were transplanted with ESC-derived pNSCs. BMS was performed on these mice every week for 8 weeks. By 3 weeks postinjury, the recovery curve began to decline and complete loss of hindlimb function was observed by 6 weeks post-SCI (Fig. 10A). On fixation and extraction of the spinal cord, large growths were visible without magnification (Fig. 10B). Sectioning and examination of GFP showed extensive abnormal integration of exogenous cells and tetratoma-like formations throughout the transplanted area (Fig. 10C). Further examination showed that the abnormal cells express Nestin as well as cytokeratin 7 and cytokeratin 20, suggesting an epithelial neoplasm [43] (Fig. 10D). Furthermore, these immunohistochemical labels were not present when analyzing tissue that was transplanted with dNSCs (Supplementary Fig. S3).
FIG. 10.
Primitive neural stem cell transplantation results in teratoma formation and loss of recovery after SCI. (A) The animal that received pNSC followed an expected recovery curve as noted by the BMS score for the first 3 weeks after SCI. After this, the BMS scores begin to decrease, reaching complete loss of hind limb motor function by 6 weeks post SCI. Data represent mean±SEM, n=3. (B) Image of whole fixed spinal cord at 8 weeks after SCI with pNSC transplantation shows large outgrowths around injury and injection sites. (C) Confocal images of longitudinal sections with pNSC counterstained DAPI show numerous abnormal cell integration patterns of the exogenous GFP+ cells in a pattern indicating a teratoma formation. Scale bars represent 250 μm. (D) Immunofluorescence labeling for epithelial neoplasms markers, nestin, cytokeratin 7, and cytokeratin 20. The teratoma-like cells are positively labeled with all three markers denoting gastrointestinal carcinoma nature to the abnormal cell growth. Scale bar represents 100 μm. Color images available online at www.liebertpub.com/scd
Discussion
Summary of findings
We demonstrate that transplantation of these dNSCs promotes motor recovery in SCI mice, as measured both by a validated open-field global measure of locomotion and by an objective computer-based gait analysis. We showed that transplanted dNSCs support the structural integrity of the injured spinal cord by preventing spinal cord atrophy seen in total residual tissue area and by maintaining a shape of cord which is closer in contour to that of the noninjured cord. Confirming work previously completed by our group in noninjured mice [25], we show that dNSCs derived via the neural pathway are capable of differentiating to oligodendrocytes, astrocytes, and other neural lineage cells in vivo. Furthermore, the engrafted cells did not express markers of Schwann cells or peripheral myelin. In addition, we demonstrated the ability of these cells to express MBP, and show that MBP-expressing transplanted cells associate closely with host axons. Using transmission electron microscopy, we confirm that transplanted cells form compact myelin which ensheaths multiple host axons within the injured spinal cord. This supports remyelination as a possible mechanism of recovery but does not preclude the influence of other mechanisms such as trophic factor support or neuronal genesis.
Functional improvements after SCI with ES-dNSC
For our mouse model of thoracic SCI, we used a clip-compressive injury model, with the cell transplantation protocol adapted from previously used methods [35–37]. Unlike some injury models used in previous ESC transplantation experiments, the clip-compressive injury closely resembles the traumatic SCI observed in the clinic. It combines both the initial impact seen with many SCI static-load compression models [44,45] and a graded contusion seen in other models [46,47] to approximate a traumatic clinical SCI. Our results indicated that clear behavioral differences arose between injured and noninjured mice. Previous studies exploring similar thoracic injuries [36,37] demonstrated similar recovery profiles to our experimental data, with BMS scores gradually improving from the time of injury to an eventual plateau.
Our findings in open-field locomotion closely compared with results from previous studies investigating the transplantation of neural lineage cells. Similar to other studies using the BMS as a measure of open-field mouse locomotion [19], improvements between the cell transplantation group and the control groups were noticeable within 1 to 2 weeks after cell transplant, but were not statistically significant until at least 28 days from the time of injury, or 21 days from the transplant. Reasons for the delayed effect of dNSCs may be related to the time required for exogenous remyelination or to later elements of the secondary injury progression. This timeframe of recovery after ES-dNSC transplantation observed using the BMS is further supported by other NSC transplant studies in mouse SCI [19].
Catwalk-assisted gait analysis measurement was initiated at the approximate beginning of this plateau point (3 weeks postinjury), as a degree of locomotor recovery, such as occasional hindlimb plantar stepping, was required for many of the gait analysis parameters. Catwalk data showed that hindlimb limb swing speed was decreased in our injury model. The related measure of hindlimb stride length was also decreased, which has been shown in other contusion-only injury models [48]. Decreased hindlimb step intensity was also noted in these mice, which suggests that injured mice placed less pressure and weight support on their hindlimbs than noninjured animals [30]. The ES-dNSC treatment group performed significantly better then media-treated mice in all of the functional outcome measures mentioned earlier, demonstrating that these cells confer a benefit after SCI.
Behavioral data from Catwalk analysis at the study endpoint showed improvements in several gait parameters among ES-dNSC-transplanted mice. Stride length, swing speed, and hindlimb step intensity have each been identified as important gait parameters in injury characterization and were improved in dNSC-transplanted mice compared with injured controls. A murine transplant study using fetal NSCs reported similar findings with regard to swing speed [49]. Another study used a video camera and noncomputerized gait analysis after SCI with a transplant of oligodendroglial progenitors and found similar increases in hindlimb stride length [22]. Our study expands these previously reported gait findings and shows improved hindlimb step intensity (an indirect measure hindlimb weight support) after cell transplantation in mice. The Catwalk system also enables an objective analysis of coordination. We observed that a higher proportion of ES-dNSC-treated mice displayed coordinated stepping compared with media treated controls. For coordinated and regular footstep sequences, it is necessary for neural executors of motor function (ie, CGPs) in the hindlimb and forelimb to communicate their states between one another and regulate their activity [30]. It is possible that an effect of dNSC transplantation in the injured mouse spinal cord includes the preservation of neural connections between forelimb and hindlimb CPGs which are crucial in maintaining coordinated and regular footstep sequences.
Remyelination as mechanisms for ES-dNSC-mediated recovery
Several possible mechanisms for behavioral improvements seen in ESC transplantation have been proposed, including tissue preservation, remyelination, trophic factor support, and angiogenesis. However, specifically within the thoracic spinal cord, remyelination appears to be the principal route for neuro-recovery. A study comparing thoracic SCIs targeting the central cord, gray matter only versus a contusion injury that affected gray and white matter showed that white matter disruption is required to show functional deficit after SCI [50]. This was not seen when examining lumbar SCI where both central cord and contusion injuries resulted in significant motor deficits. Remyelination has been proposed to be a major component of the mechanism by which cell-based treatments confer a functional benefit after SCI. Our lab and others have shown evidence that remyelination occurs with NSCs from mouse and human sources. NSCs isolated from the fetal brain primarily differentiated toward oligodendrocytes in a contusion model SCI [51]. Oligodendroglial precursor engineered from ESCs remyelinated the injured spinal cord and promoted functional improvements [11,22]. iPSC-derived NSCs were shown to myelinate axons after contusive SCI [52,53]. The most compelling data supporting the critical value of remyelination in stem cell treatments for SCI compared adult tissue isolated NSCs from wild-type mice with those from Shiverer mice. The NSCs for each source were characterized and shown to be equivalent except for their ability to form compact myelin. Since only the animals treated with the myelinating NSCs showed a significant function benefit, remyelination was shown to be the mechanism of recovery [54]. Our results are supported by these studies and extend these findings by showing that NSCs derived from an ESC source using clonal expansion of free floating neurosphere also has this potential.
Our data suggest that remyelination is conferring functional benefit observed in the dNSC-transplanted group. This study demonstrates that transplanted cells are capable of differentiating primarily to cells of oligodendroglial lineage in the injured mouse spinal cord, suggesting the myelination observed to be of CNS character. This was also confirmed by a lack of labeling by the Schwann cell marker, p75, and PNS myelin marker, p0. This is consistent with a dNSC identity of the transplanted cells, rather than from a neural crest lineage that could yield these PNS elements [55]. Our lab has previously demonstrated the capacity of ES-dNSCs clonally selected from ESCs using the same methods as this project to generate myelin in vivo in noninjured shiverer mice [25] as well using adult NPCs in shiverer mice [15] and rodent SCI [38,39]. The nature and appearance of the exogenous myelination is physiologically appropriate, as it is seen to associate with host axons, is multilayer and compacted, and in areas with the proper nodal architecture and ion channel distribution. Since the shiverer mouse lacks MBP and thus compacted myelin, our data provide strong evidence that ES-dNSCs are capable of forming new myelin in vivo. Furthermore, a logarithmic relationship was found between the number of myelinated axons surviving in the subpial rim of the spinal cord after SCI and recovery of neurological function, indicating that small changes in axon number or conduction can substantially influence function [56]. Considering this, relatively few remyelinated axons restore the functionality of these bare, injured axons and evoke recovery after SCI. Even though the white matter area is not completely maintained with dNSC therapy compared with shams, remyelination still remains a possible and likely contributing mechanism of neurorepair and recovery.
Other potential mechanisms of ES-dNSC-mediated recovery
Although remyelination is a key process in recovery after SCI, it is likely that some or all of these other mechanisms play some role in behavioral benefits noted after NSC transplants. In this study, we demonstrate that tissue preservation contributes to the functional outcomes seen in our mice. This is supported by other transplantation studies that identify the potential importance of tissue sparing with cell-based therapy for SCI [19–22,39]. With regard to tissue sparing, our data show significant increases in total cord area among dNSC-transplanted mice, with increases in gray matter area likely contributing the most to a net increase in total cord volume. Our data suggest that cell transplantation provides structural support within the gray matter of the injured spinal cord and maintains the regular shape and contour of the cord, increasing the total cross-sectional area around the injury site. This is particularly apparent in viewing histological samples of dNSC-transplanted cords compared with media-only transplanted cords, which appear to be compressed and collapsed. Whether tissue sparing directly contributes to the functional benefits found in our study is difficult to assess. Similar effects on total tissue sparing have been noted in other studies, where nonstem cell transplants, including fibroblasts, have contributed to increased total cord area without providing a functional benefit [22].
There was a small subset of the transplanted ES-dNSCs that differentiated into NeuN+ neurons in vivo. This creates the possibility that the improvement in motor function was related to exogenous cells directly replacing the neurons lost after SCI. However, neuronal restricted precursor cells have limited success after thoracic SCI, with poor survival and a lack of functional recovery [57,58]. The level of SCI may be a key variable in determining the value of neuron replacement strategy for SCI treatment. The cervical and lumbar enlargement may be more receptive to this strategy, as interneurons involved in the central pattern generators are located there.
Cell-based therapy for SCI is not limited to simply replacing lost or damaged cells. The NSCs in this study, as well as others, express many potentially beneficial neurotrophic factors (Supplementary Fig. S1) [39,59]. Neurotrophins have been shown to have anti-apoptotic effects [60], enhance axonal regrowth [61], promote endogenous remyelination [62,63] and neuronal plasticity [64,65]. However, the importance of these factors expressed by NSCs after thoracic SCI has been disputed. Researchers using CNS isolated NSCs as a treatment for thoracic SCI showed that the recovery observed was lost on ablation of the transplanted cells, suggesting the long-term survival of the NSCs is required for their functional benefit to be maintained [51].
Integration of ES-NSC into the spinal tissue
A recent research report quantified the teratoma-forming potential of neural lineage cells differentiated from ESCs with EB formation [26]. The study found that residual ESCs were present after transplantation with in vitro culture to oligodendroglial progenitors, and that these cells could contribute to teratoma formation in vivo. Clonally expanding NSCs from ESCs using the default pathway dramatically reduces retention of pluripotent cells in dNSC transplants [25]. A step in generating dNSCs from ESCs involves a more primitive phase, termed primitive NSC where the cells are LIF dependent. In characterizing the generation of NSCs from ESCs using the default pathway, our group showed that pNSCs retain many of the markers found in teratoma-forming NSC-ESC (EB+) transplants [25–27]. As a part of this study, we examined the teratoma-forming potential of pNSCs in vivo. The formation of the teratoma is accompanied by a subsequent drop-off in behavioral function, as measured by the BMS. This finding is confirmed in the literature, where a similar drop in behavioral function is noted in another study exploring teratoma formation in vivo after NSC-ESC (EB+) transplant [27]. Importantly, during the histological analysis of all spinal cords transplanted with dNSCs in this study, none were found to have teratoma formation, demonstrating that the clonal expansion of dNSC in serum-free media with FGF, heparin, and B27 is sufficient to select a clinically relevant cell population for cell-based treatments. This fact—coupled with the potent teratoma-forming potential of NSC-ESC (EB+) transplants and the pNSCs transplants in this study—underscores the importance of careful culture conditions in generating NSCs from pluripotent cells for transplant and the potentially devastating effects of NSC transplants that retain undifferentiated pluripotent cells.
Conclusions
The generation of clinically relevant and safe NSCs from a pluripotent source is a critical step in moving cell-based therapies from the bench to the bedside. In this study, we have shown that one can derive dNSCs from ESC using clonal expansion of floating neurospheres that can be engrafted in the injured spinal cord and improve locomotor function without evidence of adverse teratoma formation.
Supplementary Material
Acknowledgments
This project was funded by the Canadian Institutes of Health Research (CIHR), Friedrich Flick Förderungsstiftung through Wings for Life, the Krembil Foundation, and Phillip and Peggy DeZwirek. R.A.M. received funding from the American Association of Neurosurgeons (AANS) and End MS Research and Training Network. R.P.S. received funding from CIHR Training Programs in Regenerative Medicine. M.G.F. is the Gerald and Tootsie Halbert Chair in Neural Repair and Regeneration.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Fehlings MG, Cadotte DW. and Fehlings LN. (2011). A series of systematic reviews on the treatment of acute spinal cord injury: a foundation for best medical practice. J Neurotrauma 28:1329–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sekhon LH. and Fehlings MG. (2001). Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine 26:S2–S12 [DOI] [PubMed] [Google Scholar]
- 3.Rowland JW, Hawryluk GWJ, Kwon B. and Fehlings MG. (2008). Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus 25:E2. [DOI] [PubMed] [Google Scholar]
- 4.Eftekharpour E, Karimi-Abdolrezaee S. and Fehlings MG. (2008). Current status of experimental cell replacement approaches to spinal cord injury. Neurosurg Focus 24:E19. [DOI] [PubMed] [Google Scholar]
- 5.Tetzlaff W, Okon EB, Karimi-Abdolrezaee S, Hill CE, Sparling JS, Plemel JR, Plunet WT, Tsai EC, Baptiste D, et al. (2011). A systematic review of cellular transplantation therapies for spinal cord injury. J Neurotrauma 28:1611–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hawryluk GWJ. and Fehlings MG. (2008). The center of the spinal cord may be central to its repair. Cell Stem Cell 3:230–232 [DOI] [PubMed] [Google Scholar]
- 7.Bain G, Kitchens D, Yao M, Huettner JE. and Gottlieb DI. (1995). Embryonic stem cells express neuronal properties in vitro. Dev Biol 168:342–357 [DOI] [PubMed] [Google Scholar]
- 8.Joannides AJ, Fiore-Hériché C, Battersby AA, Athauda-Arachchi P, Bouhon IA, Williams L, Westmore K, Kemp PJ, Compston A, Allen ND. and Chandran S. (2007). A scaleable and defined system for generating neural stem cells from human embryonic stem cells. Stem Cells 25:731–737 [DOI] [PubMed] [Google Scholar]
- 9.Murry CE. and Keller G. (2008). Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132:661–680 [DOI] [PubMed] [Google Scholar]
- 10.Shin S, Mitalipova M, Noggle S, Tibbitts D, Venable A, Rao R. and Stice SL. (2006). Long-term proliferation of human embryonic stem cell-derived neuroepithelial cells using defined adherent culture conditions. Stem Cells 24:125–138 [DOI] [PubMed] [Google Scholar]
- 11.McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, Gottlieb DI. and Choi DW. (1999). Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med 5:1410–1412 [DOI] [PubMed] [Google Scholar]
- 12.Shihabuddin LS, Ray J. and Gage FH. (1997). FGF-2 is sufficient to isolate progenitors found in the adult mammalian spinal cord. Exp Neurol 148:577–586 [DOI] [PubMed] [Google Scholar]
- 13.Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC. and Reynolds BA. (1996). Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci 16:7599–7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moreno-Manzano V, Rodríguez-Jiménez FJ, García-Roselló M, Laínez S, Erceg S, Calvo MT, Ronaghi M, Lloret M, Planells-Cases R, Sánchez-Puelles JM. and Stojkovic M. (2009). Activated spinal cord ependymal stem cells rescue neurological function. Stem Cells 27:733–743 [DOI] [PubMed] [Google Scholar]
- 15.Eftekharpour E, Karimi-Abdolrezaee S, Wang J, El Beheiry H, Morshead C. and Fehlings MG. (2007). Myelination of congenitally dysmyelinated spinal cord axons by adult neural precursor cells results in formation of nodes of Ranvier and improved axonal conduction. J Neurosci 27:3416–3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruff CA, Wilcox JT. and Fehlings MG. (2011). Cell-based transplantation strategies to promote plasticity following spinal cord injury. Exp Neurol 235:78–90 [DOI] [PubMed] [Google Scholar]
- 17.Chiba S, Iwasaki Y, Sekino H. and Suzuki N. (2003). Transplantation of motoneuron-enriched neural cells derived from mouse embryonic stem cells improves motor function of hemiplegic mice. Cell Transplant 12:457–468 [DOI] [PubMed] [Google Scholar]
- 18.Kerr DA, Lladó J, Shamblott MJ, Maragakis NJ, Irani DN, Crawford TO, Krishnan C, Dike S, Gearhart JD. and Rothstein JD. (2003). Human embryonic germ cell derivatives facilitate motor recovery of rats with diffuse motor neuron injury. J Neurosci 23:5131–5140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumagai G, Okada Y, Yamane J, Nagoshi N, Kitamura K, Mukaino M, Tsuji O, Fujiyoshi K, Katoh H, et al. (2009). Roles of ES cell-derived gliogenic neural stem/progenitor cells in functional recovery after spinal cord injury. PLoS One 4:e7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bottai D, Cigognini D, Madaschi L, Adami R, Nicora E, Menarini M, Di Giulio AM. and Gorio A. (2010). Embryonic stem cells promote motor recovery and affect inflammatory cell infiltration in spinal cord injured mice. Exp Neurol 223:452–463 [DOI] [PubMed] [Google Scholar]
- 21.Marques SA, Almeida FM, Fernandes AM, dos Santos Souza C, Cadilhe DV, Rehen SK. and Martinez AMB. (2010). Predifferentiated embryonic stem cells promote functional recovery after spinal cord compressive injury. Brain Res 1349:115–128 [DOI] [PubMed] [Google Scholar]
- 22.Keirstead HS, Nistor G, Bernal G, Totoiu M, Cloutier F, Sharp K. and Steward O. (2005). Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. J Neurosci 25:4694–4705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Werbowetski-Ogilvie TE, Bossé M, Stewart M, Schnerch A, Ramos-Mejia V, Rouleau A, Wynder T, Smith M-J, Dingwall S, et al. (2009). Characterization of human embryonic stem cells with features of neoplastic progression. Nat Biotechnol 27:91–97 [DOI] [PubMed] [Google Scholar]
- 24.Miura K, Okada Y, Aoi T, Okada A, Takahashi K, Okita K, Nakagawa M, Koyanagi M, Tanabe K, et al. (2009). Variation in the safety of induced pluripotent stem cell lines. Nat Biotechnol 27:743–745 [DOI] [PubMed] [Google Scholar]
- 25.Rowland JW, Lee JJ, Salewski RP, Eftekharpour E, Kooy Dvd. and Fehlings MG. (2011). Generation of neural stem cells from embryonic stem cells using the default mechanism: in vitro and in vivo characterization. Stem Cells Dev 20:1829–1845 [DOI] [PubMed] [Google Scholar]
- 26.Sadowski D, Kiel ME, Apicella M, Arriola AG, Chen CP. and McKinnon RD. (2010). Teratogenic potential in cultures optimized for oligodendrocyte development from mouse embryonic stem cells. Stem Cells Dev 19:1343–1353 [DOI] [PubMed] [Google Scholar]
- 27.Matsuda R, Yoshikawa M, Kimura H, Ouji Y, Nakase H, Nishimura F, Nonaka J, Toriumi H, Yamada S, et al. (2009). Cotransplantation of mouse embryonic stem cells and bone marrow stromal cells following spinal cord injury suppresses tumor development. Cell Transplant 18:39–54 [PubMed] [Google Scholar]
- 28.Smukler SR, Runciman SB, Xu S. and van der Kooy D. (2006). Embryonic stem cells assume a primitive neural stem cell fate in the absence of extrinsic influences. J Cell Biol 172:79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tropepe V, Hitoshi S, Sirard C, Mak TW, Rossant J. and van der Kooy D. (2001). Direct neural fate specification from embryonic stem cells: a primitive mammalian neural stem cell stage acquired through a default mechanism. Neuron 30:65–78 [DOI] [PubMed] [Google Scholar]
- 30.Hamers FPT, Koopmans GC. and Joosten EAJ. (2006). CatWalk-assisted gait analysis in the assessment of spinal cord injury. J Neurotrauma 23:537–548 [DOI] [PubMed] [Google Scholar]
- 31.Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM. and Popovich PG. (2006). Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 23:635–659 [DOI] [PubMed] [Google Scholar]
- 32.Pajoohesh-Ganji A, Byrnes KR, Fatemi G. and Faden AI. (2010). A combined scoring method to assess behavioral recovery after mouse spinal cord injury. Neurosci Res 67:117–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagy A, Rossant J, Nagy R, Abramow-Newerly W. and Roder JC. (1993). Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A 90:8424–8428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salewski RP, Buttigieg J, Mitchell RA, van der Kooy D, Nagy A. and Fehlings MG. (2012). The generation of definitive neural stem cells from piggyBac transposon induced pluripotent stem cells can be enhanced by induction of the NOTCH signalling pathway. Stem Cells Dev 22:383–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho N, Nguyen DH, Satkunendrarajah K, Branch DR. and Fehlings MG. (2012). Evaluating the role of IL-11, a novel cytokine in the IL-6 family, in a mouse model of spinal cord injury. J Neuroinflammation 9:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joshi M. and Fehlings MG. (2002). Development and characterization of a novel, graded model of clip compressive spinal cord injury in the mouse: Part 2. Quantitative neuroanatomical assessment and analysis of the relationships between axonal tracts, residual tissue, and locomotor recovery. J Neurotrauma 19:191–203 [DOI] [PubMed] [Google Scholar]
- 37.Joshi M. and Fehlings MG. (2002). Development and characterization of a novel, graded model of clip compressive spinal cord injury in the mouse: Part 1. Clip design, behavioral outcomes, and histopathology. J Neurotrauma 19:175–190 [DOI] [PubMed] [Google Scholar]
- 38.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Schut D. and Fehlings MG. (2010). Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci 30:1657–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM. and Fehlings MG. (2006). Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci 26:3377–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grabenbauer M, Geerts WJ, Fernadez-Rodriguez J, Hoenger A, Koster AJ. and Nilsson T. (2005). Correlative microscopy and electron tomography of GFP through photooxidation. Nat Methods 2:857–862 [DOI] [PubMed] [Google Scholar]
- 41.Schurch W, Seemayer TA, Lagace R. and Gabbiani G. (1984). The intermediate filament cytoskeleton of myofibroblasts: an immunofluorescence and ultrastructural study. Virchows Arch A Pathol Anat Histopathol 403:323–336 [DOI] [PubMed] [Google Scholar]
- 42.Hofstetter CP, Holmstrom NA, Lilja JA, Schweinhardt P, Hao J, Spenger C, Wiesenfeld-Hallin Z, Kurpad SN, Frisen J. and Olson L. (2005). Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci 8:346–353 [DOI] [PubMed] [Google Scholar]
- 43.Chu P, Wu E. and Weiss LM. (2000). Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol 13:962–972 [DOI] [PubMed] [Google Scholar]
- 44.Farooque MM. (2000). Spinal cord compression injury in the mouse: presentation of a model including assessment of motor dysfunction. Acta Neuropathol 100:13–22 [DOI] [PubMed] [Google Scholar]
- 45.Scheff SW, Rabchevsky AG, Fugaccia I, Main JA. and Lumpp JE. (2003). Experimental modeling of spinal cord injury: characterization of a force-defined injury device. J Neurotrauma 20:179–193 [DOI] [PubMed] [Google Scholar]
- 46.Jakeman LB, Guan Z, Wei P, Ponnappan R, Dzwonczyk R, Popovich PG. and Stokes BT. (2000). Traumatic spinal cord injury produced by controlled contusion in mouse. J Neurotrauma 17:299–319 [DOI] [PubMed] [Google Scholar]
- 47.Kuhn PL. and Wrathall JR. (1998). A mouse model of graded contusive spinal cord injury. J Neurotrauma 15:125–140 [DOI] [PubMed] [Google Scholar]
- 48.Hamers FP, Lankhorst AJ, van Laar TJ, Veldhuis WB. and Gispen WH. (2001). Automated quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries. J Neurotrauma 18:187–201 [DOI] [PubMed] [Google Scholar]
- 49.Salazar DL, Uchida N, Hamers FPT, Cummings BJ. and Anderson AJ. (2010). Human neural stem cells differentiate and promote locomotor recovery in an early chronic spinal cord injury NOD-scid mouse model. PLoS One 5:e12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Magnuson DS, Trinder TC, Zhang YP, Burke D, Morassutti DJ. and Shields CB. (1999). Comparing deficits following excitotoxic and contusion injuries in the thoracic and lumbar spinal cord of the adult rat. Exp Neurol 156:191–204 [DOI] [PubMed] [Google Scholar]
- 51.Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, Gage FH. and Anderson AJ. (2005). Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci U S A 102:14069–14074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nori S, Okada Y, Yasuda A, Tsuji O, Takahashi Y, Kobayashi Y, Fujiyoshi K, Koike M, Uchiyama Y, et al. (2011). Grafted human-induced pluripotent stem-cell-derived neurospheres promote motor functional recovery after spinal cord injury in mice. Proc Natl Acad Sci U S A 108:16825–16830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsuji O, Miura K, Okada Y, Fujiyoshi K, Mukaino M, Nagoshi N, Kitamura K, Kumagai G, Nishino M, et al. (2010). Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci U S A 107:12704–12709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yasuda A, Tsuji O, Shibata S, Nori S, Takano M, Kobayashi Y, Takahashi Y, Fujiyoshi K, Hara CM, et al. (2011). Significance of remyelination by neural stem/progenitor cells transplanted into the injured spinal cord. Stem Cells 29:1983–1994 [DOI] [PubMed] [Google Scholar]
- 55.Woodhoo A. and Sommer L. (2008). Development of the Schwann cell lineage: from the neural crest to the myelinated nerve. Glia 56:1481–1490 [DOI] [PubMed] [Google Scholar]
- 56.Fehlings MG. and Tator CH. (1995). The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp Neurol 132:220–228 [DOI] [PubMed] [Google Scholar]
- 57.Cao QL, Howard RM, Dennison JB. and Whittemore SR. (2002). Differentiation of engrafted neuronal-restricted precursor cells is inhibited in the traumatically injured spinal cord. Exp Neurol 177:349–359 [DOI] [PubMed] [Google Scholar]
- 58.Lepore AC. and Fischer I. (2005). Lineage-restricted neural precursors survive, migrate, and differentiate following transplantation into the injured adult spinal cord. Exp Neurol 194:230–242 [DOI] [PubMed] [Google Scholar]
- 59.Hawryluk GW, Mothe A, Wang J, Wang S, Tator C. and Fehlings MG. (2012). An in vivo characterization of trophic factor production following neural precursor cell or bone marrow stromal cell transplantation for spinal cord injury. Stem Cells Dev 21:2222–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koda M, Murakami M, Ino H, Yoshinaga K, Ikeda O, Hashimoto M, Yamazaki M, Nakayama C. and Moriya H. (2002). Brain-derived neurotrophic factor suppresses delayed apoptosis of oligodendrocytes after spinal cord injury in rats. J Neurotrauma 19:777–785 [DOI] [PubMed] [Google Scholar]
- 61.Neuhuber B, Timothy Himes B, Shumsky JS, Gallo G. and Fischer I. (2005). Axon growth and recovery of function supported by human bone marrow stromal cells in the injured spinal cord exhibit donor variations. Brain Res 1035:73–85 [DOI] [PubMed] [Google Scholar]
- 62.Tolwani RJ, Cosgaya JM, Varma S, Jacob R, Kuo LE. and Shooter EM. (2004). BDNF overexpression produces a long-term increase in myelin formation in the peripheral nervous system. J Neurosci Res 77:662–669 [DOI] [PubMed] [Google Scholar]
- 63.Hempstead BL. and Salzer JL. (2002). Neurobiology. A glial spin on neurotrophins. Science 298:1184–1186 [DOI] [PubMed] [Google Scholar]
- 64.Nakamura M, Okano H, Toyama Y, Dai HN, Finn TP. and Bregman BS. (2005). Transplantation of embryonic spinal cord-derived neurospheres support growth of supraspinal projections and functional recovery after spinal cord injury in the neonatal rat. J Neurosci Res 81:457–468 [DOI] [PubMed] [Google Scholar]
- 65.Chen Q, Zhou L. and Shine HD. (2006). Expression of neurotrophin-3 promotes axonal plasticity in the acute but not chronic injured spinal cord. J Neurotrauma 23:1254–1260 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.