Abstract
Major depressive disorder (MDD) is prevalent after traumatic brain injury (TBI); however, there is a lack of evidence regarding effective treatment approaches. We conducted a choice-stratified randomized controlled trial in 100 adults with MDD within 10 years of complicated mild to severe TBI to test the effectiveness of brief cognitive behavioral therapy administered over the telephone (CBT-T) (n=40) or in-person (CBT-IP) (n=18), compared with usual care (UC) (n=42). Participants were recruited from clinical and community settings throughout the United States. The main outcomes were change in depression severity on the clinician-rated 17 item Hamilton Depression Rating Scale (HAMD-17) and the patient-reported Symptom Checklist-20 (SCL-20) over 16 weeks. There was no significant difference between the combined CBT and UC groups over 16 weeks on the HAMD-17 (treatment effect=1.2, 95% CI: −1.5–4.0; p=0.37) and a nonsignificant trend favoring CBT on the SCL-20 (treatment effect=0.28, 95% CI: −0.03–0.59; p=0.074). In follow-up comparisons, the CBT-T group had significantly more improvement on the SCL-20 than the UC group (treatment effect=0.36, 95% CI: 0.01–0.70; p=0.043) and completers of eight or more CBT sessions had significantly improved SCL-20 scores compared with the UC group (treatment effect=0.43, 95% CI: 0.10–0.76; p=0.011). CBT participants reported significantly more symptom improvement (p=0.010) and greater satisfaction with depression care (p<0.001), than did the UC group. In-person and telephone-administered CBT are acceptable and feasible in persons with TBI. Although further research is warranted, telephone CBT holds particular promise for enhancing access and adherence to effective depression treatment.
Key words: : behavior, clinical trial, head trauma, rehabilitation, TBI
Introduction
Traumatic brain injury (TBI) occurs in >3,500,000 people in the United States, and 10,000,000 people worldwide annually.1,2 With TBI being the “signature injury” of the conflicts in Iraq and Afghanistan, the need for effective treatments for the sequelae of TBI is increasing significantly.3,4 Rehabilitation aims to help TBI survivors resume their roles in work or school, with family or friends, and in the larger community. However, mental health problems such as depression, anxiety, and substance abuse are common, and may interfere with successful recovery.5–9 Psychosocial problems are often more predictive of poor outcomes than the physical sequelae of TBI in both civilian10 and military4 populations. Major depressive disorder (MDD) is the most prevalent psychiatric disorder accompanying TBI7,8 and is associated with poorer health status,11–13 including physical complaints,11 cognitive14–18 and social14,19,20 problems, and increased costs21 among persons with TBI.
Despite the prevalence and adverse impact of depression after TBI, the science and practice of treating depression in this population lack a solid evidence base. Depression is undertreated in this population, with only 20% of those with MDD receiving counseling, and 41% receiving antidepressants during the 1st year after injury.22 In order to decrease morbidity and improve functional outcomes after TBI, effective treatments for MDD must be developed, tested, and disseminated. Recent reviews of depression treatment literature in people with TBI conclude that serotonergic antidepressants and cognitive behavioral therapy (CBT) appear to be the most promising approaches to treating depression following TBI; however, there is an absence of high quality depression treatment trials and no published psychotherapy trials for MDD.23–25 Important theoretical and preliminary work has described how CBT could be adapted for people with TBI.26–29 Structural equation modeling has shown that post-TBI depression is consistent with cognitive behavioral theory,30 and preliminary CBT trials have been promising for decreasing depressive symptoms.23,28,31,32
The model of treatment delivery is crucial, because of the tremendous barriers to receipt of adequate mental health services.33–37 Barriers for people with TBI include stigma; difficulty coordinating care for multiple medical and psychological problems; limited transportation (e.g., because of cost, distance, seizure disorder with inability to drive); avoidance of trauma-focused components of mental health care; and other motivational (ambivalence), behavioral, cognitive, social, and financial problems that interfere with their ability to attend scheduled appointments. Research on telephone-delivered CBT indicates that it is effective, and overcomes barriers.38 For example, Simon and colleagues39 showed that the addition of brief telephone-based CBT resulted in reduced depression among primary care patients who were treated with antidepressants, compared with usual care controls, and that 84% of telephone CBT participants received at least four sessions. A survey of people with TBI and depression found that more people would be willing to participate in psychotherapy rather than pharmacotherapy for depression, and that 72% would be willing to receive treatment over the telephone.40 The aim of this study was to test the efficacy of a 12 session manualized CBT program to treat MDD that occurred within 10 years of complicated mild to severe TBI. We compared telephone-delivered CBT, in-person CBT, and usual care, and used a choice-stratified randomization strategy to enhance ecological validity. Our primary hypothesis was that CBT delivered via telephone or in person would lead to significantly reduced depressive symptoms, compared with usual care.
Methods
Procedures
The study was coordinated at the University of Washington between December 2008 and December 2012. The Institutional Review Boards of the University of Washington and study recruitment sites approved all study protocols. All screening, baseline, and outcome assessments were conducted over the telephone by trained study staff who were blinded to randomization status. Two pilot participants received the CBT study intervention prior to start of randomization to facilitate optimization of study procedures; their data are excluded.
We screened potentially eligible persons with TBI with the Patient Health Questionnaire-9 (PHQ-9) depression scale.41,42 Those who scored ≥8 and met other eligibility criteria were invited to participate in a baseline interview, which included the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV) (SCID).43 Those who met criteria for MDD and met other eligibility criteria were offered informed consent to participate in the randomized trial of telephone-administered CBT (CBT-T), in-person CBT (CBT-IP), or usual care (UC). The primary outcomes were assessed at 16 weeks after randomization, with an interim assessment at 8 weeks and a follow-up assessment at 24 weeks.
Participants
Participants were recruited nationally from community and clinical settings serving persons with TBI and referrals from clinicians. Specifically, patients at the University of Washington Medical Center and Harborview Medical Center (Seattle, Washington), St. Luke's Rehabilitation Institute (Spokane, Washington), Moss Rehabilitation Research Institute (Philadelphia, Pennsylvania), and University of Alabama (Birmingham, Alabama) were notified about the study through their clinicians, mailing lists, and advertisements. Furthermore, community outreach included Brain Injury Association web sites, TBI support groups, TBI clubhouses, a dedicated study web site (www.LIFTcare.org) and Facebook page, TBI conferences, and networking with community and national TBI agencies that included study information and a link to the study web site on their web page, newsletters, and listservs.
Inclusion criteria were: being ≥18 years old; having been hospitalized within the past 10 years for a complicated mild to severe TBI as indicated by Glasgow Coma Scale (GCS) score of 3–12, or documented intracranial abnormalities on imaging, or having had post-traumatic amnesia of at least 7 days (participants with GCS scores<13 and no radiological evidence of TBI were excluded if their blood alcohol levels exceeded 199 mg/dL because alcohol intoxication can decrease GCS scores);44 speaking English; meeting criteria for MDD on SCID and having scores ≥10 on PHQ-9; and residing in any of the 50 United States.
Exclusion criteria were: having no stable home or regular access to a telephone; history of diagnosis of schizophrenia; evidence of bipolar disorder, psychosis, or suicidal intent, or current (within the past month) alcohol or drug dependence on Mini International Neuropsychiatric Interview (MINI);45 currently receiving or planning to start evidence-based psychotherapy for depression within the 16-week study period (other forms of counseling such as rehabilitation counseling were permitted); antidepressant initiation within 6 weeks or dosage adjustment within 4 weeks prior to randomization, or planning to start an antidepressant within the 16 week intervention period; or severe cognitive impairment as defined by significant impairment on two or more of the following tests administered via telephone – Digit Span (cutoff below the lower 5th percentile),46 or the Hopkins Verbal Learning Test (HVLT),47 or Oral Trail Making Test (TMT) A and B48 (cutoff below the lower 1st percentile).
Randomization
We randomized the first nine participants to CBT-T. CBT-IP, or UC; thereafter, we instituted a “choice- or equipoise-stratified” approach49 in order to enhance the accessioning and ecological validity of the study. Participants were given a choice of three randomization options: 1) CBT-T versus CBT-IP versus UC; 2) CBT-IP versus UC; or 3) CBT-T versus UC. Randomization was stratified on TBI severity (complicated mild/moderate, severe) and randomization option choice. A biostatistician (J.B.) computer generated blocked randomization lists with block size equal to the number of possible treatment options available in that stratum. He also assigned participants to treatments after they were enrolled by the research coordinator (K.M.).
Measures
Demographic and injury-related characteristics were obtained from participant interviews and medical record reviews. During screening, potential depression was assessed using the PHQ-9,42 which has been used extensively in TBI populations and has been found to be valid and reliable.41,50 Dysthymic disorder, current substance abuse or dependence, as well as exclusion diagnoses of bipolar disorder or psychosis, were assessed using the MINI.45 Patients were asked about history of post-traumatic stress disorder (PTSD) diagnosis or treatment, and whether they were currently involved with or planning litigation related to their injury. Patients provided a list of current medications, and the Cornell Services Index51 was used to document concurrent counseling consisting of four or more sessions by a psychiatrist or psychologist36 and antidepressant use at a therapeutic dose52 at baseline and outcome assessments. For confounder and moderator analyses, baseline medical comorbidity was assessed using a checklist of chronic medical conditions and self-rating of environmental reward and response-contingent positive reinforcement; automatic negative thoughts and dysfunctional attitudes were assessed using the Environmental Reward Observation Scale (EROS),53 Automatic Thoughts Questionnaire (ATQ),54 and the Dysfunctional Attitudes Scale (DAS),55 respectively.
Depression outcomes
The primary depression outcome measures were the clinician-rated 17-item Hamilton Depression Rating Scale (HAMD-17)56 and the patient-reported Symptom Checklist-20 (SCL-20).57 The SCL-20 is a more unidimensional depression scale that is less dependent on somatic symptoms than the HAMD-17,58 and, therefore, more sensitive to change in the TBI population. These instruments were chosen because they are widely used depression intervention outcome measures and have previously been used in TBI populations to assess depression outcomes.59,60 We used a structured version of the HAMD-1761 for improved reliability. Secondary depression outcomes included MDD criteria based on the SCID, self-rated improvement in depression symptoms as measured by the Patient Global Impression (PGI),62 and Satisfaction with Depression Care as measured on a 1 (very satisfied) to 7 (very dissatisfied) Likert scale.63 Therapeutic alliance was assessed with the Working Alliance Inventory-Short Form.64 Interrater reliability for the HAMD-17, SCID and MINI were ≥90%.
Other secondary outcomes
Secondary outcomes included quality of life (SF-36),65 functional impairment (Sheehan Disability Scale),66 17 post-concussive symptoms (Head Injury Symptom Checklist)67 rated from 0 to 5 on frequency and bothersomeness, environmental reward, automatic negative thoughts, and dysfunctional attitudes.
CBT-TBI Intervention
Telephone and in-person CBT-TBI was adapted from Simon and Ludman's eight session structured telephone care management and CBT protocol.68,69 Although originally developed for English-speaking primary care patients, the program has been adapted for different populations and translated into multiple languages.70–74
Based on our prior TBI telephone counseling studies,59,75 the intervention protocol was tailored in several ways to individuals living with TBI. Brief care management at the beginning of each session addressed issues specific to promoting TBI rehabilitation and recovery,76 including return to work or school, substance abuse, social and interpersonal isolation, and transportation difficulties. Patients' support persons (spouse, significant other, parent, adult child, or other caregiver) were invited to attend sessions to assist with planning, implementing, and monitoring CBT-TBI activities. Motivational interviewing (MI) was used to engage participants in the treatment protocol.77
The CBT-TBI sessions occurred weekly over 12 rather than 8 weeks so that the material could be presented in smaller portions, more slowly and with greater repetition. Table 1 illustrates session content and Table 2 lists accommodations for cognitive impairments. Therapy sessions were generally kept to 30–60 min to minimize problems with mental fatigue, and began with a review of issues from the previous session.
Table 1.
Cognitive Behavioral Therapy for Traumatic Brain Injury (CBT-TBI) Treatment Session Content
| Session | Session contenta |
|---|---|
| Initial contact | Introductions, explain program, review materials, answer questions, schedule calls. |
| 1 | Depression education. Elicit how depression affects the person's feelings, body, thinking, and behavior. Motivation enhancement to strengthen engagement in therapy. Practice activity: describe best and worst parts of their days. |
| 2 | Review depression education. Review practice activity and identify what activities made the patient feel better or worse. Learn what helps others feel better. Pleasant activity identification exercise. Practice activity: choose pleasant activities. |
| 3 | Review pleasant activity preferences. Plan pleasant activity experiment. Practice activity: pleasant activity experiment. |
| 4 | Review pleasant activity experiment. What worked? What was the effect on mood? What were the barriers? Problem-solve overcoming barriers. Plan new experiments. Practice activity: pleasant activity experiments. |
| 5 | Review pleasant activity experiments. What worked? What was the effect on mood? What were the barriers? Problem-solve overcoming barriers. Add to and expand positive parts of experiments. Revise and repeat experiments. Practice activity: pleasant activity experiments. |
| 6 | Review pleasant activity experiments. What worked? What was the effect on mood? What were the barriers? Problem-solve overcoming barriers. Identify most promising activities. Make written weekly pleasant activity schedule. Write out most common barriers and best plans to overcome barriers. Practice activity: follow schedule. |
| 7 | Review practice activity. Discuss depressive thinking (e.g., guilt, self-blame). Common negative thoughts. Thought identification examples and exercise. Practice activity: keep schedule, try catching negative thoughts. |
| 8 | Review practice activity. Elicit negative thoughts and triggers. Discuss more ways to observe automatic negative thoughts and their effects. Practice activity: keep schedule; negative thought monitoring exercise. |
| 9 | Review practice activity. Discuss effects of negative thoughts. Illustrate ways of distancing from automatic negative thoughts. Practice ways of distancing. Practice activity: keep schedule; watch negative thoughts; try distancing. |
| 10 | Review practice activity. Identify examples of automatic negative thinking. Guided practice of cognitive strategies: thought stopping, distracting, reasoning, and exaggeration. Practice activity: try cognitive strategies at home; practice using cue card(s). |
| 11 | Review practice activity. Create a self-care plan, pinpoint strategies that worked best. Set long-term goals. Develop relapse prevention strategy. |
| 12 | Review most helpful parts of program. Integrate self-care plan (including medication adherence, if appropriate) into written schedule and cue cards. Review and adjust relapse prevention plan. Congratulate participant for gains made. |
Sessions begin with: soliciting participation from a support person, reviewing the Patient Health Questionnaire-9 (PHQ-9) depression scale, troubleshooting persistent symptoms, motivational enhancement to increase engagement (if needed).
Table 2.
Accommodations for Cognitive Impairments in the Course of Providing Cognitive Behavioral Therapy for Traumatic Brain Injury (CBT-TBI)
| Cognitive impairment | Accommodations |
|---|---|
| Slowed speed of information processing and responding | Present information at slower rate. |
| Speak slowly. | |
| Allow patient more time to respond. | |
| Check for understanding (e.g., “what is your understanding of what I just explained?”) | |
| Impaired attention and concentration | Minimize environmental stimulation and distractions during session. |
| Provide written summary of session beforehand. | |
| Focus on one topic at a time. | |
| Frequently repeat and summarize key points and have patient reflect them back. | |
| Conduct shorter sessions (e.g., 30–40 min) when indicated. | |
| Avoid need for multi-tasking (e.g., no note taking while listening). | |
| Provide breaks when needed. | |
| Impaired learning and recall | Provide written summary of session for patient to follow (patient workbook). |
| Review concepts and strategies from prior session at beginning of each session. | |
| Assign simple written homework between sessions. | |
| Provide written educational materials to reiterate key CBT concepts. | |
| Encourage patient to ask questions. | |
| Use compensatory tools (e.g., datebooks, smartphones, memory book) to provide reminders. | |
| Provide personalized follow-up letter summarizing key concepts, strategies, and goals from last session. | |
| Plan additional practice of CBT skills within session (overlearn skills). | |
| Focus on process and practical steps of CBT. | |
| Impaired verbal abilities | Minimize emphasis on verbally mediated aspects of CBT. |
| Emphasize behavioral activation and pleasant events scheduling over formal cognitive restructuring techniques. | |
| Impaired initiation and generalization | Include support person in treatment planning and performing homework assignments. |
| Reinforce scheduling activities. | |
| Provide two sessions devoted to generalization and relapse prevention at end of intervention. | |
| Impaired motivation | Use motivational interviewing techniques to engage patient in therapeutic model and tasks. |
| Ask at each session, “Is there anything else in the agenda you'd like to add?” | |
| Focus on depression-mitigating tasks that are intrinsically rewarding or of primary concern to the patient (e.g., care management activities aimed at return to work, school, or other meaningful roles, and finding effective rehabilitation resources in outlying areas) | |
| Make reminder call prior to each session. | |
| Maintain flexibility to accommodate patient preferences (e.g., timing and duration of sessions, spend more time on specific CBT components). |
A participant workbook included didactic material, in-session exercises, and between-session written practice (homework) exercises. Participants were asked to read the relevant workbook chapter prior to each session. During each session, the therapist followed a specific agenda. After each session, the therapist mailed a personalized follow-up letter describing mutually agreed-upon plans for between-session exercises. Following the final session, in addition to a summary follow up letter, participants received a list of local TBI and mental health resources.
The same two therapists (S.V. and J.D.) delivered both the telephone and in-person CBT. Therapists followed and completed detailed session-by-session checklists to assure fidelity to the structure and content of the intervention. All sessions were audiotaped, and a random 10% were reviewed using a fidelity checklist. Average therapist ratings for both CBT modalities were excellent and exceeded 95%. No differences in quality ratings were observed for any therapist for either modality. All recorded sessions received detailed review and corrective feedback during weekly supervision by one of the study supervisors.
Usual care
Participants assigned to usual care were notified by phone of their depression status and encouraged to continue using the rehabilitation and primary care services available to them. Patients were provided a list of local mental health and TBI resources and were free to self-refer to mental health services outside the study.
Statistical analysis
Fisher exact and Mann–Whitney U tests were used to compare demographic, clinical, and process variables among groups. Mixed-effects linear regression models were used to compare treatment efficacy of combined CBT, CBT-T, and CBT-IP compared with UC. Primary analysis included observations at baseline, and 8, 16, and 24 weeks, with 16 weeks being the prespecified time of primary interest. Eight and 24 week outcomes were included to examine early effects of treatment, and persistence of effect beyond the treatment period, respectively. Random effects were included for participants' intercepts. Fixed effects were time (categorical, with pretreatment as the reference category), assigned treatment (CBT vs. UC), interaction of time with treatment, stratification factors (acceptable treatments, severe vs. complicated mild or moderate TBI), and potential confounders and variables to improve sensitivity; that is, variables related to outcome without consideration of treatment. The primary indicator of treatment effect was the interaction of time by treatment. We calculated the mean treatment effect for the depression outcomes as the time-by-treatment interaction evaluated at 16 weeks; that is, the estimated difference between treatment groups at 16 weeks in excess of the difference at baseline. Exact logistic regression was used to compare depression response rates (≥50% decrease from baseline), PGI (percent very much or much improved) and satisfaction with treatment (percent very or moderately satisfied) at 16 weeks. Secondary outcomes were assessed using mixed-effects linear regression models, controlling for the same variables as in the primary analysis. SPSS 17.0 and SAS 9.3 statistical software were used for the analyses.
Variables examined as potential confounders included: age, sex, medical comorbidity, depression variables (MDD duration, number of prior MDD episodes, pre-injury history), PTSD history, litigation, word recall (HVLT-delayed recall score), psychomotor speed and cognitive flexibility (TMT B), and baseline EROS and DAS scores. We examined potential effect modification by the following domains: demographics, depression variables, medical comorbidity, injury characteristics (TBI severity, time since injury), and other psychiatric history (history of substance dependence, history of PTSD), cognitive function, baseline EROS and DAS, involvement of a support person in CBT, and concurrent mental health treatment (counseling or antidepressants during the 16 week study period).
Results
Demographic, injury, and clinical characteristics
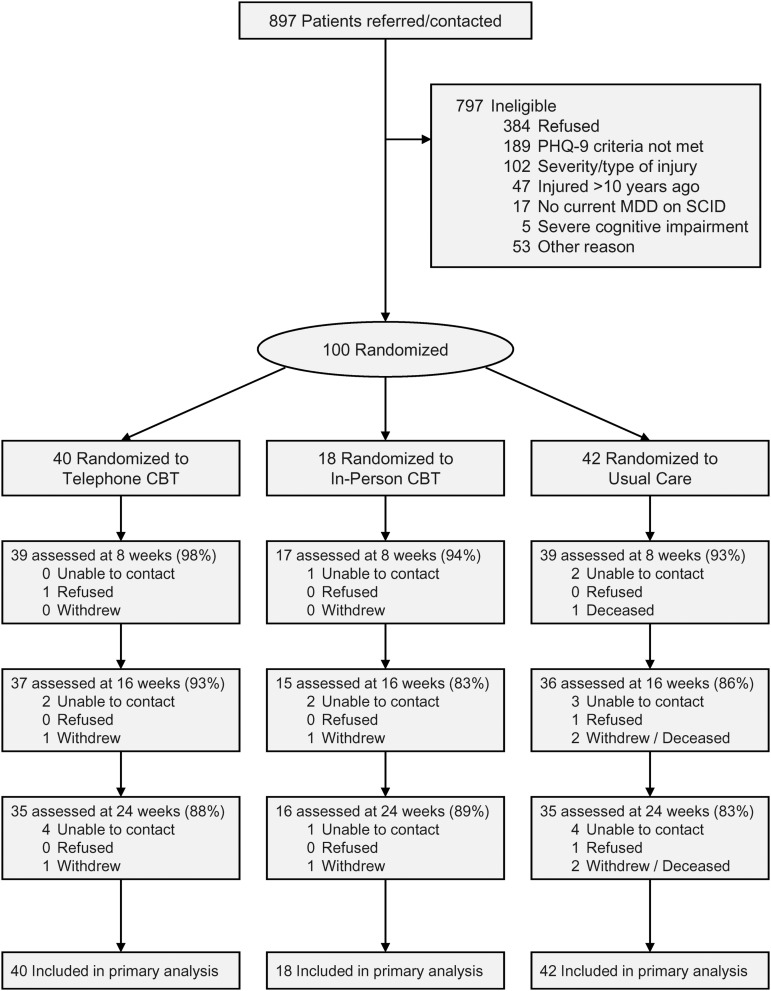
A total of 897 people with TBI were referred/contacted, and 100 met entrance criteria, consented, and were randomized (Fig. 1). Most participants opted for the three arm (n=49) or CBT-T versus UC (n=47) randomization options; 58 were randomized to CBT (40 to CBT-T, 18 to CBT-IP); and 42 to usual care. Demographic, injury, and clinical characteristics of the sample are shown in Tables 3 and 4. Mean time since injury was 3.33 (SD 2.72) years; 31% had a severe TBI; 59% reported having two or more chronic medical conditions; 20% were in litigation related to their injury; and 67% of participants had at least one, 35% had at least two, 10% had three, and 1% had four baseline neurocognitive test scores below the 5th percentile. The most frequent impairments were in the areas of executive function (44% on TMT B) and verbal learning (38% on HVLT). Mean HAMD-17 scores at baseline (17.6 [SD 4.0]) were in the moderately depressed range;78 12% did not provide 16 week outcome data.
FIG. 1.
Flow of participants in the trial. CBT, cognitive behavioral therapy; MDD, major depressive disorder; PHQ-9, Patient Health Questionnaire-9; SCID, Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV).
Table 3.
Demographic and Injury Characteristics of Participants
| Variable | All randomized subjects n=100 | Randomized to CBT n=58 | Randomized to usual care n=42 | pa |
|---|---|---|---|---|
| Randomization choice | ||||
| CBT-IP vs. UC | 4 (4%) | 1 (2%) | 3 (7%) | – |
| CBT-T vs. UC | 47 (47%) | 24 (41%) | 23 (55%) | |
| CBT-IP vs. CBT-T vs. UC | 49 (49%) | 33 (57%) | 16 (38%) | |
| Geographical region | ||||
| Western Washington | 69 (69%) | 44 (76%) | 25 (60%) | 0.224 |
| Eastern Washington | 11 (11%) | 5 (9%) | 6 (14%) | |
| Outside Washington | 20 (20%) | 9 (16%) | 11 (26%) | |
| Age - years | ||||
| Mean (SD) | 45.8 (13.3) | 45.4 (14.1) | 46.3 (12.4) | 0.512 |
| Range | (19.9, 87.3) | (21.4, 87.3) | (19.9, 75.9) | |
| Sex – n (%) | ||||
| Female | 37 (37%) | 24 (41%) | 13 (31%) | 0.304 |
| Male | 63 (63%) | 34 (59%) | 29 (69%) | |
| Race – n (%) | ||||
| Non-Hispanic white | 90 (90%) | 52 (90%) | 38 (90%) | 1.000 |
| Other | 10 (10%) | 6 (10%) | 4 (10%) | |
| Education – n (%) | ||||
| GED or less | 12 (12%) | 7 (12%) | 5 (12%) | 0.147 |
| High school diploma | 10 (10%) | 8 (14%) | 2 (5%) | |
| Tech/Voc./Some college | 52 (52%) | 31 (53%) | 21 (50%) | |
| College degree | 26 (26%) | 12 (21%) | 14 (33%) | |
| Marital status – n (%) | ||||
| Single/Never married | 30 (30%) | 18 (31%) | 12 (29%) | 0.774 |
| Married/Partnered | 24 (24%) | 11 (19%) | 13 (31%) | |
| Divorced | 39 (39%) | 24 (41%) | 15 (36%) | |
| Separated | 5 (5%) | 3 (5%) | 2 (5%) | |
| Widowed | 1 (1%) | 1 (2%) | 0 (0%) | |
| Other | 1 (1%) | 1 (2%) | 0 (0%) | |
| Years since injury – mean (SD) | 3.33 (2.72) | 3.41 (2.84) | 3.21 (2.58) | 0.961 |
| Cause of injury – n (%) | ||||
| Motor vehicle | 28 (28%) | 18 (31%) | 10 (24%) | 0.929 |
| Motorcycle | 7 (7%) | 4 (7%) | 3 (7%) | |
| Bicycle | 5 (5%) | 3 (5%) | 2 (5%) | |
| All-terrain vehicle | 3 (3%) | 2 (3%) | 1 (2%) | |
| Pedestrian | 8 (8%) | 4 (7%) | 4 (10%) | |
| Fall | 34 (34%) | 18 (31%) | 16 (38%) | |
| Hit by falling objects | 6 (6%) | 3 (5%) | 3 (7%) | |
| Recreational/Sports | 1 (1%) | 0 (0%) | 1 (2%) | |
| Assault/Blunt | 8 (8%) | 6 (10%) | 2 (5%) | |
| TBI severity | ||||
| Complicated mild/moderate | 69 (69%) | 40 (69%) | 29 (69%) | 1.000 |
| Severe | 31 (31%) | 18 (31%) | 13 (31%) | |
| Injury Severity Score (ISS) - highest non-head severity (0–5) | ||||
| Mean (SD) | 1.80 (1.41) | 1.91 (1.38) | 1.64 (1.45) | 0.344 |
| 0 | 26 (26%) | 12 (21%) | 14 (33%) | 0.344 |
| 1 | 20 (20%) | 13 (22%) | 7 (17%) | |
| ≥2 | 54 (54%) | 33 (57%) | 21 (50%) | |
| Blood alcohol level at time of injury – mg/dL | ||||
| 0 | 46 (56%) | 29 (55%) | 17 (59%) | 0.295 |
| 1–79 | 9 (11%) | 8 (15%) | 1 (3%) | |
| ≥80 | 27 (33%) | 16 (30%) | 11 (38%) | |
| Unknown | 18 | 5 | 13 | |
| Current/planned litigation related to injury - n (%) | 20 (20%) | 15 (26%) | 5 (12%) | 0.127 |
| Unknown | 2 | 1 | 1 | |
| Chronic medical conditionsb | ||||
| Mean (SD) | 2.21 (1.79) | 2.05 (1.73) | 2.43 (1.88) | 0.265 |
| 0 | 19 (19%) | 9 (16%) | 10 (24%) | 0.010 |
| 1 | 22 (22%) | 19 (33%) | 3 (7%) | |
| ≥2 | 59 (59%) | 30 (52%) | 29 (69%) | |
Significance by Fisher exact or Mann–Whitney U Test
Medical conditions reported by >10% of participants were: chronic pain (53%), hearing/vision loss (41%), hypertension (36%), epilepsy/stroke (19%), arthritis/rheumatism (18%), asthma/emphysema (13%), urinary problems (11%).
CBT, cognitive behavioral therapy, CBT-IP, in-person CBT; CBT-T, telephone CBT; TBI; traumatic brain injury; UC, usual care.
Table 4.
Clinical Characteristics of Participants
| Variable | All randomized subjects n=100 | Randomized to CBT n=58 | Randomized to usual care n=42 | pa |
|---|---|---|---|---|
| History of major depressive disorder | ||||
| None | 50 (50%) | 27 (47%) | 23 (55%) | 0.198 |
| Pre-injury | 38 (38%) | 26 (45%) | 12 (29%) | |
| Post-injury only | 12 (12%) | 5 (9%) | 7 (17%) | |
| Prior major depressive episodes – n (%) | ||||
| Median (IQR) | 0.5 (0, 3) | 1 (0, 3) | 0 (0, 2) | 0.512 |
| 0 | 50 (50%) | 27 (47%) | 23 (55%) | 0.628 |
| 1 | 16 (16%) | 9 (16%) | 7 (17%) | |
| 2+ | 34 (34%) | 22 (38%) | 12 (29%) | |
| Duration of current major depressive episode – years | ||||
| Mean (SD) | 2.1 (2.6) | 2.2 (2.8) | 2.0 (2.2) | 0.789 |
| <1 year | 43 (47%) | 25 (49%) | 18 (45%) | 0.833 |
| ≥1 year | 48 (53%) | 26 (51%) | 22 (55%) | |
| Unknown | 9 | 7 | 2 | |
| Current dysthymic disorder -n (%) | 12 (12%) | 7 (12%) | 5 (12%) | 1.000 |
| Alcohol dependence within past year – n (%) | 13 (13%) | 7 (12%) | 6 (14%) | 0.771 |
| Current alcohol abuse -n (%) | 10 (10%) | 5 (9%) | 5 (12%) | 0.738 |
| Substance dependence within past year – n (%) | 5 (5%) | 2 (3%) | 3 (7%) | 0.647 |
| Current substance abuse – n (%) | 4 (4%) | 3 (5%) | 1 (2%) | 0.637 |
| History of PTSD – n (%) | 18 (18%) | 15 (26%) | 3 (7%) | 0.017 |
| Neuropsychological tests – mean (SD) | ||||
| Mean digit span | 15.3 (3.3) | 14.9 (3.4) | 16.0 (3.2) | 0.108 |
| Mean HVLT score | 6.9 (3.1) | 6.6 (3.2) | 7.4 (2.9) | 0.246 |
| Mean oral TMT A (sec) | 8.5 (4.7) | 8.6 (5.1) | 8.3 (4.0) | 0.734 |
| Mean oral TMT B (sec) | 49.8 (36.0) | 49.2 (36.8) | 50.6 (35.3) | 0.781 |
| Treatment during weeks 0–16 | ||||
| Antidepressant at therapeutic dose | 39 (39%) | 27 (47%) | 12 (29%) | 0.096 |
| ≥4 sessions counseling | 16 (16%) | 10 (17%) | 6 (14%) | 0.787 |
| Antidepressant or counseling | 49 (49%) | 32 (55%) | 17 (40%) | 0.162 |
Significance by Fisher Exact or Mann–Whitney U Test
HVLT, Hopkins Verbal Learning Test; IQR, interquartile range; PTSD, post-traumatic stress disorder; TMT, Trail Making Test.
A total of 38% reported a history of MDD prior to their TBI, 34% had had two or more prior major depressive episodes, and 53% reported their current depressive episode having lasted≥1 year. PTSD history was imbalanced between treatment groups, but was not a confounder of the treatment effect. At the time of study screening, 23% were receiving counseling (other than evidence-based psychotherapy for depression) and 40% were receiving an antidepressant. During the course of the 16 week trial period, the number of participants who reported receiving antidepressant medication at a therapeutic dose was 29% in the usual care group and 47% in the CBT group (p=0.096), and the number who reported receiving four or more sessions of evidence-based psychotherapy for depression outside the study was 14% in the usual care group and 17% in the CBT group (p=0.787).
CBT participation and therapeutic alliance
CBT process data are shown in Table 5. Participants receiving CBT-T averaged one more session than those receiving CBT-IP, but the duration of psychotherapy sessions was significantly longer for the CBT-IP group (p=0.001); 69% of CBT recipients received at least eight sessions (defined as “completers”) and 91% received at least four sessions, which has been cited as a “minimally effective dose” of psychotherapy in some studies;36,79 there were 12% more CBT-T than CBT-IP participants in each of these categories. A support person participated in at least one session for 12 (30%) of the CBT-T participants and 8 (44%) of the CBT-IP participants; among those who had a support person participate, they did so in significantly more of the CBT-T sessions (44%) than the CBT-IP sessions (11%). The CBT-T and CBT-IP groups did not differ on overall therapeutic alliance or on any of the subscales (task agreement, therapeutic bond, goal agreement). There were no study-related adverse events.
Table 5.
Cognitive Behavioral Therapy (CBT) Process Variables
| Variable | All CBT n=58 | CBT-T n=40 | CBT-IP n=18 | pa |
|---|---|---|---|---|
| Number of CBT sessions completed – mean (SD) | 9.3 (3.8) | 9.6 (3.3) | 8.6 (4.6) | 0.905 |
| Median (IQR) | 12 (6, 12) | 11.5 (7, 12) | 12 (5, 12) | |
| Completed ≥4 – n (%) | 53 (91%) | 38 (95%) | 15 (83%) | 0.167 |
| Completed ≥8 – n (%) | 40 (69%) | 29 (73%) | 11 (61%) | 0.540 |
| CBT sessions completed by 8 week assessment – mean (SD) | 5.2 (2.0) | 5.5 (1.7) | 4.7 (2.6) | 0.300 |
| CBT session duration (minutes) – mean (SD) | 44.9 (8.7) | 42.5 (8.9) | 50.4 (5.0) | 0.001 |
| Number of participants who had a support person attend ≥1 session – n (%) | 20 (34%) | 12 (30%) | 8 (44%) | 0.373 |
| % of sessions with support person - mean (SD)b | 31 (32) | 44 (36) | 11 (6) | 0.007 |
| Therapeutic alliance (WAI-SF) at week 16 – mean (SD) | 6.2 (0.8) | 6.2 (0.7) | 6.2 (1.0) | 0.658 |
| Task subscale | 6.2 (0.9) | 6.3 (0.6) | 5.9 (1.3) | 0.565 |
| Bond subscale | 6.3 (0.8) | 6.3 (0.8) | 6.4 (0.9) | 0.461 |
| Goal subscale | 6.2 (0.9) | 6.1 (0.9) | 6.3 (1.1) | 0.435 |
IQR, interquartile range; WAI-SF=Working Alliance Inventory-Short Form (client version); CBT-T, telephone CBT; CBT-IP, in-person CBT.
CBT-T vs. CBT-IP. Significance by Fisher Exact or Mann–Whitney U Test.
Among those with a support person at one or more sessions.
Depression outcomes
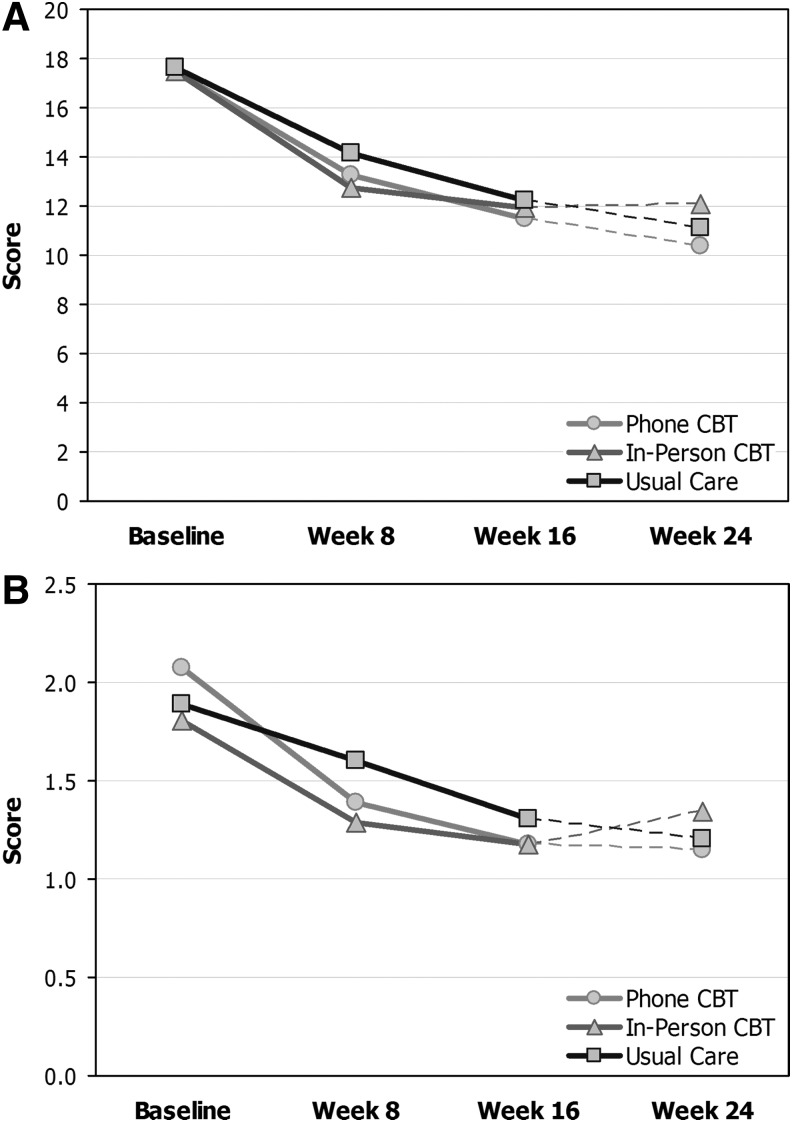
Adjusted mixed effects models of depression outcomes are shown in Table 6, and depression scores over time are shown in Figure 2. Over 16 weeks, there was no statistically significant difference between the combined CBT and UC groups on the HAMD-17 (treatment effect=1.2, 95% CI: −1.5–4.0; p=0.37), and a nonsignificant trend for improvement in the CBT versus UC groups on the SCL-20 (treatment effect=0.28, 95% CI: −0.03–0.59; p=0.074). That is, the treated participants at 16 weeks had HAMD estimated to decrease by 1.2 points more from baseline than those receiving UC, and SCL-20 score estimated to decrease by 0.28 more among treated participants. In a secondary analysis looking only at the first 8 weeks, the difference on SCL-20 was significant (p=0.001), whereas that for HAMD-17 was not (p=0.32).
Table 6.
Depression Outcomes
| Other comparisons | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | All CBT | CBT-T | CBT-IP | UC | All CBT vs. UCc | CompletersdAll CBT vs. UC | CBT-T vs. UC | CBT-IP vs. UC |
| n in each group | 58 | 40 | 18 | 42 | 58, 42 | 40, 42 | 40, 39 | 18, 19 |
| HAMD-17 scorea | ||||||||
| Baseline | 17.5 (3.9) | 17.5 (3.3) | 17.5 (5.0) | 17.6 (4.3) | – | – | – | – |
| Week 8 | 13.1 (6.1) | 13.3 (5.6) | 12.7 (7.2) | 14.1 (5.4) | 0.315 | 0.212 | 0.573 | 0.200 |
| Week 16 | 11.6 (6.1) | 11.5 (6.2) | 11.9 (6.1) | 12.2 (6.8) | 0.372 | 0.228 | 0.452 | 0.164 |
| Week 24 | 10.9 (6.9) | 10.4 (6.4) | 12.1 (7.8) | 11.1 (6.2) | 0.494 | 0.505 | 0.413 | 0.971 |
| Week 16 responseb | 17 (33%) | 12 (32%) | 5 (33%) | 11 (31%) | 0.212 | 0.202 | 0.356 | 0.191 |
| SCL-20a | ||||||||
| Baseline | 1.99 (0.55) | 2.07 (0.58) | 1.81 (0.45) | 1.89 (0.50) | – | – | – | – |
| Week 8 | 1.36 (0.65) | 1.39 (0.62) | 1.29 (0.72) | 1.60 (0.74) | 0.001 | 0.001 | 0.002 | 0.155 |
| Week 16 | 1.18 (0.72) | 1.18 (0.74) | 1.18 (0.68) | 1.30 (0.68) | 0.074 | 0.011 | 0.043 | 0.170 |
| Week 24 | 1.21 (0.77) | 1.15 (0.76) | 1.34 (0.80) | 1.20 (0.77) | 0.250 | 0.136 | 0.065 | 0.776 |
| Week 16 responseb | 23 (44%) | 17 (46%) | 6 (40%) | 10 (28%) | 0.070 | 0.042 | 0.087 | 0.075 |
| MDD negative on SCIDb | ||||||||
| Week 16 | 38 (73%) | 26 (70%) | 12 (80%) | 20 (57%) | 0.211 | 0.335 | 0.436 | 0.136 |
| Week 24 | 35 (67%) | 24 (69%) | 11 (65%) | 23 (68%) | 0.987 | 0.904 | 0.687 | 0.248 |
| Patient Global Impression (PGI) - much or very much improved – n (%) | ||||||||
| Week 16 | 32 (62%) | 25 (68%) | 7 (47%) | 14 (39%) | 0.010 | 0.007 | 0.012 | 0.133 |
| Week 24 | 29 (58%) | 23 (68%) | 6 (38%) | 14 (40%) | 0.040 | 0.044 | 0.026 | 0.633 |
| Satisfaction with depression care - moderately or very satisfied – n (%) | ||||||||
| Week 16 | 42 (84%) | 30 (83%) | 12 (86%) | 9 (26%) | <0.001 | <0.001 | <0.001 | 0.007 |
Mixed model regression using an autoregressive correlation matrix, reporting the significance of the time by treatment term.
Response defined as ≥50% reduction from baseline. Significance by exact logistic regression (using covariates from linear model).
Adjusts for strata (choice, traumatic brain injury [TBI] severity) and confounders; that is, MDD duration (all models), baseline Environmental Reward Observation Scale score (all models except HAMD-17), and medical comorbidity (HAMD-17).
Completers defined as those who completed eight or more CBT sessions.
CBT, cognitive behavioral therapy; CBT-T, telephone CBT; CBT-IP, in-person CBT; HAMD-17, 17-item Hamilton Depression Rating Scale; MDD, Major Depressive Disorder; SCID, Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM-IV); SCL-20, Symptom Checklist-20; UC, usual care.
FIG. 2.
(A) Mean HAMD-17 depression score for CBT and Usual Care groups. (B) Mean SCL-20 depression score for CBT and Usual Care groups. CBT, cognitive behavioral therapy; HAMD-17, 17 item Hamilton Depression Rating Scale; SCL-20, Symptom Checklist-20.
Follow-up comparisons indicated that the CBT-T group improved more than UC on the SCL-20 (treatment effect=0.36, 95% CI: 0.01–0.70; p=0.043), but the CBT-IP group did not (treatment effect=0.34, 95% CI: −0.15–0.84; p=0.17). Among those who completed at least 8 sessions, CBT was not superior to UC on the HAMD-17 (treatment effect=1.78, 95% CI: −1.13–4.69; p=0.23), but CBT was superior to UC on the SCL-20 (treatment effect=0.43, 95% CI: 0.10–0.76; p=0.011). Response rates did not differ significantly at 16 weeks between CBT and UC on the HAMD-17, although on the SCL-20, completers had a significantly higher response rate (49%; p=0.042), and there were nonsignificant trends (p<0.1) on the SCL-20 for higher response in the CBT group overall (44%), CBT-T (46%) and CBT-IP (40%) versus UC (28%). Many of the CBT treatment responders responded by 8 weeks: 10 (43%) in CBT who were SCL-20 responders at 16 weeks were also responders at 8 weeks, compared with 2 (20%) in UC.
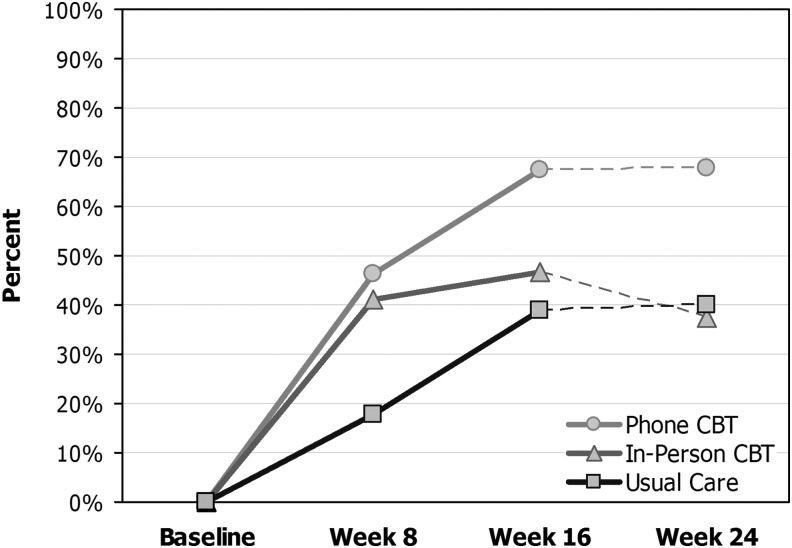
The proportions of participants negative for MDD on the SCID at 16 weeks were 70% for CBT-T, 80% for CBT-IP, and 57% for UC (p=0.25). At 16 weeks, a significantly greater proportion of CBT participants (62%) reported “much” or “very much” symptom improvement on the PGI questionnaire compared with UC (39%; p=0.010); ratings remained high at 24 weeks in the CBT-T group, whereas they fell to UC levels in the CBT-IP group (Fig. 3). A greater proportion of CBT participants (84%) reported that they were “moderately” or “very” satisfied with their overall depression care, compared with the UC group (26%, p<0.001).
FIG. 3.
Percent of participants reporting that their depression was much or very much improved on the Patient Global Impression Scale. CBT, cognitive behavioral therapy.
Secondary outcomes and effect modifiers
There were no significant group differences on health-related quality of life, functional impairment, overall post-concussive symptom score, environmental reward, automatic negative thoughts, or dysfunctional attitudes. There was a nonsignificant trend (p=0.065) for CBT-T to have improved SCL-20 depression scores at 24 weeks, compared with UC. In the moderator analysis, there was a significant interaction between treatment and pre-injury MDD (p=0.02 for HAMD-17, p=0.07 for SCL-20): for those with no pre-injury MDD, there was a significantly greater decrease in depression for those assigned to CBT compared with UC (p=0.036 for HAMD-17, p=0.008 for SCL-20), whereas for those with a pre-injury history of MDD, the groups did not differ significantly. TBI severity and level of cognitive impairment did not modify treatment effects.
Discussion
To our knowledge, this is the first published randomized controlled trial of cognitive behavioral therapy for MDD in persons with TBI. Consistent with results from preliminary studies in TBI populations,23,28,31,32 we found CBT-IP and, particularly, CBT-T are acceptable and feasible in a geographically diverse sample with complicated mild to severe TBI. There were no statistically significant differences between CBT treatments overall compared with UC on the primary outcomes. However, further comparisons indicated that completers of eight or more CBT sessions, as well as those receiving CBT-T, but not CBT-IP, reported significantly greater improvement in patient-reported depressive symptoms compared with those receiving UC. Participants who received CBT rated their global improvement over 24 weeks as significantly greater than those who received UC, and >80% in the CBT group were moderately or very satisfied with their overall depression care, compared with only 25% of the UC group. Although further enhancements to improve treatment effect are warranted, CBT-T appears to hold particular promise in this population for enhancing access and adherence to treatment without any decline in efficacy or negative impact on the therapeutic relationship.
Our finding that CBT-T may be more accessible and effective than UC is consistent with recent meta-analyses of telephone-based psychotherapy interventions for depression.38,80 Even among participants residing in close proximity to the coordinating site (Western Washington; n=69), more participants chose the CBT-T versus UC randomization option (n=17) than the CBT-IP versus UC option (n=4). Although not statistically significant, participants in the CBT-T group received a greater number of CBT sessions than did those in the CBT-IP group. When a support person was available, that person attended sessions four times more frequently in the CBT-T group. These differences may in part explain the greater effect of CBT-T on self-reported measures of depression symptoms (SCL-20) and global improvement (PGI).
By using a choice-stratified randomization strategy, we were able to recruit a study sample that was more consistent with “real-world” conditions; that is, incorporating individual preferences and practical considerations into treatment assignment, compared with a traditional three arm study. Although this approach improved ecological validity and likely enhanced the generalizability of our study findings, nearly half (49%) of the study sample reported receiving counseling or antidepressants outside the study during the 16 week study period. Two thirds of participants had significant impairment on baseline cognitive testing; however, it is encouraging that TBI severity and cognitive impairment did not modify CBT efficacy. Although the significant level of psychiatric and medical comorbidity in our sample may have conservatively influenced CBT treatment effects, it also demonstrates the feasibility of providing CBT to TBI patients with complex problems. Prior studies in medically complex populations have also shown a smaller overall effect of psychotherapy for depression compared with non-medical populations.81–84 We found that a history of prior depression was associated with a diminished treatment effect, suggesting that those with recurrent or chronic depression may require more intensive intervention; for example, combined counseling and medications, or a different psychotherapeutic approach.
Our data on the different depression outcome measures (HAMD-17, SCL-20, SCID) reveal the complexities and challenges associated with assessing depression and treatment outcomes in TBI populations. As expected, the HAMD-17, which includes a higher proportion of somatic symptoms that may be confounded with TBI (e.g., headaches, fatigue, sexual dysfunction, insomnia, general somatic symptoms, somatic anxiety, hypochondriasis, psychomotor retardation or agitation, and work and activity impairments) than the SCL-20, showed a lower treatment effect. Applying previously proposed criteria,85–87 the HAMD treatment effects fall in the clinically nonsignificant range, whereas the SCL-20 treatment effects can be interpreted as clinically meaningful. Because of the often chronic nature of TBI symptoms, these potentially confounded items would make the HAMD less sensitive to change in depression, and a potentially suboptimal intervention outcome measure in persons with TBI.
Researchers have raised concerns about the psychometric validity of including somatic symptoms in the assessment of depression in patients with TBI,88,89 and the issue becomes more complex when trying to influence somatic symptoms in populations with high medical comorbidity with relatively brief interventions.23 The HAMD has been criticized for being a multidimensional scale that is relatively insensitive to change.58 Also, evaluators may not have seen the same changes in depression using semistructured interviews, as they would from the patients' self-reports. Further research is needed in TBI populations to examine the psychometric properties and validity of depression instruments and the responsiveness to treatment of specific psychological and somatic symptoms. Patient-reported metrics that encompass depressive symptom severity, functioning, and quality of life may provide the most patient-centered approach.90
Limitations
Several study limitations should be noted. Our study sample demonstrated higher levels of medical, cognitive, and psychiatric comorbidity than other TBI populations with depression.10,22 Research has shown that populations with high levels of psychiatric and medical comorbidity tend to be more treatment resistant. This high comorbidity, as well as the modest sample size of this phase IIb study, likely affected our ability to demonstrate statistically significant differences in treatment outcomes among the study groups. Although our sample was geographically diverse and encompassed a wide age range, our sample was predominantly non-Hispanic white and well educated. Previous research suggests that the benefits of organized care programs for depression may be greater among members of racial/ethnic minorities and the uninsured.91,92 Our study design may have biased our comparisons in favor of the UC group.93 Participants assigned to UC were notified of their depression status and provided a comprehensive list of local depression and TBI resources, likely resulting in treatment that would not have occurred otherwise. A large percentage of participants in both study arms received antidepressants or counseling outside the study, which may have contributed to an underestimation of the effectiveness of CBT compared with UC outside a research setting.
Conclusion
Our research findings suggest several important points related to clinical practice. First, CBT-T may hold particular promise for enhancing access to effective depression treatment after TBI. We were able to demonstrate that the therapeutic alliance was excellent compared with counseling studies in non-TBI populations,94 and just as strong when delivering the intervention over the telephone as it was in person. Although CBT can be an effective treatment approach for some patients with TBI and depression, engagement strategies are important to maximize adherence and chances of achieving a “therapeutic dose.” Meta-analyses in medical populations have shown that including studies only providing intent-to-treat data significantly reduced the effect size of CBT.81 Many study participants were experiencing significant psychosocial stress during the course of the study; for example, job and relationship loss or bankruptcy. Such dire psychosocial stressors are common in the years following moderate to severe TBI and likely contribute significantly to depression.95 Although our CBT intervention did include brief TBI care management at the beginning of each session, this was limited, and likely not adequate in meeting many of the participants' psychosocial needs.
The trajectory of depression scores suggest that the CBT group improved more rapidly than UC during the first 8 weeks. During this period, the therapy consisted primarily of behavioral activation strategies; that is, increasing pleasant and rewarding activities and decreasing avoidance behaviors.96 Although we can speculate that behavioral activation may have provided the majority of the active treatment effect, compared with the cognitive therapy component in the latter part of the intervention, the study was not designed to dismantle specific active intervention components. Our finding that more than twice as many 16 week responders to CBT versus UC responded by 8 weeks, suggests that there is an opportunity at 8 weeks to adjust or intensify treatment for CBT nonresponders in order to achieve greater improvement.
One approach for future studies is to explore a more flexible approach to the behavioral and cognitive components of CBT, based on patient preferences, concurrent response monitoring, and treatment plan revisions, and to offer a patient-centered, multifaceted, stepped care approach that includes options for pharmacotherapy and other psychotherapeutic modalities, such as problem-solving or interpersonal therapy.23–25 Clearly, more research is needed to examine the efficacy and potential mediators of these psychotherapeutic alternatives, and the trade-offs of providing a structured, manualized therapy versus one that is less structured and more subject to variability. Recent prospective research on the temporal relationship between functional status and depression indicates that helping people with TBI improve their everyday functioning may be one key to improving mood,97 supporting the need for more intensive and structured care management and possibly occupational, vocational, speech, or physical therapy. Regular relapse prevention “booster sessions” also appear indicated, particularly for CBT-IP, given the lack of significant treatment effects at 24 weeks.
In addition to capitalizing on the promising results of telephone administered psychotherapy in this study, researchers should also explore alternative treatment delivery strategies, such as the Internet and mobile technologies, to improve reach, adherence, and follow-up in TBI populations. Larger phase III studies with greater statistical power, including those conducted in real-world settings, will be needed to determine how to best target particular treatment approaches to patients with specific demographic and clinical characteristics.98,99 Ultimately, interventions that simultaneously address common comorbid conditions and that are integrated into a patient's routine medical or rehabilitation care may hold the most promise, but require further investigation to determine feasibility and cost effectiveness.
Acknowledgments
This study was supported by the National Institutes of Health (grant R21HD53736) and the Department of Education, National Institute on Disability and Rehabilitation Research (grant H133G070016). We thank Douglas Weeks at St. Luke's Rehabilitation Institute, Tessa Hart, at Moss Rehabilitation Research Institute, and Thomas Novack at the University of Alabama, Birmingham for assistance with study recruitment.
Trial registration: clinicaltrials.gov identifier: NCT00878150.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Coronado V.G., McGuire L.C., Sarmiento K., Bell J., Lionbarger M.R., Jones C.D., Geller A.I., Khoury N., and Xu L. (2012). Trends in traumatic brain injury in the U.S. and the public health response: 1995–2009. J. Safety Res. 43, 299–307 [DOI] [PubMed] [Google Scholar]
- 2.Hyder A.A., Wunderlich C.A., Puvanachandra P., Gururaj G., and Kobusingye O.C. (2007). The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation 22, 341–353 [PubMed] [Google Scholar]
- 3.Faul M., Xu L., Wald M., and Coronado V. (2010). Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta [Google Scholar]
- 4.Hoge C.W., McGurk D., Thomas J.L., Cox A.L., Engel C.C., and Castro C.A. (2008). Mild traumatic brain injury in U.S. Soldiers returning from Iraq. N. Engl. J. Med. 358, 453–463 [DOI] [PubMed] [Google Scholar]
- 5.NIH Consensus Development Panel on Rehabilitation of Persons With Traumatic Brain Injury (1999) Consensus conference. Rehabilitation of persons with traumatic brain injury. JAMA 282, 974–983 [PubMed] [Google Scholar]
- 6.Deb S., Lyons I., Koutzoukis C., Ali I., and McCarthy G. (1999). Rate of psychiatric illness 1 year after traumatic brain injury. Am. J. Psychiatry 156, 374–378 [DOI] [PubMed] [Google Scholar]
- 7.Hibbard M.R., Uysal S., Kepler K., Bogdany J., and Silver J. (1998). Axis I psychopathology in individuals with traumatic brain injury. J. Head Trauma Rehabil. 13, 24–39 [DOI] [PubMed] [Google Scholar]
- 8.Whelan–Goodinson R., Ponsford J., Johnston L., and Grant F. (2009). Psychiatric disorders following traumatic brain injury: their nature and frequency. J. Head Trauma Rehabil. 24, 324–332 [DOI] [PubMed] [Google Scholar]
- 9.Whelan–Goodinson R., Ponsford J., and Schonberger M. (2008). Association between psychiatric state and outcome following traumatic brain injury. J. Rehabil Med. 40, 850–857 [DOI] [PubMed] [Google Scholar]
- 10.Hart T., Brenner L., Clark A.N., Bogner J.A., Novack T.A., Chervoneva I., Nakase–Richardson R., and Arango–Lasprilla J.C. (2011). Major and minor depression after traumatic brain injury. Arch. Phys. Med. Rehabil. 92, 1211–1219 [DOI] [PubMed] [Google Scholar]
- 11.Fann J.R., Katon W.J., Umoto J.M., and Esselmann P.C. (1995). Psychiatric disorders and functional disability in outpatients with traumatic brain injuries. Am. J. Psychiatry 152, 1493–1499 [DOI] [PubMed] [Google Scholar]
- 12.Satz P., Forney D.L., Zaucha K., Asarnow R.R., Light R., McCleary C., Levin H., Kelly D., Bergsneider M., Hovda D., Martin N., Namerow N., and Becker D. (1998). Depression, cognition, and functional correlates of recovery outcome after traumatic brain injury. Brain Inj. 12, 537–553 [DOI] [PubMed] [Google Scholar]
- 13.Christensen A.L., and Uzzell B.P. (1994). Brain Injury and Neuropsychological Rehabilitation: International Perspectives. Erlbaum Associates, Inc.: Hillsdale [Google Scholar]
- 14.Jorge R.E., Robinson R.G., Moser D., Tateno A., Crespo–Facorro B., and Arndt S. (2004). Major depression following traumatic brain injury. Arch. Gen. Psychiatry 61, 42–50 [DOI] [PubMed] [Google Scholar]
- 15.Rintala D.H., Hanover D., Alexander J.L., Sanson–Fisher R.W., Willems E.P., and Halstead L.S. (1986). Team care: an analysis of verbal behavior during patient rounds in a rehabilitation hospital. Arch. Phys. Med. Rehabil. 67, 118–122 [DOI] [PubMed] [Google Scholar]
- 16.Kuny S., and Stassen H.H. (1995). Cognitive performance in patients recovering from depression. Psychopathology 28, 190–207 [DOI] [PubMed] [Google Scholar]
- 17.Downhill J.E., Jr., and Robinson R.G. (1994). Longitudinal assessment of depression and cognitive impairment following stroke. J. Nerv. Ment. Dis. 182, 425–431 [DOI] [PubMed] [Google Scholar]
- 18.Sweet J. (1992). Significance of depression in clincal neuropsychological assessment. Clin. Psychol. Rev. 12, 21–45 [Google Scholar]
- 19.Gomez–Hernandez R., Max J.E., Kosier T., Paradiso S., and Robinson R.G. (1997). Social impairment and depression after traumatic brain injury. Arch. Phys. Med. Rehabil. 78, 1321–1326 [DOI] [PubMed] [Google Scholar]
- 20.Jorge R.E., Robinson R.G., Starkstein S.E., and Arndt S.V. (1994). Influence of major depression on 1-year outcome in patients with traumatic brain injury. J. Neurosurg. 81, 726–733 [DOI] [PubMed] [Google Scholar]
- 21.Rockhill C.M., Jaffe K., Zhou C., Fan M.Y., Katon W., and Fann J.R. (2012). Health care costs associated with traumatic brain injury and psychiatric illness in adults. J. Neurotrauma 29, 1038–1046 [DOI] [PubMed] [Google Scholar]
- 22.Bombardier C.H., Fann J.R., Temkin N.R., Esselman P.C., Barber J., and Dikmen S.S. (2010). Rates of major depressive disorder and clinical outcomes following traumatic brain injury. JAMA 303, 1938–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fann J.R., Hart T., and Schomer K.G. (2009). Treatment for depression after traumatic brain injury: a systematic review. J. Neurotrauma 26, 2383–2402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stalder–Luthy F., Messerli–Burgy N., Hofer H., Frischknecht E., Znoj H., and Barth J. (2013). Effect of psychological interventions on depressive symptoms in long-term rehabilitation after an acquired brain injury: a systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 94, 1386–1397 [DOI] [PubMed] [Google Scholar]
- 25.Barker–Collo S., Starkey N., and Theadom A. (2013). Treatment for depression following mild traumatic brain injury in adults: a meta-analysis. Brain Inj. 27, 1124–1133 [DOI] [PubMed] [Google Scholar]
- 26.Hibbard M.K., Rendon D., Charatz H., and Kothera L. (2005). CBT in individuals with traumatic brain injury, in: Cognitive Behavior Therapy in Nursing Practice. Freeman S.M. and Freeman A., (eds.). Springer Publishing Company: New York: pp. 189–220 [Google Scholar]
- 27.Hibbard M.R., Ruckdeschel M., Gordon W.A., Egelko S., and Langer K. (1987). Issues in the diagnosis and cognitive therapy of depression in brain-damaged individuals, in: Cognitive Therapy: Applications in Psychiatric and Medical Settings. Freeman A., and Greenwood V. (eds.). Human Sciences Press, Inc.: New York, pps. 183–198 [Google Scholar]
- 28.Bradbury C.L., Christensen B.K., Lau M.A., Ruttan L.A., Arundine A.L., and Green R.E. (2008). The efficacy of cognitive behavior therapy in the treatment of emotional distress after acquired brain injury. Arch. Phys. Med. Rehabil. 89, S61–68 [DOI] [PubMed] [Google Scholar]
- 29.Block C.K., and West S.E. (2013). Psychotherapeutic treatment of survivors of traumatic brain injury: review of the literature and special considerations. Brain Inj. 27, 775–788 [DOI] [PubMed] [Google Scholar]
- 30.Malec J.F., Brown A.W., Moessner A.M., Stump T.E., and Monahan P. (2010). A preliminary model for posttraumatic brain injury depression. Arch. Phys. Med. Rehabil. 91, 1087–1097 [DOI] [PubMed] [Google Scholar]
- 31.Topolovec–Vranic J., Cullen N., Michalak A., Ouchterlony D., Bhalerao S., Masanic C., and Cusimano M.D. (2010). Evaluation of an online cognitive behavioural therapy program by patients with traumatic brain injury and depression. Brain Inj. 24, 762–772 [DOI] [PubMed] [Google Scholar]
- 32.Bedard M., Felteau M., Marshall S., Cullen N., Gibbons C., Dubois S., Maxwell H., Mazmanian D., Weaver B., Rees L., Gainer R., Klein R., and Moustgaard A. (2013). Mindfulness-based cognitive therapy reduces symptoms of depression in people with a traumatic brain injury: results from a randomized controlled trial. J. Head Trauma Rehabil. 29, E13–E22 [DOI] [PubMed] [Google Scholar]
- 33.Simon G.E., Von Korff M., Rutter C.M., and Peterson D.A. (2001). Treatment process and outcomes for managed care patients receiving new antidepressant prescriptions from psychiatrists and primary care physicians. Arch. Gen. Psychiatry 58, 395–401 [DOI] [PubMed] [Google Scholar]
- 34.Horvath A., and Greenberg L. (1986). The development of the Working Alliance Inventory, in: The Psychotherapeutic Process: A Research Handbook. Greenberg L., and Pinsof W. (eds.). Guilford: New York, pp. 529–556 [Google Scholar]
- 35.Young A.S., Klap R., Sherbourne C.D., and Wells K.B. (2001). The quality of care for depressive and anxiety disorders in the United States. Arch. Gen. Psychiatry 58, 55–61 [DOI] [PubMed] [Google Scholar]
- 36.Horvitz–Lennon M., Normand S.L., Frank R.G., and Goldman H.H. (2003). “Usual care” for major depression in the 1990s: characteristics and expert-estimated outcomes. Am. J. Psychiatry 160, 720–726 [DOI] [PubMed] [Google Scholar]
- 37.Wang P.S., Berglund P., and Kessler R.C. (2000). Recent care of common mental disorders in the United States: prevalence and conformance with evidence-based recommendations. J. Gen. Intern. Med. 15, 284–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohr D.C., Vella L., Hart S., Heckman T., and Simon G. (2008). The effect of telephone–administered psychotherapy on symptoms of depression and attrition: a meta-analysis. Clin. Psychol (New York) 15, 243–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simon G.E., Ralston J.D., Savarino J., Pabiniak C., Wentzel C., and Operskalski B.H. (2011). Randomized trial of depression follow-up care by online messaging. J. Gen. Intern. Med. 26, 698–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fann J.R., Jones A.L., Dikmen S.S., Temkin N.R., Esselman P.C., and Bombardier C.H. (2009). Depression treatment preferences after traumatic brain injury. J. Head Trauma Rehabil. 24, 272–278 [DOI] [PubMed] [Google Scholar]
- 41.Fann J.R., Bombardier C.H., Dikmen S., Esselman P., Warms C.A., Pelzer E., Rau H., and Temkin N. (2005). Validity of the Patient Health Questionnaire-9 in assessing depression following traumatic brain injury. J. Head Trauma Rehabil. 20, 501–511 [DOI] [PubMed] [Google Scholar]
- 42.Kroenke K., Spitzer R.L., and Williams J.B. (2001). The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 16, 606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.First M., Gibbon M., Spitzer R., and Williams B. (2001). User's Guide for the Structured Clinical Interview for DSM IV TR Axis I Disorders–Research Version. Biometrics Research Department, New York State Psychiatric Institute: New York [Google Scholar]
- 44.Jagger J., Fife D., Vernberg K., and Jane J.A. (1984). Effect of alcohol intoxication on the diagnosis and apparent severity of brain injury. Neurosurgery 15, 303–306 [DOI] [PubMed] [Google Scholar]
- 45.Sheehan D.V., Lecrubier Y., Sheehan K.H., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., and Dunbar G.C. (1998). The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59, Suppl. 20, 22–57 [PubMed] [Google Scholar]
- 46.Wechsler D. (1997). Wechsler Adult Intelligence Scale III. The Psychological Corp: San Antonio [Google Scholar]
- 47.Benedict R., Schretlen D., Groninger L., and Brandt J. (1998). Hopkins Verbal Learning Test Revised: normative data and analysis of inter-form and test–retest reliability. Clin. Neuropharmacol. 12, 43–55 [Google Scholar]
- 48.Ricker J.H., and Axelrod B.N. (1994). Analysis of an oral paradigm for the Trail Making Test. Assessment 1, 47–52 [DOI] [PubMed] [Google Scholar]
- 49.Lavori P.W., Rush A.J., Wisniewski S.R., Alpert J., Fava M., Kupfer D.J., Nierenberg A., Quitkin F.M., Sackeim H.A., Thase M.E., and Trivedi M. (2001). Strengthening clinical effectiveness trials: equipoise-stratified randomization. Biol. Psychiatry 50, 792–801 [DOI] [PubMed] [Google Scholar]
- 50.Cook K.F., Bombardier C.H., Bamer A.M., Choi S.W., Kroenke K., and Fann J.R. (2011). Do somatic and cognitive symptoms of traumatic brain injury confound depression screening? Arch. Phys. Med. Rehabil. 92, 818–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sirey J.A., Meyers B.S., Teresi J.A., Bruce M.L., Ramirez M., Raue P.J., Perlick D.A., and Holmes D. (2005). The Cornell Service Index as a measure of health service use. Psychiatr. Serv. 56, 1564–1569 [DOI] [PubMed] [Google Scholar]
- 52.Fava M. (2003). Diagnosis and definition of treatment-resistant depression. Biol. Psychiatry. 53, 649–659 [DOI] [PubMed] [Google Scholar]
- 53.Armento M.E., and Hopko D.R. (2007). The Environmental Reward Observation Scale (EROS): development, validity, and reliability. Behav. Ther. 38, 107–119 [DOI] [PubMed] [Google Scholar]
- 54.Hollon S., and Kendall P. (1980). Cognitive self-statements in depression: development of an automatic thoughts questionnaire. Cogn. Therapy Res. 4, 383–395 [Google Scholar]
- 55.Weissman M. (1979). Dysfunctional attitude scale: a validation study [Ph.D. dissertation]. University of Pennsylvania, Philadelphia [Google Scholar]
- 56.Hamilton M.A. (1960). A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 12, 56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Derogatis L.R., Lipman R.S., and Covi L. (1973). SCL-90: an outpatient psychiatric rating scale—preliminary report. Psychopharmacol. Bull. 9, 13–28 [PubMed] [Google Scholar]
- 58.Bagby R.M., Ryder A.G., Schuller D.R., and Marshall M.B. (2004). The Hamilton Depression Rating Scale: has the gold standard become a lead weight? Am. J. Psychiatry 161, 2163–2177 [DOI] [PubMed] [Google Scholar]
- 59.Bombardier C.H., Bell K.R., Temkin N.R., Fann J.R., Hoffman J., and Dikmen S. (2009). The efficacy of a scheduled telephone intervention for ameliorating depressive symptoms during the first year after traumatic brain injury. J. Head Trauma Rehabil. 24, 230–238 [DOI] [PubMed] [Google Scholar]
- 60.Ashman T.A., Cantor J.B., Gordon W.A., Spielman L., Flanagan S., Ginsberg A., Engmann C., Egan M., Ambrose F., and Greenwald B. (2009). A randomized controlled trial of sertraline for the treatment of depression in persons with traumatic brain injury. Arch. Phys. Med. Rehabil. 90, 733–740 [DOI] [PubMed] [Google Scholar]
- 61.Williams J.B. (1988). A structured interview guide for the Hamilton Depression Rating Scale. Arch. Gen. Psychiatry 45, 742–747 [DOI] [PubMed] [Google Scholar]
- 62.Guy W. (1976). ECDEU: Assessment Manual for Psychopharmacology. Government Printing Office: Washington, DC [Google Scholar]
- 63.Katon W., Von Korff, M., Lin E., Walker E., Simon G.E., Bush T., Robinson P., and Russo J. (1995). Collaborative management to achieve treatment guidelines. Impact on depression in primary care. JAMA 273, 1026–1031 [PubMed] [Google Scholar]
- 64.Horvath A.O., and Greenberg L.S. (1994). The Working Alliance: Theory Research and Practice. John Wiley & Sons: New York [Google Scholar]
- 65.Ware J.E., Jr., and Sherbourne C.D. (1992). The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 30, 473–483 [PubMed] [Google Scholar]
- 66.Sheehan D.V., Harnett–Sheehan K., and Raj B.A. (1996). The measurement of disability. Int. Clin. Psychopharmacol. 11, Suppl. 3, 89–95 [DOI] [PubMed] [Google Scholar]
- 67.McLean A., Jr., Dikmen S.S., and Temkin N.R. (1993). Psychosocial recovery after head injury. Arch. Phys. Med. Rehabil. 74, 1041–1046 [DOI] [PubMed] [Google Scholar]
- 68.Simon G.E., Ludman E.J., Tutty S., Operskalski B., and Von Korff M. (2004). Telephone psychotherapy and telephone care management for primary care patients starting antidepressant treatment: a randomized controlled trial. JAMA 292, 935–942 [DOI] [PubMed] [Google Scholar]
- 69.Ludman E.J., Simon G.E., Tutty S., and Von Korff M. (2007). A randomized trial of telephone psychotherapy and pharmacotherapy for depression: continuation and durability of effects. J. Consult. Clin. Psychol. 75, 257–266 [DOI] [PubMed] [Google Scholar]
- 70.Tutty S., Spangler D.L., Poppleton L.E., Ludman E.J., and Simon G.E. (2010). Evaluating the effectiveness of cognitive-behavioral teletherapy in depressed adults. Behav. Ther. 41, 229–236 [DOI] [PubMed] [Google Scholar]
- 71.Lerner D., Adler D., Hermann R.C., Chang H., Ludman E.J., Greenhill A., Perch K., McPeck W.C., and Rogers W.H. (2012). Impact of a work-focused intervention on the productivity and symptoms of employees with depression. J. Occup. Environ. Med. 54, 128–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Furukawa T.A., Horikoshi M., Kawakami N., Kadota M., Sasaki M., Sekiya Y., Hosogoshi H., Kashimura M., Asano K., Terashima H., Iwasa K., Nagasaku M., and Grothaus L.C. (2012). Telephone cognitive-behavioral therapy for subthreshold depression and presenteeism in workplace: a randomized controlled trial. PLoS One 7,e35330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Linde J.A., Simon G.E., Ludman E.J., Ichikawa L.E., Operskalski B.H., Arterburn D., Rohde P., Finch E.A., and Jeffery R.W. (2011). A randomized controlled trial of behavioral weight loss treatment versus combined weight loss/depression treatment among women with comorbid obesity and depression. Ann. Behav. Med. 41, 119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dwight–Johnson M., Aisenberg E., Golinelli D., Hong S., O'Brien M., and Ludman E. (2011). Telephone-based cognitive-behavioral therapy for Latino patients living in rural areas: a randomized pilot study. Psychiatr. Serv. 62, 936–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bell K.R., Temkin N.R., Esselman P.C., Doctor J.N., Bombardier C.H., Fraser R.T., Hoffman J.M., Powell J.M., and Dikmen S. (2005). The effect of a scheduled telephone intervention on outcome after moderate to severe traumatic brain injury: a randomized trial. Arch. Phys. Med. Rehabil. 86, 851–856 [DOI] [PubMed] [Google Scholar]
- 76.Bell K.R., Hoffman J.M., Doctor J.N., Powell J.M., Esselman P., Bombardier C., Fraser R., and Dikmen S. (2004). Development of a telephone follow-up program for individuals following traumatic brain injury. J. Head Trauma Rehabil. 19, 502–512 [DOI] [PubMed] [Google Scholar]
- 77.Burke B.L., Arkowitz H., and Menchola M. (2003). The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J. Consult. Clin. Psychol. 71, 843–861 [DOI] [PubMed] [Google Scholar]
- 78.Zimmerman M., Martinez J.H., Young D., Chelminski I., and Dalrymple K. (2013). Severity classification on the Hamilton Depression Rating Scale. J. Affect. Disord. 150, 384–388 [DOI] [PubMed] [Google Scholar]
- 79.Baldwin S.A., Berkeljon A., Atkins D.C., Olsen J.A., and Nielsen S.L. (2009). Rates of change in naturalistic psychotherapy: contrasting dose-effect and good-enough level models of change. J. Consult. Clin. Psychol. 77, 203–211 [DOI] [PubMed] [Google Scholar]
- 80.Osenbach J.E., O'Brien K.M., Mishkind M., and Smolenski D.J. (2013). Synchronous telehealth technologies in psychotherapy for depression: a meta-analysis. Depress. Anxiety 30, 1058–1067 [DOI] [PubMed] [Google Scholar]
- 81.Beltman M.W., Voshaar R.C., and Speckens A.E. (2010). Cognitive-behavioural therapy for depression in people with a somatic disease: meta-analysis of randomised controlled trials. Br. J. Psychiatry 197, 11–19 [DOI] [PubMed] [Google Scholar]
- 82.Iosifescu D.V. (2007). Treating depression in the medically ill. Psychiatr. Clin. North Am. 30, 77–90 [DOI] [PubMed] [Google Scholar]
- 83.Baumeister H., Hutter N., and Bengel J. (2011). Psychological and pharmacological interventions for depression in patients with coronary artery disease. Cochrane Database Syst. Rev. 9,CD008012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Baumeister H., Hutter N., and Bengel J. (2012). Psychological and pharmacological interventions for depression in patients with diabetes mellitus and depression. Cochrane Database Syst. Rev. 12,CD008381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Montgomery S.A. (1994). Clinically relevant effect sizes in depression. Eur. Neuropsychopharmacol. 4, 283–284 [Google Scholar]
- 86.Furukawa T.A., Akechi T., Azuma H., Okuyama T., and Higuchi T. (2007). Evidence-based guidelines for interpretation of the Hamilton Rating Scale for Depression. J. Clin. Psychopharmacol. 27, 531–534 [DOI] [PubMed] [Google Scholar]
- 87.Lowe B., Unutzer J., Callahan C.M., Perkins A.J., and Kroenke K. (2004). Monitoring depression treatment outcomes with the patient health questionnaire-9. Med. Care 42, 1194–1201 [DOI] [PubMed] [Google Scholar]
- 88.Jorge R.E., Robinson R.G., and Arndt S. (1993). Are there symptoms that are specific for depressed mood in patients with traumatic brain injury? J. Nerv. Ment. Dis. 181, 91–99 [DOI] [PubMed] [Google Scholar]
- 89.Kim E., Lauterbach E.C., Reeve A., Arciniegas D.B., Coburn K.L., Mendez M.F., Rummans T.A., and Coffey E.C. (2007). Neuropsychiatric complications of traumatic brain injury: a critical review of the literature (a report by the ANPA Committee on Research). J. Neuropsychiatry Clin. Neurosci. 19, 106–127 [DOI] [PubMed] [Google Scholar]
- 90.Cohen R.M., Greenberg J.M., and IsHak W.W. (2013). Incorporating multidimensional patient-reported outcomes of symptom severity, functioning, and quality of life in the Individual Burden of Illness Index for Depression to measure treatment impact and recovery in MDD. JAMA Psychiatry 70, 343–350 [DOI] [PubMed] [Google Scholar]
- 91.Miranda J., Duan N., Sherbourne C., Schoenbaum M., Lagomasino I., Jackson–Triche M., and Wells K.B. (2003). Improving care for minorities: can quality improvement interventions improve care and outcomes for depressed minorities? Results of a randomized, controlled trial. Health Serv. Res. 38, 613–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smith J.L., Rost K.M., Nutting P.A., and Elliott C.E. (2001). Resolving disparities in antidepressant treatment and quality-of-life outcomes between uninsured and insured primary care patients with depression. Med. Care 39, 910–922 [DOI] [PubMed] [Google Scholar]
- 93.Hart T., Fann J.R., and Novack T.A. (2008). The dilemma of the control condition in experience-based cognitive and behavioural treatment research. Neuropsychol. Rehabil. 18, 1–21 [DOI] [PubMed] [Google Scholar]
- 94.Busseri M.A., and Tyler J.D. (2003). Interchangeability of the Working Alliance Inventory and Working Alliance Inventory, Short Form. Psychol. Assess. 15, 193–197 [DOI] [PubMed] [Google Scholar]
- 95.Pagulayan K.F., Hoffman J.M., Temkin N.R., Machamer J.E., and Dikmen S.S. (2008). Functional limitations and depression after traumatic brain injury: examination of the temporal relationship. Arch. Phys. Med. Rehabil. 89, 1887–1892 [DOI] [PubMed] [Google Scholar]
- 96.Dimidjian S., Barrera M., Jr., Martell C., Munoz R.F., and Lewinsohn P.M. (2011). The origins and current status of behavioral activation treatments for depression. Annu. Rev. Clin. Psychol. 7, 1–38 [DOI] [PubMed] [Google Scholar]
- 97.Schonberger M., Ponsford J., Gould K.R., and Johnston L. (2011). The temporal relationship between depression, axnxiety, and functional status after traumatic brain injury: a cross-lagged analysis. J. Int. Neuropsychol. Soc. 17, 781–787 [DOI] [PubMed] [Google Scholar]
- 98.Wallace M.L., Frank E., and Kraemer H.C. (2013). A novel approach for developing and interpreting treatment moderator profiles in randomized clinical trials. JAMA Psychiatry 70, 1241–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McGrath C.L., Kelley M.E., Holtzheimer P.E., Dunlop B.W., Craighead W.E., Franco A.R., Craddock R.C., and Mayberg H.S. (2013). Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry 70, 821–829 [DOI] [PMC free article] [PubMed] [Google Scholar]