Abstract
Choline acetyltransferase (EC 2.3.1.6) isolated from the head ganglia of squid could be purified by use of mercurial-Sepharose columns as well as Sepharose columns to which the enzyme inhibitor p-(m-bromophenyl)vinyl pyridinium had been attached. These columns, in conjunction with 30-55% ammonium sulfate precipitation, 40-30% ammonium sulfate extraction, chromatography on sulfopropyl-Sephadex and on cellulose phosphate and hydroxylapatite columns, led to the isolation of three factions of choline acetyltransferase ranging in activity from 1000 to 4000 μmole/mg of protein/per hr. Polyacrylamide gel electrophoresis suggests that two of these fractions are homogeneous. The squid choline acetyltransferase is different from the mammalian-brain enzymes in having a larger molecular weight under the conditions used and in being relatively poorly inhibited by styryl pyridinium compounds.
Keywords: (p-(m-bromophenyl)vinyl pyridinium, mercurial-Sepharose columns
Full text
PDF
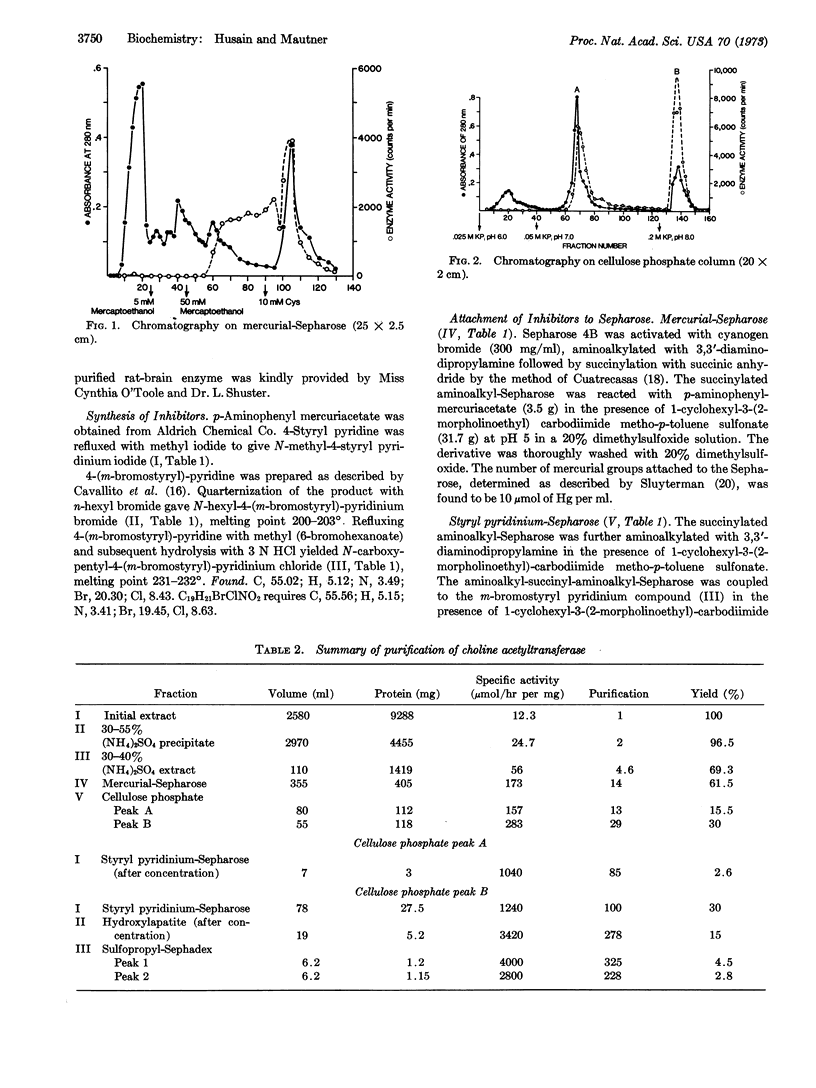

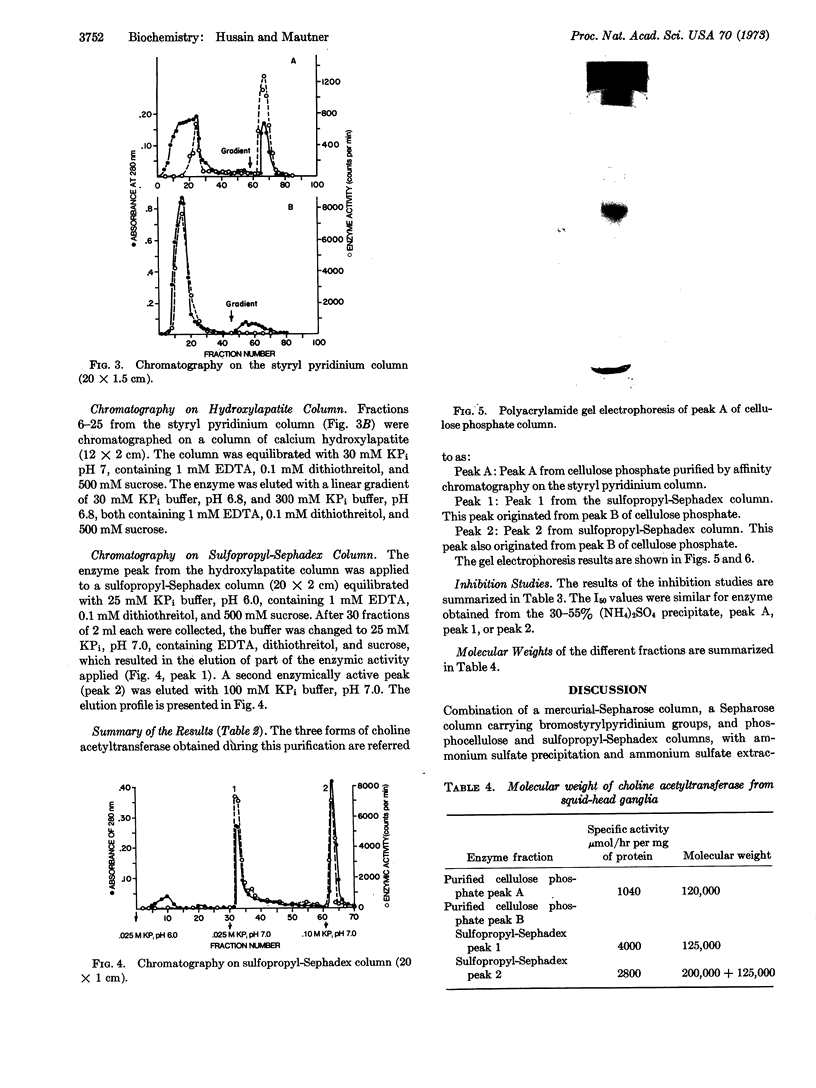

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of molecular size and molecular weights of biological compounds by gel filtration. Methods Biochem Anal. 1970;18:1–53. [PubMed] [Google Scholar]
- Cavallito C. J., Yun H. S., Smith J. C., Foldes F. F. Choline acetyltransferase inhibitors. Configurational and electronic features of styrylpyridine analogs. J Med Chem. 1969 Jan;12(1):134–138. doi: 10.1021/jm00301a034. [DOI] [PubMed] [Google Scholar]
- Chao L. P., Wolfgram F. Purification and some properties of choline acetyltransferase (EC 2.3.1.6) from bovine brain. J Neurochem. 1973 Apr;20(4):1075–1081. doi: 10.1111/j.1471-4159.1973.tb00078.x. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Affinity chromatography. Annu Rev Biochem. 1971;40:259–278. doi: 10.1146/annurev.bi.40.070171.001355. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dudai Y., Silman I., Shinitzky M., Blumberg S. Purification by affinity chromatography of the molecular forms of acetylcholinesterase present in fresh electric-organ tissue of electric eel. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2400–2403. doi: 10.1073/pnas.69.9.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover V. A., Potter L. T. Purification and properties of choline acetyltransferase from ox brain striate nuclei. J Neurochem. 1971 Apr;18(4):571–580. doi: 10.1111/j.1471-4159.1971.tb11987.x. [DOI] [PubMed] [Google Scholar]
- Hebb C. Biosynthesis of acetylcholine in nervous tissue. Physiol Rev. 1972 Oct;52(4):918–957. doi: 10.1152/physrev.1972.52.4.918. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leuzinger W., Baker A. L., Cauvin E. Acetylcholinesterase. II. Crystallization, absorption spectra, isoionic point. Proc Natl Acad Sci U S A. 1968 Feb;59(2):620–623. doi: 10.1073/pnas.59.2.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malthe-Sorenssen D., Fonnum F. Multiple forms of choline acetyltransferase in several species demonstrated by isoelectric focusing. Biochem J. 1972 Mar;127(1):229–236. doi: 10.1042/bj1270229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoulié J., Rieger F., Tsuji S. Solubilisation de l'acetylcholinestérase des organes électriques de gymnote. Action de la trypsine. Eur J Biochem. 1970 Jul;14(3):430–439. doi: 10.1111/j.1432-1033.1970.tb00307.x. [DOI] [PubMed] [Google Scholar]
- Morris D., Maneckjee A., Hebb C. The kinetic properties of human placental choline acetyltransferase. Biochem J. 1971 Dec;125(3):857–863. doi: 10.1042/bj1250857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NACHMANSOHN D. Metabolism and function of the nerve cell. Harvey Lect. 1953;49:57–99. [PubMed] [Google Scholar]
- NACHMANSOHN D., WILSON I. B. The enzymic hydrolysis and synthesis of acetylcholine. Adv Enzymol Relat Subj Biochem. 1951;12:259–339. doi: 10.1002/9780470122570.ch5. [DOI] [PubMed] [Google Scholar]
- Potter L. T., Glover V. A., Saelens J. K. Choline acetyltransferase from rat brain. J Biol Chem. 1968 Jul 25;243(14):3864–3870. [PubMed] [Google Scholar]
- Prince A. K., Hide E. G. Activation of choline acetyltransferase by salts. Nat New Biol. 1971 Sep 15;234(50):222–223. doi: 10.1038/newbio234222a0. [DOI] [PubMed] [Google Scholar]
- Prince A. K. Properties of choline acetyltransferase isolated from squid ganglia. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1117–1122. doi: 10.1073/pnas.57.4.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REISBERG R. B. Properties and biological significance of choline acetylase. Yale J Biol Med. 1957 Feb;29(4):403–435. [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Carrillo A., Allfrey V. G. A method for the purification of histone fraction F3 by affinity chromatography. Arch Biochem Biophys. 1973 Jan;154(1):185–191. doi: 10.1016/0003-9861(73)90047-7. [DOI] [PubMed] [Google Scholar]
- Sastry B. V., Henderson G. I. Kinetic mechanisms of human placental choline acetyltransferase. Biochem Pharmacol. 1972 Mar 15;21(6):787–802. doi: 10.1016/0006-2952(72)90122-0. [DOI] [PubMed] [Google Scholar]
- Schrier B. K., Shuster L. A simplified radiochemical assay for choline acetyltransferase. J Neurochem. 1967 Oct;14(10):977–985. doi: 10.1111/j.1471-4159.1967.tb09509.x. [DOI] [PubMed] [Google Scholar]
- Sluyterman L. A., Wijdenes J. An agarose mercurial column for the separation of mercaptopapain and nonmercaptopapain. Biochim Biophys Acta. 1970 Mar 31;200(3):593–595. doi: 10.1016/0005-2795(70)90122-4. [DOI] [PubMed] [Google Scholar]