Abstract
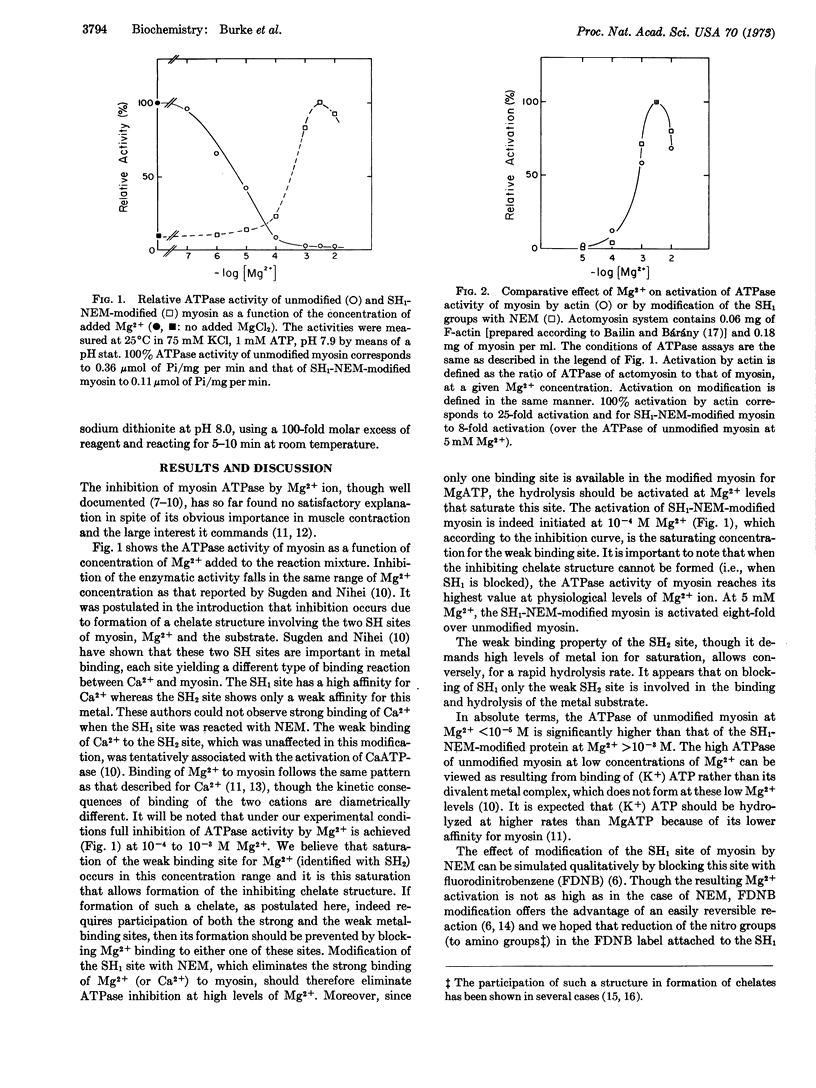
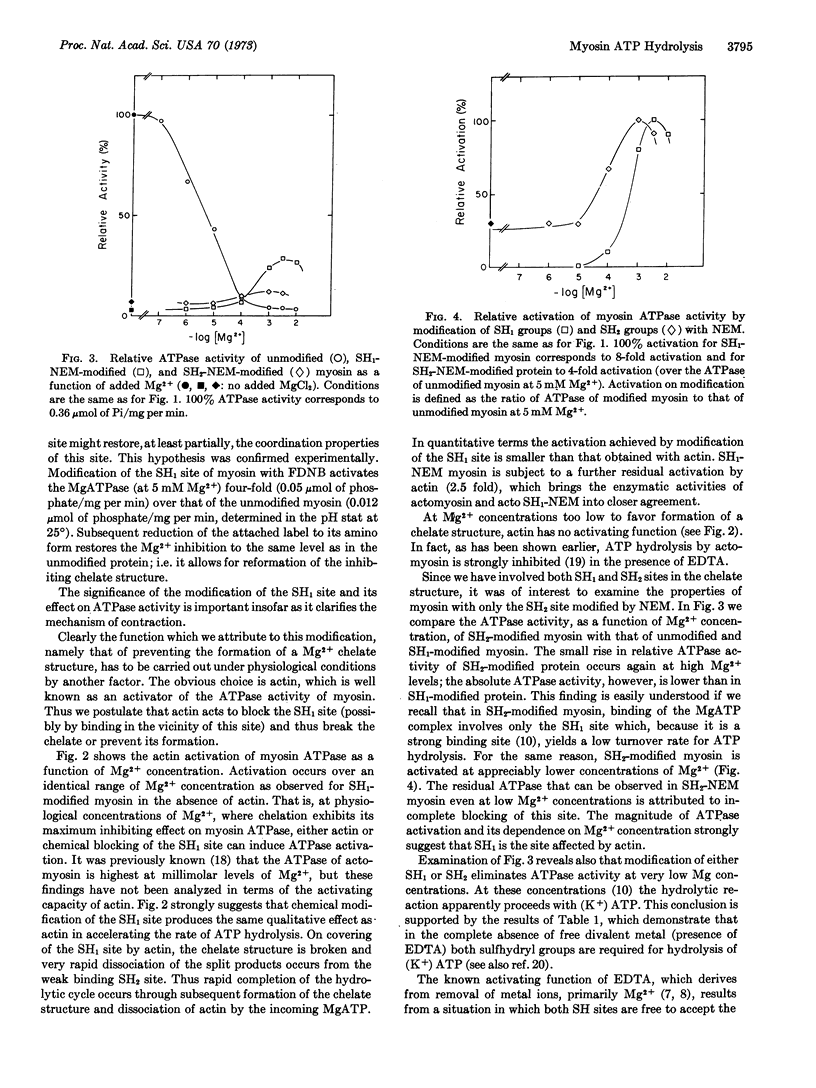
It is suggested that under physiological conditions (> 1 mM Mg2+) MgATP binds to myosin to form a chelate involving the two reactive sulfhydryl sites (SH1 and SH2). The stability of the chelate structure results in marked inhibition of the myosin ATPase in the presence of millimolar magnesium ion. The inhibitory effect of magnesium ion can be eliminated chemically by blocking either the SH1 or SH2 site since this precludes formation of the chelate. In muscle, actin apparently behaves in a similar fashion in that its interaction with myosin causes a disruption of the chelate structure.
Keywords: muscle, sulfhydryl sites, ATPase, actin
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailin G., Bárány M. A simple procedure for the preparation of tropomyosin-free F-actin. J Mechanochem Cell Motil. 1972 Dec;1(4):189–190. [PubMed] [Google Scholar]
- Bailin G., Bárány M. Thiolysis of dinitrophenylated myosin with restoration of adenosine triphosphatase activity. J Biol Chem. 1972 Dec 10;247(23):7815–7821. [PubMed] [Google Scholar]
- KIELLEY W. W., BRADLEY L. B. The relationship between sulfhydryl groups and the activation of myosin adenosinetriphosphatase. J Biol Chem. 1956 Feb;218(2):653–659. [PubMed] [Google Scholar]
- KIELLEY W. W., KALCKAR H. M., BRADLEY L. B. The hydrolysis of purine and pyrimidine nucleoside triphosphates by myosin. J Biol Chem. 1956 Mar;219(1):95–101. [PubMed] [Google Scholar]
- Kiely B., Martonosi A. Kinetics and substrate binding of myosin adenosine triphosphatase. J Biol Chem. 1968 May 10;243(9):2273–2278. [PubMed] [Google Scholar]
- Kiely B., Martonosi A. The binding of ADP to myosin. Biochim Biophys Acta. 1969 Jan 14;172(1):158–170. doi: 10.1016/0005-2728(69)90101-7. [DOI] [PubMed] [Google Scholar]
- MUEHLRAD A., FABIAN F., BIRO N. A. ON THE ACTIVATION OF MYOSIN ATPASE BY EDTA. Biochim Biophys Acta. 1964 Jul 8;89:186–188. [PubMed] [Google Scholar]
- Malik M. N., Marchioli L., Martonosi A. The effect of divalent metal ions on the ATPase activity and ADP binding of H-meromyosin. Arch Biochem Biophys. 1972 Nov;153(1):147–154. doi: 10.1016/0003-9861(72)90430-4. [DOI] [PubMed] [Google Scholar]
- OFFER G. W. THE ANTAGONISTIC ACTION OF MAGNESIUM IONS AND ETHYLENEDIAMINETETRAACETATE ON MYOSIN A ATPASE (POTASSIUM ACTIVATED). Biochim Biophys Acta. 1964 Sep 18;89:566–569. doi: 10.1016/0926-6569(64)90090-2. [DOI] [PubMed] [Google Scholar]
- Rizzino A. A., Barouch W. W., Eisenberg E., Moos C. Actin-heavy meromyosin biding. Determination of binding stoichiometry from adenosine triphosphatase kinetic measurements. Biochemistry. 1970 Jun 9;9(12):2402–2408. doi: 10.1021/bi00814a003. [DOI] [PubMed] [Google Scholar]
- Seidel J. C. Similar effects on enzymic activity due to chemical modification of either of two sulfhydryl groups of myosin. Biochim Biophys Acta. 1969 May;180(1):216–219. doi: 10.1016/0005-2728(69)90215-1. [DOI] [PubMed] [Google Scholar]
- Seidel J. C. The effects of monovalent and divalent cations on the ATPase activity of myosin. Biochim Biophys Acta. 1969 Oct 21;189(2):162–170. doi: 10.1016/0005-2728(69)90044-9. [DOI] [PubMed] [Google Scholar]
- Shaltiel S. Thiolysis of some dinitrophenyl derivatives of amino acids. Biochem Biophys Res Commun. 1967 Oct 26;29(2):178–183. doi: 10.1016/0006-291x(67)90583-9. [DOI] [PubMed] [Google Scholar]
- Silverman R., Eisenberg E., Kielley W. W. Interaction of SH 1 -blocked HMM with actin and ATP. Nat New Biol. 1972 Dec 13;240(102):207–208. doi: 10.1038/newbio240207a0. [DOI] [PubMed] [Google Scholar]
- Sugden E. A., Nihei T. The effects of calcium and magnesium ions on the adenosine triphosphatase and inosine triphosphatase activities of myosin A. Biochem J. 1969 Aug;113(5):821–827. doi: 10.1042/bj1130821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER A. On the role of calcium in the activity of adenosine 5'-triphosphate hydrolysis by actomyosin. J Biol Chem. 1959 Oct;234:2764–2769. [PubMed] [Google Scholar]
- Yamaguchi M., Sekine T. Sulfhydryl groups involved in the active site of myosin A adenosine triphosphatase. I. Specific blocking of the SH group responsible for the inhibitory phase in "B phasic response" of the catalytic activity. J Biochem. 1966 Jan;59(1):24–33. doi: 10.1093/oxfordjournals.jbchem.a128254. [DOI] [PubMed] [Google Scholar]
- Yazawa M., Morita F., Yagi K. Role of divalent metal cations in heavy meromyosin-adenosine triphosphate complex. J Biochem. 1972 Feb;71(2):301–310. doi: 10.1093/oxfordjournals.jbchem.a129767. [DOI] [PubMed] [Google Scholar]
- Young M. The molecular basis of muscle contraction. Annu Rev Biochem. 1969;38:913–950. doi: 10.1146/annurev.bi.38.070169.004405. [DOI] [PubMed] [Google Scholar]



