Significance
Antibody (IgG) plays an important role in defense against infection and in the pathogenesis of autoimmune diseases such as systemic lupus erythematosus (SLE). Low affinity-activating fragment crystallizable gamma receptors (FcγRs) that bind IgG immune complexes (ICs) mediate many effector functions of antibody and are controlled by an inhibitory receptor, FcγRIIb. Here we show a previously unappreciated role for IC in driving an expansion of lymphatic conduits within lymph nodes. This was dependent on macrophage VEGF-A production and inhibited by FcγRIIb. Lymphangiogenesis and VEGF-A were increased in the lymph nodes of mice with arthritis and SLE and in macrophages obtained from people with a SLE-associated, defunctioning polymorphism in FCGR2B. These findings have implications for the pathogenesis and treatment of autoimmune diseases.
Keywords: Fc gamma receptors, autoimmunity, VEGF-A, lupus, lymphangiogenesis
Abstract
IgG immune complexes (ICs) are generated during immune responses to infection and self-antigen and have been implicated in the pathogenesis of autoimmune diseases such as systemic lupus erythematosus (SLE). Their role, and that of the fragment crystallizable (Fc) receptors that bind them, in driving local inflammation is not fully understood. Low affinity-activating Fcγ receptors (FcγRs) that bind immune complexes are controlled by a single inhibitory receptor, FcγRIIb (CD32b). We investigated whether FcγR cross-linking by IC might induce VEGF-A and lymph node lymphangiogenesis. Murine macrophages and dendritic cells (DCs) stimulated with ICs produced VEGF-A, and this was inhibited by coligation of FcγRIIb. Similarly, IC-induced VEGF-A production by B cells was inhibited by FcγRIIb. In vivo, IC generation resulted in VEGF-A–dependent intranodal lymphangiogenesis and increased DC number. We sought to determine the relevance of these findings to autoimmunity because elevated serum VEGF-A has been observed in patients with SLE; we found that lymphangiogenesis and VEGF-A were increased in the lymph nodes of mice with collagen-induced arthritis and SLE. In humans, a SLE-associated polymorphism (rs1050501) results in a dysfunctional FcγRIIBT232 receptor. Monocyte-derived macrophages from subjects with the FcγRIIBT/T232 genotype showed increased FcγR-mediated VEGF-A production, demonstrating a similar process is likely to occur in humans. Thus, ICs contribute to inflammation through VEGF-A–driven lymph node lymphangiogenesis, which is controlled by FcγRIIb. These findings have implications for the pathogenesis, and perhaps future treatment, of autoimmune diseases.
Antibodies are important for defense against infection but may also be pathogenic in some autoimmune diseases. Many effector functions of antibody are mediated via fragment crystallizable gamma receptors (FcγRs) that bind to the Fc portion of IgG. FcγRs may be activating (in mice FcγRI, III, and IV) or inhibitory (FcγRIIb) and are found on most cells of the immune system (1). Following stimulation with IgG-opsonized antigen, the inhibitory receptor FcγRIIb negatively regulates B-cell activation, macrophage phagocytosis and proinflammatory cytokine release, and antigen presentation by dendritic cells (DCs). Mice deficient in FcγRIIb demonstrate hyperactive immune responses and are susceptible to antibody-mediated autoimmune diseases (2). In humans, a single nucleotide polymorphism in FCGR2B (rs1050501) results in an amino acid substitution (a threonine for an isoleucine) within the transmembrane domain of the receptor. This substitution is associated with receptor dysfunction and confers susceptibility to the autoimmune disease systemic lupus erythematosus (SLE) (3–5) but may enhance protective responses against some pathogens (6, 7).
An adaptive immune response requires the anatomical colocalization of antigen or antigen-loaded antigen presenting cells (APCs), such as DCs, with rare antigen-specific B and T cells. These interactions take place within secondary lymphoid organs (spleen and lymph nodes), in which the microanatomical arrangement of immune cells and stromal cell networks optimizes the likelihood of such encounters (8). Lymphatic vessels transport antigen and DCs from peripheral tissues and provide a distribution network within lymph nodes, providing access lanes to the T-cell area (9). During tissue inflammation, there is an expansion of lymphatic vasculature (lymphangiogenesis) within draining lymph nodes (10, 11). This increases the available conduits through which antigen or DCs may travel, enhancing transit to, and distribution within, draining lymph nodes. Vascular endothelial growth factor A (VEGF-A) appears to be particularly important in mediating this process, via its receptor VEGFR2 (10–13). Lymph node-resident B cells provide an important source (10, 13) but macrophages and stromal cells can also produce VEGF-A (11, 14, 15). A variety of stimuli result in VEGF-A production, including proinflammatory cytokines such as TNF-α (16), toll-like receptor (TLR) agonists, and in B cells, B-cell receptor (BCR) cross-linking (10). We sought to determine whether FcγR cross-linking with IgG immune complexes (ICs) would stimulate VEGF-A production in lymph node immune cells, resulting in intranodal lymphangiogenesis. This would identify a novel effector function for IgG and an additional process, which might be negatively regulated by FcγRIIb. We demonstrate that ICs drive VEGF-A production by immune cells, and hence intranodal lymphangiogenesis. This is controlled by FcγRIIb in mice and also in humans, indicating that FcγRIIb could potentially limit immunoreactivity via control of lymphatics and suggests an additional therapeutic target in autoimmune disease.
Results and Discussion
IgG Immune Complex-Induced VEGF-A Production by Macrophages and DCs Is Inhibited by FcγRIIb.
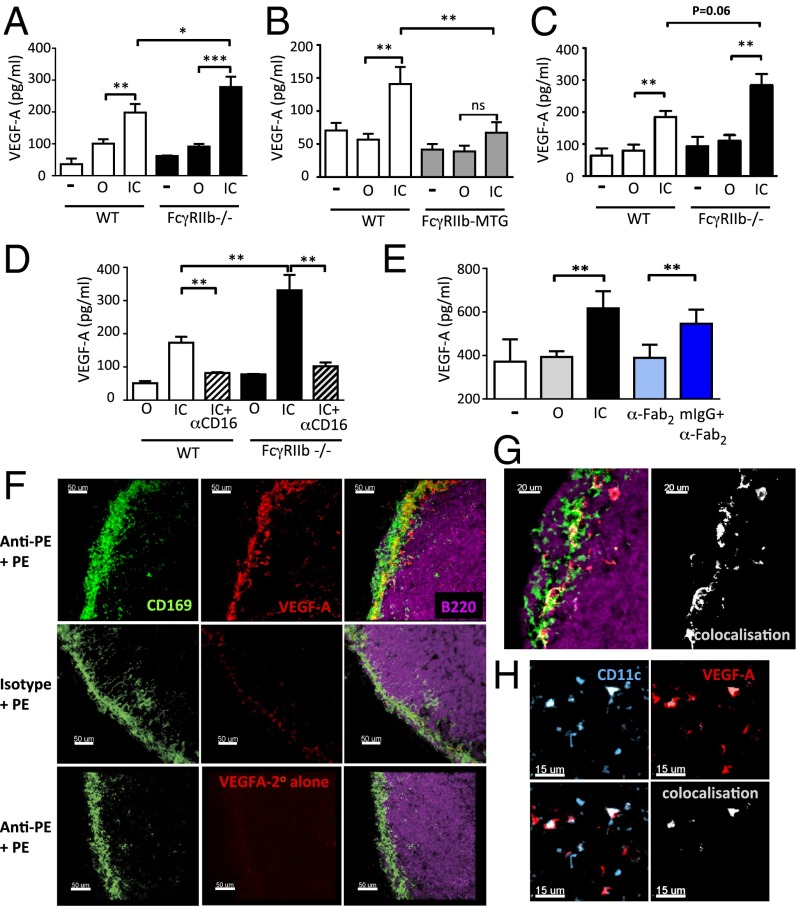
Because FcγRs mediate many effector functions of antibody, and VEGF-A is critical for driving intranodal lymphatic expansion (10, 13), we sought to determine whether FcγR cross-linking by IgG immune complexes could induce VEGF-A secretion by macrophages and DCs. Following incubation of peritoneal macrophages with immune complexes [ovalbumin opsonized with rabbit anti-OVA IgG (IC)] for 24 h, a significantly higher concentration of VEGF-A was detectable within culture supernatants compared with macrophages cultured with ovalbumin alone (O) and this was more marked in FcγRIIb-deficient macrophages (Fig. 1A). Conversely, FcγR ligation on macrophages obtained from transgenic mice with macrophage-specific overexpression of FcγRIIb (FcγRIIb-MTG) (17) resulted in significantly lower VEGF-A production compared with nontransgenic littermates (Fig. 1B). Similarly, in bone-marrow–derived DCs (BMDCs), culture with IC induced VEGF-A production, which was regulated by FcγRIIb (Fig. 1C). The time course of immune-complex–induced VEGF-A production in macrophages was investigated, demonstrating VEGF-A in culture supernatants within 6 hours and its production increased to 36 h (Fig. S1A). IC-induced VEGF-A production by mouse and human macrophages was significantly attenuated by blocking activating FcγRs (Fig. 1D and Fig. S1B). VEGF-A production was also observed in murine macrophages and DCs following stimulation with syngeneic murine IgG IC (Fig. 1E and Fig. S2 A and B) and in human monocyte-derived macrophages following stimulation with human IC (Fig. S2C).
Fig. 1.
IgG immune complex-induced VEGF-A production by macrophages and DCs is inhibited by FcγRIIb. VEGF-A concentration in culture supernatants following 24-h incubation with media alone (−), ovalbumin (O), or IgG-opsonized ovalbumin (IC) using (A) peritoneal macrophages from WT and Fcgr2b−/− mice, (B) peritoneal macrophages from nontransgenic (NTG) and FcγRIIb-MTG mice, and (C) bone-marrow–derived DCs from WT and Fcgr2b−/− mice. (D) VEGF-A concentration in culture supernatants of murine macrophages stimulated with O, OVA-IC, and isotype (IC), or OVA-IC and anti-CD16 (FcγRIII) antibody (IC+αCD16). (E) VEGF-A production by unstimulated (US) bone-marrow–derived macrophages or those stimulated with O, IC, anti-mouse F(ab′)2,(αFab2), or anti-mouse F(ab′)2/mouse IgG immune complexes (mIgG+αFab2). Graphs show the mean and SE of mean. P values calculated using a Student's t test. Graphs show representative experiments from three to five repeats. (F) Confocal micrograph of inguinal lymph node section obtained from WT mice following passive immunization with an anti-PE antibody or isotype control, followed by s.c. PE. Lymph nodes harvested at 24 h and stained for CD169 (green), VEGF-A (red), and B220 (purple). (Lower) Secondary antibody alone for VEGF-A. Images representative of three lymph node sections. (G) High power image showing extent of colocalization of CD169 and VEGF-A. (H) Confocal image of lymph node stained with CD11c antibody (blue) and VEGF-A (red). Colocalization is shown in white.
To determine if macrophages within lymph nodes produce VEGF-A in response to FcγR cross-linking, we passively transferred antiphycoerythrin (anti-PE) IgG to mice and immunized them s.c. with PE 12 hours later to generate PE-immune complexes within draining lymph nodes, as described previously (18). Subcapsular sinus (SCS) macrophages are a specialized subset of macrophages specifically positioned at the periphery of lymph nodes to filter incoming lymphatic fluid. They prevent viral dissemination (19) and capture incoming ICs (18). At 24 h following immunization, colocalization of VEGF-A staining was noted with CD169+ SCS macrophages in the draining lymph nodes of mice that received an anti-PE antibody (Fig. 1 F, Upper and G and Fig. S3 A and B) but not in animals that received isotype control antibody (Fig. 1F, Middle). VEGF-A was also detectable in CD169− cells at the edge of the B-cell follicle (Fig. 1G). Less frequently, colocalization was observed with CD11c, indicating DC production of VEGF-A (Fig. 1H and Fig. S3 C–F). Thus, immune complexes induce VEGF-A production by macrophages and DCs, both in vitro and in vivo.
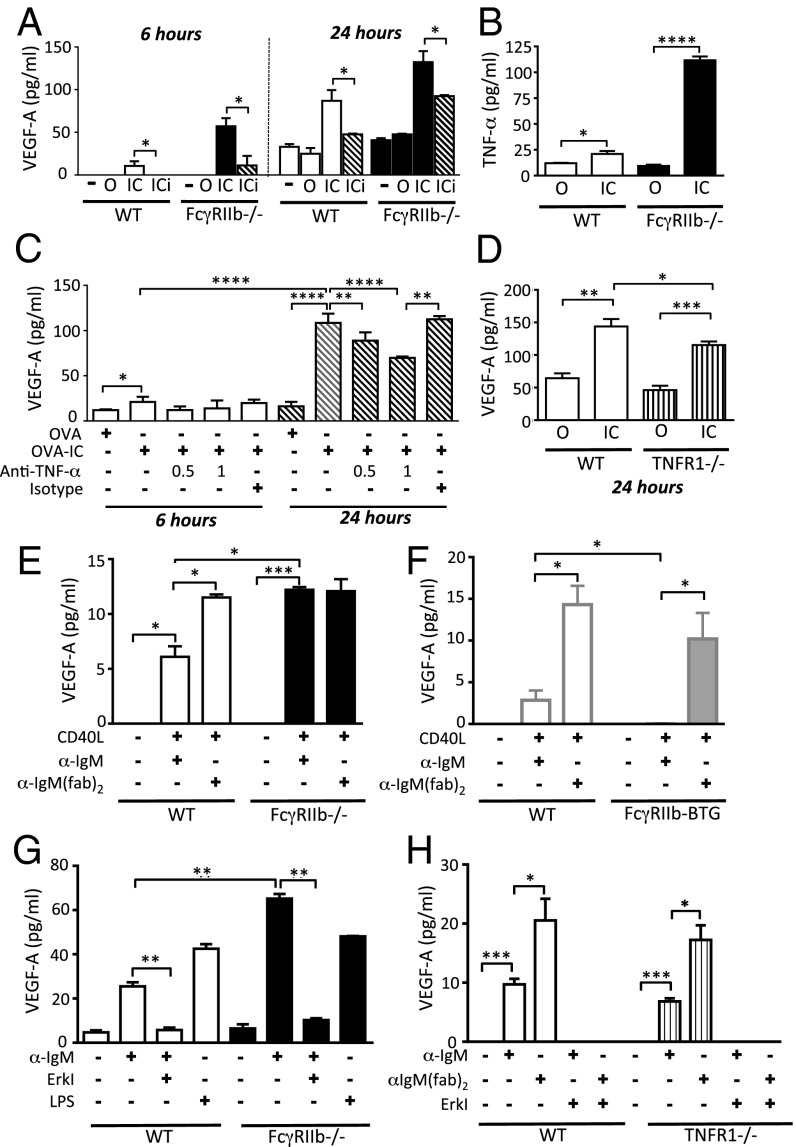
Early immune complex-induced VEGF-A production is a direct effect of FcγR signaling, but is indirectly enhanced by TNF-α at later time points. VEGF-A expression has been shown to be increased by a number of inflammatory cytokines including TNF-α. Because activating FcγR cross-linking can induce significant TNF-α production in macrophages (6), we reasoned that the FcγR-mediated VEGF-A production we had observed might be a direct result of FcγR cross-linking or might occur indirectly, through the effects of TNF-α. As noted above, VEGF-A was detectable in culture supernatants within 6 h of macrophage stimulation, in support of a direct effect by FcγR cross-linking (Fig. S1A). Furthermore, inhibition of extracellular signal-regulated kinase (Erk), a kinase located downstream of activating FcγRs (20), using the MEK1/2 inhibitor U0126 attenuated VEGF-A production to baseline at this early time point in both macrophages (Fig. 2A) and DCs (Fig. S4). Inhibition of other pathways, including TLR signaling using an IRAK1/4 inhibitor, and calcineurin blockade had no impact on IC-induced VEGF-A production (Fig. S5). At 24 h, TNF-α was detectable in macrophage culture supernatants following immune complex stimulation (Fig. 2B) and TNF-α blockade partially abrogated IC-induced VEGF-A production (Fig. 2C). Similarly, there was significantly reduced VEGF-A production by TNFR1-deficient macrophages following FcγR cross-linking at 24 h (Fig. 2D). These data suggest that VEGF-A production occurs both as a direct result of immune complex stimulation but can also be amplified by the indirect effects of the TNF-α induced by FcγR cross-linking.
Fig. 2.
IC-induced VEGF-A production abrogated by Erk inhibition. VEGF-A concentration in culture supernatants following incubation with media alone (−), ovalbumin (OVA), IgG-opsonized ovalbumin (OVA-IC), or OVA-IC with the addition of the MEK1/2 inhibitor U0126 (OVA-ICi, hatched bars) using (A) peritoneal macrophages from WT and Fcgr2b −/− mice (B) TNF-α concentration in culture supernatants following incubation of peritoneal macrophages from WT and Fcgr2b−/− mice with O or IC for 24 h. (C) VEGF-A concentration in culture supernatants following incubation of peritoneal macrophages from WT mice with OVA or OVA-IC for 6 h (white bars) and 24 h (hatched bars) with and without an anti–TNF-α blocking antibody at 0.5 or 1 μg/mL or an isotype control antibody. (D) VEGF-A concentration in culture supernatants following 24-h incubation of peritoneal macrophages from WT (white bars) and Tnfr1−/− (striped bars) mice with O or IC. VEGF-A concentration in culture supernatants harvested 48 h following BCR cross-linking [with anti-IgM or anti-IgMF(ab′)2] together with CD40L of splenic B cells obtained from (E) WT and Fcgr2b−/− mice and (F) FcγRIIb B-cell transgenic mice (FcγRIIb-BTG, gray bars) and littermate controls (NTG, white bars). (G) WT and Fcgr2b−/− B cells stimulated with LPS or IgM ± MEK1/2 inhibitor U0126 (Erki). (H) WT (white bars) and Tnfr1−/− (striped bars) B cells stimulated with IgM or anti-IgM(fab)2 ± Erki. Graphs show the mean and SE of mean. Results are representative of two to four independent experiments. P values were calculated using a Student t test. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
VEGF-A Production by B Cells Limited by Coligation of FcγRIIb.
Angeli et al. demonstrated that B cells produce VEGF-A following BCR cross-linking (10), but it is not known whether this can be controlled by coligation of FcγRIIb. We confirmed VEGF-A secretion by B cells in response to BCR cross-linking with anti-IgMF(ab′)2 together with CD40 costimulation (Fig. 2 E and F). Coligation of the BCR and FcγRIIb with anti-IgM attenuated VEGF-A production in WT B cells but not in FcγRIIb-deficient B cells (Fig. 2E). In keeping with these data, anti-IgM stimulation of B cells obtained from transgenic mice with supranormal expression of FcγRIIb on B cells (17) (FcγRIIb-BTG), resulted in the abrogation of VEGF-A secretion (Fig. 2F). Of note, the quantity of VEGF-A produced by B cells in vitro in response to immune complexes was 100-fold less than that observed in macrophages and DCs. Erk pathway inhibition reduced VEGF-A production to baseline (Fig. 2 G and H), whereas there was equivalent secretion of VEGF-A by TNFR1-deficient B cells compared with WT B cells, both following anti-IgMF(ab′)2 and anti-IgM stimulation (Fig. 3H). Thus, BCR crosslinking directly induces Erk-dependent VEGF-A production that can be inhibited by coligating FcγRIIb.
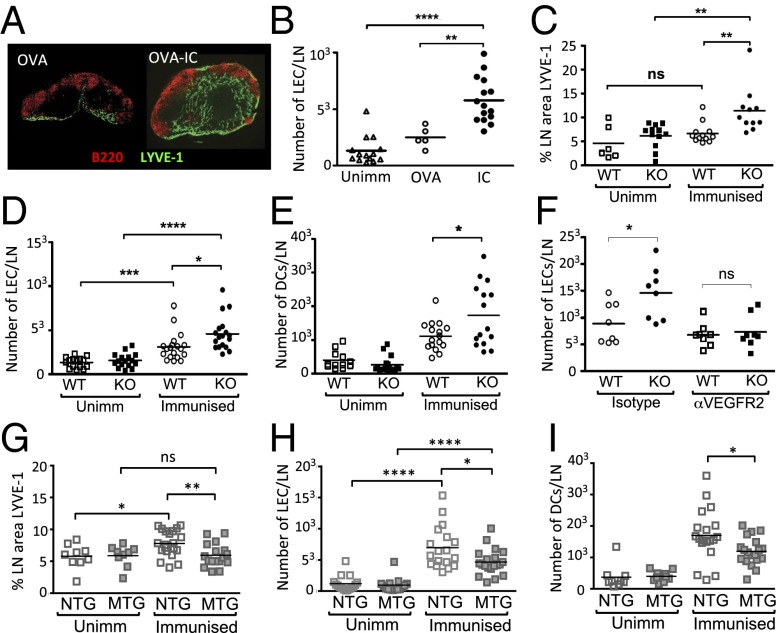
Fig. 3.
Immune complexes induce lymph node lymphangiogenesis in vivo, that is mediated via VEGFR2 and attenuated by macrophage expression of FcγRIIb. (A) Representative images of inguinal lymph nodes showing Lyve-1 positive lymphatic endothelial cells (LECs, green) and B cells (red) from mice immunized with OVA (OVA) s.c. in the flank (Left) or mice primed with OVA-Alum IP to generate anti-OVA IgG, and boosted 3 wk later with s.c. OVA (OVA-IC) (Right). (B) Quantification of inguinal lymph node LECs by flow cytometry in unimmunized mice (Unimm) or mice immunized s.c. with OVA or OVA-IC, as decribed in A. (C) Percentage of cross-sectional area of inguinal lymph node occupied by lymphatic endothelium and (D) number of LECs per lymph node and (E) number of myeloid DCs per lymph node (gating on live, CD11c positive, B220 negative cells) in unimmunized or OVA-IC immunized WT and Fcgr2b−/− (KO) mice. (F) Number of LECs per lymph node in OVA-IC immunized WT and Fcgr2b−/− (KO) mice following treatment with the VEGFR2 blocking antibody DC101 or an isotype control on the day of boost and 48 h later. (G) Percentage of cross-sectional area of inguinal lymph node occupied by lymphatic endothelium and (H) number of LECs per lymph node and (I) number of myeloid DCs per lymph node in unimmunized or OVA-IC immunized FcγRIIB macrophage transgenic (MTG) mice and littermate NTG controls. In all cases, each point represents a single lymph node. Combined results are of three independent experiments. P values were calculated using a Student t test. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. ns, nonsignificant.
Immune Complexes Induce Lymph Node Lymphangiogenesis in Vivo, Which Is Attenuated by FcγRIIb and Mediated via VEGFR2.
Given our in vitro data demonstrating IC-induced VEGF-A production in macrophages, DCs, and B cells, including those in lymph nodes, we hypothesized that ICs may stimulate intranodal lymphangiogenesis. To test this hypothesis, we immunized mice with ovalbumin in alum (OVA-Alum) intraperitoneally (leading to the production of anti-OVA IgG), followed 21 d later by a s.c. boost to generate immune-complexed antigen in immunized tissues and draining lymph nodes. Lymph nodes were harvested 3 d later and lymphatic vessels assessed both by immunohistochemistry (using an anti–Lyve-1 antibody to identify lymphatic endothelial cells, LECs) and by flow cytometry (CD45-negative, CD31/podoplanin double positive cells, as used previously to identify LECs) (11). In unimmunized mice, LECs were observed in the medulla and interlobar area of the inguinal lymph node. Following immunization and boost, an expansion of LECs was observed in draining lymph nodes, whereas only minimal lymphatic expansion occurred in the absence of primary immunization (Fig. 3 A and B). IC-induced lymphangiogenesis was particularly prominent in subcapsular sinus, the medulla, interfollicular regions, and in the cortical sinus (Fig. 3A, Right). IC-induced lymphangiogenesis was also observed in a second model in which mice passively immunized intraperitoneally with an anti-PE antibody were subsequently challenged with PE s.c. (Fig. S6A).
Given that the inhibitory receptor FcγRIIb negatively regulates VEGF-A production in vitro in macrophages, DCs, and B cells (Figs. 1 A–D and 2 A and B), we hypothesized that intranodal lymphangiogenesis would be increased in mice lacking the inhibitory receptor. At baseline, both the cross-sectional area of lymph node composed of lymphatic vessels (determined by histological analysis) and the number of LECs (determined by flow cytometry) were similar in WT and FcγRIIb-deficient mice (Fig. 3 C and D). Following OVA immunization and boost, a modest increase in lymph node lymphatics was observed in WT mice, but this was significantly greater in Fcgr2b-knockout animals (Fig. 3 C and D). Previous reports demonstrated that inflammation-associated lymphangiogenesis leads to increased DC accumulation in lymph nodes (10). Similarly, we found a significantly higher number of DCs in Fcgr2b−/− mice following immunization and boost (Fig. 3E), in keeping with the increased expansion of lymph node lymphatic vessels observed in these mice.
Inhibition of VEGF-A in Vivo Eliminates Differences in Lymphangiogenesis Between WT and FcγRIIb-Deficient Mice.
We hypothesized that IC-induced intranodal lymphangiogenesis is mediated via VEGF-A and that the heightened lymphangiogenesis observed in FcγRIIb-deficient mice occurred due to an increase in VEGF-A in these mice. To test this hypothesis, we used a VEGFR2 antibody that prevents VEGF-A binding to its receptor. Treatment of animals with a VEGFR2 blocking antibody reduced IC-induced lymph node lymphangiogenesis in WT and FcγRIIb-deficient mice, compared with those treated with isotype control antibody. This reduction was more marked in the Fcgr2b−/− mice, and indeed the number of lymphatic endothelial cells observed in anti–VEGFR2-treated Fcgr2b−/− mice was no different from that observed in WT mice (Fig. 3F).
FcγRIIb Expression on Macrophages Rather than B Cells Moderates Lymph Node Lymphangiogenesis in Vivo.
Previous reports have shown that B cells play a critical role in inflammation-induced intranodal lymphangiogenesis (10, 13). Because our data showed that FcγRIIb modulates IC-mediated BCR activation in B cells and VEGF-A production in vitro (Fig. 2), we used the FcγRIIb-BTG mice to test the role of B cells in lymphatic expansion in vivo. Following immunization and boost, less lymphatic expansion was observed in the draining lymph nodes of FcγRIIb-BTG mice compared with littermate controls (Fig. S6 B and C), but this did not reach statistical significance and there was a nonsignificant reduction in DC numbers in FcγRIIb-BTG mice (Fig. S6D). In contrast, in mice overexpressing FcγRIIb on macrophages (FcγRIIb-MTG), there was a significant reduction in lymph node lymphangiogenesis (Fig. 3 G and H) and DC number (Fig. 3I) following immunization and boost. These data suggest that FcγRIIb attenuates IC-induced intranodal lymphangiogenesis principally by its effects on macrophage activation.
Increased Lymphangiogenesis and VEGF-A in Autoimmunity.
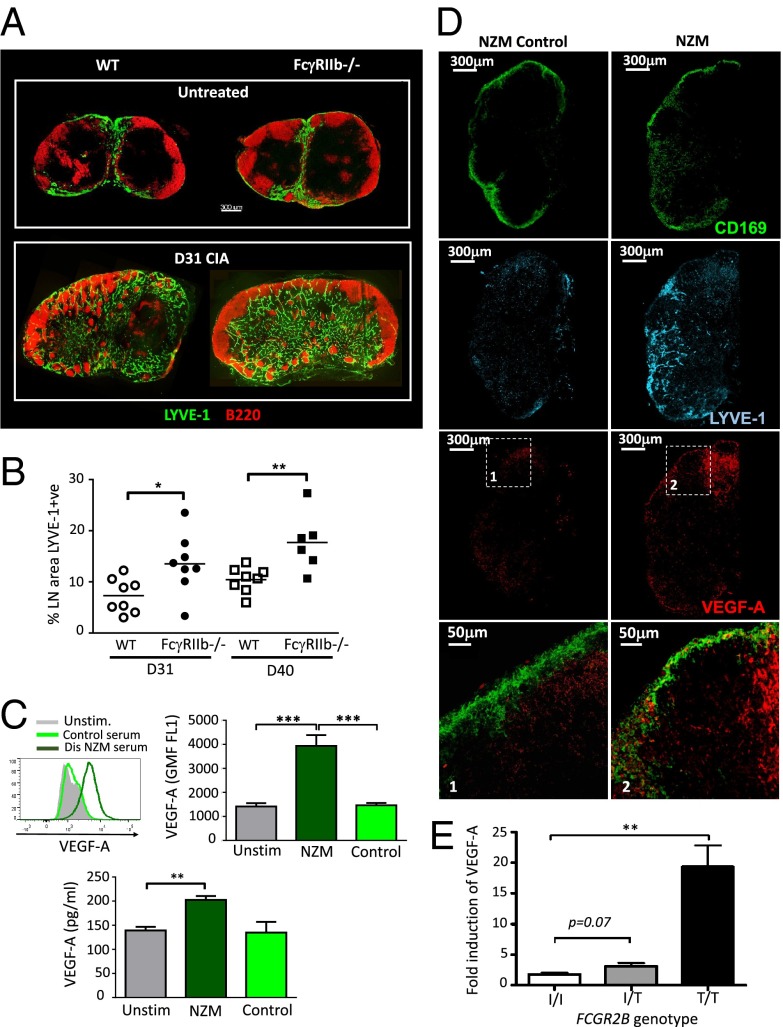
We next sought to determine whether these observations were relevant to autoimmune diseases in which autoantibodies are known to be pathogenic. Collagen-induced arthritis (CIA) is a model of inflammatory arthritis with some similarities to human rheumatoid arthritis. Autoantibodies against type II collagen play an important role in CIA pathogenesis via FcγR ligation, and deficiency of FcγRIIb on myeloid cells results in increased disease severity (21). Of note, VEGF-A inhibition ameliorates disease (22). We examined the draining lymph nodes (inguinal, brachial, and axillary) of mice with active arthritis and observed a marked expansion in LECs in mice with disease (Fig. 4A). Lymph node lymphangiogenesis was greater in Fcgr2b−/− mice compared with WT mice (Fig. 4B), and in keeping with previous studies, Fcgr2b−/− mice also had a more severe arthritis (data not shown).
Fig. 4.
Lymphangiogenesis and VEGF-A production occurs in autoimmune lymph nodes and immune complex-induced VEGF-A is increased in individuals with the lupus-associated FcγRIIBT/T232 polymorphism. (A) Representative confocal micrographs of inguinal lymph node sections obtained from WT and Fcgr2b−/− mice 31 d following induction of collagen-induced arthritis (CIA). Lymph node sections were stained for Lyve-1 (green) and B220 (red). (B) Percentage of cross-sectional area of inguinal lymph node occupied by lymphatic endothelium in WT and Fcgr2b−/− mice. Each point represents one lymph node (inguinal, axillary, or brachial) at days 31 and 40 following induction of CIA. (C) Representative flow cytometric histogram (Upper Left) and quantification (Upper Right) of intracellular VEGF-A staining of murine peritoneal macrophages following stimulation for 24 h with heat-inactivated serum obtained from NZM2410 mice with lupus nephritis (dark green) or from control mice without disease (light green). (Lower) Quantification of VEGF-A in culture supernatants obtained from the same experiments. Graphs shown are mean and SE of mean of triplicates (SEM). (D) Confocal micrograph of inguinal lymph node section obtained from NZM2410 mice with and without autoimmune diseases (lupus nephritis). Lymph node sections were stained for CD169 (green), VEGF-A (red), and LYVE-1 (cyan). High power images are shown (Lower panels 1 and 2). (E) VEGF-A concentration in culture supernatants following FcγR cross-linking in primary human monocyte-derived macrophages from individuals with differing FCGR2B genotypes (FcγRIIBI/I232, FcγRIIBI/T232, and FcγRIIBT/T232) after 48-h culture. Graphs show the mean and SEM. Combined results are of three independent experiments. All P values were calculated using a Student t test. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
SLE is a systemic autoimmune disease characterized by the presence of circulating autoantibodies, in which the deposition of ICs results in tissue inflammation. VEGF-A blockade has been used in murine models of lupus nephritis, but any effect on renal outcome was confounded by effects on podocyte integrity (23). Peritoneal macrophages stimulated with autoantibody-containing, heat-inactivated serum obtained from New Zealand mixed (NZM)2410 mice with lupus nephritis demonstrated significantly increased VEGF-A production compared with macrophages stimulated with serum obtained from healthy control mice without disease (Fig. 4C). In addition, we investigated lymphangiogenesis and VEGF-A production in lymph nodes in diseased NZM2410 mice. We observed significantly increased numbers of LYVE-1+ LECs and VEGF-A+ cells in the lymph nodes of NZM mice with lupus compared with age-matched controls without disease (Fig. 4D). Of note, VEGF-A staining was observed in CD169+ SCS macrophages in diseased NZM mice, but not in those without disease.
Immune Complex-Induced VEGF-A Production by Human Monocyte-Derived Macrophages is Increased in Individuals with the Lupus-Associated FcγRIIbT/T232 Genotype.
Elevated VEGF-A has been noted in patients with rheumatoid arthritis and SLE and correlates with disease activity (24, 25). In humans, a nonsynonymous SNP in FCGR2B (rs1050501) (isoleucine to threonine at position 232) leads to receptor dysfunction and confers susceptibility to SLE (5, 26). We hypothesized that rs1050501 would affect VEGF-A production in individuals with the SLE-associated FcγRIIBT232 receptor. To test this assertion, peripheral blood monocytes from individuals with FcγRIIBI/I232 (the wild-type receptor), FcγRIIBI/T232, and FcγRIIBT/T232 were treated with macrophage colony-stimulating factor (M-CSF) to generate macrophages and were subsequently stimulated with ICs. IC-induced VEGF-A production was significantly higher in individuals with FcγRIIBT/T232 compared with FcγRIIBI/I232 and FcγRIIBI/T232 (Fig. 4E), raising the possibility that reduced FcγRIIb function may have a similar effect on human lymph node lymphatics, potentially propagating autoimmunity. We did not assess the effect of immune complex size and glycosylation state on VEGF-A production, but these factors are known to alter FcγR binding (27) and may well impact on IC-induced VEGF-A production.
In summary, previous studies have shown that macrophages produce VEGF-A in response to innate stimuli such as TLR agonists and proinflammatory cytokines. Here we find that VEGF-A is also secreted in an adaptive immune response via engagement of FcγR by IgG ICs and that this is limited by FcγRIIb. Our study demonstrates a previously unappreciated immune-enhancing effect of IgG in increasing VEGF-A–induced intranodal lymphangiogenesis. Because FcγRIIb inhibits IC-induced VEGF-A production, it indirectly controls immune responses by limiting the available lymphatic channels in lymph nodes through which antigen and APCs travel. This may have additional implications, because intranodal LECs also provide a source of sphingosine1-phosphate (S1P), enabling lymphocyte egress from lymph nodes (28). These observations may also be relevant to vaccine design, in that FcγRIIb blockade during boost might enhance lymphangiogenesis and promote DC and antigen delivery to the relevant area of the lymph nodes. Our data also support the concept that VEGF-A inhibition may be a potential treatment target for antibody-mediated autoimmune diseases.
Materials and Methods
Mice.
FcγRIIb-deficient BALB/c and C57BL/6 mice were kindly provided by Jeff Ravetch and Silvia Bolland, The Rockefeller University, New York. FcγRIIb–B-cell and macrophage transgenic mice and nontransgenic controls were produced in-house (17). NZM2410 mice were purchased from Jax and TNFR1-deficient mice were obtained from Jean Langhorne, National Institute for Medical Research, Mill Hill, London. All procedures were conducted in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986.
Immunization.
Mice were immunized with 200 μg ovalbumin (Invitrogen) in 200 μL alum (Imject) intraperitoneally and subsequently boosted with 100 μg ovalbumin in 100 μL alum s.c. 3–4 wk later. Alternatively, mice were passively immunized intraperitoneally with antiphycoerythrin antibody (Rockland) followed by s.c. administration of 10 μg of phycoerythrin (Invitrogen Molecular Probes) as described previously (18).
Collagen-Induced Arthritis.
The protocol followed to induce CIA in C57BL/6 mice was reported previously (17).
In Vivo Inhibition of VEGF-A.
VEGF-A was inhibited using a VEGFR-2 rat monoclonal antibody, clone DC101 (ImClone Systems) (10).
Immunohistochemistry.
Immunohistochemistry methodology is described in SI Materials and Methods.
Histological Quantification of Lymphatics.
The proportion of the total lymph node cross-section occupied by LYVE-1 positive cells was quantified using Velocity Software (Perkin-Elmer). Colocalization analysis was performed using Imaris software.
Flow Cytometric Quantification of Lymphatic Endothelium.
Inguinal lymph nodes were harvested, weighed, counted, and stained. Details of the antibodies used are found in SI Materials and Methods.
Macrophage, Dendritic Cell, and B-Cell in Vitro Assays.
Peritoneal macrophages, BMDCs, and splenic B cells were harvested (details in SI Materials and Methods) and stimulated with OVA (Invitrogen) or OVA opsonized with rabbit polyclonal anti-ovalbumin antibody (Sigma). A total of 10 μM 1,4-diamino-2,3-dicyano-1,4-bis[2-aminophenylthio]butadiene (U0126; Sigma) was used to inhibit ERK. Anti-TNFα antibody (polyclonal goat anti-mouse antibody; R&D Systems) was used at 0.5 and 1 μg/mL. B cells were stimulated with goat anti-mouse IgM μ-chain–specific F(ab′)2 or intact IgG (Jackson ImmunoResearch Laboratories) at 10 μg/mL and CD40L (Peprotech), or 10 μg/mL of LPS (Salmonella typhimurium; Sigma).
Primary Human Cells.
Primary human monocyte-derived macrophages were obtained from peripheral blood obtained from healthy volunteers who had given informed consent, via the Cambridge BioResource (www.cambridgebioresource.org.uk). Macrophages were generated by culturing monocytes for 7 d with M-CSF (400 ng/mL; Peprotech).
Cytokine and VEGF-A Measurement.
VEGF-A and TNF-α concentrations in culture supernatants were measured by ELISA (Duokit; R&D Systems) according to the manufacturer’s instructions.
Statistics.
Statistical comparisons were made using GraphPad PRISM software. A two-tailed Student t test was applied, unless otherwise indicated. Results are expressed as means and SE of mean. All experiments were subject to at least three replicates per experimental parameter.
Supplementary Material
Acknowledgments
We thank Jean Langhorne for providing bone marrow from TNFR1−/− mice and Cambridge BioResource for assistance. This work was supported by a Wellcome Trust Intermediate Fellowship (WT081020) to M.R.C., a Wellcome Trust Programme Grant (083650/Z/07/Z), a Lister Prize Fellowship (to K.G.C.S.), and the National Institute for Health Research Cambridge Biomedical Research Centre.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1413915111/-/DCSupplemental.
References
- 1.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8(1):34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 2.Smith KGC, Clatworthy MR. FcgammaRIIB in autoimmunity and infection: Evolutionary and therapeutic implications. Nat Rev Immunol. 2010;10(5):328–343. doi: 10.1038/nri2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Floto RA, et al. Loss of function of a lupus-associated FcgammaRIIb polymorphism through exclusion from lipid rafts. Nat Med. 2005;11(10):1056–1058. doi: 10.1038/nm1288. [DOI] [PubMed] [Google Scholar]
- 4.Kono H, et al. FcgammaRIIB Ile232Thr transmembrane polymorphism associated with human systemic lupus erythematosus decreases affinity to lipid rafts and attenuates inhibitory effects on B cell receptor signaling. Hum Mol Genet. 2005;14(19):2881–2892. doi: 10.1093/hmg/ddi320. [DOI] [PubMed] [Google Scholar]
- 5.Willcocks LC, et al. A defunctioning polymorphism in FCGR2B is associated with protection against malaria but susceptibility to systemic lupus erythematosus. Proc Natl Acad Sci USA. 2010;107(17):7881–7885. doi: 10.1073/pnas.0915133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clatworthy MR, Smith KGC. FcgammaRIIb balances efficient pathogen clearance and the cytokine-mediated consequences of sepsis. J Exp Med. 2004;199(5):717–723. doi: 10.1084/jem.20032197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clatworthy MR, et al. Systemic lupus erythematosus-associated defects in the inhibitory receptor FcgammaRIIb reduce susceptibility to malaria. Proc Natl Acad Sci USA. 2007;104(17):7169–7174. doi: 10.1073/pnas.0608889104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bajénoff M, et al. Highways, byways and breadcrumbs: Directing lymphocyte traffic in the lymph node. Trends Immunol. 2007;28(8):346–352. doi: 10.1016/j.it.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 9.Randolph GJ, Angeli V, Swartz MA. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5(8):617–628. doi: 10.1038/nri1670. [DOI] [PubMed] [Google Scholar]
- 10.Angeli V, et al. B cell-driven lymphangiogenesis in inflamed lymph nodes enhances dendritic cell mobilization. Immunity. 2006;24(2):203–215. doi: 10.1016/j.immuni.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Halin C, Tobler NE, Vigl B, Brown LF, Detmar M. VEGF-A produced by chronically inflamed tissue induces lymphangiogenesis in draining lymph nodes. Blood. 2007;110(9):3158–3167. doi: 10.1182/blood-2007-01-066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirakawa S, et al. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201(7):1089–1099. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shrestha B, et al. B cell-derived vascular endothelial growth factor A promotes lymphangiogenesis and high endothelial venule expansion in lymph nodes. J Immunol. 2010;184(9):4819–4826. doi: 10.4049/jimmunol.0903063. [DOI] [PubMed] [Google Scholar]
- 14.Chyou S, et al. Fibroblast-type reticular stromal cells regulate the lymph node vasculature. J Immunol. 2008;181(6):3887–3896. doi: 10.4049/jimmunol.181.6.3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kataru RP, et al. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009;113(22):5650–5659. doi: 10.1182/blood-2008-09-176776. [DOI] [PubMed] [Google Scholar]
- 16.Haneda Y, et al. Leukotriene D4 enhances tumor necrosis factor-α-induced vascular endothelial growth factor production in human monocytes/macrophages. Cytokine. 2011;55(1):24–28. doi: 10.1016/j.cyto.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Brownlie RJ, et al. Distinct cell-specific control of autoimmunity and infection by FcgammaRIIb. J Exp Med. 2008;205(4):883–895. doi: 10.1084/jem.20072565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phan TG, Green JA, Gray EE, Xu Y, Cyster JG. Immune complex relay by subcapsular sinus macrophages and noncognate B cells drives antibody affinity maturation. Nat Immunol. 2009;10(7):786–793. doi: 10.1038/ni.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Junt T, et al. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450(7166):110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 20.Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. doi: 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- 21.Yilmaz-Elis AS, et al. FcγRIIb on myeloid cells rather than on B cells protects from collagen-induced arthritis. J Immunol. 2014;192(12):5540–5547. doi: 10.4049/jimmunol.1303272. [DOI] [PubMed] [Google Scholar]
- 22.Sone H, et al. Neutralization of vascular endothelial growth factor prevents collagen-induced arthritis and ameliorates established disease in mice. Biochem Biophys Res Commun. 2001;281(2):562–568. doi: 10.1006/bbrc.2001.4395. [DOI] [PubMed] [Google Scholar]
- 23.Watanabe H, et al. Anti-vascular endothelial growth factor receptor-2 antibody accelerates renal disease in the NZB/W F1 murine systemic lupus erythematosus model. Clin Cancer Res. 2005;11(1):407–409. [PubMed] [Google Scholar]
- 24.Kurosaka D, et al. Clinical significance of serum levels of vascular endothelial growth factor, angiopoietin-1, and angiopoietin-2 in patients with rheumatoid arthritis. J Rheumatol. 2010;37(6):1121–1128. doi: 10.3899/jrheum.090941. [DOI] [PubMed] [Google Scholar]
- 25.Navarro C, et al. Vascular endothelial growth factor plasma levels in patients with systemic lupus erythematosus and primary antiphospholipid syndrome. Lupus. 2002;11(1):21–24. doi: 10.1191/0961203302lu131oa. [DOI] [PubMed] [Google Scholar]
- 26.Siriboonrit U, et al. Association of Fcgamma receptor IIb and IIIb polymorphisms with susceptibility to systemic lupus erythematosus in Thais. Tissue Antigens. 2003;61(5):374–383. doi: 10.1034/j.1399-0039.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 27.Lux A, Yu X, Scanlan CN, Nimmerjahn F. Impact of immune complex size and glycosylation on IgG binding to human FcγRs. J Immunol. 2013;190(8):4315–4323. doi: 10.4049/jimmunol.1200501. [DOI] [PubMed] [Google Scholar]
- 28.Pham TH, et al. Lymphatic endothelial cell sphingosine kinase activity is required for lymphocyte egress and lymphatic patterning. J Exp Med. 2010;207(1):17–27. doi: 10.1084/jem.20091619. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.