Significance
The fin-to-limb transition and acquisition of sterna were critical steps in the evolution of tetrapods, but despite the importance of the sternum in enabling quadrupedal locomotion and avian flight, the mechanisms controlling acquisition and evolutionary adaptation of sterna are not understood. Furthermore, the mechanisms that underlie sternum development and sternal defects are not known. We describe T-box transcription factor gene Tbx5 function in sternum formation, how disruption of TBX5 causes human sternum defects, and how modulation of Tbx5 was key to acquisition and adaptation of sterna in vertebrates. We also demonstrate a quantitative correlation between sternum dimensions and forelimb use in avian species.
Keywords: sternum development, sternum adaptation, sternum defects, Tbx5
Abstract
The sternum bone lies at the ventral midline of the thorax where it provides a critical attachment for the pectoral muscles that allow the forelimbs to raise the body from the ground. Among tetrapods, sternum morphology is correlated with the mode of locomotion: Avians that fly have a ventral extension, or keel, on their sterna, which provides an increased area for flight muscle attachment. The sternum is fused with the ribs attaching on either side; however, unlike the ribs, the sternal precursors do not originate from the somites. Despite the crucial role of the sternum in tetrapod locomotion, little attention has been given to its acquisition, evolution, and embryological development. We demonstrate an essential role for the T-box transcription factor gene Tbx5 in sternum and forelimb formation and show that both structures share an embryological origin within the lateral plate mesoderm. Consistent with this shared origin and role of Tbx5, sternum defects are a characteristic feature of Holt–Oram Syndrome (OMIM 142900) caused by mutations in TBX5. We demonstrate a link between sternum size and forelimb use across avians and provide evidence that modulation of Tbx5 expression underlies the reduction in sternum and wing size in a flightless bird, the emu. We demonstrate that Tbx5 is a common node in the genetic pathways regulating forelimb and sternum development, enabling specific adaptations of these features without affecting other skeletal elements and can also explain the linked adaptation of sternum and forelimb morphology correlated with mode of locomotion.
The evolutionary transition from fins to limbs during the colonization of land was a key innovation that enabled extended radiation of the vertebrate clade. Changes in the shape and positioning of the bones of the limb and shoulder girdle during this event have been investigated extensively, but little attention has been given to the acquisition of the sternum, a feature considered characteristic of virtually all terrestrial vertebrates, and which is mandatory for tetrapod locomotion (1).
The sternum is a thin flat bone lying at the ventral midline of the thorax that provides a crucial attachment site for the pectoral muscles, allowing the forelimbs to raise the body up from the ground. The sternum forms direct connections with the clavicles and the distal tips of the ribs and, in doing so, strengthens the ribcage and helps protect internal organs such as the heart and lungs. At the caudal extremity, the xiphoid process is an attachment site for the tendons of the diaphragm. Among tetrapods, there is variation in sternal morphology and a clear link between sternum morphology and the mode of locomotion used. This correlation is demonstrated particularly well in avians, which we therefore chose for further study. For example, birds that use their forelimbs (wings) for flight have an adaptation to their sterna in the form of a large ventral extension, known as the keel, which provides an increased surface area for flight muscle attachment (2). In flightless birds, however, both the wings and sternum are reduced in size and the sternal keel is flattened.
Despite its critical role in tetrapod locomotion, the mechanisms controlling sternum development are not understood. In the mouse, the sternum is first visible as two condensing mesenchymal strips in the ventrolateral body wall at embryonic day (E) 12 (3). These bands move toward the midline and fuse together, with the ribs attaching on either side. It was originally proposed that the sternum is formed from the distal tips of the ribs, which are somite-derived, but explant experiments demonstrated that the sternal precursors instead originate from the lateral plate mesoderm (LPM) (1). The sternum is therefore unusual in that its progenitors move medially from a lateral position to their final location at the midline, where they fuse with axial tissues derived from a distinct embryological origin.
Relatively late onset sternal defects have been reported in transgenic mouse models and in humans, such as failure of sternal band fusion, which can cause the heart to bulge out from the chest (ectopia cordis) (4, 5). Alternatively, the sternum can protrude out (pectus carinatum) or be sunken (pectus excavatum) (6). The origin of these defects has been ascribed to a failure of proper fusion of the sternal bands at the midline. However, earlier steps in sternum formation have not been studied and, in particular, how the sternal bands form and from where they arise.
We demonstrate an essential role for the T-box transcription factor gene Tbx5 in sternum and forelimb formation and show that both structures share an embryological origin within the LPM. Consistent with this dual function, sternum defects are a characteristic feature of Holt–Oram Syndrome (HOS; OMIM 142900) caused by mutations in TBX5 (4–7). We demonstrate a link between sternum size and forelimb use across avians and provide evidence that modulation of Tbx5 expression underlies the reduction in sternum and wing size in a flightless bird, the emu. We demonstrate that Tbx5 is a common genetic node in the molecular pathways regulating forelimb and sternum development, enabling adaptations of these features specifically, without affecting the hindlimbs or other skeletal elements.
Results
Avian Sternum Dimensions Are Correlated with Forelimb Use.
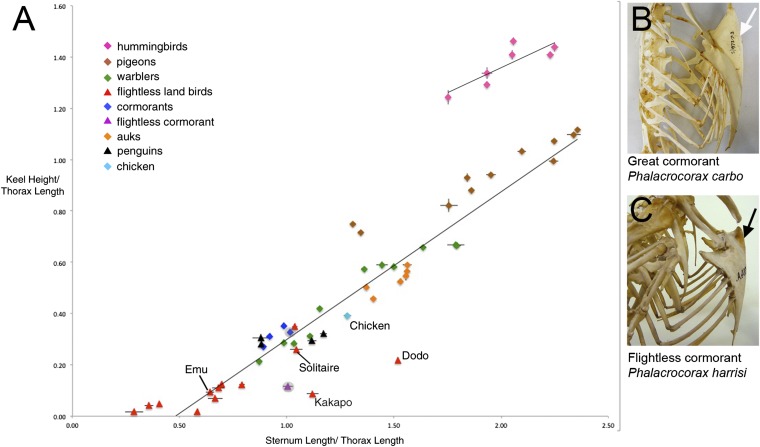
The force exerted by the forelimbs to lift the body from the ground is generated by the pectoral muscles, which attach to the sternum. Sternal morphology is correlated with the mode of locomotion, with birds providing a particularly striking example. A ventral protrusion, the sternal keel, provides an increased surface area for the attachment of large flight muscles, whereas in flightless birds, both the wings and sternum are reduced (1, 8). We quantitatively examined the relationship between forelimb use and sternum size by measuring sterna in a range of skeletons from avian species by using different forms of locomotion. Specimens from bird families were chosen on the basis of general assumptions of their flight ability. For example, hummingbirds (Trochilidae) were selected as examples of highly specialized fliers that generate high-frequency wing strokes, can hover and fly backward. At the other extreme, flightless land birds such as the Dodo (Raphus cucullatus) and moa (Dinornis sp.) were chosen. Species of warblers and pigeons were chosen as examples of birds with intermediate flight abilities. Measurements of sternum length, width, and keel height were taken and normalized for bird size. We observed a strong positive correlation between sternum length, width, and keel height with these dimensions increasing in line with our estimate of increasing flight ability. Together these results quantitatively demonstrate the link between the use of the forelimbs in locomotion and the dimensions of the sternum, and in particular the height of the keel, the sternal adaptation found in most avians. These results demonstrate a link between forelimb use and sternum dimensions (Fig. 1, Fig. S1, Table S1, and discussed in greater detail in SI Results) and suggest the two structures may share common mechanisms in their formation.
Fig. 1.
Sternum length and keel height are correlated with mode of locomotion in avians. (A) Scatterplot of measurements for sternum length and keel height, normalized for bird size by dividing by thorax length, for a range of bird groups. Each point on the graph represents one species. When possible, multiple specimens were measured per species. Error bars show SE between multiple specimen measurements. Flying species are represented as diamonds; flightless species as triangles. Thoracic skeletons of volant cormorant (Phalacrocorax carbo) with the keel indicated (white arrow) (B) and flightless cormorant (Phalacrocorax harissi) with the reduced keel indicated (black arrow) (C).
Fate Mapping the Sternum Precursor Cells.
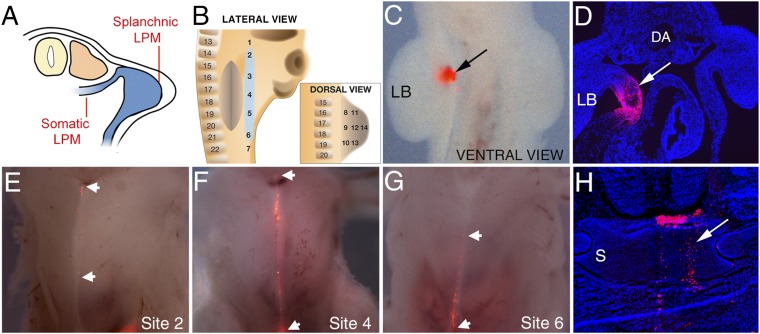
In addition to a functional link between the sternum and forelimbs, we investigated the embryological relationship underlying the formation of both these structures. Whereas the precursor cells of the forelimbs originate in the LPM (3), the origin of the sternum precursors and the path by which they reach the ventral midline has not been established. Grafting experiments have demonstrated that the sternal precursors do not reside within the somites, which led to the suggestion that they may originate in the LPM (3). We generated a fate map of the sternum precursor cells by using lineage tracer dye (DiI) to label sites in the LPM within and around the forelimb bud in Hamburger and Hamilton (HH)20 chicken embryos (Fig. 2 A and B). DiI-labeled LPM cells ventral to the limb bud at the level of somites 14–21 resulted in DiI within the sternum at stage HH36, as seen in whole mount and confirmed in section (Fig. 2 C–H and Fig. S2 A–G and J). Labeling sites rostral or caudal to this region, or within the limb bud proper, resulted in no detectable DiI at the midline, demonstrating the sternum precursor population does not extend rostral or caudal of LPM adjacent to somites 14–21 and does not extend into the limb bud itself (Fig. 2B). From this location, sternal precursors move medially to form the sternal bands that fuse at the midline by HH36 (data not shown). In a complementary approach, to confirm that no sternum precursors are present in the forelimb bud proper, we carried out homotypic grafts of entire forelimb buds from HH20 GFP-expressing transgenic embryos into wild-type hosts at the same embryological stage (9). In operated embryos at HH36, GFP-positive cells were present within the limb and pectoral muscle, but not in the sternum, demonstrating that sternal precursors are not present within the forelimb bud at HH20 (Fig. S2 H and I; n = 5).
Fig. 2.
The sternum precursor cells reside in the LPM, ventral to the forelimb bud. (A) Schematic of transverse section through HH20 chick showing LPM subdivision into somatic and splanchnic domains. (B) Schematic of DiI injection sites and adjacent somites with sternum precursor population highlighted (blue). Ventral whole-mount view (C) and transverse section of HH20 embryos showing DiI-labeling (arrows) (D) following injection into site 4. Limb bud is labeled LB; dorsal aorta is labeled DA. (E–G) Ventral whole-mount view of harvested, skinned HH36 embryos showing DiI-labeled cells at the midline (boundaries of population shown by white arrowheads) following injection into HH20 embryos at sites 2, 4, and 6, respectively. (H) Transverse section through a harvested embryo showing DiI-labeling in the sternum (arrow). S, sternum.
The Essential, Early Requirement for Tbx5 in Sternum Development.
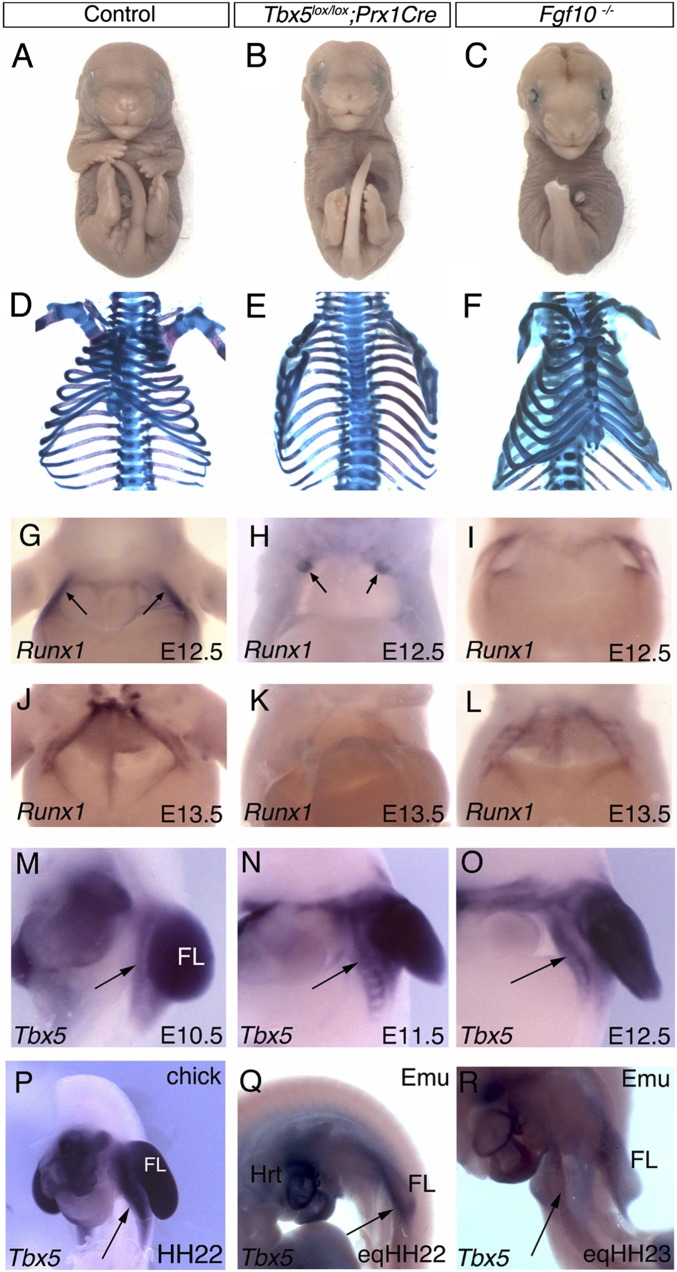
The T-box gene, Tbx5, has a restricted expression domain in the rostral LPM in amniotes (10) and is essential for the formation of the forelimbs (11). We conditionally deleted Tbx5 in this region in the mouse by using the Prx1Cre transgene, which is active in regions of the LPM including the limb buds and the sternal precursor domains (12, 13). Tbx5lox/lox;Prx1Cre mice completely lack both forelimbs and a sternum (Fig. 3 B and E). In the absence of a sternum, the ribcage fails to close and there is often associated herniation of internal organs and failure of abdominal body wall closure (Fig. 3 H and K). This striking phenotype demonstrates that Tbx5 plays an essential role in both sternum and forelimb development and reveals Tbx5 as a common, genetic link in the programs regulating forelimb and sternum formation.
Fig. 3.
The sternal bands and forelimbs fail to form in the absence of Tbx5. Ventral views of control (A and D), Tbx5 conditional mutant (Tbx5lox/lox;Prx1Cre) (B and E), and Fgf10 mutant (Fgf10−/−) (C and F) embryos at E17.5. In whole-mount (A–C) and Alcian Blue/Alizarin Red (D–F) skeletal preparations. The most distal forelimb structures have been cropped in the control image. (G–L) Ventral views of Runx1 expression in the sternal precursors (arrows) at E12.5 and E13.5 in control, Tbx5lox/lox;Prx1Cre, and Fgf10−/− mouse embryos. Herniation of the internal organs following the failure of body wall closure present in H and K. (M–O) Ventrolateral view showing Tbx5 expression in the forelimb and ventral body wall detected by in situ hybridization in E10.5, 11.5, and 12.5 mouse embryos..(P) Ventrolateral view of HH22 chick showing Tbx5 expression in the forelimb bud (FL) and LPM ventral to the limb bud (black arrow). (Q and R) Ventrolateral views of Emu embryos at eqHH22 and eqHH23, respectively, showing expression of Tbx5 in the small forelimb bud (FL) and LPM ventral to the limb bud (black arrows).
A downstream target of Tbx5 in the forelimb is Fgf10 (14, 15), but this factor is not required for sternum development because Fgf10 mutant mice (Fgf10−/−) form a normal sternum despite lacking forelimbs (Fig. 3 C and F), indicating that in its role(s) in sternum formation, Tbx5 must act through downstream targets other than Fgf10. We explored the connection between the forelimbs and the sternum further by examining Tbx5 expression in limb bud stage mouse embryos. Whole-mount in situ hybridization shows that the Tbx5 expression domain extends ventrally beyond the forelimb bud from E10.5 onwards (Fig. 3 M–O) into the sternum precursor region, as determined in the chick (Fig. 2B). In contrast, Fgf10 expression is restricted to the limb buds only and does not extend into the thorax (16), consistent with Fgf10 not acting downstream of Tbx5 in sternum development. Equivalent expression pattern was also observed in the chick (Fig. 3P).
To visualize what happens to the sternal precursors in the absence of Tbx5, we used Runx1 as a marker of the sternal bands. Runx1 has a role in the ossification of the sternum but is not required for sternum development before this step (17). Whole-mount in situ hybridization for Runx1 in control mouse embryos shows specific staining in the sternal bands on either side of the thorax at E12.5, which move closer to the midline by E13.5 (Fig. 3 G and J). In Tbx5 conditional mutant embryos (Tbx5lox/lox;Prx1Cre) the sternal bands do not form (Fig. 3 H and K). At E12.5 however, a patch of Runx1-positive cells are visible in the anterior ventral body wall (Fig. 3H; n = 4), indicating that some sternal precursors are specified. By E13.5, no Runx1-positive cells are detectable (Fig. 3K; n = 5), indicating that by this stage the sternal precursors have been either lost through cell death or no longer express Runx1.
Forelimb bud formation is completely blocked in Fgf10−/− mice, but the sternum forms normally and Runx1 expression is unaffected (Fig. 3 I and L), demonstrating that the sternum formation program can operate independently of the forelimb program despite both being Tbx5-dependent. Our genetic data demonstrate that Tbx5 is acting early in sternum development, possibly being required for sternum precursor migration to form the sternal bands.
Modulation of Tbx5 Expression Accompanies Emu Forelimb and Sternum Reduction.
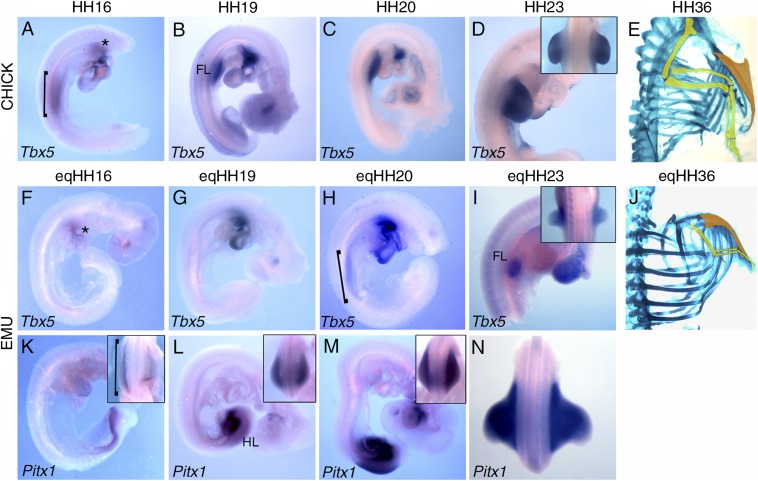
Birds that have lost the ability to fly have a smaller sternum and wings compared with flying birds (Fig. 1 and Fig. S1). The mechanisms that drove these changes over the course of evolution are unclear, but it is likely to involve adaptations in regulatory pathways that operate during embryonic development. We used the emu, Dromaius novaehollandiae, as a model flightless bird, and the chicken, Gallus gallus, as a flighted bird for comparison to investigate the genetic mechanisms that underlie the reduction in forelimb and sternum size. The forelimbs and sternum are smaller (relatively) in late stage emu embryos compared with the chick (Fig. 4 E and J). Emu forelimb budding is delayed compared with that of the hindlimb (18), in contrast to the chick where the forelimb emerges slightly ahead of the hindlimb (19). This reversal of heterochrony suggests that changes have arisen early in the emu forelimb development program, so we investigated modulation of Tbx5 expression as a candidate for driving these adaptations.
Fig. 4.
Modulation of Tbx5 expression accompanies forelimb and sternum adaptation in the emu. (A–I) In situ hybridization showing Tbx5 expression in the forelimb-forming region (bracket), forelimb (FL), and heart (*) of chick (A–D) and emu (F–I) embryos. (K–N) In situ hybridization showing Pitx1 expression in the emu hindlimb forming region (bracket) and hindlimb (HL). (E and J) Lateral view of Alcian Blue/Alizarin Red-stained chick and emu skeletons at days 10 and 27, respectively, highlighting forelimbs (yellow) and sterna (orange). (A–M) Lateral views. (N and Inset) Dorsal views.
We analyzed the expression of Tbx5 in the emu and chick compared to the expression of a marker of hindlimb initiation, Pitx1 (20). Because there is no established normal staging system for the emu, embryos were staged according to hindlimb and head morphology, matched with the equivalent chick Hamburger/Hamilton stages (19) and assigned a Hamburger/Hamilton equivalent stage (eqHH). Up to eqHH19, Tbx5 is not expressed in the emu LPM, although it is detectable in the heart (Fig. 4 F and G). At the same stages, Pitx1 expression can already be seen in the hindlimb-forming region and early hindlimb bud (Fig. 4 K and L), and in the chick, Tbx5 is clearly detectable in the forelimb-forming LPM at HH16 (Fig. 4A). Tbx5 expression is first detectable in the emu forelimb-forming LPM at eqHH20, where it spans a rostro-caudal domain of comparable size to that in a HH16 chick (Fig. 4H). By eqHH23, Tbx5 and Pitx1 are expressed in the emu forelimb and hindlimb, respectively (Fig. 4 I and N). From HH22 (and eqHH22) onwards, Tbx5 expression in both the chick and the emu expands beyond the emu forelimb and into the ventral body wall (Fig. 3 P–R), encompassing the region in which the sternal precursors reside, as demonstrated in the chick (Fig. 2B). The emu forelimb bud, however, is reduced in size, spanning only 2.5 to 3 somites compared with 6 somites when the chick forelimb bud first appears (Fig. 4I) (19). Therefore, delayed onset of Tbx5 expression, but not reduction in the size of the Tbx5 expression domain, accompanies the reduction in emu wing and sternum size compared with the chick.
Discussion
The fin-to-limb transition and acquisition of a sternum were critical steps in the evolution of tetrapods. However, despite the importance of the sternum in enabling quadrupedal locomotion and avian flight, the acquisition and adaptation of the sternum has often been overlooked. Here we demonstrate the shared embryological origins of the forelimbs and sternum and reveal a common requirement for Tbx5 activity for forelimb and sternum development. Further, we show that Tbx5 represents a common regulatory node in the molecular pathways regulating forelimb and sternum development, providing a mechanistic explanation for how these structures have adapted in concert in different tetrapod lineages.
We establish the sternum as a component of the appendicular skeleton, originating in the LPM. Other elements of the pectoral and pelvic girdles also originate in the LPM, such as the pelvis, clavicle, and a majority of the scapula (11). The sternum precursor cells differentiate within an LPM-derived connective tissue environment, placing the sternum within the abaxial patterning domain, along with the pectoral muscle and the limb (21). Acting as a brace to the axial skeleton, the sternum is situated at the lateral-somitic frontier, forming an interface with the sternal ribs, which belong to the primaxial patterning domain, having differentiated within the somitic compartment (with the exception of the first rib) (11). This boundary between the primaxial and abaxial domains is often the site of evolutionary modifications (21, 22).
Our data indicate that Tbx5 is required at the earliest stages of sternum development, because there is a failure of sternal band formation before E12.5 in Tbx5lox/lox;Prx1Cre mouse embryos and conditional deletion after E11.5 does not produce sternum defects. These observations have clinical relevance in explaining the etiology of HOS sternal defects, which are caused by mutations in TBX5. It has been suggested that TBX5 may regulate the patterning of the sternum via Connexin 40 (Cx40), and that HOS defects occur as a result of reduced Cx40 levels (23). However, Cx40 is expressed in the sternal bands from E13.5 onwards, suggesting that any alteration in Cx40 expression would arise too late to explain HOS sternal defects. Another potential downstream target of Tbx5 during sternum formation is Fgf10. In the limb, Tbx5 activates an FGF signaling feedback loop that drives limb outgrowth (13–15). We show that Fgf10 is not required for sternum formation and so it can be excluded as a candidate Tbx5 target in this process. The direct downstream target(s) of Tbx5 in sternum development remain to be identified.
We do not directly assess whether the requirement for Tbx5 in sternum development is autonomous to the sternal precursors. The use of the Prx1Cre line generates embryos lacking Tbx5 in all LPM-derived tissues, including the sternal precursors and the surrounding connective tissue. The presence of Runx1-expressing cells in Tbx5 mutant mice suggests that (at least some) sternum precursors are initially specified in the absence of Tbx5, indicating a nonautonomous role for Tbx5 in instructing or laying down a path for migrating sternal precursors. We have described a role for Tbx5 acting in the limb connective tissue to pattern muscle and tendons (24), which supports a model in which Tbx5 acts in the abaxial thoracic connective tissue during sternum development.
Despite the apparent divergence in the downstream targets of Tbx5 in sternum and forelimb development, our work suggests that modulation of Tbx5 expression allows changes to be made specifically to the forelimb and sternum developmental programs, without affecting the hindlimbs or other LPM-derived structures. Previous work has also suggested Tbx5 modulation as a mechanism to generate limb-type–specific morphological changes on an evolutionary scale (25). Experiments in chick have demonstrated that the recruitment of lateral plate mesoderm cells to initiate limb bud formation occurs during a temporal window of competence. Our results are consistent with a delay in Tbx5 expression resulting in recruitment of sternum and limb progenitors only at the latter part of this window. This delay leads to the recruitment of a smaller pool of progenitors that ultimately can only support production of a smaller sternum and smaller limb elements with missing digits. Our results also suggest that temporal modulation of Tbx5 can explain the adaptation of wing and sternum programs and, in particular, by tempering the action of Tbx5 in recruiting forelimb bud and sternal precursors, this modulation can lead to the linked reduction in limb and sternum elements.
Together, our results provide an embryological and genetic framework to understand the origins of sternum defects. Specifically in the case of HOS, our genetic deletion in the mouse and fate mapping in the chick indicate that the associated sternum abnormalities arise as a result of disrupted migration of precursors rather than being due to failure to specify this population of cells or from disruption of sternal band fusion. More broadly, our results predict that disruption in the migration of sternum precursors or the substrate they move over can be causative.
Methods
Mouse Lines.
Mouse embryos were staged according to Kaufman (26). Noon on the day a vaginal plug was observed was taken to be E0.5 of development. Tbx5lox/lox mice (27) were crossed to Tbx5lox/+; Prx1Cre (12). Fgf10−/− mice have been described (15).
Avian Embryos and Operations.
Fertilized chicken eggs (Winter’s Farm) and transgenic chicken eggs ubiquitously expressing GFP (Roslin Institute) were incubated at 38 °C and staged according to Hamburger and Hamilton (HH) (19). Fertilized emu eggs (Denbury Farm and Leicestershire Emus and Rheas) were incubated at 37.5 °C, rotating 90° twice daily. Emu eggs were windowed by using a Dremel 8000 drill.
Embryos were exposed by opening the egg and removing membranes using forceps. CM-DiI (Molecular Probes) at 2 mg/mL in 100% EtOH was diluted 1/10 in fresh 15% (wt/vol) sucrose solution. DiI solution was administered to the desired location by mouth pipetting using a pulled glass needle and 50 μL of pen/strep antibiotic (Gembio) was added.
For HH20 limb bud grafts, wild-type forelimb buds were removed in ovo by using a tungsten needle. A corresponding limb bud from a stage-matched GFP embryo was then grafted into place by using a pin made from 0.08-mm platinum wire (Goodfellow). Embryos were photographed by using an Olympus MVX10 microscope with a Hamamatsu C4742-95 camera and Openlab software.
Whole-Mount in Situ Hybridization.
Hybridization was carried out essentially as described (28). All chick and mouse probes used have been described as follows: cPitx1 (29), cTbx5 (29), mRunx1 (30), and mTbx5 (13). Emu probes were made by amplifying fragments of emu Tbx5 and Pitx1 from a limb bud stage emu embryo cDNA preparation, using primer sets designed based on avian sequence alignments of orthologous genes.
Immunofluorescence.
Detection of skeletal muscle on frozen sections was performed with mouse anti-my32 (1:800; Sigma), as described (31). Sections were stained with DAPI (1:15 000), mounted by using DAKO medium (DAKO), and photographed using a Zeiss Axioimager M1 microscope with an Axiocam MRc camera and Axiovision software.
Skeletal Preparations.
Mouse, chick, and emu skeletal preparations were stained with Alcian Blue and Alizarin Red, essentially as described (13).
Skeleton Measurements.
Avian skeletons at The Natural History Museum at Tring and the University Museum of Zoology, Cambridge, were measured by using vernier calipers. Measurements of sternum length, width, keel height, and thorax length (the distance from the first to the final thoracic vertebra) were taken. Where possible, up to four samples were measured per species and the SE calculated.
Supplementary Material
Acknowledgments
We thank Jo Cooper (Natural History Museum at Tring) and Robert Asher and Matt Lowe (Cambridge University Museum of Zoology) for making their collections of avian skeletons available and for helpful advice; Colin Meadows (Denbury Farm), Margaret Dover (Leicestershire emus and rheas), and Peter Farlie (Murdoch Children’s Research Institute) for providing ratite eggs and embryos; Marella de Bruijn and Andrew Jarratt (Weatherall Institute for Molecular Medicine) for the Runx1 clone; Sue Miller for technical help; and Anne Burke and Véronique Duboc for critical reading of the manuscript. This work was funded by Medical Research Council Grant MC_PC_13052.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1409913111/-/DCSupplemental.
References
- 1.King AS, McLelland J. Outlines of Avian Anatomy. Bailliere Tindall; London: 1975. [Google Scholar]
- 2.Chen JM. Studies on the morphogenesis of the mouse sternum. I. Normal embryonic development. J Anat. 1952;86(4):373–386. [PMC free article] [PubMed] [Google Scholar]
- 3.Seno T. The origin and evolution of the sternum. Anat Anz. 1961;110:97–101. [PubMed] [Google Scholar]
- 4.Li QY, et al. Holt-Oram syndrome is caused by mutations in TBX5, a member of the Brachyury (T) gene family. Nat Genet. 1997;15(1):21–29. doi: 10.1038/ng0197-21. [DOI] [PubMed] [Google Scholar]
- 5.Basson CT, et al. Mutations in human TBX5 [corrected] cause limb and cardiac malformation in Holt-Oram syndrome. Nat Genet. 1997;15(1):30–35. doi: 10.1038/ng0197-30. [DOI] [PubMed] [Google Scholar]
- 6.Newbury-Ecob RA, Leanage R, Raeburn JA, Young ID. Holt-Oram syndrome: A clinical genetic study. J Med Genet. 1996;33(4):300–307. doi: 10.1136/jmg.33.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelly RE., Jr Pectus excavatum: Historical background, clinical picture, preoperative evaluation and criteria for operation. Semin Pediatr Surg. 2008;17(3):181–193. doi: 10.1053/j.sempedsurg.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 8.Strickland HE, Melville AG. The Dodo and Its Kindred; or the History, Affinities and Osteology of the Dodo, Solitaire, and Other Extinct Birds of the Islands Mauritius, Rodriguez, and Bourbon. Benham and Reeve; London: 1848. [PMC free article] [PubMed] [Google Scholar]
- 9.McGrew MJ, et al. Efficient production of germline transgenic chickens using lentiviral vectors. EMBO Rep. 2004;5(7):728–733. doi: 10.1038/sj.embor.7400171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibson-Brown JJ, et al. Evidence of a role for T-box genes in the evolution of limb morphogenesis and the specification of forelimb/hindlimb identity. Mech Dev. 1996;56(1–2):93–101. doi: 10.1016/0925-4773(96)00514-x. [DOI] [PubMed] [Google Scholar]
- 11.Rallis C, et al. Tbx5 is required for forelimb bud formation and continued outgrowth. Development. 2003;130(12):2741–2751. doi: 10.1242/dev.00473. [DOI] [PubMed] [Google Scholar]
- 12.Durland JL, Sferlazzo M, Logan M, Burke AC. Visualizing the lateral somitic frontier in the Prx1Cre transgenic mouse. J Anat. 2008;212(5):590–602. doi: 10.1111/j.1469-7580.2008.00879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logan M, et al. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33(2):77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- 14.Ng JK, et al. The limb identity gene Tbx5 promotes limb initiation by interacting with Wnt2b and Fgf10. Development. 2002;129(22):5161–5170. doi: 10.1242/dev.129.22.5161. [DOI] [PubMed] [Google Scholar]
- 15.Sekine K, et al. Fgf10 is essential for limb and lung formation. Nat Genet. 1999;21(1):138–141. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- 16.Ohuchi H, et al. The mesenchymal factor, FGF10, initiates and maintains the outgrowth of the chick limb bud through interaction with FGF8, an apical ectodermal factor. Development. 1997;124(11):2235–2244. doi: 10.1242/dev.124.11.2235. [DOI] [PubMed] [Google Scholar]
- 17.Liakhovitskaia A, et al. The essential requirement for Runx1 in the development of the sternum. Dev Biol. 2010;340(2):539–546. doi: 10.1016/j.ydbio.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Nagai H, et al. Embryonic development of the emu, Dromaius novaehollandiae. Dev Dyn. 2011;240(1):162–175. doi: 10.1002/dvdy.22520. [DOI] [PubMed] [Google Scholar]
- 19.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. 1951. Dev Dyn. 1992;195(4):231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- 20.Logan M, Tabin CJ. Role of Pitx1 upstream of Tbx4 in specification of hindlimb identity. Science. 1999;283(5408):1736–1739. doi: 10.1126/science.283.5408.1736. [DOI] [PubMed] [Google Scholar]
- 21.Burke AC, Nowicki JL. A new view of patterning domains in the vertebrate mesoderm. Dev Cell. 2003;4(2):159–165. doi: 10.1016/s1534-5807(03)00033-9. [DOI] [PubMed] [Google Scholar]
- 22.Shearman RM, Burke AC. The lateral somitic frontier in ontogeny and phylogeny. J Exp Zoolog B Mol Dev Evol. 2009;312(6):603–612. doi: 10.1002/jez.b.21246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pizard A, et al. Connexin 40, a target of transcription factor Tbx5, patterns wrist, digits, and sternum. Mol Cell Biol. 2005;25(12):5073–5083. doi: 10.1128/MCB.25.12.5073-5083.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hasson P, et al. Tbx4 and tbx5 acting in connective tissue are required for limb muscle and tendon patterning. Dev Cell. 2010;18(1):148–156. doi: 10.1016/j.devcel.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duboc V, Logan MPO. Building limb morphology through integration of signalling modules. Curr Opin Genet Dev. 2009;19(5):497–503. doi: 10.1016/j.gde.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Kaufman MH. The Atlas of Mouse Development. 2nd Ed Academic; London: 1992. [Google Scholar]
- 27.Bruneau BG, et al. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell. 2001;106(6):709–721. doi: 10.1016/s0092-8674(01)00493-7. [DOI] [PubMed] [Google Scholar]
- 28.Riddle RD, Johnson RL, Laufer E, Tabin C. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell. 1993;75(7):1401–1416. doi: 10.1016/0092-8674(93)90626-2. [DOI] [PubMed] [Google Scholar]
- 29.Logan M, Simon HG, Tabin C. Differential regulation of T-box and homeobox transcription factors suggests roles in controlling chick limb-type identity. Development. 1998;125(15):2825–2835. doi: 10.1242/dev.125.15.2825. [DOI] [PubMed] [Google Scholar]
- 30.Eng SR, Lanier J, Fedtsova N, Turner EE. Coordinated regulation of gene expression by Brn3a in developing sensory ganglia. Development. 2004;131(16):3859–3870. doi: 10.1242/dev.01260. [DOI] [PubMed] [Google Scholar]
- 31.DeLaurier A, Schweitzer R, Logan M. Pitx1 determines the morphology of muscle, tendon, and bones of the hindlimb. Dev Biol. 2006;299(1):22–34. doi: 10.1016/j.ydbio.2006.06.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.