Abstract
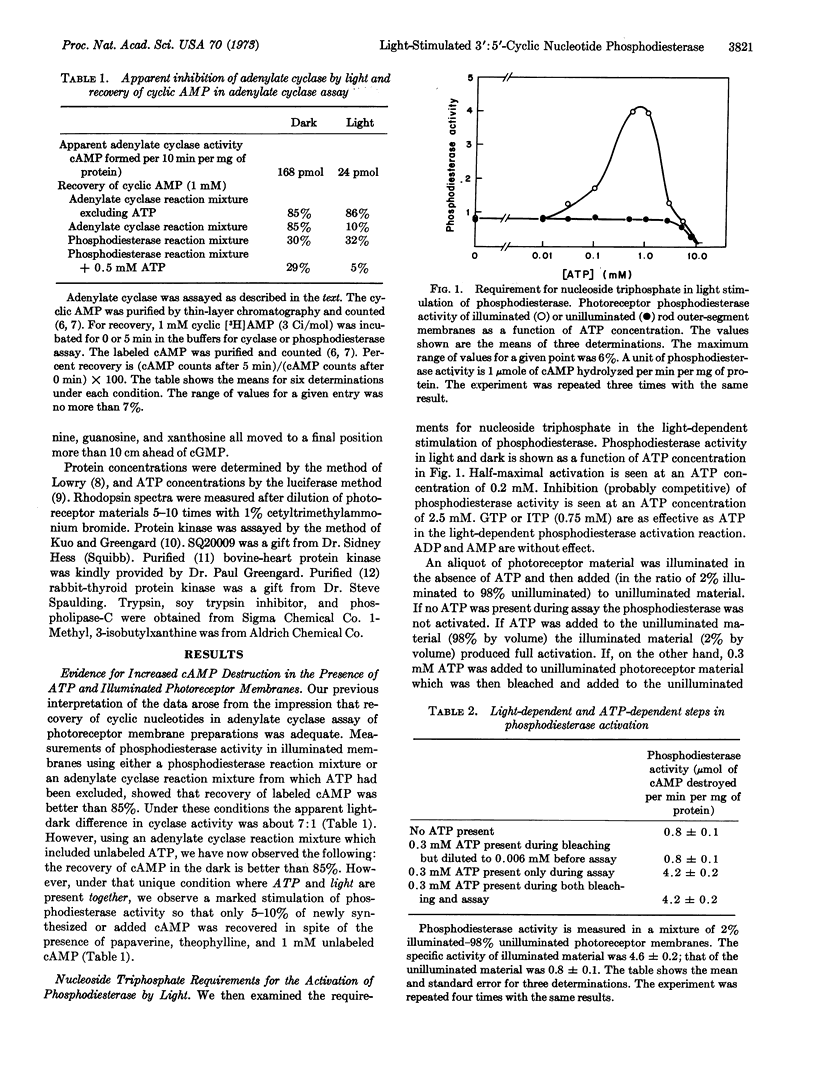
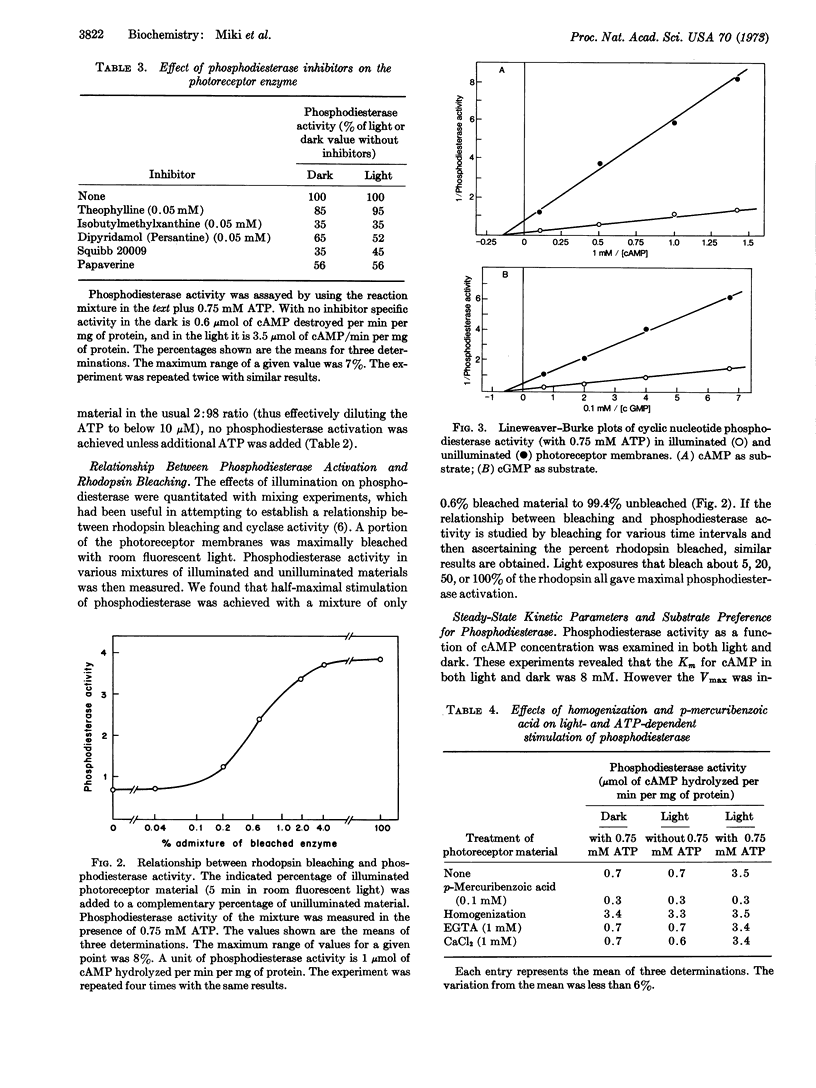
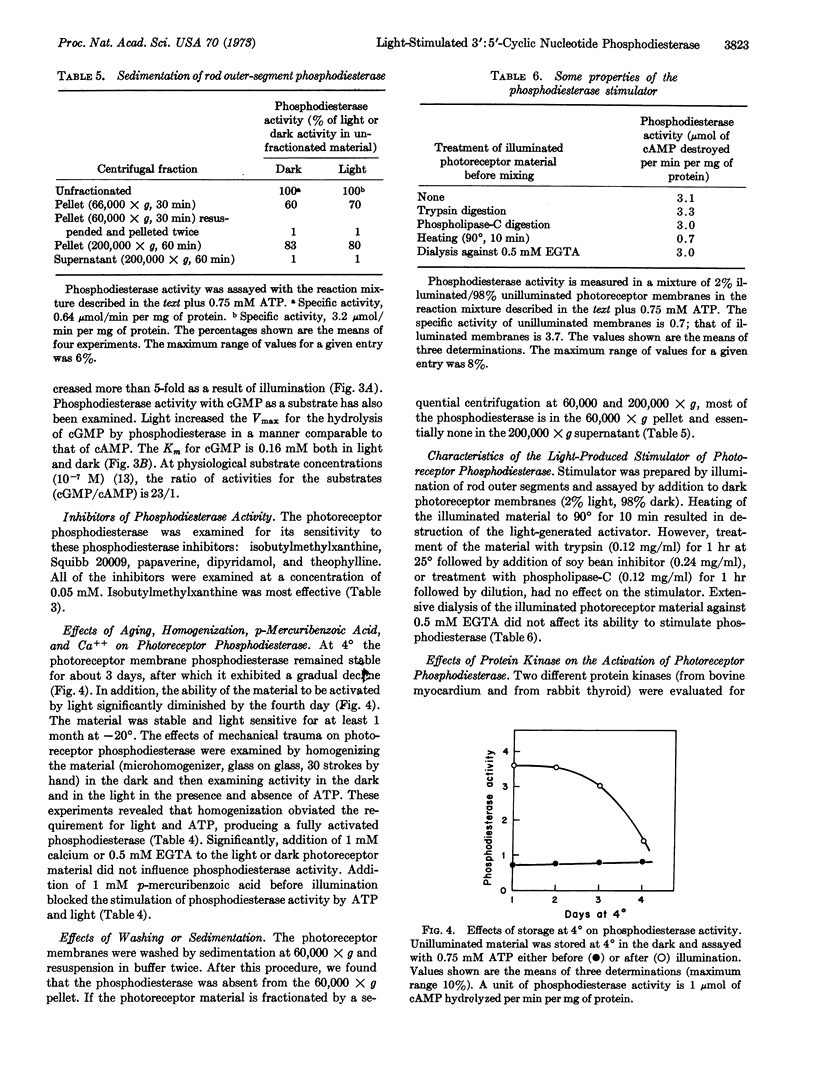
Regulation of cyclic nucleotide concentrations in rod outer segments (Rana pipiens) has been further examined. The present studies show that illumination markedly diminishes the concentration of cyclic nucleotides in suspensions of photoreceptor membranes, but the locus of regulation is cyclic nucleotide phosphodiesterase (EC 3.1.4.c) (light-stimulated) and not adenylate cyclase. There is a marked disproportionality between bleaching of rhodopsin and stimulation of phosphodiesterase. Bleaching only 0.6% of the rhodopsin produces half the stimulation produced by bleaching 100% of the rhodopsin. The process of activation of phosphodiesterase by light is in two steps, a light-dependent step followed by an ATP-dependent step. Illumination (in the absence of ATP) produces a trypsin-resistant, heat-labile, macromolecular stimulator. In the presence of 0.75 mM ATP (GTP or ITP) this stimulator produces a greater than 5-fold increases in the Vmax of photoreceptor phosphodiesterase without changing the Km. At physiological substrate concentrations (10-7 M) the rate of hydrolysis of cyclic GMP is 23 times greater than that of cyclic AMP. The light-produced stimulator appears unique to the photoreceptor membranes and does not activate phosphodiesterase in other tissues.
Keywords: cyclic AMP, cyclic GMP, adenylate cyclase, rhodopsin, retinal rods
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitensky M. W., Gorman R. E., Miller W. H. Adenyl cyclase as a link between photon capture and changes in membrane permeability of frog photoreceptors. Proc Natl Acad Sci U S A. 1971 Mar;68(3):561–562. doi: 10.1073/pnas.68.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitensky M. W., Gorman R. E., Miller W. H. Digitonin effects on photoreceptor adenylate cyclase. Science. 1972 Mar 24;175(4028):1363–1364. doi: 10.1126/science.175.4028.1363. [DOI] [PubMed] [Google Scholar]
- Bitensky M. W., Miller W. H., Gorman R. E., Neufeld A. H., Robinson R. The role of cyclic AMP in visual excitation. Adv Cyclic Nucleotide Res. 1972;1:317–335. [PubMed] [Google Scholar]
- Cheung W. Y. Cyclic 3',5'-nucleotide phosphodiesterase. Demonstration of an activator. Biochem Biophys Res Commun. 1970 Feb 6;38(3):533–538. doi: 10.1016/0006-291x(70)90747-3. [DOI] [PubMed] [Google Scholar]
- Kakiuchi S., Yamazaki R., Teshima Y. Regulation of brain phosphodiesterase activity: Ca++ plus Mg++ -dependent phosphodiesterase and its activating factor from rat brain. Adv Cyclic Nucleotide Res. 1972;1:455–477. [PubMed] [Google Scholar]
- Kreiner P. W., Keirns J. J., Bitensky M. W. A temperature-sensitive change in the energy of activation of hormone-stimulated hepatic adenylyl cyclase. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1785–1789. doi: 10.1073/pnas.70.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. VI. Isolation and partial purification of a protein kinase activated by guanosine 3',5'-monophosphate. J Biol Chem. 1970 May 25;245(10):2493–2498. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Miller W. H., Gorman R. E., Bitensky M. W. Cyclic adenosine monophosphate: function in photoreceptors. Science. 1971 Oct 15;174(4006):295–297. doi: 10.1126/science.174.4006.295. [DOI] [PubMed] [Google Scholar]
- Miyamoto E., Kuo J. F., Greengard P. Cyclic nucleotide-dependent protein kinases. 3. Purification and properties of adenosine 3',5'-monophosphate-dependent protein kinase from bovine brain. J Biol Chem. 1969 Dec 10;244(23):6395–6402. [PubMed] [Google Scholar]
- Neufeld A. H., Miller W. H., Bitensky M. W. Calcium binding to retinal rod disk membranes. Biochim Biophys Acta. 1972 Apr 14;266(1):67–71. doi: 10.1016/0005-2736(72)90120-4. [DOI] [PubMed] [Google Scholar]
- Spaulding S. W., Burrow G. N. Several adenosine 3',5'-monophosphate-dependent protein kinases in the thyroid. Endocrinology. 1972 Nov;91(5):1343–1349. doi: 10.1210/endo-91-5-1343. [DOI] [PubMed] [Google Scholar]
- Stanley P. E., Williams S. G. Use of the liquid scintillation spectrometer for determining adenosine triphosphate by the luciferase enzyme. Anal Biochem. 1969 Jun;29(3):381–392. doi: 10.1016/0003-2697(69)90323-6. [DOI] [PubMed] [Google Scholar]



