Significance
Non–image-forming opsins such as Opn4 regulate important physiological functions such as circadian photo-entrainment and affect. The recent discovery that melanopsin (Opn4) functions outside the central nervous system prompted us to explore a potential role for this receptor in blood vessel regulation. We hypothesized that Opn4-mediated signaling might explain the phenomenon of photorelaxation, for which a mechanism has remained elusive. We report the presence in blood vessels of Opn4 and demonstrate that it mediates wavelength-specific, light-dependent vascular relaxation. This photorelaxation signal transduction involves cGMP and phosphodiesterase 6, but not protein kinase G. Furthermore it is regulated by G protein-coupled receptor kinase 2 and involves vascular hyperpolarization. This receptor pathway can be harnessed for wavelength-specific light-based therapy in the treatment of diseases that involve altered vasoreactivity.
Keywords: photorelaxation, opsin, melanopsin, signal transduction, GRK2
Abstract
Melanopsin (opsin4; Opn4), a non-image-forming opsin, has been linked to a number of behavioral responses to light, including circadian photo-entrainment, light suppression of activity in nocturnal animals, and alertness in diurnal animals. We report a physiological role for Opn4 in regulating blood vessel function, particularly in the context of photorelaxation. Using PCR, we demonstrate that Opn4 (a classic G protein-coupled receptor) is expressed in blood vessels. Force-tension myography demonstrates that vessels from Opn4−/− mice fail to display photorelaxation, which is also inhibited by an Opn4-specific small-molecule inhibitor. The vasorelaxation is wavelength-specific, with a maximal response at ∼430–460 nm. Photorelaxation does not involve endothelial-, nitric oxide-, carbon monoxide-, or cytochrome p450-derived vasoactive prostanoid signaling but is associated with vascular hyperpolarization, as shown by intracellular membrane potential measurements. Signaling is both soluble guanylyl cyclase- and phosphodiesterase 6-dependent but protein kinase G-independent. β-Adrenergic receptor kinase 1 (βARK 1 or GRK2) mediates desensitization of photorelaxation, which is greatly reduced by GRK2 inhibitors. Blue light (455 nM) regulates tail artery vasoreactivity ex vivo and tail blood blood flow in vivo, supporting a potential physiological role for this signaling system. This endogenous opsin-mediated, light-activated molecular switch for vasorelaxation might be harnessed for therapy in diseases in which altered vasoreactivity is a significant pathophysiologic contributor.
Photorelaxation, the reversible relaxation of blood vessels to cold light, was initially described by Furchgott et al. in 1955 (1). Subsequent studies have attempted to define the signal transduction mechanisms responsible for this phenomenon. The process seems to be cGMP-dependent but endothelial-independent. The role of nitric oxide (NO) in photorelaxation has been controversial (2–7), with some studies showing that NOS inhibition with l-NAME not only fails to inhibit the response (2) but in some cases enhances and prolongs it (3). Moreover, several published reports examining photorelaxation demonstrate an attenuation of the response with each subsequent light stimulation. A number of investigators have proposed that NO dependence results from the photo-release of NO stores from nitrosothiols and that the endothelium and NOS are important for the repriming of these stores (stores that become depleted with each photo-stimulation); however, the source of those nitrosothiols has not as yet been clearly identified (6). Importantly, photo-release of NO occurs in the UV-A spectrum at 366 nm (4–6), a wavelength at which intravascular nitrosospecies and nitrite have the potential to release substantial quantities of NO (7). However, this wavelength is very different from that at which others have observed vascular responses. Given the controversy surrounding the photorelaxation mechanism, we postulated an entirely new mechanism: that photorelaxation is mediated by transduction through photosensitive receptors in blood vessels. These photoreceptors are part of the family of non-image-forming (NIF) opsins. We now report a signaling cascade mediating photorelaxation via Opn4, cGMP, and phosphodiesterase 6 (PDE6) that is regulated by G protein-coupled receptor kinase 2 (GRK2).
Methods
A complete description of methods is provided in SI Methods.
Vasoreactivity.
Photorelaxation of blood vessels was assessed via force-tension myography. Aorta was mounted on a myograph using a microscope, bathed in physiological Krebs buffer, and preconstricted. Light was delivered via cold light lamp (40,000–200,000 lux), light diodes [red (620–750 nm), green (495–570 nm) or blue (380–495 nm)], or a monochromator with varying wavelength.
Laser Doppler Flowmetry.
Anesthetized mice were secured in the supine position. A laser-Doppler flow probe was placed on the proximal ventral surface of the tail. The position of the fiber-optic probe was adjusted to obtain a stable flux measurement and was secured in place with glue. Relative changes in red blood cell flux were monitored with a Perimed PeriFlux System 5000 laser-Doppler Flowmeter.
RT-PCR.
To demonstrate the expression of Opn4, GRK2, PDE5A, and PDE6G, RT-PCR was performed using mRNA isolated from mouse aortas.
Adenovirus Encoding shRNA.
GRK2 was knocked down in aortic rings with shRNA. Ad-sh-Nontargeted and Ad-shGRK2 encoded viruses were generated using a pAdBLOCK-iT kit (Life Sciences).
Western Blot Analysis.
Aorta lysates were resolved by 10% (vol/vol) SDS/PAGE buffer, transferred, probed with antibodies, visualized with peroxidase, and enhanced with chemiluminescence system (Pierce).
Membrane Potential Measurements.
Endothelium-denuded segments of murine thoracic aorta were pinned in place, lumen side down, in a recoding chamber. Intracellular recordings were performed in current clamp (3.0–4.0 kHz sampling rate) mode and recorded on a chart recorder (TA240; Gould Instrument Systems).
Results
Opsin 4 Expression in Blood Vessels Mediates Photorelaxation.
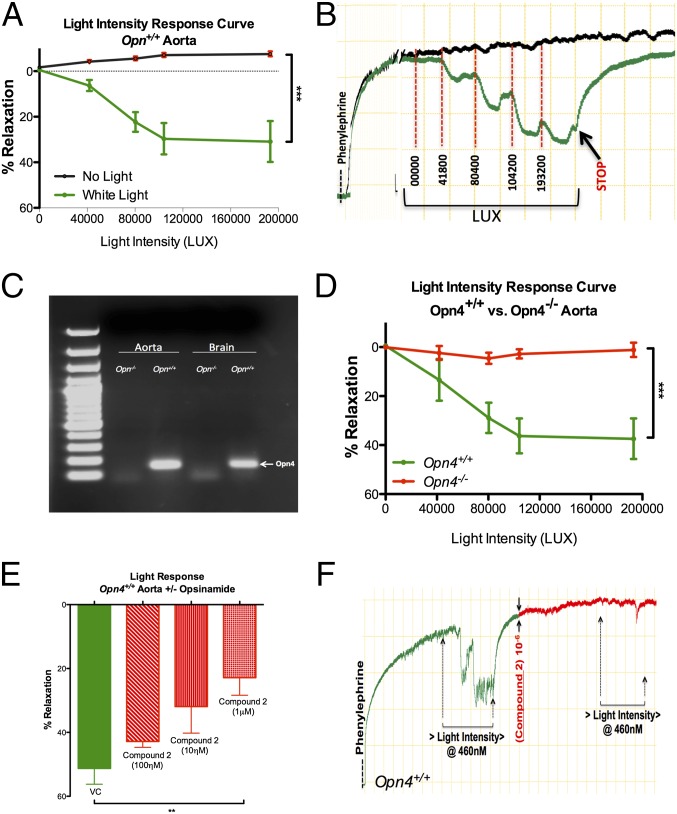
We first demonstrated photorelaxation in mouse aortic rings suspended in an organ chamber in physiological solution and preconstricted with phenylephrine (PE; 1 µM). Exposing the suspended vessels to cold white light at increasing intensities (40,000–2,000,000 lux units) produced a phasic, intensity-dependent vasorelaxation with a maximal response of ∼35% (Fig. 1 A and B). The diverse physiological roles of NIF opsins prompted us to explore their involvement in photorelaxation. Using PCR, we demonstrated expression of Opn4 in the aorta of Opn4+/+ but not in Opn4−/− mice (Fig. 1C). Furthermore, the abundance of transcript seems to be higher in aorta than in homogenates of whole brain (Fig. S1A). Expression of Opn4 was further confirmed using a second set of PCR primers (Fig. S1B). In aortic rings from Opn4−/− mice, vasorelaxant responses to light were virtually abolished (Fig. 1D and Fig. S1C), whereas relaxation to the endothelium-dependent vasodilator acetylcholine was unaltered (Fig. S1D). A small-molecule opsin inhibitor (“opsinamide” with sulfonamide structure) has recently been identified and designated Compound 2: 1-(2,5-dichloro-4-methoxy-benzenesulfonyl)-piperidine, Ki ∼7 nM (8). Compound 2 elicited a dose-dependent inhibition of photorelaxation, with an IC50 of 18.2 nM (Fig. 1 E and F), while retaining an unchanged vasorelaxant response to the NO donor sodium nitroprusside (SNP) (Emax, 101 ± 0.15% vs. 101 ± 0.12%, nonsignificant, n = 8).
Fig. 1.
Opsin 4 expression in blood vessels and its role in photorelaxation. (A) Summary data and (B) representative trace of ex vivo vasoreactivity demonstrating dose-dependent effects of cold white light on Opn4+/+ mouse aorta compared with no light exposure. Error bars denote SEM, n = 6, ***P < 0.001. (C) RT-PCR analysis of mouse aorta and brain demonstrates Opn4 mRNA expression in Opn4+/+ mice but not Opn4−/− mice. n = 6. (D) Vasoreactivity to cold white light demonstrates absence of photorelaxation in aortas from Opn4−/− mice compared with Opn4+/+ mice. Error bars denote SEM, n = 6, ***P < 0.001. (E) Dose-dependent inhibition of the photorelaxation response in presence of opsinamide, Compound 2 (an Opn4 inhibitor). Error bars denote SEM, n = 4, **P < 0.005. (F) Representative trace: vasoreactivity data showing photorelaxation response before and after pharmacological inhibition of Opn4 with 1 μM concentration of Compound 2 in Opn4+/+ aorta.
The Photorelaxation Response Is Wavelength-Specific.
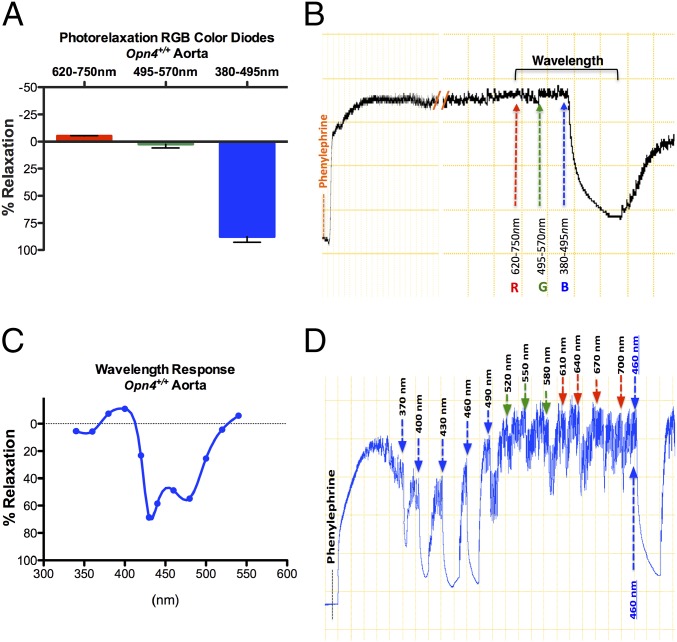
Vasorelaxation was initially observed in response to cold white light. We next examined vasorelaxation responses to a range of wavelengths with diodes that emit red (620–750 nm), green (495–570 nm), or blue (380–495 nm) light (RGB). The vessels did not respond to red or green light but displayed maximal vasorelaxation at low-intensity blue light (Fig. 2 A and B). Next, using a monochromator, we determined the precise peak wavelength spectral sensitivity by changing the wavelength at 30-nm intervals and measuring the vasorelaxant response. Responses were observed only in the blue light spectrum (400–500 nm), with a maximal response at between ∼430 and 460 nm (Fig. 2 C and D) corresponding to the optimal absorption wavelength for the mouse Opn4 receptor (9).
Fig. 2.
Determination of optimal wavelength within the visible electromagnetic spectrum mediating the photorelaxation response. (A) Vasoreactivity of Opn4+/+ mouse aorta to red (620–750 nm) to green (495–570 nm) to blue (380–495 nm) (RGB) wavelengths of the visible spectrum using light-emitting diodes. n = 4. (B) Representative trace demonstrates no vasorelaxation in Opn4+/+ aorta in the R to G spectrum but maximum vasorelaxation in the presence of B spectrum light. (C) Summary data of vasoreactivity of Opn4+/+ aorta to precise wavelength changes within blue light spectrum using monochromator (precise peak wavelength spectral changes at 30-nm intervals). n = 3. (D) Representative trace demonstrating vasoreactivity of Opn4+/+ aorta to precise 30-nm wavelength increments from 370 nm to 700 nm, followed by repeat 460-nm exposure to confirm this optimal wavelength and ensure lack of desensitization.
Photorelaxation Signal Transduction Is Endothelium- and eNOS-Independent but Involves Soluble Guanylyl Cyclase (SGC), PDE6, and Vessel Hyperpolarization.
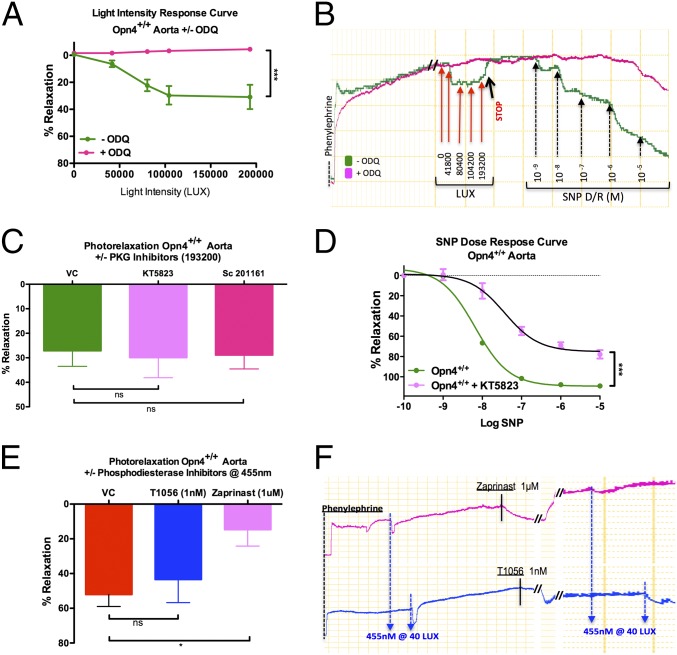
We investigated signal transduction mechanisms underlying Opn4-mediated photorelaxation. We first ascertained whether phototransduction is an endothelium-dependent phenomenon. Removal of the endothelium had no effect on photorelaxation (Fig. S2 A and B). The NOS inhibitor l-NAME had little effect, modestly enhancing responses (Fig. S2 C and D) (3). Additionally, in eNOS−/− mice aorta, endothelium-dependent relaxation responses to acetylcholine were abolished (Fig. S2E), but photorelaxation responses were not attenuated (Fig. S2F), consistent with other reports (4). To exclude other potential NO stores (nitrosothiols) as a possible mechanism contributing to light-induced relaxation, vessels were preincubated with the NO scavenger carboxy-PTIO (CPTIO) (4). Whereas CPTIO, as predicted, significantly attenuated the vasorelaxant response to endothelial dependent activation with ACh (Fig. S2 G and H), CPTIO had no effect on the vasorelaxant response to blue light (Fig. S2 I and J). Additionally, to exclude cyclooxygenase or cytochrome p450 (cyp450)-derived hyperpolarizing eicosanoids as possible mediators of the vasorelaxant response to light, vessel rings were preincubated with the cyclooxygenase inhibitor indomethacin (3 μM), the epoxyeicosatrienoic acids (EETs) antagonist 14,15-EEZE (30 μM) (10), and the cyp450 epoxygenase inhibitor N-(methylsulfonyl)-2-(2-propynyloxy)-benzenehexamide (MS-PPOH) (20 μM) (11). Moreover, to exclude carbon monoxide as a potential mediator of this effect, vessel rings from heme oxygenase 2 knockout mice (HO2−/−) mice were tested. Preincubation of aortic rings with indomethacin, 14,15-EEZE, or MS-PPOH had no effect on the vasorelaxant response to blue light (Fig. S3 A and B, C and D, and E, respectively). The vasorelaxant responses of the rings from HO2−/− mice were also no different from their WT controls (Fig. S3F). Interestingly, preincubation of mouse aortic rings with the sGC inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) completely inhibited the photorelaxation response (Fig. 3 A and B). Although the functional GC in retinal phototransduction is membrane-bound (12), signaling may be promiscuous given the abundance of sGC in vascular smooth muscle cell (VSMC). Because photorelaxation seemed to be sGC-dependent, we tested whether cGMP-dependent protein kinase (PKG) was involved. Although preincubating vessel rings with the PKG inhibitor KT5823 (0.5 μM) failed to attenuate the photorelaxation response (Fig. 3C), it significantly inhibited the response to SNP [Emax, 108 ± 0.86% vs. 75 ± 3.3%, logEC50, −8.24 ± 0.03 vs. −7.43 ± 0.13; n = 3, P < 0.05 vehicle control (VC) vs. KT5823] (Fig. 3D). We confirmed this finding using two other PKG inhibitors, sc-201161 (10 μM) and Rp-8Br-PET-cGMP (10 μM) (Fig. 3C and Fig. S4 A and B). This suggests that the vasorelaxation mechanism is sGC/cGMP-dependent but PKG-independent.
Fig. 3.
Signal transduction mechanism involved in photorelaxation. (A) Vasoreactivity in Opn4+/+ mouse aorta to cold white light demonstrates marked attenuation of photorelaxation in the vessels preincubated with the sGC inhibitor ODQ (∼−4% relaxation), whereas untreated vessels relaxed ∼31%. Error bars denote SEM, ***P < 0.001. (B) Representative trace: vasoreactivity of Opn4+/+ mouse aorta to cold white light in the presence and absence of ODQ, followed by dose–response curve with nitric oxide donor SNP to confirm sGC inhibition. (C) Vasoreactivity of Opn4+/+ mouse aorta to cold white light demonstrates no significant difference in photorelaxation in vessels preincubated with the PKG inhibitor KT5823 (0.5 μM), (n = 3) or sc-201161 (10 μM) (n = 4) compared with untreated vessels. n = 7. (D) Significant impairment in vasorelaxation to the NO donor SNP in Opn4+/+ mouse aorta preincubated with KT5823 compared with VC. n = 3, ***P < 0.001. (E) Maximum photorelaxation response to 455 nm blue light in untreated Opn4+/+ aortas compared with aorta preincubated with 1 nM T1056 (selective PDE5 inhibitor) and 1 μM Zaprinast (nonselective PDE5 and PDE6 inhibitor). Only Zaprinast-treated vessels had attenuated photorelaxation (∼52% vs. ∼14%). Error bars denote SEM, *P < 0.05. (F) Representative trace comparing photorelaxation in vessels treated with 1 nM T1056 and 1 μM Zaprinast.
We next tested whether the hyperpolarization in photorelaxation involves direct cGMP-regulated ion channels. G protein-dependent phototransduction is classically dependent on PDE6 activation and cGMP hydrolysis by PDE6 (13). Preincubation of aortic rings with the PDE inhibitor sildenafil abolished photorelaxation (Fig. S4C). Although the Ki of sildenafil for PDE5 is 4 nM, its Ki for PDE6 is also low (Ki 40 nM) (14). To discriminate PDE5 and PDE6, vessel rings were preincubated with the PDE5-selective inhibitor T0156 (Tocris Bioscience; Ki 56 nM and Ki 0.23 nM for PDE5 and 6, respectively, thus >200-fold selectivity vs. 10-fold for sildenafil). T0156 (1 nM) failed to inhibit light-induced vasorelaxation (Fig. 3 D and E). However, Zaprinast, a nonselective PDE5 and PDE6 inhibitor (IC50 for PDE5, 0.76 µM; IC50 for PDE6, 0.15 µM) (15), completely inhibited the vasorelaxant responses to light. Accordingly, it seems that PDE6 rather than PDE5 mediates photorelaxation. Using RT-PCR, we confirmed that both PDE5 and PDE6 are expressed in thoracic aorta isolated from both Opn4+/+ and Opn4−/− mice (Fig. S4C).
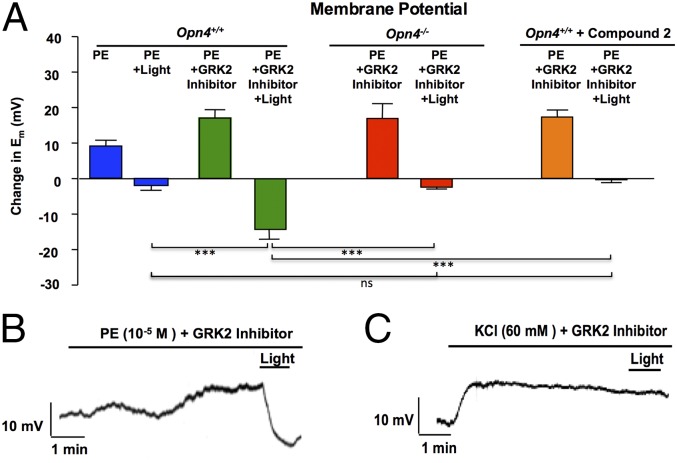
To evaluate the relationship between photorelaxation and alterations in membrane potential (Em), we made sharp electrode intracellular recordings of in situ VSMC Em in endothelium-denuded segments of mouse thoracic aorta at baseline, in the presence of a depolarizing stimulus and GRK2 inhibitor, and then as the tissue was exposed to blue light (Fig. 4A). Blue light hyperpolarized PE-depolarized vessels (Fig. 4 A and B), whereas vessels depolarized with 60 mM K+ exhibited no change in Em to blue light (Fig. 4C and Fig. S4E). These data imply that opening of K+ channels, which are blocked with 60 mM K+, mediates photorelaxation. Furthermore, hyperpolarizing responses to blue light were almost completely lost in Opn4−/− mice and in vessels from Opn4+/+ mice preincubated with the opsinamide, Compound 2 (1 μM) (Fig. 4A). Thus, mechanisms underlying photorelaxation closely recapitulate the “simple” photoreceptors in invertebrates involving hyperpolarization, PDE6, and likely cGMP-gated channels.
Fig. 4.
Sharp electrode intracellular recordings of in situ smooth muscle Em in endothelium-denuded segments of mouse thoracic aorta. n = 5–12. (A) Bar graph shows composite data (mean ± SEM) for change in Em under various conditions in aortas from Opn4+/+ mice, Opn4−/− mice, and after pretreatment with opsinamide, Compound 2 for 30 min (1 μM). Depolarizing stimuli with and without the GPK2 inhibitor methyl 5-[2-(5-nitro-2-furyl)vinyl]-2-furoate were applied for 5 min in dark conditions before subjecting the tissue to light. n = 5–12. There was a significant decrease in Em when blue light (380–495 nm) was applied to GRK2 pretreated vessels (with PE) from Opn4+/+ mice compared with vessels from Opn4−/− and after pretreatment of Opn4+/+ vessels with Compound 2. n = 5–12; ***P < 0.001. (B and C) Representative traces show the effect of blue light (380–495 nm) on Em in cells depolarized with (B) PE or (C) potassium chloride (KCl).
GRK2 Mediates Light Stimulus-Dependent Desensitization.
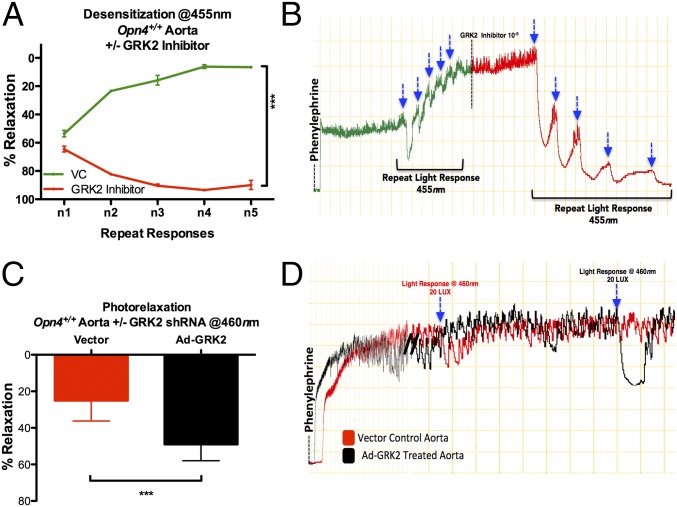
Photorelaxation desensitized with repeated rapid light stimulation but recovered after 30 min of dark exposure (Fig. S5A). Given that opsins are G protein-coupled receptors (GPCRs), we hypothesized that desensitization is mediated by GPCR kinases. Accordingly, we preincubated aortic rings with Gallein (Tocris Biosciences), which blocks the interaction between βγ subunit and GRK2 and has been shown to reduce β-AR-mediated membrane recruitment of GRK2 in isolated adult mouse cardiomyocytes and prevent pharmacologically induced heart failure in mice (16). Gallein prevented desensitization of light stimulus-dependent vasorelaxant responses (Fig. S5B). Additionally, we preincubated the vessels with the GRK2-specific inhibitor methyl 5-[2-(5-nitro-2-furyl)vinyl]-2-furoate (Calbiochem) at 0.010–1 μM. GRK2 inhibition blocked desensitization (Fig. 5 A and B) and markedly enhanced the sensitivity of the blood vessels to light. We depleted GRK2 in aortic rings with adenoviral-transduced shRNA (Fig. S5C), which was confirmed in mouse embryonic fibroblasts (Fig. S5D). GRK2 depletion in aorta significantly enhanced photorelaxation [Fig. 5 C and D (at 460 nm), Fig. S5 E and F (RGB), and Fig. S5G (white light)] and inhibited stimulus-dependent desensitization (Fig. S5 H and I).
Fig. 5.
Stimulus-dependent desensitization, photorelaxation, and its modulation. (A) Vasoreactivity: Opn4+/+ aorta demonstrate desensitization/diminished vasodilatory responses to iso-intensity repeat light stimulation (455 nm at 40 lux; ∼2-min interval) and attenuation of desensitization/enhanced vasodilatory responses to the same iso-intensity repeat light stimulation after inhibition with GPK2 inhibitor. Error bars denote SEM, n = 5, ***P < 0.001. (B) Representative trace demonstrating desensitization resulting in loss of photorelaxation, followed by resensitization of receptors with use of GPK2 inhibitor, resulting in exaggerated vasodilatory responses with blue light (455 nm at 40 lux). (C) Vasoreactivity: Opn4+/+ aortic rings, preincubated with the GRK2 shRNA adenovirus (Ad-GRK2), demonstrating enhanced wavelength-specific photorelaxation (∼49%) compared with the vector-treated vessels (∼25%). Error bars denote SEM, n = 4, *P < 0.05. (D) Representative trace demonstrating enhanced photorelaxation in Opn4+/+ aortas treated with Ad-GRK2 compared with vector control.
Role of Opsin 4 in Peripheral Vasculature and Its Importance in Vivo.
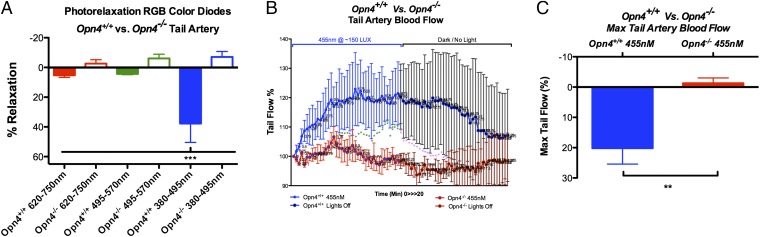
We have demonstrated a role for Opn4 in isolated blood vessels. To determine in vivo physiological consequences and evaluate vascular beds in which exposure to light is likely, we studied photorelaxation in tail arteries of Opn4+/+ and Opn4−/− mice, both ex vivo and in vivo. Using wire myography, we examined vasorelaxation responses in mouse-tail arteries, to a range of wavelengths with RGB diodes. Consistent with the data from aorta, the tail arteries of Opn4+/+ did not respond to red or green light but displayed maximal vasorelaxation at low-intensity blue light. The tail arteries of Opn4−/− mice showed no reactivity to any of the three wavelength spectrums (Fig. 6A). Using laser Doppler flowmetry, in vivo experiments were conducted to determine tail blood flow. Red blood cell flux was measured as the tail was exposed to blue light for 10 min followed by a 10-min dark period. There was a significant increase in tail blood flow in Opn4+/+ mice with exposure to blue light, which was reversed in dark. This phenomenon was not observed in Opn4−/− mice (Fig. 6 B and C).
Fig. 6.
Opsin4 in vivo peripheral blood flow and vasoreactivity. (A) Vasoreactivity of mouse-tail artery to RGB wavelength spectrum demonstrates that only blue light causes vasorelaxation in Opn4+/+ but no response in Opn4−/− tail arteries. Error bars denote SEM, n = 4, ***P < 0.001. (B) In vivo laser Doppler flowmetry of mouse tail demonstrates a significant increase in tail blood flow after 10 min of cumulative blue light (455 nm at 40 lux) exposure in Opn4+/+ but not in Opn4−/− mice. This is reversible on reexposure to dark. Error bars denote SD, *P < 0.05, **P < 0.01. (C) Maximum change in tail flow in Opn4+/+ and Opn4−/− mice. Error bars denote SEM, n = 4, **P < 0.01.
Discussion
Here we report the presence and acute regulation of GPCR Opn4 receptors in blood vessels and provide a previously unidentified mechanistic insight into the signal mechanisms by which their stimulation produces photorelaxation, a phenomenon that has been described by vascular biologists for more than 5 decades.
Behavioral and physiological adaptation to changes in the ambient light is crucial for life. The light modulation of neuroendocrine function and temporal alignment of physiology and behavior to the day/night cycle by the circadian clock are such adaptations. The best-characterized adaptation to light occurs in the eye, where NIF responses can function independently of rods and cones but depend on ocular light reception. The expression and function of the NIF GPCR Opn4 in intrinsically photosensitive retinal ganglion cells (pRGCs) and exploration of its role in major NIF responses provided fundamental understanding of mammalian adaptation to the light environment (17). Recent advances have linked Opn4 to diverse physiological and behavioral responses to light, including circadian photo-entrainment, light suppression of activity in nocturnal animals, alertness in diurnal animals, acute suppression of pineal melatonin, light modulation of sleep, light exacerbation of migraine, and the pupillary light reflex (18). Indeed, an intrinsic photosensitive iris pupillary reflex independent of neuronal circuitry has recently been described (18), suggesting that Opn4 is expressed in extraneuronal tissue. Our finding that functional Opn4 receptors are present in the vasculature is consistent with such nonvisual functions of Opn4. The finding that photoreceptors exist in blood vessels is not entirely surprising, because Opn4 seems to regulate retinal blood vessel development through a VEGF-dependent pathway (19). Moreover, there is precedence for the presence of “environment sensing” receptors in the cardiovascular system. For example, TRPV1 nociceptors are expressed in both vascular smooth muscle and endothelium and mediate opposing vasomotor effects (20). Vascular disposition of photorelaxation may represent a vestige of evolution, a function of embryologic determinism, or a novel mechanism for regulating blood flow distribution in response to changes in the light cycle. This system may be targeted for therapy in diseases that involve altered vasoreactivity.
Phylogenetic analysis reveals that mammalian melanopsin is more closely related to invertebrate opsins than to the classic vertebrate visual opsin, rhodopsin (21). Opn4 uses 11-cis-retinaldehyde as a chromophore. Light stimulation causes a conformational change that photo-isomerizes the protein to all-transretinal, resulting in a conformational change in the receptor and downstream G protein signaling. The reported peak wavelength spectral sensitivity of melanopsin ranges from 420 to 500 nm (blue–cyan range) (22), with the first successful absorption spectrum of mouse melanopsin suggesting peak absorbance at 424 nm (9). This is almost precisely the wavelength at which we observed maximal vasorelaxation in response to light (Fig. 2), further supporting the notion that Opn4 mediates photorelaxation in vessels. Genetic deletion of Opn4 and treatment with Opn4 inhibitors markedly reduced photorelaxation, also implying a main role for this opsin, although it remains possible that other NIF opsins (Opn3 and 5) are involved in vascular responses.
In classic rhodopsin signaling, light-activated opsin triggers the pertussis toxin-sensitive G protein transducin, which activates PDE6, with subsequent hydrolysis of cGMP leading to closure of the cGMP-gated ion channels and hyperpolarization of photoreceptor cells. By contrast, Opn4 signaling has not been fully elucidated (23–26). In the pRGCs, limited studies in native cells and cells in which Opn4 is overexpressed point to coupling of Opn4 to the Gg/11 G protein. Pharmacologic evidence supports a role for phospholipase C (PLC) in Opn4-driven light responses, because the PLC inhibitor U73122, but not its inactive homolog, blocks phototransduction in pRGC (27). Furthermore, the PLCβ4 isoform mediates this signaling, because photoresponses are almost entirely abolished in pRGCs from PLCβ4 knockout mice (18). Our data indicate a cGMP-dependent and PKG-independent pathway. Interestingly, signal transduction in photoreceptive neurons of invertebrates such as crayfish, sea slugs, and other molluscs (Aplysia, Onchidium, and Helix) involves a Go type G protein coupled to particulate GC and cGMP-dependent gating of K+ channels, leading to hyperpolarization. Because invertebrate photoreceptors are similar to the NIF opsins in the mammalian retina (24–26), it is likely that this promiscuous signaling is responsible for the signaling pathways we observe in blood vessels.
Like other GPCRs, Opn4 is a substrate for GRKs that mediate desensitization/regulation. GRKs are recruited to and phosphorylate ligand-occupied GPCRs on the cytoplasmic carboxyl-terminal tail. β-Arrestins bind phosphorylated GPCRs with enhanced affinity, thereby blocking recoupling of the dissociated G protein subunits to the GPCR and preventing further receptor activation (i.e., desensitization). On the basis of the complement of G protein receptor kinases present in melanopsin-expressing retinal ganglion cells, GRK2 seemed to be a strong candidate for melanopsin’s cognate GRK (28). Our finding supports this possibility that both small-molecule inhibition and knockdown of GRK2 enhance photorelaxation. Consistent with our results, GRK2 seems to be the principal GRK in regulating vascular smooth muscle-associated GPCRs (28), including α1-adrenergic (29), endothelin (30), purinergic (31), and β-adrenergic receptors (32).
Although we have inferred that the Opn4 receptors seem to be expressed on the VSMCs, we cannot absolutely exclude that the receptors are present in the adventitia and mediate vasorelaxant signaling in a paracrine fashion. This confirmation will need to await future studies in which cellular localization is determined by immunohistochemical techniques or in situ hybridization, or functional studies in which Opn4 receptors are selectively silenced in vascular smooth muscle.
The demonstration that blue light regulates blood flow has implications for understanding circadian regulation of blood flow and pressure in mammals. Blood pressure begins to rise in the morning (in humans and mice) (33), a process that is strongly correlated with the timing of adverse cardiovascular events (34). Moreover, each element of the vascular structure has an internal circadian oscillator that contributes to diurnal rhythms in vascular tone (35, 36). Although genes that regulate the output of the clock have been identified, environmental input signals have not been established. We speculate that the Opn4 receptor is one of these vascular sensors.
The characterizations of this previously unidentified light-dependent vasorelaxation pathway represents a opportunity for targeting wavelength-specific light therapy in vascular disease; specifically, vascular diseases that are associated with enhanced vasconstriction mediated by neural, humoral, or paracrine signals, as well as in sites where light will have access. This is especially the case in Raynaud’s phenomenon (RP), which is characterized by recurrent episodes of robust painful cutaneous vasoconstriction in excess of the typical response to cold temperature (37). The intense digital vasoconstriction and hypoxemia is associated with vascular dysfunction related to ischemia/reperfusion injury, systemic inflammation, increased oxidant stress, endothelial dysfunction, and an imbalance between coagulation and fibrinolysis (38, 39). Patients affected by this condition experience pain, slow-healing ulcers, and vasculopathy (40–42). The mechanisms mediating the robust cutaneous vasoconstriction to cold that is dysregulated with RP involves the intracellular translocation of α2C adrenergic receptor from the Golgi apparatus to the membrane, inducing vasoconstriction (43), and an AR-independent increase in calcium sensitivity through inhibition of myosin light chain phosphatase (44, 45). Treatment of RP can include pharmacologics targeted to the endothelium or smooth muscle to increase NO-dependent vasodilation (sildenafil) or vasoactive drugs that block vasoconstrictive pathways (endothelin-1 antagonists). However, the inconsistent response to treatment and complications and side effects of therapy provide opportunity for this potential new therapy. Additionally diabetic microvascular disease may represent a further opportunity to use light therapy for the treatment of vascular disease.
Supplementary Material
Acknowledgments
We thank Dr. King-Wai Yau (Johns Hopkins University) for providing the breeding pair of Opn4−/− mice. This study was funded by National Heart, Lung, and Blood Institute R01 Grants HL105296–02 and HL089668 (to D.E.B.) and National Institute of Mental Health Grant MH8501 to National Institute of Drug Abuse Grant DA000266 (to S.H.S.).
Footnotes
The authors declare no conflict of interest.
See Commentary on page 17704.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1420258111/-/DCSupplemental.
References
- 1.Furchgott RF, Sleator WJ, Mccaman MW, Elchlepp J. Relaxation of arterial strips by light and the influence of drugs on this photodynamic effect. J Pharmacol Exp Ther. 1955;113:22. [Google Scholar]
- 2.Matsunaga K, Furchgott RF. Interactions of light and sodium nitrite in producing relaxation of rabbit aorta. J Pharmacol Exp Ther. 1989;248(2):687–695. [PubMed] [Google Scholar]
- 3.Chen X, Gillis CN. Enhanced photorelaxation in aorta, pulmonary artery and corpus cavernosum produced by BAY K 8644 or N-nitro-L-arginine. Biochem Biophys Res Commun. 1992;186(3):1522–1527. doi: 10.1016/s0006-291x(05)81579-7. [DOI] [PubMed] [Google Scholar]
- 4.Andrews KL, McGuire JJ, Triggle CR. A photosensitive vascular smooth muscle store of nitric oxide in mouse aorta: No dependence on expression of endothelial nitric oxide synthase. Br J Pharmacol. 2003;138(5):932–940. doi: 10.1038/sj.bjp.0705115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrews KL, Triggle CR, Ellis A. NO and the vasculature: Where does it come from and what does it do? Heart Fail Rev. 2002;7(4):423–445. doi: 10.1023/a:1020702215520. [DOI] [PubMed] [Google Scholar]
- 6.Flitney FW, Megson IL. Nitric oxide and the mechanism of rat vascular smooth muscle photorelaxation. J Physiol. 2003;550(Pt 3):819–828. doi: 10.1113/jphysiol.2003.041970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez J, Maloney RE, Rassaf T, Bryan NS, Feelisch M. Chemical nature of nitric oxide storage forms in rat vascular tissue. Proc Natl Acad Sci USA. 2003;100(1):336–341. doi: 10.1073/pnas.0234600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones KA, et al. Small-molecule antagonists of melanopsin-mediated phototransduction. Nat Chem Biol. 2013;9(10):630–635. doi: 10.1038/nchembio.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman LA, Walker MT, Brown RL, Cronin TW, Robinson PR. Melanopsin forms a functional short-wavelength photopigment. Biochemistry. 2003;42(44):12734–12738. doi: 10.1021/bi035418z. [DOI] [PubMed] [Google Scholar]
- 10.Gauthier KM, Edwards EM, Falck JR, Reddy DS, Campbell WB. 14,15-epoxyeicosatrienoic acid represents a transferable endothelium-dependent relaxing factor in bovine coronary arteries. Hypertension. 2005;45(4):666–671. doi: 10.1161/01.HYP.0000153462.06604.5d. [DOI] [PubMed] [Google Scholar]
- 11.Ponnoth DS, Nayeem MA, Tilley SL, Ledent C, Jamal Mustafa S. CYP-epoxygenases contribute to A2A receptor-mediated aortic relaxation via sarcolemmal KATP channels. Am J Physiol Regul Integr Comp Physiol. 2012;303(10):R1003–R1010. doi: 10.1152/ajpregu.00335.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bondarenko VA, Hayashi F, Usukura J, Yamazaki A. Involvement of rhodopsin and ATP in the activation of membranous guanylate cyclase in retinal photoreceptor outer segments (ROS-GC) by GC-activating proteins (GCAPs): A new model for ROS-GC activation and its link to retinal diseases. Mol Cell Biochem. 2010;334(1-2):125–139. doi: 10.1007/s11010-009-0323-y. [DOI] [PubMed] [Google Scholar]
- 13.Korenbrot JI. Speed, sensitivity, and stability of the light response in rod and cone photoreceptors: facts and models. Prog Retin Eye Res. 2012;31(5):442–466. doi: 10.1016/j.preteyeres.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cote RH. Characteristics of photoreceptor PDE (PDE6): Similarities and differences to PDE5. Int J Impot Res. 2004;16(Suppl 1):S28–S33. doi: 10.1038/sj.ijir.3901212. [DOI] [PubMed] [Google Scholar]
- 15.Goto M, Ikeyama K, Tsutsumi M, Denda S, Denda M. Phosphodiesterase inhibitors block the acceleration of skin permeability barrier repair by red light. Exp Dermatol. 2011;20(7):568–571. doi: 10.1111/j.1600-0625.2011.01255.x. [DOI] [PubMed] [Google Scholar]
- 16.Casey LM, et al. Small molecule disruption of G beta gamma signaling inhibits the progression of heart failure. Circ Res. 2010;107(4):532–539. doi: 10.1161/CIRCRESAHA.110.217075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatori M, et al. Inducible ablation of melanopsin-expressing retinal ganglion cells reveals their central role in non-image forming visual responses. PLoS ONE. 2008;3(6):e2451. doi: 10.1371/journal.pone.0002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xue T, et al. Melanopsin signalling in mammalian iris and retina. Nature. 2011;479(7371):67–73. doi: 10.1038/nature10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao S, et al. A direct and melanopsin-dependent fetal light response regulates mouse eye development. Nature. 2013;494(7436):243–246. doi: 10.1038/nature11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kark T, et al. Tissue-specific regulation of microvascular diameter: Opposite functional roles of neuronal and smooth muscle located vanilloid receptor-1. Mol Pharmacol. 2008;73(5):1405–1412. doi: 10.1124/mol.107.043323. [DOI] [PubMed] [Google Scholar]
- 21.Koyanagi M, Terakita A. Gq-coupled rhodopsin subfamily composed of invertebrate visual pigment and melanopsin. Photochem Photobiol. 2008;84(4):1024–1030. doi: 10.1111/j.1751-1097.2008.00369.x. [DOI] [PubMed] [Google Scholar]
- 22.Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433(7027):741–745. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- 23.Gotow T, Nishi T. Light-dependent K(+) channels in the mollusc Onchidium simple photoreceptors are opened by cGMP. J Gen Physiol. 2002;120(4):581–597. doi: 10.1085/jgp.20028619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gotow T, Nishi T. Involvement of a Go-type G-protein coupled to guanylate cyclase in the phototransduction cGMP cascade of molluscan simple photoreceptors. Brain Res. 2007;1144:42–51. doi: 10.1016/j.brainres.2007.01.068. [DOI] [PubMed] [Google Scholar]
- 25.Gotow T, Nishi T. Simple photoreceptors in some invertebrates: Physiological properties of a new photosensory modality. Brain Res. 2008;1225:3–16. doi: 10.1016/j.brainres.2008.04.059. [DOI] [PubMed] [Google Scholar]
- 26.Gotow T, Nishi T. A new photosensory function for simple photoreceptors, the intrinsically photoresponsive neurons of the sea slug onchidium. Front Cell Neurosci. 2009;3:18. doi: 10.3389/neuro.03.018.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graham DM, et al. Melanopsin ganglion cells use a membrane-associated rhabdomeric phototransduction cascade. J Neurophysiol. 2008;99(5):2522–2532. doi: 10.1152/jn.01066.2007. [DOI] [PubMed] [Google Scholar]
- 28.Blasic JR, Jr, Lane Brown R, Robinson PR. Light-dependent phosphorylation of the carboxy tail of mouse melanopsin. Cell Mol Life Sci. 2012;69(9):1551–1562. doi: 10.1007/s00018-011-0891-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohn HI, et al. Inhibition of vascular smooth muscle G protein-coupled receptor kinase 2 enhances alpha1D-adrenergic receptor constriction. Am J Physiol Heart Circ Physiol. 2008;295(4):H1695–H1704. doi: 10.1152/ajpheart.00564.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris GE, Nelson CP, Standen NB, Challiss RA, Willets JM. Endothelin signalling in arterial smooth muscle is tightly regulated by G protein-coupled receptor kinase 2. Cardiovasc Res. 2010;85(3):424–433. doi: 10.1093/cvr/cvp310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris GE, et al. G protein-coupled receptor kinase 2 and arrestin2 regulate arterial smooth muscle P2Y-purinoceptor signalling. Cardiovasc Res. 2011;89(1):193–203. doi: 10.1093/cvr/cvq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schutzer WE, et al. Age-related β-adrenergic receptor-mediated vasorelaxation is changed by altering G protein receptor kinase 2 expression. Vascul Pharmacol. 2011;55(5-6):178–188. doi: 10.1016/j.vph.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 33.Li P, Sur SH, Mistlberger RE, Morris M. Circadian blood pressure and heart rate rhythms in mice. Am J Physiol. 1999;276(2 Pt 2):R500–R504. doi: 10.1152/ajpregu.1999.276.2.R500. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg RJ. Epidemiologic aspects of circadian patterns of cardiovascular disease and triggers of acute cardiac events. Cardiol Clin. 1996;14(2):175–184. doi: 10.1016/s0733-8651(05)70271-x. [DOI] [PubMed] [Google Scholar]
- 35.Paschos GK, FitzGerald GA. Circadian clocks and vascular function. Circ Res. 2010;106(5):833–841. doi: 10.1161/CIRCRESAHA.109.211706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudic RD. Time is of the essence: Vascular implications of the circadian clock. Circulation. 2009;120(17):1714–1721. doi: 10.1161/CIRCULATIONAHA.109.853002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levien TL. Advances in the treatment of Raynaud’s phenomenon. Vasc Health Risk Manag. 2010;6:167–177. doi: 10.2147/vhrm.s4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Block JA, Sequeira W. Raynaud’s phenomenon. Lancet. 2001;357(9273):2042–2048. doi: 10.1016/S0140-6736(00)05118-7. [DOI] [PubMed] [Google Scholar]
- 39.Cooke JP, Marshall JM. Mechanisms of Raynaud’s disease. Vasc Med. 2005;10(4):293–307. doi: 10.1191/1358863x05vm639ra. [DOI] [PubMed] [Google Scholar]
- 40.Abou-Raya A, Abou-Raya S, Helmii M. Statins: Potentially useful in therapy of systemic sclerosis-related Raynaud’s phenomenon and digital ulcers. J Rheumatol. 2008;35(9):1801–1808. [PubMed] [Google Scholar]
- 41.Herrick AL. Management of Raynaud’s phenomenon and digital ischemia. Curr Rheumatol Rep. 2013;15(1):303. doi: 10.1007/s11926-012-0303-1. [DOI] [PubMed] [Google Scholar]
- 42.Schieir O, et al. Canadian Scleroderma Research Group Prevalence, severity, and clinical correlates of pain in patients with systemic sclerosis. Arthritis Care Res (Hoboken) 2010;62(3):409–417. doi: 10.1002/acr.20108. [DOI] [PubMed] [Google Scholar]
- 43.Chotani MA, Flavahan S, Mitra S, Daunt D, Flavahan NA. Silent alpha(2C)-adrenergic receptors enable cold-induced vasoconstriction in cutaneous arteries. Am J Physiol Heart Circ Physiol. 2000;278(4):H1075–H1083. doi: 10.1152/ajpheart.2000.278.4.H1075. [DOI] [PubMed] [Google Scholar]
- 44.Bailey SR, Eid AH, Mitra S, Flavahan S, Flavahan NA. Rho kinase mediates cold-induced constriction of cutaneous arteries: Role of alpha2C-adrenoceptor translocation. Circ Res. 2004;94(10):1367–1374. doi: 10.1161/01.RES.0000128407.45014.58. [DOI] [PubMed] [Google Scholar]
- 45.Bailey SR, Mitra S, Flavahan S, Flavahan NA. Reactive oxygen species from smooth muscle mitochondria initiate cold-induced constriction of cutaneous arteries. Am J Physiol Heart Circ Physiol. 2005;289(1):H243–H250. doi: 10.1152/ajpheart.01305.2004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.