Significance
Small molecules to target oncogenic signaling cascades in cancer have achieved success in molecularly defined patient subsets. The path to approval is often protracted and plagued with failures. Repositioning Food and Drug Administration-approved drugs with known side effects has become a major focus. Bisphosphonates are a commonly prescribed therapy for osteoporosis and skeletal metastases. The drugs have also been associated with reduced tumor burden in some patients, but the mechanism is unknown. Here we provide evidence that bisphosphonates inhibit the human EGFR (HER) receptor tyrosine kinase, including the commonly mutated forms that drive nonsmall cell lung cancer, as well as a resistance mutation. This new mechanism lays the basis for the future use of bisphosphonates for the prevention and therapy of HER family-driven cancers.
Keywords: drug repurposing, cancer therapy, cancer prevention
Abstract
A variety of human cancers, including nonsmall cell lung (NSCLC), breast, and colon cancers, are driven by the human epidermal growth factor receptor (HER) family of receptor tyrosine kinases. Having shown that bisphosphonates, a class of drugs used widely for the therapy of osteoporosis and metastatic bone disease, reduce cancer cell viability by targeting HER1, we explored their potential utility in the prevention and therapy of HER-driven cancers. We show that bisphosphonates inhibit colony formation by HER1ΔE746-A750-driven HCC827 NSCLCs and HER1wt-expressing MB231 triple negative breast cancers, but not by HERlow-SW620 colon cancers. In parallel, oral gavage with bisphosphonates of mice xenografted with HCC827 or MB231 cells led to a significant reduction in tumor volume in both treatment and prevention protocols. This result was not seen with mice harboring HERlow SW620 xenografts. We next explored whether bisphosphonates can serve as adjunctive therapies to tyrosine kinase inhibitors (TKIs), namely gefitinib and erlotinib, and whether the drugs can target TKI-resistant NSCLCs. In silico docking, together with molecular dynamics and anisotropic network modeling, showed that bisphosphonates bind to TKIs within the HER1 kinase domain. As predicted from this combinatorial binding, bisphosphonates enhanced the effects of TKIs in reducing cell viability and driving tumor regression in mice. Impressively, the drugs also overcame erlotinib resistance acquired through the gatekeeper mutation T790M, thus offering an option for TKI-resistant NSCLCs. We suggest that bisphosphonates can potentially be repurposed for the prevention and adjunctive therapy of HER1-driven cancers.
Bisphosphonates are the most commonly used class of therapeutics for osteoporosis and cancer bone disease, with a proven record of efficacy and safety in people (1, 2). There is increasing evidence, however, that bisphosphonates can directly kill cancer cells (3). We recently showed that aminobisphosphonates can inactivate human epidermal growth factor receptor (HER) family of receptor tyrosine kinases (RTKs) (4). We found that the drugs directly bind the HER1/2 kinase domain and, by inhibiting downstream signaling, reduce the cell viability in HER-driven lung, breast, and colon cancers (4). Knocking down the four HER isoforms abrogate bisphosphonate action, proving a selective action through this pathway (4). Indeed, this new action might explain the reduced disseminated tumor cell burden and increased disease- and recurrence-free survival documented in early breast cancer patients (5–8). The process may also explain epidemiologic observations in which patients on oral bisphosphonates for their osteoporosis had a lower incidence of colon and breast cancer (9–11).
Lung cancer is responsible for the largest number of deaths worldwide. About 80% of lung cancers are nonsmall cell cancers (NSCLCs), of which ∼30% are driven by two activating mutations in the HER1 kinase domain (HER1ΔE746-A750 and HER1L868R) (12). Since their introduction, tyrosine kinase inhibitors (TKIs), most notably erlotinib and gefitinib, have dramatically improved survival of NSCLC patients (13). However, prolonged therapy for over 3 y invariably results in resistance (14). About 50% of the resistance arises from a second site mutation of the gatekeeper residue T790 to a methionine (15, 16). This mutation results in reduced affinity to TKIs, poor drug efficacy, and a rapid downhill clinical course. In addition, HER1/2 gene amplification and overexpression drive a significant number of breast and colon cancers.
Here, we report that bisphosphonates attenuate tumor growth in nude mice xenografted with HER1ΔE746-A750-driven NSCLCs or HER1wt-expressing MB231 breast cancer cells. Impressively, tumor growth was profoundly reduced with treatment begun at the time of grafting (prevention protocol), whereas mice harboring HERlow-SW620 colon cancers remained resistant. We also provide evidence for combinatorial binding of bisphosphonates and TKIs to the HER1 kinase domain, resulting in additive effects on tumor regression in HER1ΔE746-A750-grafted mice. We suggest that the two drugs could potentially be used in concert in NSCLC patients. Finally, bisphosphonates retain their ability to inhibit the viability of cells harboring the HER1T790M gatekeeper mutation, a prelude to their use in overcoming TKI resistance.
Results
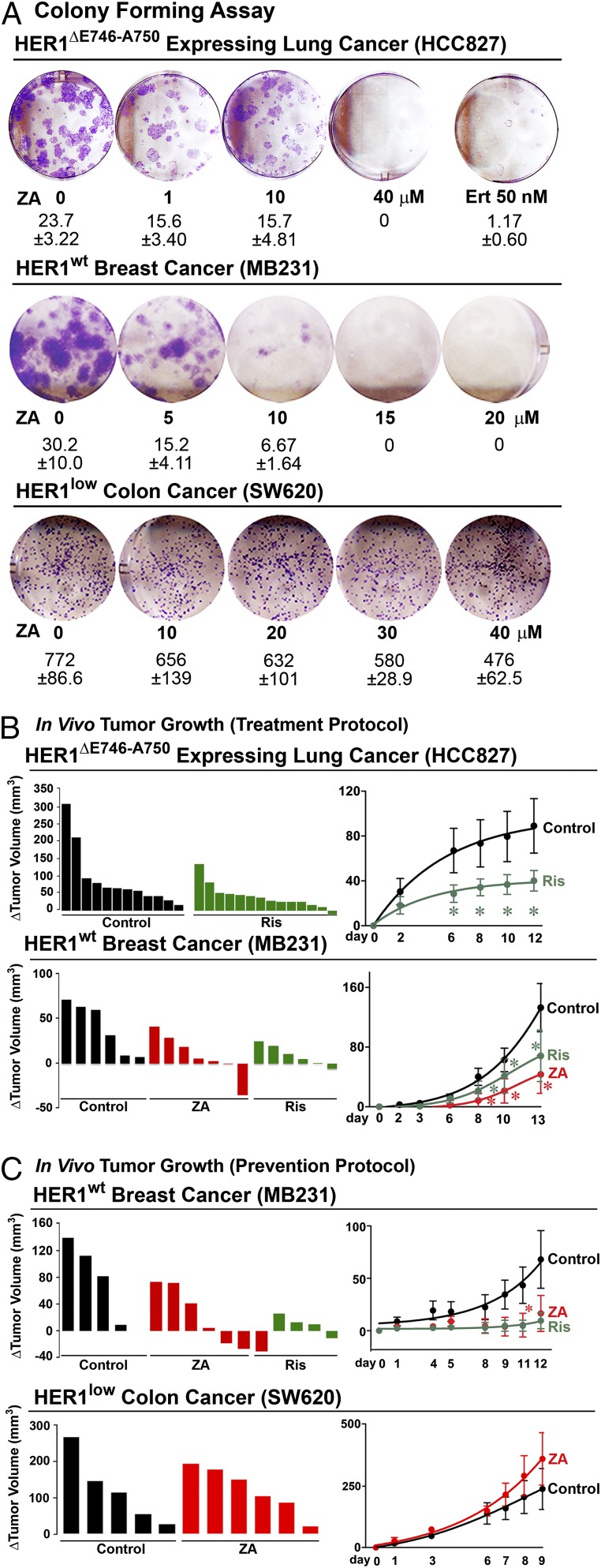
We found that zoledronic acid inhibited colony formation by HER1ΔE746-A750-driven HCC827 NSCLCs or HER1wt-expressing MB231 triple negative breast cancer cells, without effects on HERlow-SW620 colon cancer (Fig. 1A). We next tested bisphosphonate effects on tumor growth in vivo by xenografting BALB/c nu/nu mice with HCC827, MB231 or SW620 cells. Sequential measurement of tumor volume before and after daily gastric gavage with risedronate (1.42 µg/kg) or zoledronic acid (1.36 µg/kg) (Table S1), begun when HCC827 and MB231 tumors became palpable, showed significant reductions in tumor volume as early as 6 d postinitiation (Fig. 1B). In a parallel prevention protocol, when drug was given from the time of transplant, HER1wt-expressing MB231 tumors showed a dramatic and progressive reduction in volume, whereas HERlow SW620 colon cancers showed no such response to bisphosphonate (Fig. 1C). The latter finding buttresses our in vitro data (4) establishing the dependence of bisphosphonate action on HER1 expression.
Fig. 1.
Bisphosphonates inhibit HER-driven tumor growth in prevention and treatment protocols. (A) Colony formation assays performed with HCC827, MB231, and SW620 cells to study the effect of zoledronic acid (ZA; mean colony counts ± SEM; three experiments with two or three replicate wells pooled). (B and C) Tumor volume was measured sequentially following transplant of HCC827 (lung), MB231 (breast), or SW620 (colon) cancer cells into BALB/c nu/nu mice. Drugs were begun daily by oral gavage once tumors became palpable (treatment; B) or at the time of graft (prevention; C). HCC827 and MB231 tumors showed evidence of reduced growth with Ris (1.42 µg/kg, daily, gavage) or ZA (1.36 µg/kg, daily, gavage), whereas HERlow SW620 cells did not. Change (Δ) in tumor volume plotted for single mice or as group means ± SEM; statistics: ANOVA with Bonferroni’s correction; bisphosphonate- vs. vehicle-treated mice; *P < 0.05; number of mice used for the analysis corresponds to the number of animals shown in the plot for individual tumor volumes, e.g., n = 12 mice in B, Upper, control.
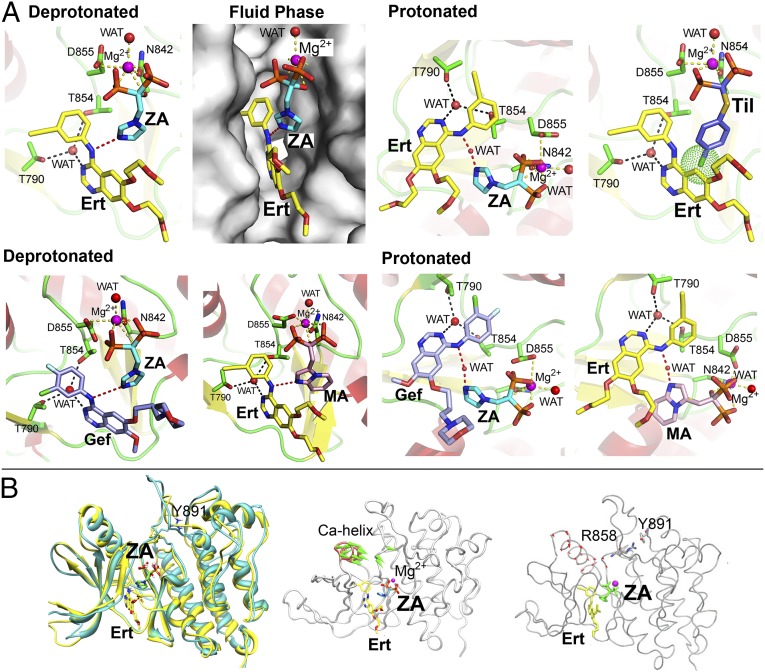
Based on initial observations of additivity between gefitinib and zoledronic acid (17), we investigated whether bisphosphonates and TKIs display combinatorial binding to the HER kinase domain. Docking protonated or deprotonated zoledronic acid into HER1wt crystal structure in complex with erlotinib (PDB ID code 1M17) revealed that there was enough space in the kinase pocket to accommodate both drugs (Fig. 2A). Furthermore, when the bisphosphonate is protonated at physiologic pH values, it binds TKIs via a conserved water bridge (Fig. 2A). Similar predicted binding modes are shown for the zoledronic acid/gefitinib and minodronic acid/erlotinib pairs (Fig. 2A). Of note, tiludronate did not display dual binding as it lacks an N-atom to make an H-bond with erlotinib and its p-chlorophenyl ring clashes with erlotinib (Fig. 2A).
Fig. 2.
Combinatorial binding of bisphosphonates and TKIs. (A) Docking of ZA in the HER1 kinase crystal structure that was cocrystallized with erlotinib (Ert) (PDB ID code 1M17). The phosphate backbone of ZA interacts with Mg2+, the removal of which prevents ZA docking. ZA also interacts with the NH group between the two aromatic rings in Ert either by itself (as a deprotonated tautomer) or via a structural water (WAT) (as a protonated tautomer). Ert associates with T790 via WAT. Fluid phase: binding mode of ZA and Ert observed from the solvent. Tiludronate (Til) does not have an imidazole ring to make an H-bond with Ert. It instead contains a p-chlorophenyl that results in severe steric clashes (green sphere) with Ert. ZA docked together with gefitinib (Gef) (PDB ID code 2ITY) and binds in a similar orientation to that observed in the ZA–Ert complex, as a deprotonated (pink dashed line) or protonated (orange dashed line) tautomer. Minodronic acid (MA) can likewise bind with Ert as a deprotonated or protonated tautomer. (B) Molecular docking reveals that the HER1L858R mutant conformation (cyan) is similar to the active state of HER1wt (yellow) in presence of Ert and ZA. However, the simultaneous binding of Ert and ZA inhibits the kinase by preventing the downstream phosphorylation because of the absence of a hydrolysable ɤ-phosphate. ANM of the HER1L858R mutant with Ert and ZA. Eigenvectors highlighting conformational fluctuations in the Cα-helix and activation loop (A loop) are shown. Of note is that, despite the presence of Ert and ZA, the Cα-helix is in a collapsed conformation. MD confirms ANM findings showing that, in presence of Ert and ZA, the interaction between R858 and Y891 locks the activation loop allowing the Cα-helix to collapse.
Molecular docking further revealed that the HER1L858R mutant conformation was similar to the active state of HER1wt in the presence of erlotinib and zoledronic acid (Fig. 2B). We used anisotropic network modeling (ANM) of the HER1L858R mutant with the two drugs to study conformational fluctuations in the Cα-helix and activation loop. We found that despite the presence of erlotinib and zoledronic acid, the Cα-helix remained in a collapsed conformation (Fig. 2B). Molecular dynamics (MD) confirmed our ANM findings showing that, in the presence of both drugs, the interaction between R858 and Y891 locked the activation loop allowing the Cα-helix to collapse (Fig. 2B). Thus, we speculate combinatorial binding of erlotinib and zoledronic acid will inhibit the HER1 kinase not by inhibiting Cα-helix collapse, but instead by preventing downstream phosphorylation because of the absence of a hydrolysable ɤ-phosphate.
We hypothesized that the biologic action of the drug pairs exceeds that of either drug; in other words, the two drugs behaved as “one big drug.” When submaximal concentrations of zoledronic acid (10 µM) and erlotinib (10 nM) were combined, the inhibition of colony formation in HCC827 cells was greater than that with either drug alone (Fig. S1A). Isobolograms showed evidence of additivity (CI or combination index ∼1) when erlotinib and zoledronic acid were combined in both HCC827 and H3255 cells (Fig. S1A). Similar results were noted with the minodronic acid/erlotinib combination, but not with tiludronate/erlotinib (Fig. S1B).
We sought to probe the cellular mechanism underlying the observed additivity. HCC827 cells treated with erlotinib showed a dramatic increase in apoptosis, as evidenced by increases in cells in the sub-G1 phase (FACS) and poly(ADP-ribose) polymerase (PARP) cleavage (Western blot), and reductions in pAKT (Fig. S1C). In contrast, zoledronic acid caused a marked cell-cycle arrest, noted as reduced cyclin B1/D1 and proliferating cell nuclear antigen (PCNA) expression (Western blot) (Fig. S1C). The two drugs together caused profoundly greater apoptosis, as evidenced by more marked increases in cells in the sub-G1 phase, as well as PARP cleavage (Fig. S1C). Importantly, the two bisphosphonates zoledronic acid and risedronate did not display additivity, either in viability inhibition (MTT) or cell-cycle arrest (FACS) (Fig. S1D). This result would not be unexpected because they bind to the same site within the HER1 kinase domain.
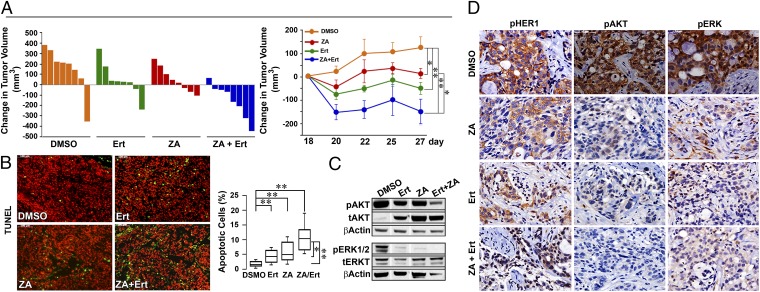
To provide proof-of-concept that bisphosphonates enhance the tumor suppressive action of erlotinib in vivo, we xenografted HCC827 cells into BALB/c nu/nu mice. Tumor volumes plotted for individual mice show that, whereas erlotinib and zoledronic acid each attenuated tumor growth (Fig. 1), combining the two drugs resulted in tumor regression (Fig. 3A). Nine days following treatment initiation, whereas each drug triggered a significant reduction in tumor volume, the effect of the two drugs surpassed the effect of either agent (Fig. 3A). Representative photomicrographs of TUNEL-labeled tumor tissue from mice displaying a median tumor volume are shown (Fig. 3B). The induction of apoptosis is both qualitatively and quantitatively greater in tissue obtained from mice treated with both drugs than with one drug alone (Fig. 3B). We also labeled tissue for phosphorylated HER1 (pHER1) and downstream molecules, pERK and pAKT. Again, whereas erlotinib and zoledronic acid both reduced phosphorylation, the effect of the two agents appeared more marked that either drug alone (Fig. 3 C and D).
Fig. 3.
Combination of bisphosphonate and TKI causes tumor regression. (A) Effect of relotinib (Ert), zoledronic acid (ZA), or Ert plus ZA on tumor volume in BALB/c nu/nu mice grafted with HCC827 cells [Waterfall plot or mean change (Δ) in tumor volume in mouse groups, versus DMSO]. Whereas Ert and ZA prevented tumor growth, the two drugs in combination caused tumors to regress. (B) Apoptotic cells stained with TUNEL (green) are shown in representative sections (Upper) and as cell number (Lower; percent of total cells; Box plots with upper and lower quartiles and range). Statistics: Two-tailed Student t test with Bonferroni’s correction; *P < 0.05, **P < 0.01; n = 8 mice per group. (C and D) Immunolabeling for pHER1, pAKT, and pERK (counterstained with hematoxylin/eosin) (D) confirmed by Western blotting for phosphorylated (p) and total (t) ERK1/2 and AKT (on representative tumor tissue displaying median volume) (C). Note: only relevant bands from Western blots are shown, with gaps introduced where irrelevant lanes are excised (SI Methods). (Magnification: B and D, 20×.)
Although TKIs result in objective treatment responses in HER1 mutation-driven lung cancers (13), resistance to therapy develops invariably, the most common mechanism being a second site mutation at the gatekeeper residue T790 (15). We therefore sought to determine, both computationally and experimentally, whether HER1-mutation–driven, TKI-resistant NSCLCs retain bisphosphonate sensitivity. If so, bisphosphonates could become a viable option for the therapy of resistant NSCLCs, which otherwise display a downhill course.
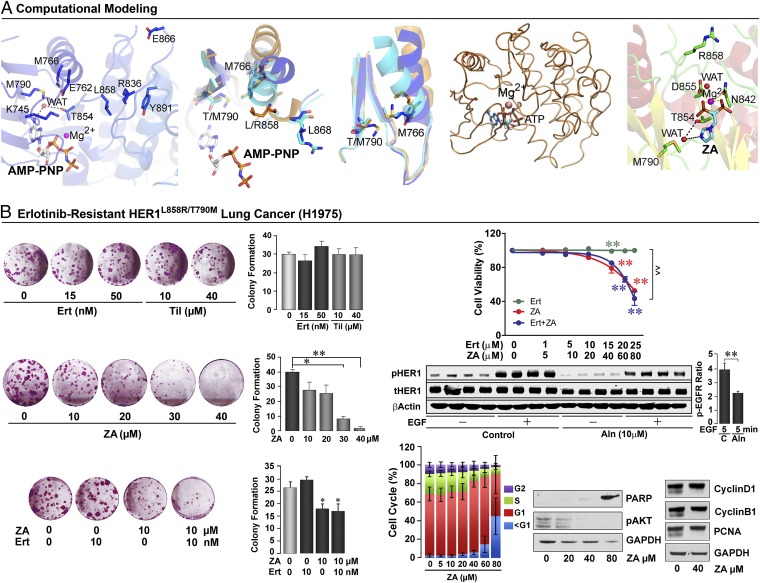
Computationally, we note that the HER1T790M mutation (PBD ID code 2JIU) causes only a partial collapse of the Cα-helix as mutant M790 acts as a wedge and interferes with the spatial positioning of the side chain of the highly conserved M766 (Fig. 4A). MD shows that, during movement of the activation loop, the Cα-helix assumes an activated conformation, with the HER1T790M mutation preventing complete collapse of the helix (Fig. 4A). However, whereas a partial collapse because of steric clashes between M790 and M793 cause resistance to erlotinib/gefitinib (16), the water molecule that bridges bisphosphonate interaction with M790 and T854 is preserved (Fig. 4A). This result predicts that bisphosphonate action will remain largely intact.
Fig. 4.
HER1 mutation T790M abolishes erlotinib but not bisphosphonate sensitivity. (A) Influence of the gatekeeper T790M mutation on the conformation of the HER1wt and HER1L858R. The HER1T790M mutation (PDB ID code 2JIU) allows only a partial collapse of the Cα-helix as mutated M790 acts like a wedge to obstruct the position of M766, a highly conserved residue throughout the kinase family. In HER1wt (orange), M766 and T790 are far apart. In HERT790M (blue), although the tilt angle of Cα-helix is ∼15°, the position of M766 is still tolerated by M790. Thus, erlotinib can bind to HER1T790M. In the HER1L858R mutant (cyan), the observed tilt angle is ∼23°; this positions M766 too close to T790. In the double-mutant HER1L858R/T790M, we therefore predict that the tilt caused by the HER1L858R activating mutation will be obstructed by M790, preventing total Cα-helix collapse. MD on HER1L858R/T790M in complex with ATP confirms that movement of the mutated activation loop allows the Cα-helix to assume an activated conformation, but prevents complete collapse of the helix over the binding site. However, the T790M mutation does not affect the binding mode of ZA’s imidazole ring by preserving the water molecule (WAT) that bridges ZA to T790 and T854. (B) Although Ert and tiludronate (Til) do not inhibit colony formation in HER1L858R/T790M (H1975) cells, ZA displays a strong inhibitory effect (mean colony counts per well ± SEM; two tailed Student t test with Bonferroni’s correction, versus zero dose; *P < 0.05, **P < 0.01; repeated three times, each in duplicate, data pooled). Furthermore, ZA inhibits H1975 cell viability (MTT assay). In contrast, Ert neither itself inhibits nor enhances the inhibitory action of ZA (unlike its effect in HER1L857R cells) (triplicate wells, done three times, data pooled; mean ± SEM; ANOVA with Bonferroni’s Correction, versus zero-dose; *P < 0.05, **P < 0.01; or combined treatment versus Ert; ^^P < 0.01). Western blots (biological quadruplicates) showing the inhibitory effect of alendronate (Aln) on EGF-induced phosphorylation of HER1L858R/T790M (pHER1) (β-actin and tHER1 as controls; versus without Aln; statistics by two-tailed Student t test; **P < 0.01, n = 4). Flow cytometry showing cell-cycle profile of H1975 cells in response to ZA, which stimulates apoptosis (repeated three times). Western blots showing the effect of ZA on PARP, pAKT, cyclin D1, cyclin B1, and PCNA (GAPDH: loading control; repeated three times).
We therefore explored the action of erlotinib and zoledronic acid in double-mutant HER1L858R/T790M lung cancer cells (H1975). Whereas erlotinib and tiludronate expectedly failed to inhibit colony formation or cell survival, zoledronic acid caused a concentration-dependent reduction in both parameters (Fig. 4B). This effect was distinct from that seen with H3255 cells in which HER1L858R drives cell proliferation, and where zoledronic acid and erlotinib are additive (compare with Fig. S1A). In H1975 cells, however, the effect of the two drugs used together was identical to that of zoledronic acid alone (MTT and colony formation) (Fig. 4B). Furthermore, as with other HER1-driven cells, zoledronic acid or alendronate inhibited HER1 phosphorylation and induced apoptosis, evidenced by an increase in cells in the sub-G1 phase, as well as enhanced PARP cleavage. There were minimal/no effects on cell cycle protein expression (Fig. 4B). Taken together, the data suggest that bisphosphonates could potentially be used to overcome TKI resistance.
Discussion
It has been unclear why only certain cancers respond to bisphosphonates and others do not. In a companion paper (4), we report that the HER family of RTKs is a bisphosphonate target and mediates the antitumor effects of bisphosphonates. Our computational predictions that aminobisphosphonates bind the HER1/2 kinase domain were confirmed in cell-free in vitro assays, notably protein thermal shift and kinase assays, using recombinant protein. Most notably, we found that the change in melting temperature of HER1 upon binding to bisphosphonates was reversed upon mutating three amino acids—K745, N842, and D855—that were predicted to mediate bisphosphonate-HER binding. Furthermore, we show that only HER-driven NSCLCs, namely HCC827, H3255, H1666, and H1703, responded to bisphosphonates in vitro in terms of cell viability inhibition; importantly, this effect was reversed upon knocking down the four HER isoforms. This latter finding established that the effect of bisphosphonates on cancer cell viability was HER-mediated.
Here, we report the effect of the two most commonly used, Food and Drug Approved-approved bisphosphonates, zoledronic acid and risedronate, on tumor growth in mice xenografted with HCC827 NSCLCs or HER1-driven MB231 breast cancer cells. The daily doses of the respective drugs (1.42 and 1.36 mg/kg) translate to approximately twice their annual cumulative exposure when used for the therapy of cancer metastases and Paget’s bone disease, respectively (Table S1). Both bisphosphonates significantly reduced tumor volume in the treatment (drug administered after tumors became palpable) and prevention (drug initiated at the time of tumor grafting) protocols. In contrast, mice grafted with HERlow SW620 colon cancer cells remained completely insensitive to bisphosphonate action. These proof-of-concept studies underscore the potential for repurposing of bisphosphonates for the prevention and treatment of HER-driven lung and other cancers, including breast, colon, gastric, and head/neck cancers (18).
Such repurposing efforts will nonetheless require purposeful pharmacokinetic, pharmacodynamic, and dose-finding studies of currently used bisphosphonates in cancer patients. Furthermore, noting that bisphosphonates were never designed as HER inhibitors, their reformulation may maximize bioavailability and improve efficacy. However, their plasma half-lives are likely to remain low as the drugs have high bone affinities and therefore rapidly disappear from plasma (1). This potential roadblock for their bedside transition might, in the future, mandate the development of a new class of HER1-avid bisphosphonates with low bone affinities.
We also offer a rationale for combining currently used bisphosphonates with TKIs to maximize therapeutic efficacy for NSCLCs. Computational predictions for combinatorial binding and experimental evidence, both in vitro and in vivo, provide compelling evidence for additivity between bisphosphonates and TKIs in inhibiting HER1 mutation-driven lung cancers. In fact, in mice grafted with HCC827 cells, although erlotinib and zoledronic acid each attenuated tumor growth, the two drugs together triggered tumor regression. Our computational and experimental evidence also suggests that the common TKI resistance mutation, T790M, does not impair the ability of bisphosphonates to interact with the HER1L858R kinase pocket, nor does it prevent their action in inhibiting cell survival. Thus, it should also be possible to use bisphosphonates for the treatment of TKI-resistant HER-driven metastatic lung cancer, underscoring a currently unmet clinical need.
Another question is whether the bisphosphonate–HER2 interaction, which we have demonstrated in thermal shift and Tyr-kinase assays (4), and in cell viability and knockdown studies (Figs. S2 and S3), is relevant clinically for the therapy of HER2-amplified breast cancers, and whether this interaction causes the reduced disseminated tumor cell burden noted in breast cancer patients treated with zoledronic acid (6–8). If such a benefit is proven, the use of bisphosphonates in a molecularly defined subgroup of HER2-amplified breast cancer patients, either alone or in combination with trastuzumab, may become a reality (19). However, although the anticancer actions of bisphosphonates appear to be dependent almost entirely on HER1/2 expression, certain other actions, such as antiangiogenic effects (20), may indeed be exerted via other RTKs, such as the VEGFR. This will depend on whether or not, as our data indicate, a bisphosphonate can fit into the kinase domain of a given RTK (4).
Materials and Methods
Computational docking, MD, and ANM studies were performed using HER crystal structures from the Protein Database. Cancer cell lines were subject to the MTT or colony formation assays as detailed in SI Methods (21). For cell-cycle assays, cells treated with bisphosphonate and erlotinib were subject to flow cytometry. For the in vivo studies, cells were injected in the flank of BALB/c nu/nu mice, with tumor sizes measured sequentially by calipers (21, 22), followed by TUNEL staining, immunohistochemistry, and Western blotting.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health Grants DK80459 (to M.Z. and L.S.), AG40132 (to M.Z.), AG23176 (to M.Z.), AR06592 (to M.Z.), and AR06066 (to M.Z.); the Italian Space Agency (A.Z.); a grant from National Science Foundation of China, Ministry of China (International Collaborative Grant to Z.B. and M.Z.); and the National Center for Advancing Translational Sciences, National Institutes of Health, through Icahn School of Medicine at Mount Sinai's Clinical and Translational Science Award (to S.I.). G.N., formerly recipient of a Howard Hughes Medical Institute Physician-Scientist Early Career Award, is a named Harrington Scholar.
Footnotes
Conflict of interest statement: M.Z., J.I., and G.N. are named inventors of a pending patent application related to the work described.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1421422111/-/DCSupplemental.
References
- 1.Russell RG. Bisphosphonates: The first 40 years. Bone. 2011;49(1):2–19. doi: 10.1016/j.bone.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 2.Wilson C, Holen I, Coleman RE. Seed, soil and secreted hormones: Potential interactions of breast cancer cells with their endocrine/paracrine microenvironment and implications for treatment with bisphosphonates. Cancer Treat Rev. 2012;38(7):877–889. doi: 10.1016/j.ctrv.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Coleman R, Gnant M, Morgan G, Clezardin P. Effects of bone-targeted agents on cancer progression and mortality. J Natl Cancer Inst. 2012;104(14):1059–1067. doi: 10.1093/jnci/djs263. [DOI] [PubMed] [Google Scholar]
- 4.Yuen T, et al. Bisphosphonates inactivate human EGFRs to exert antitumor actions. Proc Natl Acad Sci USA. 2014;111:17989–17994. doi: 10.1073/pnas.1421410111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gnant M, et al. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360(7):679–691. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 6.Coleman RE, et al. Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med. 2011;365(15):1396–1405. doi: 10.1056/NEJMoa1105195. [DOI] [PubMed] [Google Scholar]
- 7.Gnant M, et al. Long-term follow-up in ABCSG-12: Significantly improved overall survival with adjuvant zoledronic acid in premenopausal patients with endocrine-receptor–positive early breast cancer. Cancer Res. 2011;71(24) Suppl:S1–S2. [Google Scholar]
- 8.Coleman R, et al. Zoledronic acid (zoledronate) for postmenopausal women with early breast cancer receiving adjuvant letrozole (ZO-FAST study): Final 60-month results. Ann Oncol. 2013;24(2):398–405. doi: 10.1093/annonc/mds277. [DOI] [PubMed] [Google Scholar]
- 9.Pazianas M, Abrahamsen B, Eiken PA, Eastell R, Russell RG. Reduced colon cancer incidence and mortality in postmenopausal women treated with an oral bisphosphonate—Danish National Register Based Cohort Study. Osteoporos Int. 2012;23(11):2693–2701. doi: 10.1007/s00198-012-1902-4. [DOI] [PubMed] [Google Scholar]
- 10.Rennert G, Pinchev M, Rennert HS, Gruber SB. Use of bisphosphonates and reduced risk of colorectal cancer. J Clin Oncol. 2011;29(9):1146–1150. doi: 10.1200/JCO.2010.33.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sendur MA, et al. Demographic and clinico-pathological characteristics of breast cancer patients with history of oral alendronate use. Med Oncol. 2012;29(4):2601–2605. doi: 10.1007/s12032-012-0209-9. [DOI] [PubMed] [Google Scholar]
- 12.Ding L, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455(7216):1069–1075. doi: 10.1038/nature07423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med. 2008;358(11):1160–1174. doi: 10.1056/NEJMra0707704. [DOI] [PubMed] [Google Scholar]
- 14.Nishino M, et al. Imaging of lung cancer in the era of molecular medicine. Acad Radiol. 2011;18(4):424–436. doi: 10.1016/j.acra.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sequist LV, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yun CH, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci USA. 2008;105(6):2070–2075. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang JW, et al. Bisphosphonate zoledronic acid enhances the inhibitory effects of gefitinib on EGFR-mutated non-small cell lung carcinoma cells. Cancer Lett. 2009;278(1):17–26. doi: 10.1016/j.canlet.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 18.Krasinskas AM. EGFR signaling in colorectal carcinoma. Pathol Res Int. 2011;2011:932932. doi: 10.4061/2011/932932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romond EH, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 20.Di Salvatore M, et al. Anti-tumour and anti-angiogenetic effects of zoledronic acid on human non-small-cell lung cancer cell line. Cell Prolif. 2011;44(2):139–146. doi: 10.1111/j.1365-2184.2011.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sangodkar J, et al. Targeting the FOXO1/KLF6 axis regulates EGFR signaling and treatment response. J Clin Invest. 2012;122(7):2637–2651. doi: 10.1172/JCI62058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hemmi H, et al. Treml4, an Ig superfamily member, mediates presentation of several antigens to T cells in vivo, including protective immunity to HER2 protein. J Immunol. 2012;188(3):1147–1155. doi: 10.4049/jimmunol.1102541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.