Significance
Erythritol is a preferential substrate for Brucella, a common zoonotic bacterial pathogen. This four-carbon polyol is found in the reproductive organs of several affected species, a feature that may account for the characteristic viscerotropism of Brucella that leads to sterility and abortion. Although described previously as feeding glycolysis via dihydroxyacetone-phosphate, we show here that erythritol is actually converted into D-erythrose-4-phosphate through a hitherto undescribed set of reactions that involves three isomerases and that allows hexose-monophosphate synthesis and growth by feeding the pentose phosphate shunt. Elucidation of this unique carbohydrate pathway, which also applies to the Rhizobiales plant endosymbionts, opens the way for further research on the metabolic adaptation of an important facultative intracellular pathogen to target organs.
Keywords: Brucella, erythritol, alphaproteobacteria, pentose phosphate cycle, tetrose metabolism
Abstract
Erythritol is an important nutrient for several α-2 Proteobacteria, including N2-fixing plant endosymbionts and Brucella, a worldwide pathogen that finds this four-carbon polyol in genital tissues. Erythritol metabolism involves phosphorylation to l-erythritol-4-phosphate by the kinase EryA and oxidation of the latter to l-3-tetrulose 4-phosphate by the dehydrogenase EryB. It is accepted that further steps involve oxidation by the putative dehydrogenase EryC and subsequent decarboxylation to yield triose-phosphates. Accordingly, growth on erythritol as the sole C source should require aldolase and fructose-1,6-bisphosphatase to produce essential hexose-6-monophosphate. However, we observed that a mutant devoid of fructose-1,6-bisphosphatases grew normally on erythritol and that EryC, which was assumed to be a dehydrogenase, actually belongs to the xylose isomerase superfamily. Moreover, we found that TpiA2 and RpiB, distant homologs of triose phosphate isomerase and ribose 5-phosphate isomerase B, were necessary, as previously shown for Rhizobium. By using purified recombinant enzymes, we demonstrated that l-3-tetrulose-4-phosphate was converted to d-erythrose 4-phosphate through three previously unknown isomerization reactions catalyzed by EryC (tetrulose-4-phosphate racemase), TpiA2 (d-3-tetrulose-4-phosphate isomerase; renamed EryH), and RpiB (d-erythrose-4-phosphate isomerase; renamed EryI), a pathway fully consistent with the isotopomer distribution of the erythrose-4-phosphate-derived amino acids phenylalanine and tyrosine obtained from bacteria grown on 13C-labeled erythritol. d-Erythrose-4-phosphate is then converted by enzymes of the pentose phosphate pathway to glyceraldehyde 3-phosphate and fructose 6-phosphate, thus bypassing fructose-1,6-bisphosphatase. This is the first description to our knowledge of a route feeding carbohydrate metabolism exclusively via d-erythrose 4-phosphate, a pathway that may provide clues to the preferential metabolism of erythritol by Brucella and its role in pathogenicity.
Brucellosis is a widespread bacterial zoonosis caused by the facultative intracellular pathogens Brucella spp. Domestic ruminants and swine, which altogether represent more than 4 billion animals worldwide, are highly susceptible, and in this livestock, the brucellae characteristically target the genital organs and cause abortion and infertility (1). Close to parturition, these bacteria reach exceedingly high numbers (up to 1014 bacteria per conceptus) (2), thus making brucellosis abortion a highly contagious condition.
It is accepted that this tropism and extensive multiplication relate to the high erythritol concentration in the genital organs of those animals (3, 4). In vitro, this four-carbon polyol is a preferential carbon source of Brucella, promotes growth, and up-regulates the expression of major virulence factors and key enzymes of central metabolism (5–9). In vivo, it has been observed that parenteral injections of erythritol increase the numbers of Brucella in the spleens of intraperitoneally infected calves (3, 7). For these reasons, erythritol has been considered a major factor in Brucella pathogenesis.
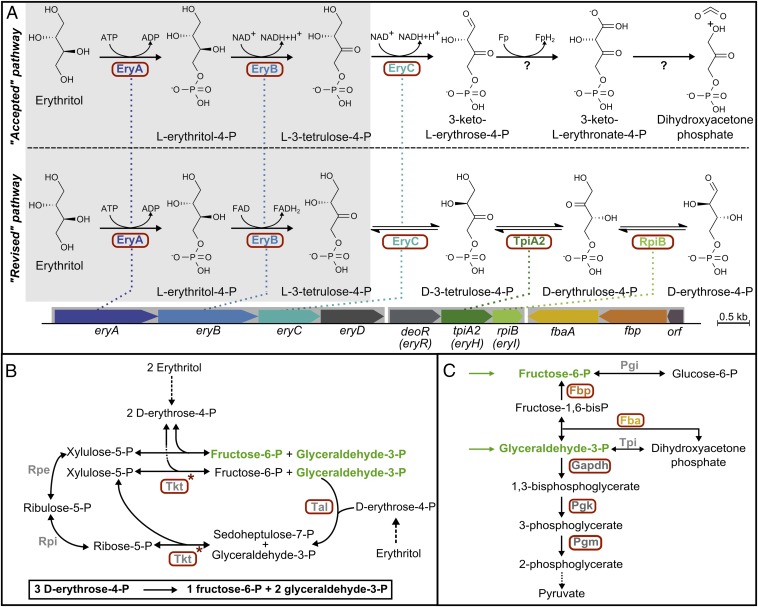
Erythritol metabolism (Fig. 1A, accepted pathway) starts with its phosphorylation by EryA to yield l-erythritol-4-P (10, 11), which is then oxidized by the dehydrogenase EryB to l-3-tetrulose-4-P (10). It is accepted that the next three steps imply the oxidation of the first carbon of l-3-tetrulose-4-P to an aldehyde by an NAD-dependent dehydrogenase, purportedly EryC (12), followed by dehydrogenation and decarboxylation reactions generating dihydroxyacetone-P and carbon dioxide as end products (10).
Fig. 1.
Erythritol catabolism by Brucella. (A) The two first reactions of the “accepted” and the “revised” pathways are similar (highlighted in gray and further characterized in Figs. S1 and S2 and Table S1). Although it was previously admitted that l-3-tetrulose-4-P is afterward oxidized to dihydroxyacetone-phosphate and CO2, we now show that this intermediate is converted to d-erythrose-4-P via three isomerization reactions catalyzed by EryC, TpiA2, and RpiB. The relevant enzyme-encoding genes are organized as illustrated in two operons also comprising two regulators (EryD and DeoR). (B) d-erythrose-4-P is further processed by enzymes of the pentose phosphate pathway to generate fructose-6-P and glyceraldehyde-3-P, two intermediates of glycolysis/gluconeogenesis (C). The proteins whose genes are induced by erythritol (9) are circled in red. The new proposed gene names are indicated in parentheses below the old designations. Fba, fructose bisphosphate aldolase; Fbp, fructose bisphosphatase; G6P, glucose-6-P; Gapdh, glyceraldehyde-3-P dehydrogenase; Pgk, phosphoglycerate kinase; Pgm, phosphoglycerate mutase; R5P, ribose-5-P; Rpe, ribulose-5-P epimerase; Rpi, ribose-5-P isomerase; Ru5P, ribulose-5-P; S7P, sedoheptulose-7-P; Tal, transaldolase; Tkt, transketolase; Tpi, triose-P isomerase; X5P, xylulose-5-P.
The use of this pathway to grow on erythritol is not restricted to brucellae or to their intracellular pathogenic lifestyle but is shared by other members of the Rhizobiales, such as Ochrobactrum anthropi (an environmental bacteria), Rhizobium leguminosarum, and Sinorhizobium meliloti (both plant symbionts) (13, 14). In all these bacteria, there is, downstream of the ery operon, a second highly conserved operon that contains three ORFs including tpiA2 and rpiB, which are homologous to triose phosphate isomerase and ribose 5-phosphate isomerase B, respectively (13). Interestingly, rpiB and tpiA2 mutants of R. leguminosarum (15) are unable to grow on erythritol as a carbon source.
Because the involvement of two putative isomerases is not considered in the accepted pathway, where a dehydrogenase and a decarboxylase are predicted to catalyze the last two steps, we reassessed the erythritol pathway. A combination of genetic, biochemical, and isotopic evidence brought us to the conclusion that erythritol metabolism involves three as-yet undescribed isomerization reactions catalyzed by EryC, TpiA2, and RpiB, which together lead to the formation of d-erythrose-4-P, rather than dihydroxyacetone-P. Erythrose-4-P can then be converted into glyceraldehyde-3-P and fructose-6-P through reactions catalyzed by enzymes of the pentose phosphate pathway (Fig. 1B).
Results
Fructose-1,6-Bisphosphatases Are Dispensable for Growth on Erythritol.
Because hexose-monophosphates are essential biomass precursors, the accepted pathway predicts that growth on erythritol as the sole carbon source requires isomerization of dihydroxyacetone-P to glyceraldehyde-3-P, triose-P condensation by aldolase, and dephosphorylation of fructose-1,6-P2 by fructose-1,6-bisphosphatase (FBPase). Brucella spp. carry only two FBPase genes [fbp and glpX (16)], and surprisingly, a double FBPase mutant (Brucella abortus 2308∆fbp∆glpx) showed no growth defect when provided with erythritol as the only carbon source (Fig. S3A), indicating that this polyol is converted not into trioses-P but into a product that can generate fructose-6-P without involvement of fructose-1,6-P2. Because d-erythrose-4-P can be converted to fructose-6-P and glyceraldehyde-3-P via the pentose phosphate pathway (Fig. 1B), we hypothesized that l-3-tetrulose-4-P is converted to d-erythrose-4-P by a series of isomerization reactions (Fig. 1A).
Three Putative Isomerases/Epimerases Are Required Instead of Dehydrogenases.
Our hypothesis was supported by sequence comparisons showing that EryC was not a dehydrogenase homolog but, rather, a member of the metal-dependent xylose isomerase-like superfamily (17). Two other hypothetical isomerases, TpiA2 and RpiB, which are homologous to triose-P isomerase and ribose-P isomerase, respectively, are required for growth of R. leguminosarum on erythritol (15). Similarly, B. abortus mutants (∆tpiA2 and ∆rpiB) in the orthologous genes, which are downstream of the ery operon, did not grow on erythritol (Fig. S3 B and C). These results, together with previous evidence on the indispensability of EryC (12), indicate that three putative isomerases are in fact involved in erythritol metabolism.
EryC, TpiA2, and RpiB Convert l-3-Tetrulose-4-P to d-Erythrose-4-P.
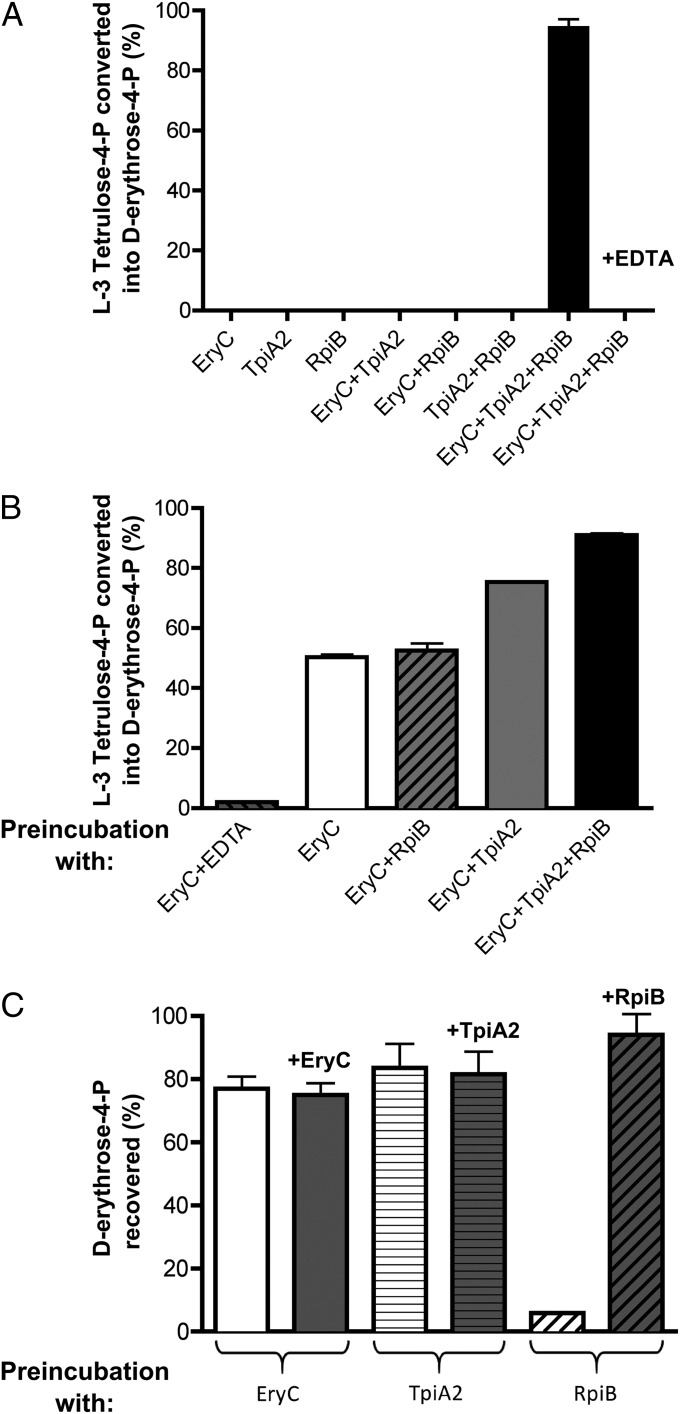
To confirm the role of EryC, TpiA2, and RpiB, l-3-tetrulose-4-P was produced from l-erythritol-4-P with EryB, purified, and tested for conversion to d-erythrose-4-P by the purified proteins. Using an assay in which d-erythrose-4-P formation was determined spectrophotometrically, with purified Escherichia coli erythrose-4-P dehydrogenase (18) as the coupling enzyme, we found that d-erythrose-4-P was formed from the product of EryB when EryC, TpiA2, and RpiB were present together in the assay mixture (Fig. 2A). No reaction was observed if any of these three proteins or the coupling enzyme was omitted or if EDTA was present, in all likelihood because of the metal-dependency of EryC (Fig. S4). These findings confirmed that all three isomerases are required and d-erythrose-4-P is the end-product of erythritol catabolism.
Fig. 2.
Conversion of l-3-tetrulose-4-P to d-erythrose-4-P: requirement of EryC, TpiA2, and RpiB (A), and determination of the sequence of their reactions (B and C). (A) l-3-tetrulose-4-P was incubated with EryC, TpiA2, and RpiB, alone or in combinations, and the formation of d-erythrose-4-P was monitored spectrophotometrically in the presence of NAD and erythrose-4-P dehydrogenase (E4PDH). (B) l-3-tetrulose-4-P was preincubated with EryC, alone or in combination with TpiA2 or RpiB or both. The preincubation was stopped with EDTA, and then TpiA2 or RpiB were added to the incubation mixtures from which they had been omitted, and the formation of d-erythrose-4-P was monitored with E4PDH. The five values shown are significantly different from each other (P < 0.05 by Student t test), with the exception of the comparison of “EryC” with “EryC+RpiB,” where there is no difference. (C) d-erythrose-4-P was incubated with EryC, TpiA2, or RpiB. These enzymes were removed from the incubation medium before spectrophotometric measurement of the remaining d-erythrose-4-P in the presence of E4PDH. Afterward, the enzyme used during the preincubation was added back, as indicated, to ensure that the metabolite had not been depleted because of an unrelated reaction (e.g., dephosphorylation by a hypothetical contaminating phosphatase). In all panels, error bars represent the SD for three distinct experiments. These error bars are too small to be visible in some cases.
Identification of the Sequence for the Three Isomerization Reactions.
We took advantage of the metal-dependency of EryC to check whether this protein acted directly on l-3-tetrulose-4-P. This compound was preincubated with EryC, the reaction was stopped with EDTA, and the formation of d-erythrose-4-P was measured in the presence of TpiA2 and RpiB (Fig. 2B, second column). We found that under these conditions, about 50% of the l-3-tetrulose-4-P had been converted by EryC during the preincubation step to a substrate for TpiA2 and RpiB, suggesting that EryC catalyzed the racemization of l-3-tetrulose-4-P to its d-isomer. Experiments in which l-3-tetrulose-4-P was preincubated with EryC and either TpiA2 or RpiB indicated that TpiA2, but not RpiB, increased the amount of tetrose-4-phosphate that became available for conversion to erythrose-4-P and erythronate-4-P in the subsequent assay (Fig. 2B, columns 3 and 4; see SI Results for a more detailed interpretation of this experiment). Accordingly, the last specific step of the pathway should be the isomerization catalyzed by RpiB. This was confirmed by showing that incubation of d-erythrose 4-phosphate with RpiB (although not with EryC or TpiA2) caused about a 90% decrease in its concentration (Fig. 2C), a result consistent with the fact that the equilibrium of the d-erythrulose-4-P/erythrose-4-P isomerization is much in favor of d-erythrulose-4-P (19).
Identification of the Intermediates with Tritiated Borohydride and Periodate Oxidation.
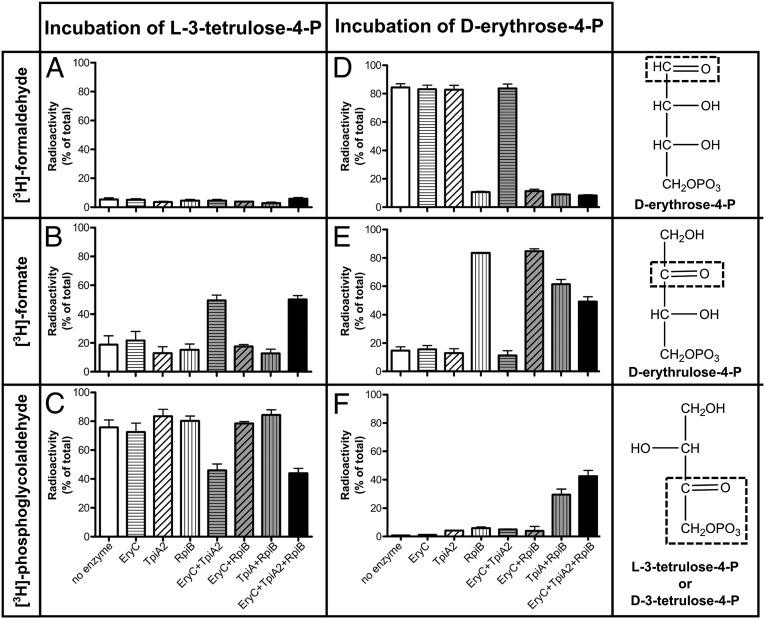
To analyze further the reactions catalyzed by the three isomerases, we examined the nature of the successively formed tetrose-4-Ps by taking advantage of the fact that reduction of erythrose-4-P, erythrulose-4-P, and 3-tetrulose-4-P with tritiated borohydride leads to specific incorporation of tritium on their C1, C2, and C3, respectively. Oxidation with periodate breaks down polyols according to well-known rules (20) predicting that the first carbon of tetritol-4-P will be converted to formaldehyde, the second one to formate, and the third and fourth ones to phosphoglycolaldehyde (see the expected patterns in Fig. S5). These breakdown products, which keep the original hydrogen–carbon linkages, can be easily separated by anion-exchange chromatography. The nature of the radiolabeled breakdown products thus informs directly on the position of the oxo group in the intermediates.
The experiments performed on the products resulting from incubations of either d-erythrose-4-P or l-3-tetrulose-4-P with the three isomerases alone or in combinations (Fig. 3) led to the following conclusions: First, EryC acts on l-3-tetrulose-4-P without moving the position of the oxo function (Fig. 3 B, C, E, and F), confirming therefore that it is a 3-tetrulose-4-P racemase (Fig. 1A). Second, TpiA2 moves reversibly the oxo group between the second and the third carbons (Fig. 3 B and C), thus interconverting a 3-tetrulose-4-P and an erythrulose-4-P. It is specific for d-3-tetrulose-4-P because it does not act directly on the product of EryB, but well after its racemization with EryC. As illustrated in Fig. 1A, it acts in the reverse direction on the isomer of erythrulose-4-P made from erythrose-4-P by RpiB (i.e., the d-isomer; Fig. 3 E and F). Not unexpectedly, the stereochemistry of the TpiA2 reaction is similar to that of the homologous enzyme triose-phosphate isomerase (TPI in Fig. S6). As shown in Fig. S7, the replacement of the highly conserved Asn10 in triose phosphate isomerase by a serine in TpiA2 presumably allows the accommodation of a larger substrate; that is, a tetrose-P instead of a triose-P. Finally, RpiB reversibly moves the oxo group between C1 and C2 (Fig. 3 D and E), thus catalyzing the isomerization of d-erythrose-4-P with d-erythrulose-4-P (Fig. 1A).
Fig. 3.
Analysis by [3H]borohydride labeling/periodate oxidation of the products made from l-3-tetrulose-4-P (A–C) and d-erythrose-4-P (D–F) by EryC, TpiA2, and RpiB. Erythrose-4-P and l-3-tetrulose-4-P were incubated alone, with EryC, TpiA2, and Rpib or in combination, as indicated. [3H]borohydride was added to the reaction mixture and the incubation pursued for 2 h. The samples were acidified with perchloric acid and neutralized. The tetritol-phosphates were purified by anion exchange chromatography and submitted to periodate oxidation. The products were then analyzed by anion-exchange chromatography to separate [3H]-formaldehyde (A and D), [3H]-formate (B and E), and [3H]-phosphoglycolaldehyde (C and F). Tritiated formaldehyde, formate, and phosphoglycolaldehyde are produced in this manner from d-erythrose-4-P, d-erythrulose-4-P, and d- or l-3-tetrulose-4-P, respectively, because of the fixation of tritium on the carbons boxed in the right panels. Mean of two experiments, with the two extremities of the error bars corresponding to the individual values.
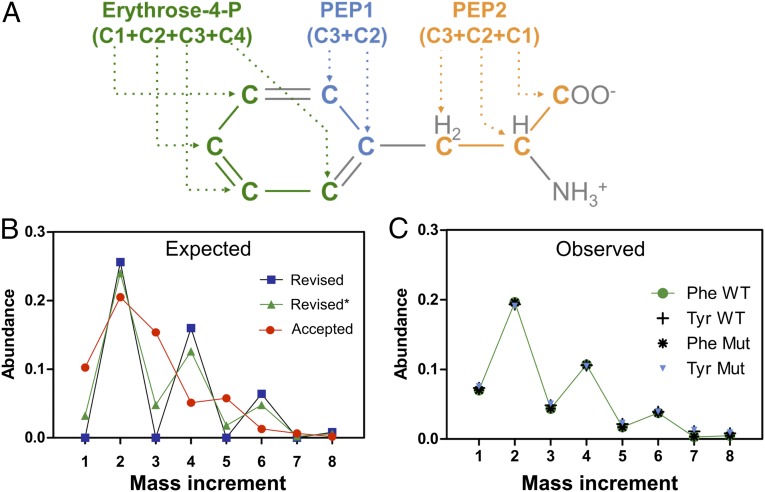
Isotopic Labeling of Phenylalanine and Tyrosine Supports the Revised Pathway.
Because phenylalanine and tyrosine are formed from one molecule of d-erythrose-4-P and two molecules of phosphoenolpyruvate (Fig. 4A), analysis of the isotopomers of these amino acids in cells grown with a mixture of 80% [12C] and 20% [U-13C] (uniformly labeled) erythritol should allow us to discriminate the “accepted” and the “revised” pathways. In the former, the M+4 isotopomer of d-erythrose-4-P directly results from [U-13C] erythritol metabolism and amounts therefore to ∼ 20% of total d-erythrose-4-P. In the latter, d-erythrose-4-P would result from recombination of [U-13C] triose-phosphate with a 13C-labeled carbon from another intermediate of the pentose phosphate pathway, thus amounting to only about 4% of total d-erythrose-4-P (Table S2). The observed distribution of the M+1 to M+8 isotopomers in carbons C2–C9 of phenylalanine and tyrosine were in much better agreement with the prediction of the revised than of the accepted pathway (Fig. 4 B and C), particularly if we took into account the possibility that part of d-[U-13C]erythrose-4-P may be converted to [2,3,4-13C] and [1-13C] erythrose-4-P through exchange reactions catalyzed by transaldolase and transketolase.
Fig. 4.
(A) Origin of the different carbons in phenylalanine and tyrosine and their expected (B) and observed (C) mass isotopomer distribution formed in wild-type (WT) and FBPase-deficient (mut) cells grown on 20% [U-13C] erythritol. The analyzed [M-159] fragment ions (SI Materials and Methods) comprise carbons C2–C9 of phenylalanine (Phe, m/z 234) or tyrosine (Tyr, m/z 364). These carbons are derived from one molecule of erythrose-4-P and from C2–C3 of two molecules of phosphoenolpyruvate. The expected distributions of isotopomers were calculated as described in SI Materials and Methods for the “accepted” pathway and the “revised” pathway, either assuming (revised*) or not (revised) 25% exchange reactions between labeled and unlabeled d-erythrose-4-P.
Discussion
This work leads to a substantial revision of the erythritol pathway, most particularly of the steps beyond l-3-tetrulose-4-P. These steps involve three enzymes that have not been described for any other species. Because the role of TpiA2 and RpiB in the metabolism of erythritol is now established, we propose to rename their genes eryH and eryI [the designations eryE, eryF, and eryG have already been proposed for the erythritol transport system of B. abortus (21)]. The gene encoding the putative regulatory protein DeoR, which precedes tpiA2 on the chromosome, has been designated eryR by Geddes and colleagues (13). Expression of these genes, including eryA,B,C,D, is induced in the presence of erythritol (9, 12). This regulation is partly based on the release by erythritol of the EryD-dependent repression of eryA,B,C,D expression (12), whereas the role of EryR remains unknown. By analogy with other members of the DeoR family, EryR binds in all likelihood a phosphate ester, presumably an intermediate of the revised erythritol pathway (15). It would thus participate, together with EryD, in the regulation of the expression of enzymes involved in erythritol catabolism.
Of note, adjacent homologs of tpiA2 (with the typical Asn to Ser substitution, Fig. S7) and rpiB are also found always downstream of a putative dihydroxyacetone kinase in bacterial genomes devoid of eryA and eryB (Fig. S8). We propose that they are involved in the metabolism of l-erythrulose (i.e., d-3-tetrulose), which would be phosphorylated by the “dihydroxyacetone kinase” to d-3-tetrulose-4-P and isomerized to d-erythrose-4-P by the TpiA2 and RpiB homologs.
The revised erythritol pathway is remarkable not only because it involves three enzymes that have never been previously identified but also because it provides the first example, to our knowledge, of a substrate that feeds carbohydrate metabolism exclusively via d-erythrose-4-P. We propose that the latter is converted to intermediates of glycolysis and gluconeogenesis by reactions involving the pentose phosphate pathway enzymes (Fig. 1B). The balance of this set of reactions is the net formation of two molecules of glyceraldehyde-3-P and one molecule of fructose-6-P from three molecules of d-erythrose-4-P.
A flow of metabolites coming from erythritol and reaching first the erythrose-4-P pool may be advantageous to Brucella. Indeed, this tetrose-P feeds, via chorismate, the synthesis of aromatic amino acids and of the siderophore 2,3-dihydroxybenzoic acid (22), the production of which is, in fact, increased by erythritol under iron-limiting conditions (23). These characteristics of the revised pathway may explain why the brucellae reach exceedingly high numbers in genital tissues. The highly abortifacient effect of some currently used brucellosis vaccines, most notably Rev1 (Revertant 1), may be related with their ability to multiply in genital organs and to consume erythritol (24). Accordingly, B. abortus S19, a vaccine that is not able to catabolize erythritol, is associated with a low risk for abortion (25). The risk for abortion is a serious drawback in mass vaccination, which is the only control strategy applicable in large areas of the world (26). A full understanding of erythritol metabolism may provide clues for developing safer vaccines. Furthermore, as none of the five enzymes involved in erythritol metabolism is shared with the host, it opens perspectives for the development of new therapeutic agents aimed at inhibiting this pathway.
Materials and Methods
Construction of Deletion Strains and Growth of B. abortus.
Clean deletion mutants of B. abortus 2308 for fbp, glpx, tpiA2, and rpiB were made by allelic replacement and complemented as described in the SI Materials and Methods. Brucella was grown at 37 °C on rich medium 2YT or on a chemically defined medium (modified Plommet medium), as detailed in SI Materials and Methods. The growth was monitored by following the OD (600 nm) during 48–72 h in an automated plate reader (Bioscreen C, Lab Systems) with continuous shaking.
Protein Purification.
Bifidus crudilactis phosphoketolase gene and Brucella eryA, eryB, eryC, tpiA2, and rpiB were inserted into pET15b vectors, which allow expression of N-terminal His-tagged proteins in E. coli Bl21pLysS. Proteins were purified by chromatography on His-trap columns to at least 80% homogeneity. A membrane pellet derived from E. coli expressing EryB was used as source of this enzyme, as it could not be purified. Details are provided in SI Materials and Methods.
Synthesis of l-Erythritol-4-P, l-3-Tetrulose-4-P, and d-Erythrose-4-P.
l-Erythritol-4-phosphate was prepared enzymatically (11), using EryA, and purified. l-3-tetrulose-4-P was prepared by oxidation of l-erythritol-4-P, using EryB and p-benzoquinone as an electron acceptor. d-Erythrose-4-P was produced enzymatically with fructose-6-P phosphoketolase from B. crudilactis. See SI Materials and Methods for details.
Enzymatic Assays.
The erythritol kinase activity of EryA was assayed at 30 °C spectrophotometrically by following ADP formation, coupled with the oxidation of NADH. The A340nm was measured either on a spectrophotometer or on a plate reader. The l-erythritol-4-P dehydrogenase activity of EryB was assayed at 30 °C by following A600nm in a mixture containing 2,6-dichlorophenolindophenol (DCPIP) as an artificial electron acceptor.
The activity of EryC, TpiA2, and RpiB were measured indirectly by measuring the formation of d-erythrose-4-P from l-3-tetrulose-4-P or the depletion of d-erythrose-4-P on incubation with the indicated enzymes. Briefly, l-3-tetrulose-4-P (0.1 mM) was incubated (30 min at 30 °C) in a mixture (100 μL) containing 25 mM Tris at pH 8.0 and 1 µg EryC, TpiA2, or RpiB (alone or in combination). EDTA was added to a 2-mM final concentration, and TpiA2 or RpiB were added to the incubation mixtures in which they were omitted and d-erythrose-4-P was quantified enzymatically. d-erythrose-4-P (0.1 mM) was incubated (30 min at 30 °C) in a mixture (800 μL) containing 25 mM Tris at pH 8.0 and 5 µg EryC, TpiA2, or RpiB (alone or in combination). Enzymes were removed by mixing with 50 μL Ni-NTA resin for 30 min at room temperature, followed by centrifugation, and d-erythrose-4-P was quantified enzymatically (SI Materials and Methods).
Characterization of Tetrose-Phosphates by [3H]borohydride Reduction and Periodate Oxidation.
l-3-tetrulose-4-P or d-erythrose-4-P (0.25 mM) was incubated at 30 °C in a mixture (100 μL) containing 25 mM Hepes at pH 7.4, 1 mM MgCl2, and 2 µg of the indicated enzyme or enzymes (see Results); after 30 min, 1 mCi tritiated sodium borohydride (final concentration, 2.5 mM) was added and reduction was allowed to proceed on ice for 2 h. Perchloric acid was then added to a final concentration of 10% (wt/vol) to destroy residual borohydride and denatured enzymes. After 2 h at room temperature, samples were neutralized and the tetritol-phosphates were purified and oxidized with periodate, and their breakdown product was analyzed, as described in SI Materials and Methods.
Isotopic Labeling Experiments and GC/MS Analysis.
B. abortus 2308 was grown in chemically defined medium with erythritol as sole carbon source composed of 80% [U-12C] and 20% [U-13C]. During exponential growth, cells were harvested, washed, and hydrolyzed with 6-M HCl at 100 °C for 24 h. Samples were then lyophilized, amino acid derivatization was carried out, and the tert-butyl-dimethylsilyl (tBDMS) derivatives were then analyzed by GC-MS (further details in SI Materials and Methods).
Supplementary Material
Acknowledgments
The E.V.S. laboratory is supported by a Welbio grant of the Walloon Region and by a grant from the Fonds de la Recherche Scientifique Médicale. J.-J.L.’s team is supported by an FNRS (Fonds National de la Recherche Scientifique) grant (Fonds de la Recherche Fondamentale Collective Grant N° 2452110) and by the Interuniversity Attraction Poles Programme initiated by the Belgian Science Policy Office. T.B. has a PhD grant as “Aspirant FNRS.” I.M.’s laboratory is supported by grants from the Ministerio de Economía y Competitividad of Spain (AGL2011-30453-C04-00) and the Fundación para la Investigación Médica Aplicada. A.Z.-R. has a postdoctoral grant from FIMA. The research team of C.W. acknowledges financial support from the German Research Foundation through Schwerpunktprogramm 1316 “Host-Pathogen Interaction.”
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1414622111/-/DCSupplemental.
References
- 1.Atluri VL, Xavier MN, de Jong MF, den Hartigh AB, Tsolis RM. Interactions of the human pathogenic Brucella species with their hosts. Annu Rev Microbiol. 2011;65:523–541. doi: 10.1146/annurev-micro-090110-102905. [DOI] [PubMed] [Google Scholar]
- 2.Corner LA. Three aspects of bovine brucellosis: Epidemiology, the role of bulls and vaccines. New South Wales Vet Proc. 1983;19:47–48. [Google Scholar]
- 3.Smith H, et al. Foetal erythritol: A cause of the localization of Brucella abortus in bovine contagious abortion. Nature. 1962;193:47–49. doi: 10.1038/193047a0. [DOI] [PubMed] [Google Scholar]
- 4.Keppie J, Williams AE, Witt K, Smith H. The role of erythritol in the tissue localization of the brucellae. Br J Exp Pathol. 1965;46:104–108. [PMC free article] [PubMed] [Google Scholar]
- 5.McCullough NB, Beal GA. Growth and manometric studies on carbohydrate utilization of Brucella. J Infect Dis. 1951;89(3):266–271. doi: 10.1093/infdis/89.3.266. [DOI] [PubMed] [Google Scholar]
- 6.Anderson JD, Smith H. The metabolism of erythritol by Brucella abortus. J Gen Microbiol. 1965;38:109–124. doi: 10.1099/00221287-38-1-109. [DOI] [PubMed] [Google Scholar]
- 7.Williams AE, Keppie J, Smith H. The chemical basis of the virulence of Brucella abortus. III. Foetal erythritol a cause of the localisation of Brucella abortus in pregnant cows. Br J Exp Pathol. 1962;43:530–537. [PMC free article] [PubMed] [Google Scholar]
- 8.Petersen E, et al. Erythritol triggers expression of virulence traits in Brucella melitensis. Microbes Infect. 2013;15(6-7):440–449. doi: 10.1016/j.micinf.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodríguez MC, et al. Evaluation of the effects of erythritol on gene expression in Brucella abortus. PLoS ONE. 2012;7(12):e50876. doi: 10.1371/journal.pone.0050876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sperry JF, Robertson DC. Erythritol catabolism by Brucella abortus. J Bacteriol. 1975;121(2):619–630. doi: 10.1128/jb.121.2.619-630.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lillo AM, Tetzlaff CN, Sangari FJ, Cane DE. Functional expression and characterization of EryA, the erythritol kinase of Brucella abortus, and enzymatic synthesis of L-erythritol-4-phosphate. Bioorg Med Chem Lett. 2003;13(4):737–739. doi: 10.1016/s0960-894x(02)01032-6. [DOI] [PubMed] [Google Scholar]
- 12.Sangari FJ, Agüero J, García-Lobo JM. The genes for erythritol catabolism are organized as an inducible operon in Brucella abortus. Microbiology. 2000;146(Pt 2):487–495. doi: 10.1099/00221287-146-2-487. [DOI] [PubMed] [Google Scholar]
- 13.Geddes BA, Hausner G, Oresnik IJ. Phylogenetic analysis of erythritol catabolic loci within the Rhizobiales and proteobacteria. BMC Microbiol. 2013;13:46. doi: 10.1186/1471-2180-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geddes BA, Oresnik IJ. Genetic characterization of a complex locus necessary for the transport and catabolism of erythritol, adonitol and L-arabitol in Sinorhizobium meliloti. Microbiology. 2012;158(Pt 8):2180–2191. doi: 10.1099/mic.0.057877-0. [DOI] [PubMed] [Google Scholar]
- 15.Yost CK, Rath AM, Noel TC, Hynes MF. Characterization of genes involved in erythritol catabolism in Rhizobium leguminosarum bv. viciae. Microbiology. 2006;152(Pt 7):2061–2074. doi: 10.1099/mic.0.28938-0. [DOI] [PubMed] [Google Scholar]
- 16.Zúñiga-Ripa A, et al. Brucella abortus depends on pyruvate phosphate dikinase and malic enzyme but not on Fbp and GlpX fructose-1,6-bisphosphatases for full virulence in laboratory models. J Bacteriol. 2014;196(16):3045–3057. doi: 10.1128/JB.01663-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee CY, Bagdasarian M, Meng MH, Zeikus JG. Catalytic mechanism of xylose (glucose) isomerase from Clostridium thermosulfurogenes. Characterization of the structural gene and function of active site histidine. J Biol Chem. 1990;265(31):19082–19090. [PubMed] [Google Scholar]
- 18.Boschi-Muller S, Azza S, Pollastro D, Corbier C, Branlant G. Comparative enzymatic properties of GapB-encoded erythrose-4-phosphate dehydrogenase of Escherichia coli and phosphorylating glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1997;272(24):15106–15112. doi: 10.1074/jbc.272.24.15106. [DOI] [PubMed] [Google Scholar]
- 19.Ohashi K, et al. Enzymatic isomerization and epimerization of D-erythrose 4-phosphate and its quantitative analysis by gas chromatography/mass spectrometry. Eur J Biochem. 1984;142(2):347–353. doi: 10.1111/j.1432-1033.1984.tb08293.x. [DOI] [PubMed] [Google Scholar]
- 20.Perlin AS. Glycol-cleavage oxidation. Adv Carbohydr Chem Biochem. 2006;60:183–250. doi: 10.1016/S0065-2318(06)60005-X. [DOI] [PubMed] [Google Scholar]
- 21.Crasta OR, et al. Genome sequence of Brucella abortus vaccine strain S19 compared to virulent strains yields candidate virulence genes. PLoS ONE. 2008;3(5):e2193. doi: 10.1371/journal.pone.0002193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.López-Goñi I, Moriyón I, Neilands JB. Identification of 2,3-dihydroxybenzoic acid as a Brucella abortus siderophore. Infect Immun. 1992;60(11):4496–4503. doi: 10.1128/iai.60.11.4496-4503.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellaire BH, Elzer PH, Baldwin CL, Roop RM., 2nd Production of the siderophore 2,3-dihydroxybenzoic acid is required for wild-type growth of Brucella abortus in the presence of erythritol under low-iron conditions in vitro. Infect Immun. 2003;71(5):2927–832. doi: 10.1128/IAI.71.5.2927-2932.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Croch D, Elberg SS. Response of the vaccine strain of Brucella melitensis Rev I to erythritol. J Bacteriol. 1967;94(5):1793–1794. doi: 10.1128/jb.94.5.1793-1794.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreno E, Moriyon I. In: The genus Brucella. The Prokaryotes. Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E, editors. Springer; New York: 2006. pp. 315–456. [Google Scholar]
- 26.Blasco JM, Molina-Flores B. Control and eradication of Brucella melitensis infection in sheep and goats. Vet Clin North Am Food Anim Pract. 2011;27(1):95–104. doi: 10.1016/j.cvfa.2010.10.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.