Significance
To design an effective subunit vaccine, it is essential to identify the most relevant protective antigen. One way to achieve this goal is to analyze, at the clonal level, the human antibody response and identify the molecules targeted by the most effective neutralizing antibodies. Here we provide an example of this approach in the case of human cytomegalovirus (HCMV), a pathogen causing severe disease in newborns and immunosuppressed individuals. Through the analysis of the human antibody response to HCMV, we identified the gHgLpUL128L pentamer as the target of the most potent neutralizing antibodies and demonstrated that a pentamer vaccine elicited in mice extremely high levels of HCMV neutralizing antibodies. This example illustrates a general approach to develop subunit vaccines against complex pathogens.
Keywords: vaccine, human cytomegalovirus, analytic vaccinology
Abstract
The use of neutralizing antibodies to identify the most effective antigen has been proposed as a strategy to design vaccines capable of eliciting protective B-cell immunity. In this study, we analyzed the human antibody response to cytomegalovirus (human cytomegalovirus, HCMV) infection and found that antibodies to glycoprotein (g)B, a surface glycoprotein that has been developed as a HCMV vaccine, were primarily nonneutralizing. In contrast, most of the antibodies to the complex formed by gH, gL, protein (p)UL128, pUL130, and pUL131 (the gHgLpUL128L pentamer) neutralized HCMV infection with high potency. Based on this analysis, we developed a single polycistronic vector encoding the five pentamer genes separated by “self-cleaving” 2A peptides to generate a stably transfected CHO cell line constitutively secreting high levels of recombinant pentamer that displayed the functional antigenic sites targeted by human neutralizing antibodies. Immunization of mice with the pentamer formulated with different adjuvants elicited HCMV neutralizing antibody titers that persisted to high levels over time and that were a hundred- to thousand-fold higher than those found in individuals that recovered from primary HCMV infection. Sera from mice immunized with the pentamer vaccine neutralized infection of both epithelial cells and fibroblasts and prevented cell-to-cell spread and viral dissemination from endothelial cells to leukocytes. Neutralizing monoclonal antibodies from immunized mice showed the same potency as human antibodies and targeted the same as well as additional sites on the pentamer. These results illustrate with a relevant example a general and practical approach of analytic vaccinology for the development of subunit vaccines against complex pathogens.
Human cytomegalovirus (HCMV) is a ubiquitously distributed member of the Herpesviridae family that establishes a lifelong infection and represents a major threat for human health. Primary infection during pregnancy is the most frequent cause of congenital birth defects, with an overall 0.6% incidence, whereas severe infections develop in immunocompromised patients (1, 2). In addition, HCMV has been proposed as an agent associated with immune senescence (3) and atherosclerosis (4).
HCMV has a broad cell tropism and exploits multiple glycoprotein complexes present on the virion envelope for binding and fusion with host cells. Some glycoproteins (g), such as gM/gN and gB, are used to infect several cell types, whereas glycoprotein complexes containing gH and gL mediate cell type-specific virus entry (5, 6). A pentameric complex comprising gH, gL, protein (p)UL128, pUL130, and pUL131 [gHgLpUL128locus (L)] was shown to be required by clinical HCMV isolates to infect endothelial, epithelial, and myeloid cells (7–10). In vitro cultured HCMV viruses with mutations in the UL128–131 locus lose tropism for endothelial and epithelial cells but retain the expression of the gHgL-containing complex, which is sufficient to infect fibroblasts (11).
Because of the high incidence rate of HCMV infections and its impact on public health, considerable efforts have been made in the last decade to develop treatments or vaccines capable of preventing HCMV infection (12). The major target populations for a HCMV vaccine are seronegative women of childbearing age, whereas infants represent another potential population contributing to viral dissemination (13). In addition, patients on a list for organ transplantation (especially those with HCMV-seronegative who are at risk for life-threatening HCMV disease) would benefit from a HCMV vaccine. The administration of the HCMV-attenuated Towne vaccine prevented the development of disease in kidney transplant recipients, although it did not prevent infection (14).
The abundant virion protein gB was shown to elicit vigorous T-cell and antibody responses and represents the basis of most vaccines developed so far (15). However, in recent phase II trials, a MF59-adjuvanted gB vaccine showed modest efficacy in preventing infection (16) and reducing duration of viremia in transplant recipients (17). These findings may be explained by the finding that most antibodies induced by the vaccines lack virus-neutralizing activity (18), whereas those that neutralized did not block efficiently infection of epithelial cells (19). Therefore, a HCMV vaccine capable of eliciting neutralizing antibodies that prevent the infection of multiple cellular targets and block viral dissemination is considered a high priority (20).
Passively administered polyclonal antibodies isolated from seropositive donors were suggested to be effective in preventing infection of the fetus (21). These findings were not confirmed in a recent randomized study where the same antibody preparation showed a modest, not significant, effect on the rate of congenital HCMV infection, possibly due to the low level of neutralizing antibodies contained in Ig preparation (22).
We previously isolated from HCMV immune donors antibodies that bound to conformational epitopes on the gHgLpUL128L pentameric complex and were extraordinarily potent in neutralizing HCMV infection of epithelial, endothelial, and myeloid cells (23). The pentamer-specific antibodies neutralized viral infection at picomolar concentrations and were a thousand-fold more potent than antibodies to gB, gH, or gMgN complex (23). More recently, we showed that an early antibody response to the pentamer was associated with lack of viral transmission to the fetus from HCMV-infected pregnant mothers, suggesting that pentamer-specific antibodies are responsible for the inhibition of viral spread in vivo (24).
In this study, we report a systematic analysis of the human antibody response to HCMV infection, which indicates that the gHgLpUL128L pentamer is the target of the most effective neutralizing antibodies. Based on this information, we developed a novel process to produce in a secreted form a recombinant pentamer vaccine from a mammalian CHO cell line stably transfected by a single polycistronic vector encoding the five different HCMV pentamer genes separated by autonomous “self-cleaving” 2A peptides. We found that this vaccine can elicit in mice titers of neutralizing antibodies 100–1,000-fold higher than those induced by natural infection. These antibodies neutralized infection of both epithelial cells and fibroblasts and prevented viral dissemination from endothelial cells to leukocytes.
Results
Analysis of the Neutralizing Antibody Response in HCMV-Infected Donors.
To investigate the specificity and properties of the antibodies produced following natural HCMV infection, we immortalized memory B cells from HCMV-immune donors (25) and tested the culture supernatants for the presence of antibodies that bound to HCMV-infected cells or to recombinant gB, gHgL dimer, and gHgLpUL128L pentamer expressed in HEK293F cells (24) (Fig. S1). In the four donors analyzed, the frequency of memory B cells producing antibodies that stained HCMV-infected cells ranged from 1.56–3.89% of total IgG+ B cells (Table 1). However, only a minor fraction of HCMV-specific IgG antibodies bound to gB, gHgL dimer, or the gHgLpUL128L pentamer (3.5 ± 1.5%, 1.6 ± 0.9%, and 2.9 ± 1.4%, respectively). These findings are consistent with the notion that the majority of the antibodies produced following viral infection are not neutralizing, being directed against internal, denatured, or postfusion proteins.
Table 1.
Antigenic specificity and neutralizing activity of human mAbs produced by IgG+ memory B cells of HCMV-immune donors
| Antibody specificity | Donor | bAbs/total Abs, % | nAbs/bAbs, n | nAbs/bAbs, % |
| HCMV cells* | 1 | 1.91 | 6/55 | 11 |
| 2 | 2.11 | 7/61 | 11 | |
| 3 | 3.89 | 32/112 | 26 | |
| 4 | 1.56 | 7/45 | 16 | |
| gB† | 1 | 0.090 | 2/13 | 15 |
| 2 | 0.118 | 3/17 | 18 | |
| 3 | 0.201 | 8/29 | 28 | |
| 4 | 0.049 | 3/7 | 43 | |
| gHgL† | 1 | 0.055 | 5/8 | 63 |
| 2 | 0.035 | 2/5 | 40 | |
| 3 | 0.125 | 15/18 | 83 | |
| 4 | 0.027 | 2/4 | 50 | |
| gHgLpUL128L† | 1 | 0.083 | 6/12 | 50 |
| 2 | 0.111 | 13/16 | 81 | |
| 3 | 0.229 | 31/33 | 94 | |
| 4 | 0.042 | 4/6 | 67 |
bAb, binding antibody; nAb, neutralizing antibody.
HCMV-positive antibodies were identified by staining of HCMV-infected cells.
Antibodies specific for gB, gHgL, and gHgLpUL128L were identified by ELISA using recombinant glycoproteins.
We then tested the monoclonal antibodies (mAbs) that bound to the surface glycoproteins for their capacity to neutralize infection of epithelial cells by the clinical HCMV strain VR1814. Consistently with a previous report (18), only a minor fraction of the gB-binding antibodies had neutralizing activity (26.0 ± 12.6%; range, 15–43%; Table 1). In contrast, a significantly higher proportion of the antibodies to gHgL (59.0 ± 18.6%; range, 40–83%) and, even more, of the antibodies to gHgLpUL128L (73.0 ± 18.9%; range, 50–94%) had HCMV-neutralizing activity. These findings indicate that following natural HCMV infection a large proportion of the antibodies produced are not neutralizing and that, among the target of neutralizing antibodies, the HCMV pentamer stands out as the antigen that preferentially elicits neutralizing over nonneutralizing antibodies. Consequently, the antipentamer response can be defined as having a high “specific activity.”
Analysis of the Antibody Response in Mice Immunized with gB, gHgL, and gHgLpUL128L Pentamer.
To test whether the same hierarchy of immunogenicity found in naturally infected individuals could be observed following immunization with recombinant proteins, we immunized mice with soluble recombinant gB, gHgL dimer, or gHgLpUL128L pentamer. BALB/c mice were immunized by s.c. injections of 5 µg of proteins in 0.5% carbopol polymer, boosted after 2 and 4 wk with soluble proteins, and sera were analyzed on day 40. The carbopol adjuvant was chosen for its ability to preserve native protein conformation (26). Immunization with soluble gB induced serum antibodies that bound to gB, with an average ED50 of 103.9, and neutralized HCMV infection of epithelial cells and fibroblasts, with an average ID50 of 103.5 and 102.2, respectively (Fig. 1 A and D). The difference in neutralizing titers reflects the different susceptibility to HCMV infection of epithelial cells versus fibroblasts. Immunization with soluble gHgL dimer induced serum antibodies that bound to gHgL and gHgLpUL128L (with ED50 of 102.7 and 103.4) and neutralized infection of epithelial cells and fibroblasts in the same range as gB-induced antibodies (ID50 of 103.5 and 102.7, respectively) (Fig. 1 B–D). Remarkably, the gHgLpUL128L pentamer induced antibodies that bound to the dimer as well as the pentamer (ED50 of 103.1 and 103.9) and showed much higher neutralizing activity, with an ID50 of 105.8 on epithelial cells and 103.9 on fibroblasts (Fig. 1 B–D).
Fig. 1.
Serum binding and neutralizing titers in mice immunized with gB, gHgL dimer, or gHgLpUL128L pentamer. Mice were immunized with 5 μg of HEK293F-produced soluble gB, gHgL dimer, or gHgLpUL128L pentamer in carbopol 0.5% and boosted on day 14 and 28, and sera were collected on day 40. (A–C) Inverse IgG serum antibody titers binding (1/ED50) to gB (A), dimer (B), and pentamer (C) measured by ELISA. Error bars show 95% confidence interval of the geometric mean values. (D) Inverse neutralizing serum antibody titers (1/ID50) measured on epithelial cells (ARPE-19, black circles) or fibroblasts (MRC-9, white circles).
To determine the specific activity of the antibody response induced by soluble gB or gHgLpUL128L pentamer, we characterized mAbs isolated from immunized mice. A total of 378 gB-binding mAbs and 246 pentamer-binding mAbs were obtained from four gB-immunized and four pentamer-immunized mice, respectively (Fig. S2 A and B). Similarly to that observed with human mAbs isolated from HCMV-infected donors, only a minor fraction of gB-specific mAbs (19.9 ± 4.2%) neutralized virus infection. In contrast, most of the pentamer-specific mAbs (75.7 ± 11.5%) showed neutralizing activity (Fig. S2A). Neutralizing mAbs displayed a broad range of OD values in ELISA, comparable to those of mAbs that bound but did not neutralize (Fig. S2B), indicating that neutralizing activity does not correlate with high binding. Interestingly, a large fraction (67%) of mAbs induced by immunization with the pentamer recognized epitopes present on the gHgL dimer and neutralized infection of both epithelial cells and fibroblasts, with IC80 values in the nanomolar range (0.08–1.5 µg/mL) (Fig. S2C and Table 2). In contrast, the remaining mAbs neutralized infection of epithelial cells but not fibroblasts, but were much more potent, displaying IC80 values in the picomolar range (0.0001–0.08 µg/mL). A subset of the latter antibodies was mapped by staining cells transfected with various combinations of the pentamer subunits and by cross-competition with human mAbs of known epitope specificity (23) (Table 2). Interestingly, the low-potency mAbs that neutralized infection of both epithelial cells and fibroblasts bound to at least two nonoverlapping sites on gH, whereas the highly potent mAbs that specifically neutralized epithelial cell infection targeted five previously described sites targeted by human antibodies on the pentamer (sites 1, 2, 3, 5, and 7) plus at least one additional site. Taken together, the above analysis performed at the clonal level reveals a striking parallel between the antibody response of infected individuals and that of mice immunized with recombinant proteins. In both cases, the gHgLpUL128L pentamer shows the highest capacity to elicit potent neutralizing antibodies that inhibit infection of different cell types.
Table 2.
Characterization of mAbs from mice immunized with soluble pentamer
| mAb, h/m | Neutr, E/F | LogIC80, M | Target antigen* | Competed hu-mAb† |
| P25, m | E | −12.1 | pUL128pUL130pUL131 | —‡ |
| P40, m | E | −11.4 | pUL128pUL130pUL131 | — |
| P38, m | E | −11.3 | pUL128pUL130pUL131 | — |
| P39, m | E | −11.2 | pUL128pUL130pUL131 | — |
| P53, m | E | −10.9 | pUL128pUL130pUL131 | — |
| P31, m | E | −10.9 | pUL128pUL130pUL131 | — |
| P42, m | E | −10.6 | pUL128pUL130pUL131 | 8J16 |
| P2, m | E | −10.8 | gHgLpUL128 | 15D8 |
| P30, m | E | −10.4 | pUL130pUL131 | 4I22 |
| P37, m | E | −10.4 | gHgLpUL128 | 15D8 |
| P46, m | E | −9.5 | pUL128pUL130pUL131 | 4I22 |
| P7, m | E | −9.5 | pUL128pUL130pUL131 | — |
| P16, m | E | −9.3 | pUL128 | 5A2/8I21 |
| D1, m | E+F | −9.3 | gH | 13H11 |
| D7, m | E+F | −8.9 | gH | — |
| D12, m | E+F | −8.9 | gH | — |
| D13, m | E+F | −8.4 | gH | — |
| 8J16, h | E | −12.3 | pUL128pUL130pUL131 | — |
| 8L13, h | E | −11.6 | pUL130pUL131 | — |
| 7I13, h | E | −11.0 | pUL128pUL130pUL131 | 10P3/15D8 |
| 15D8, h | E | −11.0 | pUL128 | 7I13 |
| 10P3, h | E | −10.5 | pUL130pUL131 | 7I13 |
| 5A2, h | E | −10.0 | pUL130pUL131 | 8I21 |
| 8I21, h | E | −9.5 | gHgLpUL128pUL130 | 5A2 |
| 13H11, h | E+F | −8.6 | gH | — |
Mouse (m) and human (h) mAbs are grouped according to their ability to neutralize HCMV infection of epithelial cells only (E) or epithelial cells and fibroblasts (E+F). Shown are the log IC80 values, corresponding to the concentration that inhibits 80% infection.
The target antigens recognized by each antibody were determined using HEK293T cells transfected with a different combination of HCMV genes, as previously described (23).
Cross-competition ELISAs were performed to identify the mouse mAbs that bound to the same sites recognized by a panel of human mAbs as previously isolated (23).
“—” indicates that the antibodies recognize novel sites on the pentamer.
Production of a Soluble gHgLpUL128L Pentamer by CHO Cells Stably Transfected with a Single Polycistronic Vector.
To produce a soluble gHgLpUL128L pentameric complex that could be developed as a candidate HCMV vaccine, we designed a single polycistronic vector encoding five genes (gH, gL, UL128, UL130, and UL131), each separated by self-cleaving 2A peptides (27). This system was designed to achieve a stoichiometric production of the five subunits for an efficient assembly of the pentameric complex. CHOK1-SV cells were stably transfected with the polycistronic vector, and a high-producer clone, named CHO-K1SV-5mer, was selected after two rounds of subcloning. This clone produced, under laboratory scale, batch conditions in protein-free chemically defined medium up to 50 mg/L of pentamer. The preparation of purified, tag-free, soluble pentamer was monodisperse with no signs of aggregation (Fig. S3 A and B). The pentamer was mainly alpha helical, as assessed by circular dichroism (CD), and showed good thermal stability (Tm ∼60 °C) (Fig. S3 C and D). The correct folding and display of relevant antigenic sites was confirmed by sandwich ELISA, using a panel of human mAbs targeting eight distinct conformational epitopes on the HCMV glycoproteins (23) (Table S1).
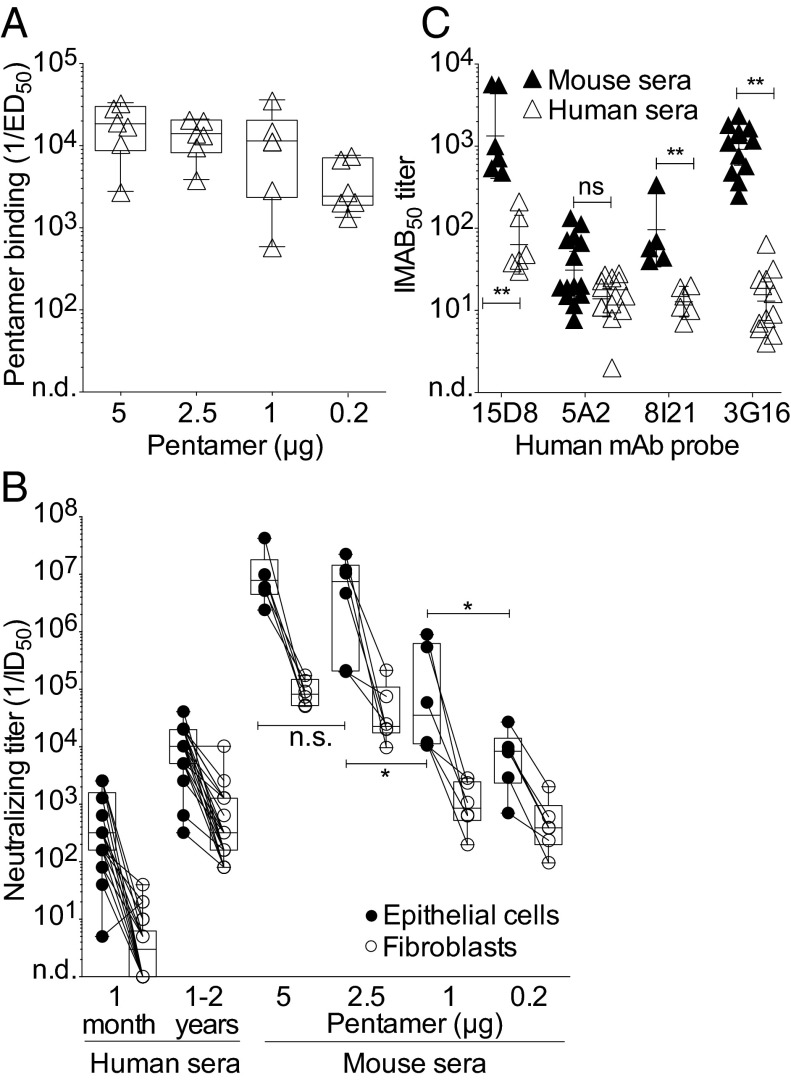
To assess the immunogenicity of the pentamer produced by the CHO-K1SV-5mer clone, we immunized mice with different doses of the pentamer formulated in Ribi, a TLR4-based adjuvant, which is equivalent to the AS01 adjuvant approved for human use (28). High serum binding and neutralizing titers were detected in mice immunized with 5 and 2.5 µg of pentamer, whereas lower responses were detected in mice immunized with 1 and 0.2 µg (Fig. 2 A and B). Remarkably, the neutralizing titers against epithelial cells and fibroblasts elicited by the 5 µg dose (IC50 of 106.9 and 104.9, respectively) were ∼16,000- and ∼40,000-fold higher that those found in the sera of donors 1 mo and ∼1,000- and ∼300-fold higher that those found 1–2 y after HCMV infection.
Fig. 2.
Binding and neutralizing antibody titers in mice immunized with different doses of pentamer produced in CHO cells. (A) Binding antibody titers to gHgLpUL128L pentamer measured by ELISA in the sera of mice immunized with different doses of pentamer (day 40). Box and whiskers plot, box containing 50% with median and whiskers minimum to maximum values. *P < 0.05; **P < 0.01. (B) HCMV-neutralizing titers (1/ID50) of human sera collected 1 mo or 1–2 y after HCMV infection (Left) and of mouse sera collected on day 40 (Right) after immunization with the indicated dose of pentamer in Ribi adjuvant. Neutralization titers were measured on epithelial cells (black circle) and fibroblasts (white circles). (C) Sera from mice immunized with 5 μg of pentamer in Ribi (black triangles) or from HCMV-infected donors 1–2 y after infection (white triangles) were tested for their capacity to inhibit the binding to the pentamer of four human mAb probes that bind to distinct sites (probes: 15D8, anti-pUL128; 5A2, anti–pUL130pUL131; 8I121, anti-gHgLpUL128pUL130; 3G16, anti-gH). Shown is the 50% Inhibition of MAb binding titer (IMAB50).
To compare the antibody response elicited by immunization of mice with the pentamer to that of humans infected with HCMV, we measured the ability of mouse and human sera to inhibit the binding of a panel of human mAbs targeting four distinct antigenic sites on the pentamer (29) (Fig. 2C). This analysis showed that mouse sera blocked the binding of all of the four antibodies tested, indicating that the antibody response elicited in mice by the CHO-derived pentamer is directed against multiple antigenic sites. The highest titers of antibodies were directed against pUL128 and gH, a finding that is consistent with the high neutralizing titers directed against both epithelial cells and fibroblasts.
Magnitude and Duration of the Serum Antibodies Elicited by Different Adjuvants.
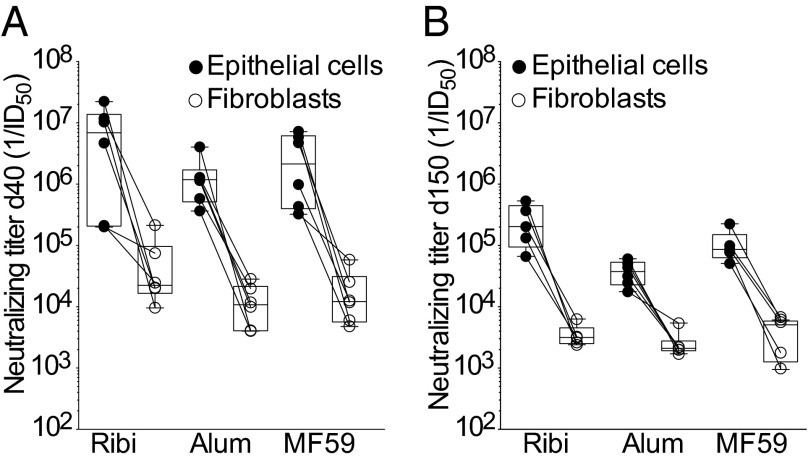
To determine the optimal adjuvant to be combined with this antigen, we immunized mice with 2.5 µg of pentamer formulated with each of the three adjuvants, which are approved for human use, namely, alum, MF59, and Ribi. Importantly, the three adjuvants were comparable in their capacity to elicit serum-neutralizing antibody titers (Fig. 3A). Mice immunized with 2.5 µg of pentamer formulated with either of the three adjuvants were bled 5 mo after the last immunization, and high binding and neutralizing antibody titers were still present after 5 mo without booster injection (Fig. 3B). There was a trend for Ribi to be more effective in conferring slightly higher and more sustained neutralizing antibody responses.
Fig. 3.
Persistence of neutralizing antibodies in mice immunized with pentamer produced in CHO cells and formulated with different adjuvants. Neutralizing antibody titers measured on epithelial cells (black circles) and fibroblasts (white circles) in the sera of mice collected on day 40 (A) or 150 (B) after immunization with 2.5 μg of pentamer formulated with Ribi, Alum, or MF59. Box and whiskers plot, box containing 50% with median and whiskers minimum to maximum values.
Vaccination with Pentamer Induces a High Level of Dissemination-Inhibiting Antibodies.
To measure the dissemination-inhibiting activities of the antibodies induced by the pentamer vaccine, the sera of immunized mice were tested for their ability to block plaque formation (i.e., cell-to-cell virus spreading) as well as virus transfer from infected endothelial cells to leukocytes. As shown in Fig. 4, sera from pentamer-immunized mice displayed a high potency in both assays, being able to inhibit plaque formation in ARPE-19 cells at dilutions ranging from 103 and 103.5 and HCMV transfer to leukocytes at dilutions ranging from 103.5 and 104.5. These titers are definitely higher than those observed in human subjects 1 y after infection. Thus, the CHO-derived pentamer is able to elicit antibodies that block in vitro activities that well recapitulate the routes of HCMV dissemination in vivo. Importantly, sera from gB- and gHgL-immunized mice showed a very weak activity (50% inhibiting titer ≤102) in blocking both cell-to-cell spreading and virus transfer to leukocytes (Fig. 4). Collectively, these results indicate that the CHO-derived pentamer is a very potent vaccine that induces antibodies that target multiple sites, protect different cellular targets, and inhibit mechanisms of virus spreading.
Fig. 4.
Sera from pentamer-immunized mice block HCMV cell-to-cell spreading in epithelial cells and virus transfer from infected endothelial cells to leukocytes. Serum titers inhibiting plaque formation (i.e., cell-to-cell virus spreading) in ARPE-19 cells (A) and HCMV transfer to leukocytes from infected endothelial cells (B). Mouse sera (white circles) were collected on day 40 following immunization with 5 μg of soluble gB, dimer, or pentamer. Shown are also sera from five donors collected 1–2 y after onset of HCMV infection (white triangles). Error bars show 95% confidence interval of the geometric mean values.
Discussion
In this study, we provide an example of a general approach to vaccine design that starts from the analysis of the human neutralizing antibody response elicited by the pathogen to identify, produce, and formulate a recombinant subunit vaccine capable of eliciting the most effective antibody response. We have referred to this process as “analytic vaccinology” (30).
Our work is founded on the original observation that antibodies that bind to the HCMV gHgLpUL128L pentamer are extremely potent in neutralizing infection of epithelial, endothelial, and myeloid cells by clinical HCMV isolates (23). This observation has been confirmed in three recent studies. The Merck group characterized the antibody response of rabbits vaccinated with an experimental HCMV vaccine virus in which the expression of the pentamer complex was restored and reported that the most potent neutralizing antibodies elicited by the vaccine were specific for the pentamer and not for gB (31). Diamond and coworkers reported that a modified vaccinia Ankara vector expressing rhesus gHgLpUL128L could reduce viral titers following intradermal challenge with rhesus CMV (32). More recently, the Novartis group tested in mice the immunogenicity of gHgL dimers and gHgLpUL128L pentamers administered as viral replicon particles or as proteins with MF59 and found that the pentamer induced higher serum antibodies compared with the dimer with 1/ID50 values of 0.5–2 × 105 on ARPE-19 and 103 on MRC-9 (33).
In this study, we engineered a soluble pentamer produced by stably transfected CHO cells and found that mice immunized with this vaccine produced HCMV neutralizing antibodies at titers that were much higher as compared to previous reports, i.e., with 1/ID50 > 107 on ARPE-19 and 105 on MRC-9. In addition, we found that all three adjuvants that are licensed for human use are compatible with the pentamer, with Ribi conferring a slightly higher and more sustained antibody response. Importantly, the CHO-derived pentamer induced high titers of antibodies not only against the products of the UL128 locus but also against gH, as determined by the capacity of the immune sera to inhibit binding of specific mAbs. These findings explain the high capacity of the immune sera to neutralize HCMV infection of both epithelial cells and fibroblasts. Moreover, we found that the immune mouse sera were able to inhibit in vitro virus spreading in epithelial cells and virus transfer from endothelial cells to leukocytes, thus showing a potential activity of the pentamer-elicited antibodies in blocking virus dissemination in vivo. In conclusion, to the best of our knowledge, we have produced a vaccine that is able to induce a HCMV-neutralizing antibody response of unprecedented potency and breadth, acting at multiple levels to block HCMV infection.
We have recently shown that in pregnant women infected by HCMV, the early production of antipentamer antibodies correlates with protection of the fetus, thus providing evidence for an in vivo protective role of such antibodies (24). The neutralizing antibody concentration required to block infection in vivo is not known. However, in several cases, it has been shown that in vivo protection requires neutralizing titers that exceed by at least 100-fold the ID90 values (34). It is important to underline that high titers of neutralizing antibodies are reached only 1–2 y after infection and even not in all patients. It is therefore encouraging to see that the pentamer vaccine produced in CHO cells can induce in mice HCMV-neutralizing titers that are 1,000 times higher than those found in the sera of convalescent donors. Furthermore, although decreasing as expected, neutralizing antibody titers persist in mouse sera on day 150 at levels at least 50-fold higher than those found in convalescent donors. Along the same line, it is tempting to suggest that the modest and uncertain efficacy of HCMV-immune globulins (22) may be explained by the insufficient amount of neutralizing antibodies administered.
It is well appreciated that the immune response elicited by live viruses is dominated by nonneutralizing antibodies, which are mainly directed against internal proteins or surface proteins in a denatured or postfusion conformation. Although nonneutralizing antibodies have been suggested in some systems to contribute to protection, there is a general consensus that protection requires neutralizing antibodies that block the interaction of the virus with its receptors on target cells or that interfere with the virus fusion machinery (35). Consequently, a desired property of a vaccine is to elicit an antibody response of high specific activity—that is, to preferentially induce neutralizing over nonneutralizing antibodies. The clonal analysis of the antibody response in HCMV-infected individuals indicates that only a small fraction of HCMV-specific B cells produces neutralizing antibodies. This low specific activity is not surprising, in view of the fact that the antiviral response is known to be directed to a large extent against internal proteins. It is, however, of interest that the response to surface glycoproteins has different profiles. We have shown that most pentamer-specific antibodies produced by infected humans or immunized mice were neutralizing, indicating that this antigen has the capacity to induce an antibody response of high specific activity. In contrast, and consistent with a previous report (18), we found that only a small fraction of anti-gB antibodies produced have neutralizing activity. It is tempting to speculate that gB, which is thought to be involved in the fusion mechanism (36), has a metastable conformation, as shown for the herpes simplex virus 1 gB (37). Therefore, the low specific activity of gB-specific antibodies may be due to the fact that the gB proteins used so far may be in the postfusion conformation, and hence, it is expected to induce mostly nonneutralizing antibodies, thus limiting the efficacy of current gB constructs as vaccines. In this case, it would be important to produce a stabilized prefusion gB, as most neutralizing antibodies are specific for the prefusion conformation, as recently shown in the case of RSV F protein (38, 39). In contrast, the pentamer, which functions as a docking or triggering receptor (40, 41), may not undergo conformational changes, explaining why most of the binding antibodies are endowed with neutralizing activity.
In summary, our data suggest that the CHO-derived pentamer alone, or possibly in combination with a stabilized prefusion form of gB, represents a promising candidate for a subunit HCMV vaccine. It is reasonable to hypothesize that vaccines capable of eliciting an antibody response of high specific activity and able to limit the spreading of both cell-free and cell-associated virus may effectively control HCMV infection in immunocompromised patients and provide sterilizing immunity in healthy individuals.
Materials and Methods
Protein Expression by Transient Transfection of 293F Cells.
Intronless full-length UL128, UL130, UL131, gH (deprived of the transmembrane portion and the cytoplasmic domains), gL, and gO of VR1814 were cloned into pcDNA3 vectors (Invitrogen) as previously described (24). Soluble gB was generated as a truncated ectodomain (amino acids 1–690, accession no. ACZ79977). The correct reactivity of gB protein was assessed by ELISA using a panel of neutralizing mAbs previously described (23). Protein purity was verified by SDS/PAGE followed by SimplyBlue staining (Invitrogen).
Expression of the gHgLpUL128 Pentamer in CHO Cells.
To produce a soluble gHgLpUL128L pentameric complex that could be developed as a candidate HCMV vaccine, we designed a single polycistronic vector encoding five genes (gH, gL, UL128, UL130, and UL131), each separated by self-cleaving 2A peptides. The gHgL and pUL subunits were cloned, respectively, in pEE6.4 and pEE12.4 vectors (Lonza Biologics). Additional modifications were added to optimize the secretion and purification of the pentamer. The signal peptide of gH was replaced by an IgG leader sequence (MGWSCIILFLVATATGVHS), and a TEV protease sequence followed by two Strep-Tag was added downstream of UL131 (ENLYFQGSGSGWSHPQFEKGSGSWSHPQFEK). The CHO stable cell line secreting the HCMV pentamer was generated by nucleofection of the vector encoding for the five subunits of the pentamer into the CHO-K1SV line (GS-System, Lonza). For protein expression, CHO cells were seeded at 1 × 106/mL in ProCho5 (Lonza); after 10 d, supernatants were harvested and loaded on affinity column StrepTactin II (Qiagen). Strep-Tag was removed by treatment with the TEV protease. Protease and uncleaved proteins were removed by negative chromatography. Finally, proteins were further purified by size-exclusion chromatography (Superdex 200 from GE Healthcare).
Protein Characterization.
Protein purity and identity was verified by SDS/PAGE followed by coomassie staining or transferred on a PVDF (polyvinylidene difluoride) membrane, which was then incubated with antibodies against gB, gH, gL, pUL128, pUL130, or pUL131. Antibody binding was detected with an anti-human or anti-rabbit HRP-conjugated antibody (Sigma) in combination with the enhanced chemiluminescence Western blotting detection reagent (GE Healthcare). CD experiments were performed as described (38).
ELISA.
Antibodies to gB, dimer, or pentamer were measured using a sandwich ELISA as described (24). To measure the amount of serum antibodies directed against a particular site, we used a competitive ELISA as described (29). Briefly, plates with captured recombinant proteins were incubated with serial dilutions of human or mouse serum followed by biotinylated mAbs of known specificity that were used as probes (23). The amount of probe bound was quantified using alkaline phosphatase-conjugated streptavidin.
Viral Neutralization, Cell-to-Cell Spreading, and Leukocyte Transfer.
Confluent layers of ARPE-19 or MRC-9 cells in 96-well plates were infected with virus (MOI of 1) premixed with serial dilutions of mouse serum (inactivated for 20 min at 56 °C). After 40 h, infection was detected using an anti-p72 mAb, and data were acquired using an automated immunofluorescence imaging system (BD Pathway Bioimaging System) as described (23). A plaque inhibition assay on ARPE-19 that had adsorbed HCMV was used to measure cell-to-cell spread as previously described (29, 42). To measure inhibition of HCMV transfer to leukocytes, monolayers of VR1814-infected HUVEC (24-well microplates 96 h p.i.) were incubated with serial dilutions of immune sera for 2 h at 37 °C followed by addition of leukocytes (29, 42). After overnight coculture, the leukocytes were fixed, permeabilized, and stained with a pool of pp65-specific murine mAbs, and the titer resulting in 50% reduction of pp65-positive leukocytes was calculated.
Human Memory B-Cell Immortalization and Screening.
Peripheral blood was obtained after informed consent from patients 1 mo and 1–2 y after primary HCMV infection. IgG+ memory B cells were isolated and immortalized by EBV and CpG as described, and supernatants were screened using both binding and neutralization assays (25).
Vaccination and Serological Analysis.
Carbopol 974P NF polymer was kindly provided by Lubrizol Advanced Materials. Gel (2% wt/vol) was prepared from carbopol powder in endotoxin-free H2O, neutralized to pH 7.2–7.4 with 10 M NaOH, and stored at –20 °C until use. Alum Hydroxide (Sigma), MF59 (Addavax, Invivogen), and RIBI base (Sigma Adjuvant System, Sigma) were used according to the manufacturer’s instruction. Female BALB/c mice 6–9 wk of age were obtained from Harlan Laboratories Inc. All procedures were performed in accordance with guidelines of the Swiss Federal Veterinary Office and after obtaining local ethical approval. Mice were immunized s.c. into flank with 100 µL of immunogen formulation on day 0, 14, and 28. Mice were bled on day 40 and 150, and antigen- and site-specific IgG titers were measured in the serum by sandwich and competitive ELISA. Neutralizing titers were also determined on ARPE-19 and MRC-9 cells. In some experiments, hybridomas were isolated from immune mice according to standard methods, and the mAbs were characterized for binding and neutralization as described above.
Statistical Analysis.
Data were analyzed with Prism 5 (GraphPad Software) using the two-tailed nonparametric Mann–Whitney U test for comparison of two groups or Kruskall–Wallis test (and Dunn’s posttest) when three or more groups were compared.
Supplementary Material
Acknowledgments
We thank Enrica Mira Catò, Andrea D’Ercole, and Roger Geiger (Institute for Research in Biomedicine) for help in performing animal studies and technical help, and Maria Grazia Revello (Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Policlinico San Matteo) and Fabio Grassi (Institute for Research in Biomedicine) for critical reading and comments. This work was partially supported by Fondazione CARIPLO Grant 93043/A (to G.G. and A.L.), Fondazione Carlo Denegri (to G.G.), Swiss National Science Foundation Grant 141254 (to A.L.), Ministero della Salute Grant RF-2010-GR-2010-2311329 (to D.L.), and Mäxi Foundation. A.L. is supported by the Helmut Horten Foundation.
Footnotes
Conflict of interest statement: A.L. is the scientific founder of Humabs BioMed SA. F.S. and A.L. hold shares in Humabs BioMed SA. G.A. and D.C. are employees of Humabs Biomed, a commercial company that might like to commercialize this technological approach.
This article is a PNAS Direct Submission.
Data deposition: The reagents described in this paper can be obtained through a material transfer agreement.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1415310111/-/DCSupplemental.
References
- 1.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17(4):253–276. doi: 10.1002/rmv.535. [DOI] [PubMed] [Google Scholar]
- 2.Streblow DN, Orloff SL, Nelson JA. Acceleration of allograft failure by cytomegalovirus. Curr Opin Immunol. 2007;19(5):577–582. doi: 10.1016/j.coi.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moss P. The emerging role of cytomegalovirus in driving immune senescence: A novel therapeutic opportunity for improving health in the elderly. Curr Opin Immunol. 2010;22(4):529–534. doi: 10.1016/j.coi.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Streblow DN, Dumortier J, Moses AV, Orloff SL, Nelson JA. Mechanisms of cytomegalovirus-accelerated vascular disease: Induction of paracrine factors that promote angiogenesis and wound healing. Curr Top Microbiol Immunol. 2008;325:397–415. doi: 10.1007/978-3-540-77349-8_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Compton T. Receptors and immune sensors: The complex entry path of human cytomegalovirus. Trends Cell Biol. 2004;14(1):5–8. doi: 10.1016/j.tcb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Ryckman BJ, et al. Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells. J Virol. 2008;82(1):60–70. doi: 10.1128/JVI.01910-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dargan DJ, et al. Sequential mutations associated with adaptation of human cytomegalovirus to growth in cell culture. J Gen Virol. 2010;91(Pt 6):1535–1546. doi: 10.1099/vir.0.018994-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerna G, et al. Dendritic-cell infection by human cytomegalovirus is restricted to strains carrying functional UL131-128 genes and mediates efficient viral antigen presentation to CD8+ T cells. J Gen Virol. 2005;86(Pt 2):275–284. doi: 10.1099/vir.0.80474-0. [DOI] [PubMed] [Google Scholar]
- 9.Hahn G, et al. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J Virol. 2004;78(18):10023–10033. doi: 10.1128/JVI.78.18.10023-10033.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D, Shenk T. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci USA. 2005;102(50):18153–18158. doi: 10.1073/pnas.0509201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanarsdall AL, Chase MC, Johnson DC. Human cytomegalovirus glycoprotein gO complexes with gH/gL, promoting interference with viral entry into human fibroblasts but not entry into epithelial cells. J Virol. 2011;85(22):11638–11645. doi: 10.1128/JVI.05659-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krause PR, et al. Priorities for CMV vaccine development. Vaccine. 2013;32(1):4–10. doi: 10.1016/j.vaccine.2013.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffiths P, et al. Desirability and feasibility of a vaccine against cytomegalovirus. Vaccine. 2013;31(Suppl 2):B197–B203. doi: 10.1016/j.vaccine.2012.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Plotkin SA, et al. Towne-vaccine-induced prevention of cytomegalovirus disease after renal transplants. Lancet. 1984;1(8376):528–530. doi: 10.1016/s0140-6736(84)90930-9. [DOI] [PubMed] [Google Scholar]
- 15.Lilja AE, Mason PW. The next generation recombinant human cytomegalovirus vaccine candidates-beyond gB. Vaccine. 2012;30(49):6980–6990. doi: 10.1016/j.vaccine.2012.09.056. [DOI] [PubMed] [Google Scholar]
- 16.Pass RF. Development and evidence for efficacy of CMV glycoprotein B vaccine with MF59 adjuvant. J Clin Virol. 2009;46(Suppl 4):S73–S76. doi: 10.1016/j.jcv.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffiths PD, et al. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: A phase 2 randomised placebo-controlled trial. Lancet. 2011;377(9773):1256–1263. doi: 10.1016/S0140-6736(11)60136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pötzsch S, et al. B cell repertoire analysis identifies new antigenic domains on glycoprotein B of human cytomegalovirus which are target of neutralizing antibodies. PLoS Pathog. 2011;7(8):e1002172. doi: 10.1371/journal.ppat.1002172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui X, Meza BP, Adler SP, McVoy MA. Cytomegalovirus vaccines fail to induce epithelial entry neutralizing antibodies comparable to natural infection. Vaccine. 2008;26(45):5760–5766. doi: 10.1016/j.vaccine.2008.07.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R. National Vaccine Advisory Committee Vaccine development to prevent cytomegalovirus disease: Report from the National Vaccine Advisory Committee. Clin Infect Dis. 2004;39(2):233–239. doi: 10.1086/421999. [DOI] [PubMed] [Google Scholar]
- 21.Nigro G, Adler SP, La Torre R, Best AM. Congenital Cytomegalovirus Collaborating Group Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med. 2005;353(13):1350–1362. doi: 10.1056/NEJMoa043337. [DOI] [PubMed] [Google Scholar]
- 22.Revello MG, et al. CHIP Study Group A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med. 2014;370(14):1316–1326. doi: 10.1056/NEJMoa1310214. [DOI] [PubMed] [Google Scholar]
- 23.Macagno A, et al. Isolation of human monoclonal antibodies that potently neutralize human cytomegalovirus infection by targeting different epitopes on the gH/gL/UL128-131A complex. J Virol. 2010;84(2):1005–1013. doi: 10.1128/JVI.01809-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lilleri D, et al. Fetal human cytomegalovirus transmission correlates with delayed maternal antibodies to gH/gL/pUL128-130-131 complex during primary infection. PLoS ONE. 2013;8(3):e59863. doi: 10.1371/journal.pone.0059863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Traggiai E, et al. An efficient method to make human monoclonal antibodies from memory B cells: Potent neutralization of SARS coronavirus. Nat Med. 2004;10(8):871–875. doi: 10.1038/nm1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai RPJ, et al. Mixed adjuvant formulations reveal a new combination that elicit antibody response comparable to Freund’s adjuvants. PLoS ONE. 2012;7(4):e35083. doi: 10.1371/journal.pone.0035083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szymczak AL, et al. Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol. 2004;22(5):589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- 28.Garçon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev Vaccines. 2011;10(4):471–486. doi: 10.1586/erv.11.29. [DOI] [PubMed] [Google Scholar]
- 29.Lilleri D, Kabanova A, Lanzavecchia A, Gerna G. Antibodies against neutralization epitopes of human cytomegalovirus gH/gL/pUL128-130-131 complex and virus spreading may correlate with virus control in vivo. J Clin Immunol. 2012;32(6):1324–1331. doi: 10.1007/s10875-012-9739-3. [DOI] [PubMed] [Google Scholar]
- 30.Lanzavecchia A, et al. Understanding and making use of human memory B cells. Immunol Rev. 2006;211:303–309. doi: 10.1111/j.0105-2896.2006.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Freed DC, et al. Pentameric complex of viral glycoprotein H is the primary target for potent neutralization by a human cytomegalovirus vaccine. Proc Natl Acad Sci USA. 2013;110(51):E4997–E5005. doi: 10.1073/pnas.1316517110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wussow F, et al. A vaccine based on the rhesus cytomegalovirus UL128 complex induces broadly neutralizing antibodies in rhesus macaques. J Virol. 2013;87(3):1322–1332. doi: 10.1128/JVI.01669-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wen Y, et al. Human cytomegalovirus gH/gL/UL128/UL130/UL131A complex elicits potently neutralizing antibodies in mice. Vaccine. 2014;32(30):3796–3804. doi: 10.1016/j.vaccine.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Hessell AJ, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature. 2007;449(7158):101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 35.Corti D, Lanzavecchia A. Broadly neutralizing antiviral antibodies. Annu Rev Immunol. 2013;31:705–742. doi: 10.1146/annurev-immunol-032712-095916. [DOI] [PubMed] [Google Scholar]
- 36.Wille PT, Knoche AJ, Nelson JA, Jarvis MA, Johnson DC. A human cytomegalovirus gO-null mutant fails to incorporate gH/gL into the virion envelope and is unable to enter fibroblasts and epithelial and endothelial cells. J Virol. 2010;84(5):2585–2596. doi: 10.1128/JVI.02249-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heldwein EE, et al. Crystal structure of glycoprotein B from herpes simplex virus 1. Science. 2006;313(5784):217–220. doi: 10.1126/science.1126548. [DOI] [PubMed] [Google Scholar]
- 38.Corti D, et al. Cross-neutralization of four paramyxoviruses by a human monoclonal antibody. Nature. 2013;501(7467):439–443. doi: 10.1038/nature12442. [DOI] [PubMed] [Google Scholar]
- 39.McLellan JS, et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science. 2013;342(6158):592–598. doi: 10.1126/science.1243283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eisenberg RJ, et al. Herpes virus fusion and entry: A story with many characters. Viruses. 2012;4(5):800–832. doi: 10.3390/v4050800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carlson C, Britt WJ, Compton T. Expression, purification, and characterization of a soluble form of human cytomegalovirus glycoprotein B. Virology. 1997;239(1):198–205. doi: 10.1006/viro.1997.8892. [DOI] [PubMed] [Google Scholar]
- 42.Gerna G, et al. Human cytomegalovirus serum neutralizing antibodies block virus infection of endothelial/epithelial cells, but not fibroblasts, early during primary infection. J Gen Virol. 2008;89(Pt 4):853–865. doi: 10.1099/vir.0.83523-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.