Significance
It has been shown that males of Caenorhabditis elegans are more likely to be attracted to females and hermaphrodites that do not harbor sperm, and that females and hermaphrodites behave differently depending on whether or not they harbor mature sperm, but the mechanism that links sperm status to behavior and attraction remains unknown. In this paper, we show that oocyte–somatic communication is responsible for controlling the production of a mate-attracting pheromone by C. elegans hermaphrodites. This volatile pheromone, whose production is suppressed by oocyte–somatic communication, is the first volatile pheromone described in C. elegans. Our study of its control provides a detailed description of pheromone regulation in a nematode.
Keywords: fertilization, sex pheromones, egg–soma communication
Abstract
Males of the androdioecious species Caenorhabditis elegans are more likely to attempt to mate with and successfully inseminate C. elegans hermaphrodites that do not concurrently harbor sperm. Although a small number of genes have been implicated in this effect, the mechanism by which it arises remains unknown. In the context of the battle of the sexes, it is also unknown whether this effect is to the benefit of the male, the hermaphrodite, or both. We report that successful contact between mature sperm and oocyte in the C. elegans gonad at the start of fertilization causes the oocyte to release a signal that is transmitted to somatic cells in its mother, with the ultimate effect of reducing her attractiveness to males. Changes in hermaphrodite attractiveness are tied to the production of a volatile pheromone, the first such pheromone described in C. elegans.
Its properties of self-fertilization and rapid generation, along with its extensive library of mutants, make Caenorhabditis elegans an excellent system in which to study reproductive events. The generation time of C. elegans is under 3 d, and a single hermaphroditic worm can use its sperm to fertilize its own eggs, without the need for mating (1). C. elegans and related nematodes have a robust sperm sensation pathway that limits unfruitful oocyte maturation and ovulation (2). Both self-sperm and non–self-sperm secrete protein ligands, known as major sperm proteins (MSPs), that activate signal transduction pathways in both unfertilized oocytes, leading to activation of mitogen-activated protein kinase (MPK-1), and the somatic gonad, involving transcription factor CEH-18. The result of this signaling is the release of oocytes from prophase I arrest and the ovulation of unarrested oocytes into the uterus (3, 4).
Several behaviors of female and hermaphroditic nematodes have been demonstrated to correlate with either the presence of sperm or the recentness of mating. C. elegans hermaphrodites that have exhausted their supply of self-sperm are more likely to elicit a mating response from males of their species, and less likely to resist an attempted mating. Mutant C. elegans hermaphrodites that develop without self-sperm also elicit more mating attempts, and this increase in attractiveness vanishes after a successful mating (5, 6). In the gonochoristic species Caenorhabditis brenneri and Caenorhabditis remanei, males are attracted to a volatile pheromone produced only by females that have not recently mated (7). The mechanisms that link these behaviors to sperm status remain unknown.
Pheromones have been shown to exist in dozens of nematode species (8–10) and have been positively identified in several, including C. elegans (11–14). Although HPLC-MS studies have led to the identification of more than 140 pheromones and pheromone-related metabolites in C. elegans (15), little is known about how production of such pheromones is regulated. Life stage and environmental conditions have been shown to affect pheromone output, but the detailed mechanisms remain elusive (16).
Results
Chasnov et al. (7) showed that females of C. brenneri and C. remanei produce an unknown volatile pheromone only if they have not recently been inseminated by conspecific males. C. elegans hermaphrodites produce no such pheromone; however, hermaphrodites of C. elegans reach adulthood already containing enough sperm to last nearly 3 d (17). We hypothesized that a novel pheromone may be observed in C. elegans only when aging hermaphrodites exhaust their supply of self-sperm, and set out to identify how sperm status regulates hermaphrodite behavior.
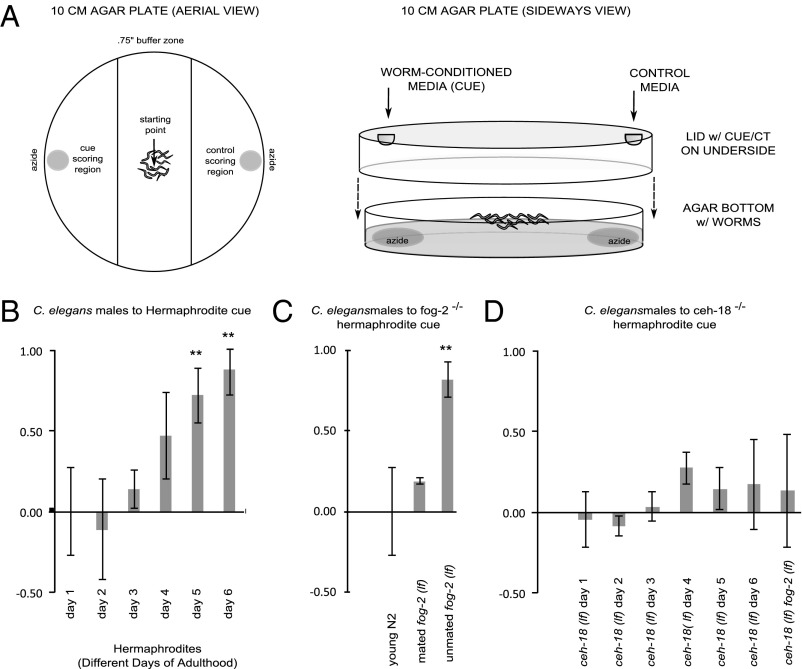
To test our hypothesis, we generated a synchronized population of larval hermaphrodites and allowed them to mature into adults. On the day on which adulthood was reached and over the next 5 d, a fraction of aging worms was incubated in a buffered medium to collect pheromones. On completion of collection, the worms were removed, yielding worm-conditioned media (WCM). To test for the presence of volatile pheromone, we subjected C. elegans males to a chemotaxis assay in which the WCM was not permitted to come into direct or indirect physical contact with the males (Fig. 1A). Any attraction to the WCM would necessarily result from sensation of volatile compounds. Our results indicate that males are attracted to a volatile compound produced by hermaphrodites, but only on the fifth to sixth day of adulthood (Fig. 1B). Previous research has shown that sperm exhaustion occurs on the third day (17), consistent with our own observations. Control experiments confirmed that the volatile pheromone is neither attractive to hermaphrodites nor produced by males (see Fig. 3 A and B).
Fig. 1.
Volatile chemotaxis assays. The y-axis values represent the chemotaxis index (CI). (A) Schematic of the volatile chemotaxis assay. (B) Volatile chemotaxis assay of C. elegans males to adult hermaphrodites of varying age. Significance was determined by ANOVA: F(5,17) = 9.460; P = 0.001. CI to WCM collected on days 5 and 6 is significantly greater (P < 0.01) than that on day 1, as determined by Dunnett’s posttest. (C) Volatile chemotaxis assay of C. elegans males to mated fog-2 mutants vs. unmated mutants and WT hermaphrodites. Attraction to mated fog-2 mutants is significantly less than attraction to unmated mutants (P < 0.0005), but statistically indistinguishable from attraction to WT hermaphrodites (P > 0.1). Significance was determined by the one-tailed homoscedastic Student t test. (D) Chemotaxis assay of young adult males to ceh-18 mutant hermaphrodites in the first to sixth days of adulthood and pseudofemales in the first day of adulthood. No significant differences are seen.
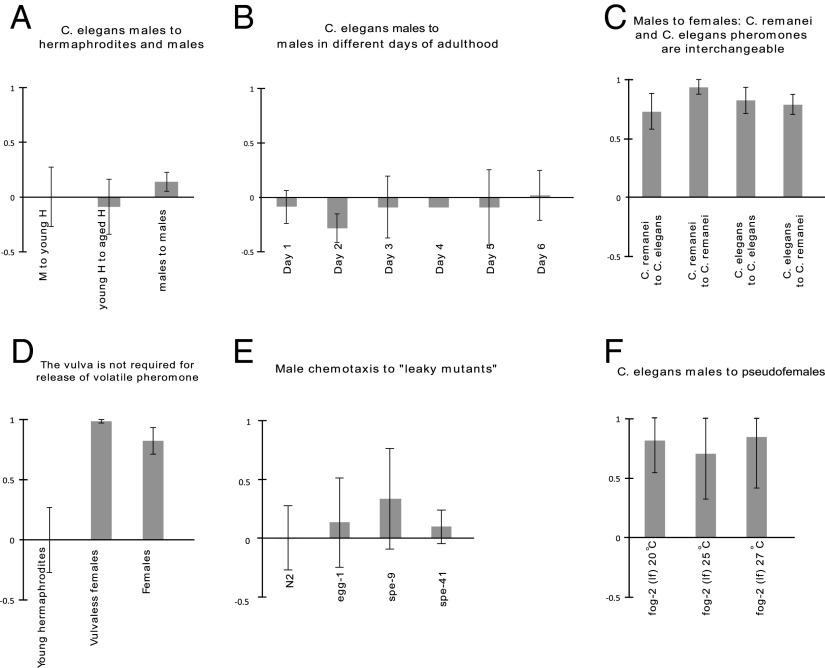
Fig. 3.
Controls and other minor experiments. The y-axis values represent the chemotaxis index. (A) Chemotaxis of young adult males to young adult hermaphrodites, young hermaphrodites to aged hermaphrodites, and young adult males to young adult males. No significant differences were found in any pairwise comparison. (B) Chemotaxis of young adult males to males in the first to sixth day of adulthood. No significant differences were found. (Error bar for day 4 is not shown, owing to the very small SD of 0.016.). (C) Chemotaxis of young adult males of C. remanei and C. elegans to females and aged hermaphrodites of C. remanei and C. elegans. No significant differences were found. (D) Attraction of young adult males to vulvaless pseudofemales is not significantly less than that to otherwise normal pseudofemales. (E) Attraction of young adult males to incompletely sterile mutants defective for fertilization. No significant differences were found compared with attraction to young adult hermaphrodites. (F) Attraction of young adult males to pseudofemales raised at varying temperatures. No significant differences were found.
To confirm that our findings resulted from loss of sperm rather than any other feature of aging, we collected WCM from hermaphrodites bearing a mutation that eliminates hermaphrodite sperm production (fog-2) (18). We also collected WCM from feminized hermaphrodites that had been mated to fertile males. Non–self-sperm are also capable of triggering suppression of pheromone production, causing the mutant hermaphrodites to attract only 19% of males, compared with 82% attracted by unmated mutants (Fig. 1C). We also performed interspecies chemotaxis assays with C. remanei to investigate whether the C. elegans pheromone would function across species. We found that the two species’ volatiles are interchangeable; males of each species are attracted to females of each species (see Fig. 3C).
These initial experiments indicate that a volatile pheromone is produced by hermaphrodites and attracts males (Fig. 1B). This pheromone is produced only after a hermaphrodite’s self-sperm supply has been exhausted. Because fog-2 mutants, which lack self-sperm, produce the pheromone on the first day of adulthood, we can infer that pheromone production is linked to sperm status rather than to age (Fig. 1C). The finding that mated fog-2 mutants do not attract males suggests that initiation of pheromone production is reversible.
This correlation between spermlessness and pheromone production in C. elegans hermaphrodites is inconsistent with the findings of Chasnov et al. (7), who also prepared WCM from aged hermaphrodites and spermless mutants. However, our WCM preparation protocol calls for 20 times as many worms per unit volume compared with that of Chasnov et al., and collection continues for four times as long, potentially yielding a far more concentrated product. Our chemotaxis assays also differ markedly.
To test the similarity of our volatile cue to the unknown mating cue suggested by Morsci et al. (6), which also correlates with age, we collected WCM from hermaphrodites and feminized worms mutant for ceh-18, a transcription factor gene known to be essential for production of this cue (6). We found no detectable volatile attraction of males to ceh-18 mutant hermaphrodites or females of any age (Fig. 1D); thus, we conclude that the pheromone production is dependent on CEH-18. However, because ceh-18 is a poorly understood gene expressed in multiple cell types, we cannot draw more detailed conclusions from this result.
To study the regulation of the volatile pheromone, we tested a series of mutant strains defective in various aspects of reproduction. We chose mutations that cause germ line development and reproduction to cease at a defined point, so that we could determine which specific aspect of the reproductive process regulates production of the volatile cue. Our results show a striking anticorrelation between sperm–egg contact and volatile pheromone production (Fig. 2). How specific reproductive phenotypes correlate with pheromone production reveals details about the regulation of this pheromone (Table 1). Mutations that induced inappropriate pheromone production include those in the genes glp-1, fem-2, fog-2, spe-8, and egg-3, as well as the oma-1;oma-2 double mutant. Tested mutations that did not induce pheromone production include those in genes spe-42, mbk-2, chs-1, mei-1, and pos-1, as well as the egg-4;egg-5 double mutant.
Fig. 2.
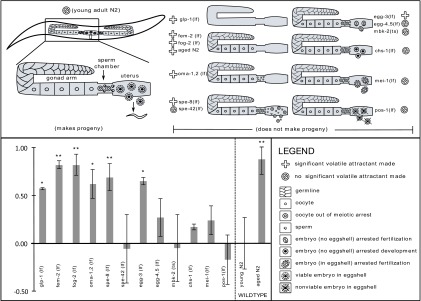
Relationship of germ line status to pheromone output. Shown are the results of volatile chemotaxis assays of C. elegans males to hermaphrodites of various genotypes, accompanied by a diagram of germ line status in each of the strains used in the mutant survey. Significance of the dataset was determined by ANOVA: F(13,50) = 9.319; P < 0.0001, with significant differences from WT determined by Dunnett’s posttest. *P < 0.05; **P < 0.01. In general, fertilization and volatile pheromone production are anticorrelated. WCM for all mutant strains except aged N2 hermaphrodites was prepared from young adults. Chemotaxis assays for spe-42 and egg-4;egg-5 double mutant were performed six times instead of three times, owing to high variance.
Table 1.
Results and implications of Fig. 2
| Strain | Sperm status | Oocyte status | Volatile pheromone | Implications |
| glp-1 | Very few, normal | None | Yes | Pheromone is produced by the soma and suppressed by the germ line. |
| fem-2 | None | Normal | Yes | Sperm depletion eliminates suppression without aging. |
| fog-2 | None | Normal | Yes | Same as fem-2. |
| Aged N2 | None | Normal | Yes | Aging of WT worms eliminates suppression. |
| oma-1,2 | Normal | Cannot be ovulated | Yes | Sperm presence is insufficient. |
| spe-8 | Immobile | Normal, ovulated | Yes | Oocyte activation/ovulation is insufficient. |
| spe-42 | Cannot fuse | Normal, ovulated | No | Sperm–oocyte fusion is unnecessary. |
| egg-3 | Normal | Oocyte cannot react to sperm | Yes | EGG-3 is necessary. |
| egg-4,5 | Normal | Oocyte cannot react to sperm | No | Known EGG-3–binding partners are unnecessary. |
| mbk-2 | Normal | No eggshell, meiosis incomplete | No | Known downstream effectors of EGG-3 are unnecessary. |
| chs-1 | Normal | No eggshell | No | Eggshell deposition is unnecessary. |
| mei-1 | Normal | Meiosis incomplete | No | Completion of meiosis is unnecessary. |
| pos-1 | Normal | Resulting embryo dies | No | Embryonic viability is unnecessary. |
| Young N2 | Normal | Normal | No | Young WT worms suppress pheromone output. |
The terms “insufficient”, “necessary,” and “unnecessary” are always in reference to the suppression of pheromone production. Overall, we conclude that the pheromone is produced in the soma and suppressed by a signal originating in the germ line. EGG-3 and sperm–oocyte contact are necessary for suppression. Eggshell deposition, complete meiosis, known EGG-3 effectors, known EGG-3–binding partners, and embryonic viability are unnecessary for suppression. Sperm presence, oocyte activation, ovulation, and egg/oocyte-laying are insufficient for suppression.
The results of this mutant survey reveal that the phenotype of the worm may be predicted by the developmental time at which the mutated gene acts. C. elegans germ line development begins with two unsexed, primordial germ cells (PGCs) (19). These cells divide mitotically to produce cells that will eventually develop into spermatocytes during the fourth larval stage and oocytes during adulthood. Oocytes arrest during prophase I and do not continue meiosis at high rates until activated by MSPs secreted by spermatozoa. The oocytes reinitiate meiosis at the same time that they are ovulated in preparation for sperm entry. Activated oocytes rearrest if they are not fertilized by spermatozoa. Ovulated oocytes that are not fertilized are laid by the hermaphrodite as if they were eggs (2).
We first examined a mutant with complete failure of germ line proliferation, leading to adults with virtually no gametes of either sex (glp-1) (20). The production of pheromone by glp-1 mutants in the absence of any appreciable germ line implies that the pheromone is produced by the soma; the default state of the soma is to produce, and a normal germ line suppresses volatile pheromone production. We then tested a mutation that feminizes the germ line, leading to adults with normal oocytes but no sperm (fem-2) (21). As in the previous experiment using fog-2 mutants, we found that fem-2 mutant hermaphrodites produce pheromones in young adulthood, corroborating our finding that absence of sperm is sufficient to trigger pheromone production, and aging is unnecessary.
Along with looking at the mere presence of sperm, we examined three strains in which both sperm and oocytes are present but their interactions are impaired. In the first strain, the oma-1;oma-2 double mutant, ovulation never occurs and so the two types of gametes never meet (22). The production of pheromone by the oma-1;oma-2 double mutant indicates that the presence of normal sperm is insufficient to suppress pheromone production. In the second strain, defective in spe-8, sperm are immobile and cannot fertilize oocytes, although they are fully capable of stimulating meiotic activation, ovulation, and egg-laying (23). Because this strain also produces pheromone, we know that activation of the canonical sperm sensation pathway is insufficient to suppress pheromone production. Finally, we tested a strain defective in spe-42 containing superficially normal sperm and oocytes but in which fertilization does not continue past sperm–egg contact (24). This strain failed to produce pheromone in young adulthood, making sperm–egg fusion the earliest failure in reproduction that still permits pheromone suppression.
We also examined the complex of genes known to regulate oocyte activities during fertilization. Strains bearing these mutations produce and lay eggs that fail to undergo pronuclear fusion and/or have no shells. The strains studied included egg-3, simultaneous mutation of egg-4 and egg-5, mei-1, mbk-2, and chs-1 (25–29). We also studied pos-1, a strain that exhibits completely normal fertilization but whose embryos fail soon thereafter (30).
On sperm–egg fusion in a WT worm, the presence of the sperm is signaled by a complex whose components are encoded by egg-3, egg-4, and egg-5. This complex triggers the completion of meiosis and is also required for proper assembly of the eggshell (2). Our data demonstrate that EGG-3 is required for pheromone suppression, but tht its two binding partners as well as its known substrates, MBK-2 and CHS-1, are dispensable for pheromone suppression. The genes mei-1 and pos-1, required for meiosis and embryonic cell determination, respectively, are also unnecessary for suppression of volatile pheromone production.
To test whether EGG-3 has an undescribed role in sperm, we also collected WCM from egg-3−/− hermaphrodites that had been mated to fertile males, so that they would contain a supply of egg-3+/+ sperm. These animals continued to produce volatile pheromone, showing that egg-3+/+ sperm are insufficient to rescue the attraction phenotype of egg-3−/− hermaphrodites (Fig. S1).
To determine whether the vulva is required for volatile pheromone release, we produced feminized animals that lack a vulva [a let-23(sy1); lin-18(e620) double mutant treated with fem-1 RNAi]. This strain continued to produce and release volatile pheromone (Fig. 3D).
In an effort to identify genes involved in sperm–oocyte communication, we assayed genes that code for transmembrane proteins required for fertilization: spe-9, spe-41, and egg-1. In all three cases, no pheromone production was detected (Fig. 3E).
Discussion
Our data indicate that the simultaneous presence of mature sperm and activated oocytes in the spermatheca (the portion of the gonad in which sperm are stored and fertilization occurs), which occurs just before fertilization, is necessary for suppression of the volatile pheromone. Membrane fusion is not required. The signal to suppress volatile pheromone production originates from the activated oocyte in an EGG-3–dependent manner and is transmitted to the maternal soma. The mere presence of sperm, activation and ovulation of oocytes, egg-laying, and sperm–oocyte contact are insufficient for pheromone suppression, and complete meiosis, embryonic viability, and the known effectors and binding partners of EGG-3 are unnecessary for pheromone suppression (Table 1).
Our data cannot distinguish between a scenario in which mature sperm and oocyte signal simultaneously to somatic cells and one in which mature sperm and oocyte communicate with one another before just a single germ cell type signals somatic tissue. In an effort to investigate the communication scheme, we tested a series of mutants that are defective for transmembrane proteins involved in fertilization but are not known to significantly impact oocyte or sperm development. A failure of any of these strains to suppress pheromone would suggest that the mutant gene is in the suppression pathway, and might lead to an understanding of how sperm status is communicated. The mutations studied, spe-9, spe-41, and egg-1, reduce successful fertilization to very low rates (31–33). None of these mutations were found to prevent the suppression of volatile pheromone production (Fig. 3E). We hypothesize that if sperm surface proteins are necessary for suppression, then this is accomplished through surface ligands that have not been implicated in fertilization. Given that the mutations for spe-9 and egg-1 are temperature-sensitive rather than nulls, as is the case for spe-41, they may retain limited function even at the nonpermissive temperature. A low rate of successful sperm–oocyte contact might have masked the requirement for either spe-9 or egg-1 in pheromone suppression. Our data provide no suggestions as to the nature of the oocyte–soma or sperm–soma signals.
These results are especially confusing in light of the dependence of pheromone suppression on the action of EGG-3. The inability of sperm to fuse to oocytes should interfere with the known functions of EGG-3. Furthermore, EGG-3’s known functions are dependent on the presence of either EGG-4 or EGG-5, as well as downstream proteins MEI-1, CHS-1, and MBK-2. The finding that none of the proteins tested in this pathway influences pheromone production, regardless of whether they reside in the sperm or the egg, suggests a unique and independent function for EGG-3. This issue is complicated even further by the fact that localization of EGG-3 to the oocyte cortex is dependent on the presence of CHS-1 (25). If EGG-3 has an undescribed function in pheromone regulation, it is a function that does not require EGG-3’s typical localization. Unfortunately, this leaves us with no clear hypothesis to explain how EGG-3 regulates pheromone production.
The similarity of our results to those of Morsci et al. (6) on the mating preference cue leads us to further speculate that the volatile pheromone and the mating preference cue are regulated by the same process, and may be the same. We naturally question what drove the evolution of oocyte-to-soma communication, which previously had been reported only in mammals, with communication between somatic cells and oocytes or embryos critical to embryonic survival. Along with influencing events that occur in both uterine and embryonic tissue shortly after fertilization, oocyte–uterine signaling plays a large role in modifying fetal nutrition and development (34, 35). In nematodes, however, the mother plays no role in the embryo's nutrition and development after fertilization, and thus such communication would serve no purpose to the developing embryo. Our findings suggest a novel utility for oocyte–uterine signaling—one that may be related to the exclusive benefit of the mother.
A growing body of research is demonstrating that in the genus Caenorhabditis, the presence of males and the act of mating may be hazardous to the health of females and hermaphrodites. Mating between hermaphrodites and males, and even mere exposure to male secretions, has been shown to shorten the lifespan of C. elegans hermaphrodites (36, 37). Male sperm and seminal fluid also trigger physiological changes in mated hermaphrodites (38). It also has been shown that the act of mating is followed by the appearance of cuticular damage around the hermaphrodite vulva (39). Unfortunately, whether any of these physiological and lifespan alterations are detrimental to hermaphrodite fecundity or the fitness of their offspring is unclear. It is also unclear whether these changes provide any benefit to the male mating partner or its offspring. A clear danger of mating has been demonstrated in other species of Caenorhabditis, however; for example, C. nigoni males have been shown to sterilize and kill hermaphrodites and females of other species through the action of their sperm (40).
We hypothesize that because of the potential harms of mating, it benefits the mother to recognize when successful fertilization has occurred, therefore shutting off further production of volatile pheromone. Although we have presented a scenario in which mating benefits males and harms hermaphrodites (if they already have sperm), it also may be the case that the male benefits from this arrangement by minimizing the possibility of sperm competition. There also may be reasons to shut off pheromone production unrelated to mating control, such as limiting the number of worms who may encroach on the hermaphrodite’s food source.
It is interesting to note that previous work has shown that gravid hermaphrodites produce nonvolatile mate-attracting pheromones (41). We cannot fully explain why, if production of mating pheromones is disadvantageous to hermaphrodites, they continue to produce nonvolatile attractants. It is possible that the loss of production of volatile attractants serves to reduce the range of attraction rather than abolish it completely; however, it is difficult to speculate on the significance of the volatility of C. elegans pheromones, because the environment in which the species evolved remains unclear (42). It is also possible that the nonvolatile attractants are indispensable for reasons other than mate attraction; indeed, some of these attractants induce dauer formation (41). It is also possible that output of nonvolatile attractants is reduced as a result of sperm depletion; however, the appropriate experiments to investigate this have not been performed. Nonetheless, we have provided the most detailed characterization yet of the regulation of pheromone output in a nematode, and hope that this will lead to a greater understanding of how nematodes communicate through chemical signals.
Materials and Methods
Strains.
Provided by the Caenorhabditis Genomics Center (complete genotypes available at www.cgc.cbs.umn.edu/): N2 (C. elegans WT), EM464 (C. remanei WT), BA671 (spe-9), PS4330 (spe-41), AD186 (egg-1), GR1034 (ceh-18), BA785 (spe-8), JK574 (fog-2), DG1604 (fog-2; ceh-18), VC2876 (egg-3), AD266 (egg-4; egg-5), JJ462 (pos-1), TX183 (oma-1; oma-2), SL1138 (spe-42), RB1189 (chs-1), TH48 (mbk-2), VC1530 (mei-1), and CB1490 (him-5). Provided by H. Robert Horvitz: MT11436 (fem-2(e2105ts,mat) III/sC1 III [s2023 dpy-1(s2170) III]). Provided by Paul Minor (Sternberg laboratory): PS6345 [let-23(sy1); lin-18(e620)].
Strain Maintenance.
Worms were raised on NG-agar plates that had been seeded with Escherichia coli OP50. The males used for chemotaxis assays were collected from strain CB1490. Strains TH48, AD186, and BA671 were grown at the nonpermissive temperature of 25 °C for the purpose of WCM collection and at 15 °C otherwise. All other strains were grown at room temperature. Control experiments confirmed that raising worms at high temperature does not interfere with pheromone production (Fig. 3F).
Synchronization.
Males for chemotaxis assays and hermaphrodites for WCM preparation were synchronized by time of hatching before other procedures. Synchronization was carried out over two generations. In the first generation, a plate of worms was rinsed with M9 buffer, removing larvae and adults but leaving behind eggs. The roughly synchronized larvae resulting from these eggs were permitted to grow to adulthood and lay eggs of their own. As the second generation of larvae began to hatch from these eggs, all worms were removed from the plate in M9. The adults and arbitrarily synchronized larvae were separated from one another by relative buoyancy, after which the larvae were replated for later use.
WCM.
WCM was prepared using synchronized young adult hermaphrodites and pseudofemales. In the case of strains that segregate genotypes, the desired hermaphrodites were separated from the origin strain by picking. For strains that did not produce males, worms were picked on the day of collection. For strains that also produced males, worms were picked the day before collection as L4s. On the day of collection, worms were rinsed from their plate in M9 buffer and permitted to settle to the bottom of a microcentrifuge tube. After settling, the supernatant was removed, and the worms were resuspended (or rinsed) in fresh M9. This rinsing step was performed a total of four times to remove bacteria from the worms. When the rinsing was complete, the worms were placed in the inverted cap of a microcentrifuge tube, one worm per microliter, 75 worms per cap. The cap was sealed and permitted to sit at room temperature for 24 h, after which the worms and any eggs or larvae deposited in the WCM were removed by centrifugation. WCM was stored at −20 °C.
Chemotaxis Assays.
Chemotaxis assays were performed with synchronized males unless noted otherwise. Males were separated from hermaphrodites by picking during the young adult stage, and were permitted to feed for another 24 h. This segregation procedure is meant to reduce the influence of recent mating on male chemotaxis behavior. The males were then rinsed from their plate with M9 buffer and transferred to a microcentrifuge tube. Rinsing was performed in the same manner as with WCM preparation, but only twice with M9 to remove bacteria and then twice with ddH2O to remove salts. The chemotaxis assay was performed in a 10-cm Petri dish prepared with chemotaxis agar (1.5% agar, 75 mM NH4Cl, 10 mM Mops pH 7.2 with NH4OH, and 0.25% Tween 20). During the final rinse of the males, 1 μL of sodium azide was dropped on each far side of the plate (referred to as east and west), a diameter apart. Sodium azide functions as a rapidly lethal neurotoxin when encountered by C. elegans, allowing us to record which position the male was most attracted to, rather than which position he was more likely to linger at if sampling without initial bias. Because the azide is added to both sides of the plate and has not had time to diffuse through the agar when the assays begin, we do not believe it has any effect on experimental outcomes.
Once the final rinse was complete, 100 males were pipetted onto the center of the plate and then gently spread north and south to speed up the absorption of the excess water into the agar. As the water began to absorb, 10 μL of WCM was pipetted to the inside lid of the Petri dish above one spot of azide, and 10 μL of M9 was pipetted to the other. While thawing and pipetting the WCM, we were careful to pipette from the top of the media and to not disturb or mix the media. The plate was then placed in a small box to eliminate the influence of light, and the box was placed onto a vibration-resistant platform to reduce the influence of vibrations. The assay was run until ≥95% of the males were dead, as determined by periodic checking. This generally took between 15 min and 4 h. At the conclusion of the assay, the males were tallied on the east and west sides of the plate, and all those within a 0.75-inch buffer zone along the north–south axis were ignored. The chemotaxis index was computed as (cue – control)/(cue + control). All chemotaxis assays were repeated three times unless noted otherwise.
Hermaphrodite Aging.
A population of 15,000 synchronized N2 larvae was permitted to reach young adulthood, split across several plates. On the day that young adulthood was reached, a portion of the worms was allocated to WCM collection, and the remaining worms were replated by rinsing onto fresh food. On each subsequent day until the sixth day, this procedure was repeated but with an additional step of separating the adults from eggs and larvae by relative buoyancy. On the sixth day, all remaining adults were put into WCM collection.
Mating Assay.
The testing of mated vs. unmated fog-2(lf) animals was performed with strain JK574. For the unmated population, 150 L4 pseudofemales were segregated by picking to a separate plate and permitted to grow for another 24 h, at which point the resulting adults were put into WCM collection. For the mated population, 150 L4 pseudofemales and an equal number of otherwise isogenic males were permitted to grow on the same plate for 24 h. At the conclusion of the mating period, males were discarded, and the pseudofemales were put into WCM collection. The testing of mated vs. unmated egg-3(lf) animals was performed in otherwise identical fashion.
Supplementary Material
Acknowledgments
We thank Asher Cutter and Eric Haag for communicating unpublished results; Ashley Hebard for assisting with some of the experiments; Mihoko Kato and Hillel Schwartz for helpful discussions; and H. Robert Horvitz, Paul Minor, and the Caenorhabditis Genetics Center for sharing worm strains. D.H.W.L. was supported by a National Institutes of Health US Public Health Service Training Grant (T32GM07616). This research was supported by the Howard Hughes Medical Institute, for which P.W.S. is an investigator.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1420439111/-/DCSupplemental.
References
- 1.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singaravelu G, Singson A. New insights into the mechanism of fertilization in nematodes. In: Kwang WJ, editor. International Review of Cell and Molecular Biology. Vol 289. Academic Press; San Diego: 2011. pp. 211–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller MA, et al. A sperm cytoskeletal protein that signals oocyte meiotic maturation and ovulation. Science. 2001;291(5511):2144–2147. doi: 10.1126/science.1057586. [DOI] [PubMed] [Google Scholar]
- 4.Miller MA, Ruest PJ, Kosinski M, Hanks SK, Greenstein D. An Eph receptor sperm-sensing control mechanism for oocyte meiotic maturation in Caenorhabditis elegans. Genes Dev. 2003;17(2):187–200. doi: 10.1101/gad.1028303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kleemann GA, Basolo AL. Facultative decrease in mating resistance in hermaphroditic Caenorhabditis elegans with self-sperm depletion. Anim Behav. 2007;74:1339–1347. [Google Scholar]
- 6.Morsci NS, Haas LA, Barr MM. Sperm status regulates sexual attraction in Caenorhabditis elegans. Genetics. 2011;189(4):1341–1346. doi: 10.1534/genetics.111.133603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chasnov JR, So WK, Chan CM, Chow KL. The species, sex, and stage specificity of a Caenorhabditis sex pheromone. Proc Natl Acad Sci USA. 2007;104(16):6730–6735. doi: 10.1073/pnas.0608050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackinnon BM. Sex attractants in nematodes. Parasitol Today. 1987;3(5):156–158. doi: 10.1016/0169-4758(87)90201-8. [DOI] [PubMed] [Google Scholar]
- 9.Green CD. Nematode sex attractants. Helminthological Abstr Ser B. 1980;49:81–93. [Google Scholar]
- 10.Choe A, et al. Ascaroside signaling is widely conserved among nematodes. Curr Biol. 2012;22(9):772–780. doi: 10.1016/j.cub.2012.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jaffe H, Huettel RN, Demilo AB, Hayes DK, Rebois RV. Isolation and identification of a compound from soybean cyst nematode, Heterodera glycines, with sex pheromone activity. J Chem Ecol. 1989;15(7):2031–2043. doi: 10.1007/BF01207435. [DOI] [PubMed] [Google Scholar]
- 12.Choe A, et al. Sex-specific mating pheromones in the nematode Panagrellus redivivus. Proc Natl Acad Sci USA. 2012;109(51):20949–20954. doi: 10.1073/pnas.1218302109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noguez JH, et al. A novel ascaroside controls the parasitic life cycle of the entomopathogenic nematode Heterorhabditis bacteriophora. ACS Chem Biol. 2012;7(6):961–966. doi: 10.1021/cb300056q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeong P-Y, et al. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature. 2005;433(7025):541–545. doi: 10.1038/nature03201. [DOI] [PubMed] [Google Scholar]
- 15.von Reuss SH, et al. Comparative metabolomics reveals biogenesis of ascarosides, a modular library of small-molecule signals in C. elegans. J Am Chem Soc. 2012;134(3):1817–1824. doi: 10.1021/ja210202y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan F, et al. Ascaroside expression in Caenorhabditis elegans is strongly dependent on diet and developmental stage. PLoS ONE. 2011;6(3):e17804. doi: 10.1371/journal.pone.0017804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Byerly L, Cassada RC, Russell RL. The life cycle of the nematode Caenorhabditis elegans, I: Wild-type growth and reproduction. Dev Biol. 1976;51(1):23–33. doi: 10.1016/0012-1606(76)90119-6. [DOI] [PubMed] [Google Scholar]
- 18.Schedl T, Kimble J. fog-2, a germ line-specific sex determination gene required for hermaphrodite spermatogenesis in Caenorhabditis elegans. Genetics. 1988;119(1):43–61. doi: 10.1093/genetics/119.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hubbard EJA, Greenstein D. Introduction to the germ line. Kimble J, Strome S, editors. The Germ Line. 2005 Available at wormbook.org/toc_germline.html. Accessed November 20, 2014.
- 20.Austin J, Kimble J. glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell. 1987;51(4):589–599. doi: 10.1016/0092-8674(87)90128-0. [DOI] [PubMed] [Google Scholar]
- 21.Pilgrim D, McGregor A, Jäckle P, Johnson T, Hansen D. The C. elegans sex-determining gene fem-2 encodes a putative protein phosphatase. Mol Biol Cell. 1995;6(9):1159–1171. doi: 10.1091/mbc.6.9.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Detwiler MR, Reuben M, Li X, Rogers E, Lin R. Two zinc finger proteins, OMA-1 and OMA-2, are redundantly required for oocyte maturation in C. elegans. Dev Cell. 2001;1(2):187–199. doi: 10.1016/s1534-5807(01)00026-0. [DOI] [PubMed] [Google Scholar]
- 23.L’Hernault SW, Shakes DC, Ward S. Developmental genetics of chromosome I spermatogenesis-defective mutants in the nematode Caenorhabditis elegans. Genetics. 1988;120(2):435–452. doi: 10.1093/genetics/120.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kroft TL, Gleason EJ, L’Hernault SW. The spe-42 gene is required for sperm-egg interactions during C. elegans fertilization and encodes a sperm-specific transmembrane protein. Dev Biol. 2005;286(1):169–181. doi: 10.1016/j.ydbio.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 25.Maruyama R, et al. EGG-3 regulates cell-surface and cortex rearrangements during egg activation in Caenorhabditis elegans. Curr Biol. 2007;17(18):1555–1560. doi: 10.1016/j.cub.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Parry JM, et al. EGG-4 and EGG-5 link events of the oocyte-to-embryo transition with meiotic progression in C. elegans. Curr Biol. 2009;19(20):1752–1757. doi: 10.1016/j.cub.2009.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Foster JM, Nelson LS, Ma D, Carlow CKS. The chitin synthase genes chs-1 and chs-2 are essential for C. elegans development and responsible for chitin deposition in the eggshell and pharynx, respectively. Dev Biol. 2005;285(2):330–339. doi: 10.1016/j.ydbio.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 28.Clark-Maguire S, Mains PE. mei-1, a gene required for meiotic spindle formation in Caenorhabditis elegans, is a member of a family of ATPases. Genetics. 1994;136(2):533–546. doi: 10.1093/genetics/136.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pellettieri J, Reinke V, Kim SK, Seydoux G. Coordinate activation of maternal protein degradation during the egg-to-embryo transition in C. elegans. Dev Cell. 2003;5(3):451–462. doi: 10.1016/s1534-5807(03)00231-4. [DOI] [PubMed] [Google Scholar]
- 30.Tabara H, Hill RJ, Mello CC, Priess JR, Kohara Y. pos-1 encodes a cytoplasmic zinc-finger protein essential for germline specification in C. elegans. Development. 1999;126(1):1–11. doi: 10.1242/dev.126.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Xu XZS, Sternberg PW. A C. elegans sperm TRP protein required for sperm–egg interactions during fertilization. Cell. 2003;114(3):285–297. doi: 10.1016/s0092-8674(03)00565-8. [DOI] [PubMed] [Google Scholar]
- 32.Singson A, Mercer KB, L’Hernault SW. The C. elegans spe-9 gene encodes a sperm transmembrane protein that contains EGF-like repeats and is required for fertilization. Cell. 1998;93(1):71–79. doi: 10.1016/s0092-8674(00)81147-2. [DOI] [PubMed] [Google Scholar]
- 33.Kadandale P, et al. The egg surface LDL receptor repeat-containing proteins EGG-1 and EGG-2 are required for fertilization in Caenorhabditis elegans. Curr Biol. 2005;15(24):2222–2229. doi: 10.1016/j.cub.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 34.van Mourik MSM, Macklon NS, Heijnen CJ. Embryonic implantation: Cytokines, adhesion molecules, and immune cells in establishing an implantation environment. J Leukoc Biol. 2009;85(1):4–19. doi: 10.1189/jlb.0708395. [DOI] [PubMed] [Google Scholar]
- 35.Murphy VE, Smith R, Giles WB, Clifton VL. Endocrine regulation of human fetal growth: the role of the mother, placenta, and fetus. Endocr Rev. 2006;27(2):141–169. doi: 10.1210/er.2005-0011. [DOI] [PubMed] [Google Scholar]
- 36.Gems D, Riddle DL. Longevity in Caenorhabditis elegans reduced by mating but not gamete production. Nature. 1996;379(6567):723–725. doi: 10.1038/379723a0. [DOI] [PubMed] [Google Scholar]
- 37.Maures TJ, et al. Males shorten the life span of C. elegans hermaphrodites via secreted compounds. Science. 2014;343(6170):541–544. doi: 10.1126/science.1244160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi C, Murphy CT. Mating induces shrinking and death in Caenorhabditis mothers. Science. 2014;343(6170):536–540. doi: 10.1126/science.1242958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodruff GC, Knauss CM, Maugel TK, Haag ES. Mating damages the cuticle of C. elegans hermaphrodites. PLoS ONE. 2014;9(8):e104456. doi: 10.1371/journal.pone.0104456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ting JJ, et al. Intense sperm-mediated sexual conflict promotes reproductive isolation in Caenorhabditis nematodes. PLoS Biol. 2014;12(7):e1001915. doi: 10.1371/journal.pbio.1001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srinivasan J, et al. A blend of small molecules regulates both mating and development in Caenorhabditis elegans. Nature. 2008;454(7208):1115–1118. doi: 10.1038/nature07168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caswell-Chen EP, et al. Revising the standard wisdom of C. elegans natural history: ecology of longevity. Sci SAGE KE. 2005;2005(40):pe30. doi: 10.1126/sageke.2005.40.pe30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.