Abstract
Background
Chemotherapy has improved the outcome of patients with newly diagnosed osteosarcoma, but its role in relapsed disease is unclear.
Methods
We reviewed the records of all patients who were treated for relapsed high-grade osteosarcoma at our institution between 1970 and 2004. Post-relapse event-free survival (PREFS) and post-relapse survival (PRS) were estimated, and outcome comparisons were made using the exact log-rank test.
Results
The 10-year PREFS and PRS of the 110 patients were 11.8% ± 3.5% and 17.0% ± 4.3%, respectively. Metastasis at initial diagnosis (14%), and relapse in lung only (75%) were not significantly associated with PREFS or PRS. Time from initial diagnosis to first relapse (RL1) ≥18 months (43%), surgery at RL1 (76%), and ability to achieve second complete remission (CR2, 56%) were favorably associated with PREFS and PRS (p≤0.0002). In patients without CR2, chemotherapy at RL1 was favorably associated with PREFS (p=0.01) but not with PRS. In patients with lung relapse only, unilateral relapse and number of nodules (≤3) were associated with better PREFS and PRS (p≤0.0005); no patients with bilateral relapse survived 10 years. The median PREFS after treatment with cisplatin, doxorubicin, methotrexate, and ifosfamide was 3.5 months (95% CI, 2.1-5.2) and median PRS 8.2 months (95% CI, 5.2-15.1).
Conclusions
Late relapse, surgical resection, and unilateral involvement (in lung relapse only) favorably impact outcome after relapse. Surgery is essential for survival; chemotherapy may slow disease progression in patients without CR2. These data are useful for designing clinical trials that evaluate novel agents.
Keywords: osteosarcoma, relapse, chemotherapy, outcome, survival, prognostic factors
INTRODUCTION
Osteosarcoma (OS) is the most common malignant bone tumor in children and young adults. Therapy consisting of surgery and chemotherapy with at least three active agents has improved the probability of long-term survival for patients with localized disease to approximately 70%. [1-3] However, a significant number of patients who achieve a complete response to upfront therapy develop disease relapse, and the probability of survival after relapse is much lower (approximately 20%). [4-10] While some studies have suggested that chemotherapy[8, 9] and radiation therapy[8, 11]may be beneficial in the setting of relapse, other studies have failed to show significant benefit of any treatment other than surgery in heavily pretreated patients. [4, 5, 7, 10]
It is clear that there is a need to identify novel agents that are effective against osteosarcoma; these agents are usually evaluated initially in patients with relapsed osteosarcoma. While the emergence of molecularly targeted therapy offers promising novel agents that could be potentially effective and less toxic than cytotoxic chemotherapy, these agents may not be appropriately evaluated using the traditional Phase II clinical trial design that assesses response rate. Historical data on prognostic factors and event-free survival after relapse are needed to inform the design of trials that assess the efficacy of new agents especially those agents with a cytostatic rather than cytotoxic mechanism of action.
We reviewed the records of all patients who were treated at our institution for relapsed osteosarcoma between 1970 and 2004 in order to identify prognostic factors and evaluate the role of chemotherapy at relapse. This report presents our experience with a large cohort of patients with relapsed osteosarcoma, and provides important data that can be used in the design of trials of novel agents and patient stratification.
Patients and Methods
We conducted a search of our solid tumor database to identify all patients who were treated for relapsed osteosarcoma at St. Jude Children’s Research Hospital between June 1970 and March 2004. The study was approved by our Institutional Review Board.
Inclusion criteria were a diagnosis of high-grade osteosarcoma confirmed by pathological report, achievement of complete remission (CR) after initial treatment with surgery and chemotherapy, and subsequent relapse documented by histologic confirmation or radiological imaging. Patients were excluded if they had low-grade osteosarcoma. CR was defined as disappearance of macroscopic tumor at all sites of disease.
Records were reviewed to obtain information regarding patient, disease, and treatment characteristics. Treatment characteristics included any surgery, chemotherapy or radiation therapy given before and after relapse. The date of diagnosis was defined as the date of initial biopsy. The date of relapse was defined as the date of surgery for patients who underwent biopsy or tumor resection before receiving any further therapy at relapse, or as the date of radiological imaging that confirmed the relapse for patients who did not undergo biopsy or tumor resection at relapse. In patients with metastases to the lung, the number of nodules was the number of nodules that showed osteosarcoma by histology for patients who underwent thoracotomy and the number of nodules on imaging studies for those who did not.
Patients treated at our institution are routinely followed in the clinic for at least 10 years after diagnosis and until they reach adult age, whichever occurs later. After patients are no longer seen in clinic, they are followed by mailed questionnaires and phone calls (if necessary). No patients in this study were lost to follow-up.
Statistical Methods
Post-relapse survival (PRS) was defined as the time interval from relapse date to date of death from any cause or to date of last follow-up for patients who were still alive. Post-relapse event-free survival (PREFS) was defined as the time interval from relapse date to date of next event or to date of last follow-up for patients without additional events. Post-relapse events included second relapse for patients who achieved a second CR, progressive disease for patients who did not achieve a second CR, second malignancy, or death from any cause. PREFS and PRS were estimated using the method of Kaplan and Meier. Differences in distributions of PRS and PREFS were investigated using the exact log-rank test. The exact Wilcoxon rank sum test was used to analyze time to first recurrence according to presence of metastatic disease at initial diagnosis.
RESULTS
Patient Characteristics
We identified a total of 110 patients with relapsed osteosarcoma who met our inclusion criteria. The median age of these patients was 14.2 years (range, 5.9 – 25.1 years) at initial diagnosis and 15.7 years (range, 7.5 – 27.8 years) at the time of first relapse. Most patients were Caucasian (n=82, 75%) with a male predominance (n=61; 55%). One hundred and five (96%) patients had extremity tumors and 15 (14%) had metastatic disease at initial diagnosis. Patients received a median number of three agents at initial diagnosis (range, 0 to 4). The median time interval from diagnosis to first CR was 27 days (range, 0 – 426 days).
Outcome after Relapse
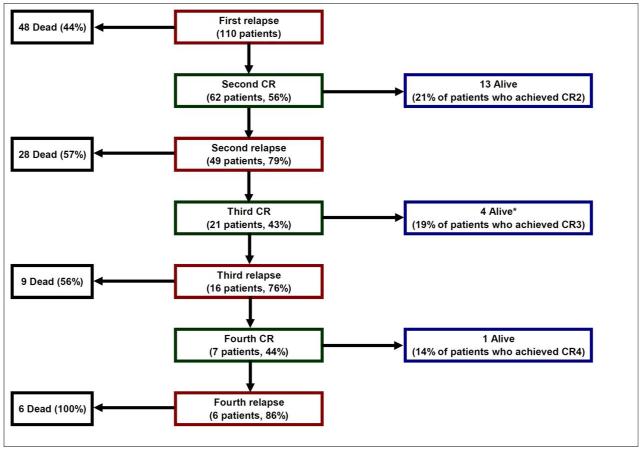
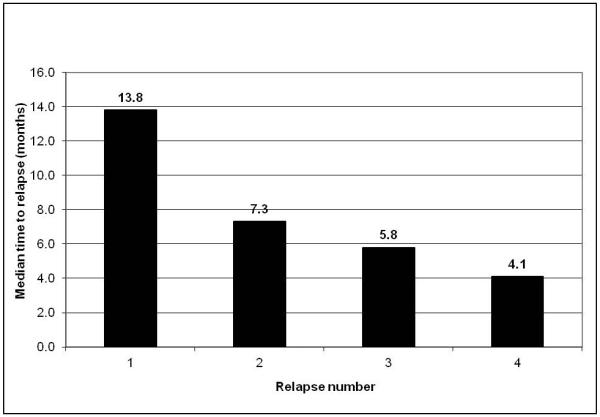
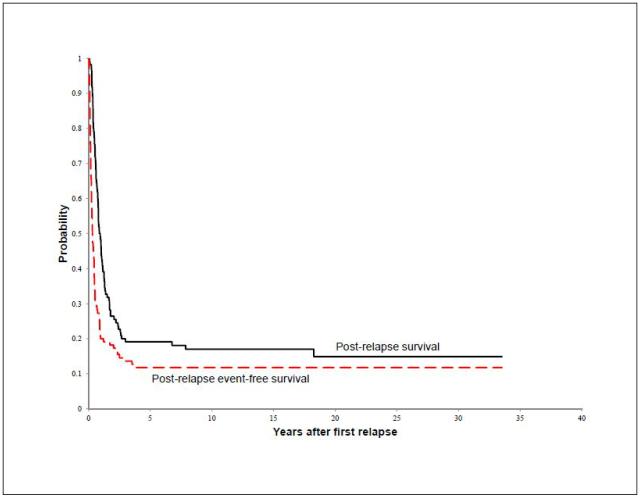
Eighteen patients (16.4%) were alive after relapse with a median follow-up of 13.7 years (range, 4.6 – 33.5 years) after relapse. Causes of death for the remaining 92 patients included osteosarcoma (n=86), complications during treatment of relapsed disease (n=4), second cancer (glioblastoma multiforme, n=1), and brain aneurysm (n=1). Figure 1 displays the 110 patients according to number of relapse, achievement of CR, and life status. Among patients who achieved a CR after relapse, the median time from CR to next relapse was shorter with subsequent relapses (Figure 2). The 10-year estimates of PREFS and PRS for all 110 patients were 11.8% ± 3.5% and 17.0% ± 4.3% (Figure 3).
Figure 1.
Number of relapse, number of complete remission (CR), and life status of all 110 patients with relapsed osteosarcoma. *Five patients were long-term survivors in CR3, but one patient died of a cerebral aneurysm 18 years after diagnosis of osteosarcoma.
Figure 2.
Median time from complete remission to subsequent relapse according to number of relapse in patients with relapsed osteosarcoma.
Figure 3.
Post-relapse survival (PRS) and event-free survival (PREFS) for all 110 patients with relapsed osteosarcoma.
Characteristics at first relapse
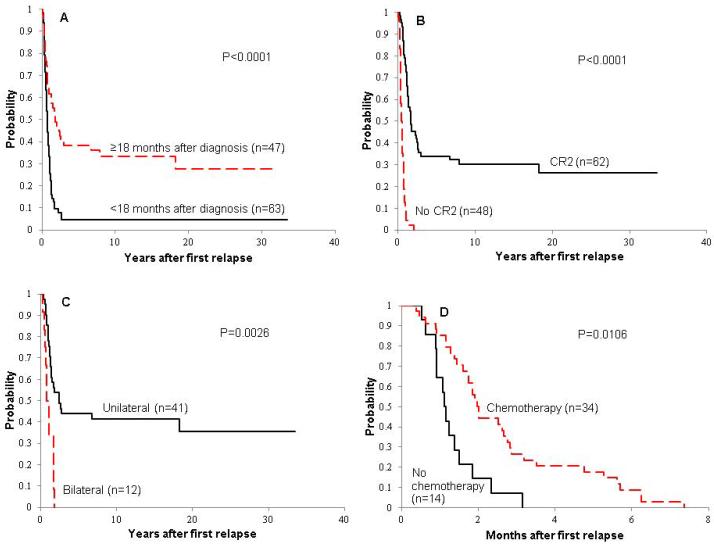
The median time from diagnosis to first relapse was 16.0 months (range, 1.7 months – 6.6 years). Most patients (n=66; 60%) had relapse while they were off treatment; 37 patients (34%) while on treatment, and the remaining 7 (6%) patients at the end of treatment. Most patients experienced isolated distant relapse (n=100; 90.9%); 5 patients had isolated local recurrence (4.6%), and the remaining patients had local and distant relapse (n=4; 3.6%) or regional lymph node and distant relapse (n=1; 0.9%). Of the 105 patients with distant relapse, 90 had a relapse in the lung (82 lung alone, 4 lung and bone, 4 lung and other site), 12 in bone only, and 3 in other sites. Sixty-two of the 110 patients (56%) achieved a surgical second CR and 48 (44%) did not. The median time to second CR from first relapse was 0 days (range, 0 – 125 days). Forty of the 62 patients who achieved a second CR had second CR the same date as the first relapse date; these patients had complete surgical resection at the time of diagnosis of relapse. Patient outcome was not significantly related to age, gender, race, presence of metastasis at initial diagnosis, number of chemotherapy agents given at initial diagnosis, or site of relapse (Table 1). Time to first recurrence was not associated with presence of metastatic disease at diagnosis (15.9 months for the 15 patients with metastasis versus 16.2 months for the 95 patients without metastasis, p=0.85). For the 58 patients in whom the histologic subtype was specified (32 osteoblastic, 14 chondroblastic, 7 telangectatic, and 5 fibroblastic), the histologic subtype was not associated with PREFS (p=0.33) or PRS (p=0.34). Prolonged time to relapse (Figure 4A) and ability to achieve a second surgical CR (Figure 4B) predicted favorable outcome after relapse.
Table 1. Ten-year estimates of post-relapse survival (PRS) and post-relapse event-free survival (PREFS) for 110 patients with relapsed osteosarcoma according to patient and disease characteristics and treatment.
| PRS ± SE (%) | PREFS ± SE (%) | ||||
|---|---|---|---|---|---|
|
| |||||
| Factor | n | 10-Year Estimate |
P-value | 10-Year Estimate |
P-value |
| Age at Diagnosis | |||||
| ≤14 years | 53 | 14.0 ± 6.5 | 0.8187 | 13.2 ± 6.2 | 0.6023 |
| >14 years | 57 | 19.3 ± 5.5 | 10.5 ± 3.8 | ||
| Gender | |||||
| Female | 49 | 20.4 ± 7.4 | 0.9818 | 16.3 ± 6.7 | 0.4063 |
| Male | 61 | 14.6 ± 4.8 | 8.2 ± 3.2 | ||
| Race | |||||
| White | 82 | 16.6 ± 5.0 | 0.8961 | 11.0 ± 4.2 | 0.9402 |
| Black or Hispanic | 28 | 17.9 ± 7.2 | 14.3 ± 5.9 | ||
| Stage at diagnosis | |||||
| Localized | 95 | 17.5 ± 4.6 | 0.5326 | 11.6 ± 3.6 | 0.4047 |
| Metastatic | 15 | 13.3 ± 8.8 | 13.3 ± 8.8 | ||
| Chemotherapy at diagnosis | |||||
| 2 or 3 agents | 72 | 15.2 ± 4.7 | 0.118 | 9.1 ± 3.5 | 0.197 |
| 4 agents | 38 | 20.7 ± 8.3 | 15.8 ± 7.2 | ||
| Time to Relapse | |||||
| <18 months | 63 | 4.8 ± 2.7 | <0.0001 | 3.2 ± 1.8 | <0.0001 |
| ≥ 18 months | 47 | 33.3 ± 8.2 | 23.4 ± 7.2 | ||
| Site of Relapse | |||||
| Lung only | 82 | 20.7 ± 5.3 | 0.0842 | 13.4 ± 4.2 | 0.4996 |
| Other sites (± lung) | 28 | 5.4 ± 3.7 | 7.1 ± 4.9 | ||
| Ability to Achieve a 2nd CR | |||||
| Achieved CR2 | 62 | 30.2 ± 7.0 | <0.0001 | 21.0 ± 5.9 | <0.0001 |
| Did not achieve CR2 | 48 | 0 ± 0 | 0 ± 0 | ||
|
| |||||
| Treatment at First Relapse | |||||
|
| |||||
| Surgery at First Relapse | |||||
| No | 26 | 0 ± 0 | <0.0001 | 0 ± 0 | 0.0002 |
| Yes | 84 | 22.3 ± 5.4 | 15.5 ± 4.5 | ||
| Chemotherapy at First Relapse | |||||
| No | 60 | 26.7 ± 6.9 | 0.0004 | 18.3 ± 5.5 | 0.0111 |
| Yes | 50 | 5.3 ± 3.0 | 4.0 ± 2.8 | ||
| Radiation at First Relapse | |||||
| No | 107 | 17.5 ± 4.4 | 0.6486 | 12.1 ± 3.6 | 0.6125 |
| Yes | 3 | 0 ± 0 | 0 ± 0 | ||
|
| |||||
| Treatment at any Relapse | |||||
|
| |||||
| Chemotherapy at any Relapse | |||||
| No | 30 | 46.7 ± 10.8 | 0.0001 | 36.7 ± 9.7 | <0.0001 |
| Yes | 80 | 5.5 ± 2.7 | 2.5 ± 1.7 | ||
| Radiation at any Relapse | |||||
| No | 89 | 21.0 ± 5.2 | 0.506 | 14.6 ± 4.3 | 0.2326 |
| Yes | 21 | 0 ± 0 | 0 ± 0 | ||
Figure 4.
Post-relapse survival (PRS) distributions according to time to first relapse (A) and ability to achieve second complete remission (B) for all 110 patients with relapsed osteosarcoma, and according to laterality of relapse for patients with lung relapse only who achieved second complete remission (CR2) (C). Post-relapse event-free survival (PREFS) distributions according to chemotherapy use at first relapse for patients with relapsed osteosarcoma who did not achieve CR2 (D).
Treatment after Relapse
At the time of first relapse, 84 of 110 patients (76%) had surgery, 50 (45%) received chemotherapy and 3 (3%) received radiation therapy (Table 1). After disease progression or second relapse, an additional 30 patients received chemotherapy and an additional 18 received radiation therapy for second and any subsequent relapse(s). A variety of chemotherapy agents were used at relapse. The most common agents used at first relapse were methotrexate in 24 patients (in combination with other agents in 7), cisplatin in 10 (in combination with other agents in 9), ifosfamide in 6 (in combination with other agents in 3), and cyclophosphamide in combination with etoposide in 6. Multiple other agents were used in five or fewer patients. In general, patients received different agents at relapse than those given at initial diagnosis.
Surgery was strongly associated with better PREFS and PRS (p≤0.0002) (Table 1). Patients who received chemotherapy had a worse outcome than patients who did not receive chemotherapy, and use of chemotherapy at relapse was significantly associated with inability to achieve CR (p<0.0001). Therefore, we evaluated the relationship of treatment after relapse with outcome according to ability to achieve a second CR.
For patients with second CR (n=62), the median PREFS was 8.4 months (95% CI, 5.3 – 11.2 months). The use of adjuvant chemotherapy at first relapse was not associated with outcome; treatment with chemotherapy at first and any subsequent relapse was associated with worse outcome (Table 2). This association is clearly confounded by indication for therapy; patients who remained in CR after treatment for first relapse did not receive additional therapy of any type, whereas patients who experienced second or subsequent relapse may have received chemotherapy.
Table 2. Patients who achieved a second complete remission (CR2, n=62).
| PRS ± SE (%) | PREFS ± SE (%) | ||||
|---|---|---|---|---|---|
|
| |||||
| Factor | N | 10-Year Estimate |
P-value | 10-Year Estimate |
P-value |
| Chemotherapy at First Relapse | |||||
| No | 46 | 34.8 ± 8.5 | 0.4030 | 23.9 ± 7.0 | 0.5936 |
| Yes | 16 | 16.7 ± 8.8 | 12.5 ± 8.3 | ||
| Radiation at First Relapse | |||||
| No | 62 | 30.2 ± 7.0 | - | 21.0 ± 5.9 | - |
| Yes | 0 | -- | -- | ||
| Chemotherapy at any Relapse | |||||
| No | 23 | 60.9 ± 12.0 | 0.0006 | 47.8 ± 11.5 | <0.0001 |
| Yes | 39 | 11.2 ± 5.3 | 5.1 ± 3.5 | ||
| Radiation at any Relapse | |||||
| No | 48 | 39.0 ± 8.8 | 0.0292 | 27.1 ± 7.3 | 0.0108 |
| Yes | 14 | 0 ± 0 | 0 ± 0 | ||
For patients who failed to achieve a second CR (n=48), the median PREFS was 1.8 months (95% CI, 1.3 – 2.0 months), and all patients died within 25 months of relapse. In this subset of patients, the use of chemotherapy at first relapse was associated with improved PREFS (p=0.011) (Figure 4D), but not PRS (Table 3). When considering treatment at first relapse and any subsequent relapse, there was a trend toward improved outcome with the use of chemotherapy (0.05<p<0.10).
Table 3. Patients who did not achieve second complete remission (CR2, n=48).
| PRS ± SE (%) | PREFS ± SE (%) | ||||
|---|---|---|---|---|---|
|
| |||||
| Factor | n | 6-Month Estimate |
P-value | 6-Month Estimate |
P-value |
| Chemotherapy at First Relapse | |||||
| No | 14 | 57.1 ± 12.5 | 0.9681 | 0 ± 0 | 0.0106 |
| Yes | 34 | 47.1 ± 8.3 | 8.8 ± 4.2 | ||
| Radiation at First Relapse | |||||
| No | 45 | 48.9 ± 7.3 | 0.0318 | 6.7 ± 3.2 | 0.1102 |
| Yes | 3 | 66.7 ± 22.2 | 0 ± 0 | ||
| Chemotherapy at any Relapse | |||||
| No | 7 | 28.6 ± 13.9 | 0.0936 | 0 ± 0 | 0.0742 |
| Yes | 41 | 53.7 ± 7.6 | 7.3 ± 3.5 | ||
| Radiation at any Relapse | |||||
| No | 41 | 48.8 ± 7.6 | 0.3288 | 7.3 ± 3.5 | 0.6092 |
| Yes | 7 | 57.1 ± 16.7 | 0 ± 0 | ||
Of the 82 patients who had a relapse in lung only, 70 (85%) underwent thoracotomy for tumor removal. Fifty-one patients had unilateral lung relapse, and 41 (80%) of these achieved CR2. Thirty-one patients had bilateral lung relapse, and 12 (39%) of these achieved CR2. Patients with unilateral disease were more likely to achieve CR2 (p=0.0002). Unilateral relapse (n=51) and smaller number of nodules (≤3, n=44) were each associated with better PREFS and PRS (p≤0.0005). The 10-year PREFS and PRS for patients with unilateral relapse were 21.6% ± 6.4% and 33.3% ± 7.9%, respectively, and for patients with bilateral relapse, 0% ± 0%. Similarly, in the subset of patients who had a relapse in the lung only and achieved CR2 (n=53), unilateral relapse and smaller number of nodules (≤3) were each associated with better PREFS and PRS (p≤0.019). The 10-year PREFS and PRS for patients with unilateral disease were 26.8% ± 7.6% and 41.5% ± 9.2%, respectively, and for patients with bilateral disease, 0% ± 0% (Figure 4C).
There were 16 patients with distant bone recurrence (12 bone only, 4 lung and bone). Five of these achieved a second surgical CR, one of whom is alive at the time of analysis (more than 20 years after recurrence).
Thirty-eight of the 110 patients (35%) received all 4 agents known to be active against osteosarcoma (cisplatin, doxorubicin, ifosfamide, and methotrexate).[12, 13] This subset of patients included those who received all four active agents at diagnosis (n=13) and those who received some agents at diagnosis and the remainder at relapse (n=25). Four of these 38 patients did not experience subsequent relapses and were alive at the time of analysis. The 10-year estimates of both PREFS and PRS were 10.5% ± 5.0%. The median PREFS after treatment with cisplatin, doxorubicin, ifosfamide, and methotrexate was 3.5 months (95% CI, 2.1-5.2) and median PRS was 8.2 months (95% CI, 5.2-15.1); the median PREFS was 2.7 months (95% CI, 1.5 – 3.9 months) in the 32 patients without surgical remission, and 2 of the 6 patients with surgical remission had relapse at 20.2 months and 24.4 months.
DISCUSSION
Treatment of recurrent osteosarcoma remains an important clinical challenge. In this retrospective study, we reviewed our experience with 110 patients with first and any subsequent relapse. We found that with first or second relapse, approximately 20% of patients who achieve a CR survive (Figure 1). We did not see a trend toward worsening outcome for patients who continued to have resectable disease as has been reported by others [10]. We did, however, see shortening time to relapse with each subsequent relapse, a trend that was also seen in the cohort of Cooperative Osteosarcoma Study Group patients with multiple recurrences.[14]
Table 5 compares our results with those of other studies of patients with recurrent osteosarcoma previously reported.[4-10, 15] As in most other reports,[4, 5, 7, 8, 15] we also found time to recurrence to be a significant predictor of PRS. This finding raises the question of whether late recurrence may represent a biologically different mechanism of recurrence or merely an indication of less aggressive disease. Very late recurrence may actually represent a second primary osteosarcoma, namely when recurrence is in bone rather than lung, as was the case in two of the long-term survivors in our study.
Considering treatment after relapse, we and others[4, 5, 7-9, 15] found that surgical resection and the ability to achieve a CR2 are essential for survival; none of our patients who did not undergo surgery or did not achieve CR2 survived. The relapse characteristics varied among the cohorts reported. Isolated relapse in the lung occurred in 51%[5] to 88%[9] of the patients. The percentage of patients who achieved a CR2 also varied widely from 47%[9] to 81%[4]. This may be related to variations in aggressiveness at surgical resection and surgical expertise among centers, upfront treatment, and study inclusion criteria. Achievement of pulmonary CR2 is largely dependent on the number and size of the pulmonary nodules, their location and proximity to cardiovascular structures in addition to disease-free interval. In general, lower number of nodules, smaller nodule size (<2.5 cm), peripheral location, and disease-free interval more than 6 months were considered favorable factors when assessing candidacy of an individual patient for surgery at our institution. It has been reported that more aggressive upfront chemotherapy has changed the pattern of relapse to result in fewer pulmonary nodules as well as later time to recurrence.[16]
There is little controversy that aggressive surgical management for relapsed osteosarcoma is warranted if CR2 may be achieved. The controversy arises in cases with very short interval between relapses. On the other hand, it is much more controversial whether chemotherapy is beneficial. Forty-five percent of patients in our study received chemotherapy at first relapse and the percentage of patients who received chemotherapy in the previously reported studies was extremely variable, ranging from 23%[4] to 91%.[5] The selection of chemotherapy agents at relapse is usually based on agent activity and prior patient treatment. Because of the variety of chemotherapy regimens used at relapse, our study was not powered to evaluate the efficacy of specific agents, numbers of agents, or combinations of agents. Our study did not show a benefit from using chemotherapy in patients who achieved CR2; the worse outcome for patients who underwent chemotherapy likely reflects a selection bias to administer chemotherapy in patients with poor prognostic features and high risk for relapse. On the other hand, chemotherapy appeared to be associated with an improved PREFS in patients unable to achieve a CR2 with a trend (p=0.0936) toward improved PRS. Approximately 9% of patients without CR2 who received second line chemotherapy survived event-free for 6 months compared to 0% of those who did not. Other studies[8, 9, 15] have also suggested a beneficial effect of chemotherapy in this group, and the largest study of 576 patients demonstrated benefit even in the group able to achieve CR2 (improved event-free survival but not overall survival).[8] The data suggest that the benefit from using chemotherapy in relapsed osteosarcoma is modest and requires large studies with adequate power to be demonstrated. It is also possible that the effect of chemotherapy is blunted in these retrospective reports due to a selection bias where patients with worse disease were more likely to have received chemotherapy. Whether the modest benefit of chemotherapy is clinically significant can be debated, and the effect on quality of life must be assessed carefully when making treatment decisions. In some institutions, treatment with radioisotopes and/or fractionated radiotherapy is used much more frequently than in our institution, and was reported to be associated with moderately prolonged survival in patients without CR.[8] The paucity of use of radiation therapy in our cohort makes any evaluation of its association with survival in our study impossible.
This study and all other studies reported in Table 4 are limited by their retrospective nature, lack of randomization or control group, and strong possibility of selection bias. A large collaborative randomized trial can evaluate definitively the value of chemotherapy in relapsed osteosarcoma. However, such a trial is difficult to prioritize in view of the relative paucity of effective chemotherapy agents and the desperate need to identify novel effective therapies, the rarity of the disease, and high cost of clinical trials. Considering the high mortality rate among patients with relapsed osteosarcoma, these patients are excellent candidates for prospective trials to evaluate novel therapeutic agents. Such trials should include biology and pharmacodynamic studies to characterize relapsed disease at the molecular level and elucidate the effects of novel agents on the tumor and the host. Our study provides useful historical data that can be used in the design of prospective trials evaluating new agents in patients with recurrent osteosarcoma. Since surgical remission is critical for survival, patients have to be segregated according to their ability to achieve surgical remission or tumor resectability. Patients who cannot achieve CR2 are candidates for phase II trials with endpoints of tumor response or preferably time to progression since it is very difficult to measure tumor response in osteosarcoma.[4, 7, 8] In our cohort, the median PREFS in this subset of patients was 1.8 months. On the other hand, patients who achieve a CR2 are ideal candidates for trials that evaluate the efficacy of novel agents that are likely to be effective in the setting of microscopic residual disease. In our cohort, the median time to second relapse in this subset of patients was 8.4 months and shorter to subsequent relapses. Therefore, it may be feasible to use the time to next relapse as a measure of efficacy.
Table 4. Summary of studies of patients with relapsed osteosarcoma.
| Author (study site) |
n | Study years | Inclusion criteria | Median months of follow-up |
Median age in years (range) |
CR2 | Lung only |
Chemo at RL1 |
Surgery at RL1 |
Post- relapse outcome |
Factors associated with PRS |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Leary (St Jude) |
110 | 1970-2004 | RL after chemo, CR |
164 | 14 (6-25) | 56% | 75% | 46% | 76% | 10 years: PRS 17% PREFS 12% |
CR2, TTR ≥18 months, chemo in no CR2 (PREFS), unilateral lung |
| Crompton (UCSF)6 |
37 | 1974-1996 | extremity primary, PR or CR |
16 (5-31) | 60% of lung only |
68% | 68% | 62% | 10 years: PRS 15% PREFS 5% |
none | |
| Kempf- Bielack (Germany)8 |
576 | 1979-1998 | RL after chemo, CR |
50 | 16 | 59% | 65% | 69% | 74% | 10 years: PRS 18% |
CR2, TTR >18 months, chemo, solitary lesion |
| Bacci (Italy)1 |
235 | 1986-1998 | extremity primary, RL after neo-adjuvant chemo, CR |
mean 72 | 14 | 74% | 80% | 23% | 74% | 5 years: PRS 29% PREFS 28% |
TTR >24 months (PREFS), < 3 lung lesions metastases, treating institution |
| Chou (MSKCC)5 |
43 | 1990-2004 | RL after chemo including 3 active agents*, CR |
15 | 15 (5-31) | 60% | 51% | 91% | 81% | 3 years: PRS 35% PREFS 14% |
CR2, TTR>24 months |
| Hawkins and Arndt(Seattle/ Mayo Clinic)7 |
59 | 1990-2000 | RL after chemo, CR |
58 | 15 (5-23) | 68% | 61% | 64% | 79% | 4 years: PRS 23% PREFS 6% |
CR2, TTR>24 months unilateral lung RL, solitary lung nodule, |
| Saeter(Norway)9 | 60 | 1975-1993 | systemic RL after CR of extremity primary |
47% | 88% | 54% | 5 years: PRS 24% |
CR2, chemo, solitary lesion |
|||
| Tabone (France)10 |
42 | 1981-1993 | non-metastatic at diagnosis, RL after chemo |
39 | 12 (4-18) | 81% | 48% | 57% | 81% | 3 years: PRS 36% PREFS 27% |
CR2, local or lung RL, first relapse |
Chemo = chemotherapy; CR = complete remission; PR = partial remission; PREFS = post-relapse event-free survival; PRS = post-relapse survival; RL = relapse; TTR = Time to relapse
Active agents included high-dose methotrexate, cisplatin, doxorubicin, and ifosfamide
In summary, survival after relapse of osteosarcoma remains dismal. Relapse ≥18 months after diagnosis, surgical resection, and unilateral involvement in lung relapse predict a favorable outcome. Our experience supports the continued use of aggressive surgical management, the consideration of chemotherapy in patients with unresectable tumors, and patient segregation in clinical trials of novel therapies according to presence or absence of macroscopic disease.
Acknowledgments
Supported in part by Cancer Center Support (CORE) grant CA21765 and grant CA23099 from the National Cancer Institute and by the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
There are no financial disclosures from any authors.
REFERENCES
- 1.Bacci G, et al. Adjuvant and neoadjuvant chemotherapy for osteosarcoma of the extremities: 27 year experience at Rizzoli Institute, Italy. Eur J Cancer. 2005;41(18):2836–45. doi: 10.1016/j.ejca.2005.08.026. [DOI] [PubMed] [Google Scholar]
- 2.Longhi A, et al. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev. 2006;32(6):423–36. doi: 10.1016/j.ctrv.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Meyer WH, et al. Carboplatin/ifosfamide window therapy for osteosarcoma: results of the St Jude Children’s Research Hospital OS-91 trial. J Clin Oncol. 2001;19(1):171–82. doi: 10.1200/JCO.2001.19.1.171. [DOI] [PubMed] [Google Scholar]
- 4.Bacci G, et al. Treatment and outcome of recurrent osteosarcoma: experience at Rizzoli in 235 patients initially treated with neoadjuvant chemotherapy. Acta Oncol. 2005;44(7):748–55. doi: 10.1080/02841860500327503. [DOI] [PubMed] [Google Scholar]
- 5.Chou AJ, et al. Treatment of osteosarcoma at first recurrence after contemporary therapy: the Memorial Sloan-Kettering Cancer Center experience. Cancer. 2005;104(10):2214–21. doi: 10.1002/cncr.21417. [DOI] [PubMed] [Google Scholar]
- 6.Crompton BD, et al. Survival after recurrence of osteosarcoma: a 20-year experience at a single institution. Pediatr Blood Cancer. 2006;47(3):255–9. doi: 10.1002/pbc.20580. [DOI] [PubMed] [Google Scholar]
- 7.Hawkins DS, Arndt CA. Pattern of disease recurrence and prognostic factors in patients with osteosarcoma treated with contemporary chemotherapy. Cancer. 2003;98(11):2447–56. doi: 10.1002/cncr.11799. [DOI] [PubMed] [Google Scholar]
- 8.Kempf-Bielack B, et al. Osteosarcoma relapse after combined modality therapy: an analysis of unselected patients in the Cooperative Osteosarcoma Study Group (COSS) J Clin Oncol. 2005;23(3):559–68. doi: 10.1200/JCO.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 9.Saeter G, et al. Systemic relapse of patients with osteogenic sarcoma. Prognostic factors for long term survival. Cancer. 1995;75(5):1084–93. doi: 10.1002/1097-0142(19950301)75:5<1084::aid-cncr2820750506>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 10.Tabone MD, et al. Osteosarcoma recurrences in pediatric patients previously treated with intensive chemotherapy. J Clin Oncol. 1994;12:2614–20. doi: 10.1200/JCO.1994.12.12.2614. [DOI] [PubMed] [Google Scholar]
- 11.Mahajan A, et al. Multimodality treatment of osteosarcoma: radiation in a high-risk cohort. Pediatr Blood Cancer. 2008;50(5):976–82. doi: 10.1002/pbc.21451. [DOI] [PubMed] [Google Scholar]
- 12.Longhi A, et al. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer treatment reviews. 2006;32(6):423–36. doi: 10.1016/j.ctrv.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Meyer WH, et al. Carboplatin/ifosfamide window therapy for osteosarcoma: results of the St Jude Children’s Research Hospital OS-91 trial. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2001;19(1):171–82. doi: 10.1200/JCO.2001.19.1.171. [DOI] [PubMed] [Google Scholar]
- 14.Bielack SS, et al. Second and subsequent recurrences of osteosarcoma: presentation, treatment, and outcomes of 249 consecutive cooperative osteosarcoma study group patients. J Clin Oncol. 2009;27(4):557–65. doi: 10.1200/JCO.2008.16.2305. [DOI] [PubMed] [Google Scholar]
- 15.Ferrari S, et al. Postrelapse survival in osteosarcoma of the extremities: prognostic factors for long-term survival. J Clin Oncol. 2003;21(4):710–5. doi: 10.1200/JCO.2003.03.141. [DOI] [PubMed] [Google Scholar]
- 16.Bacci G, et al. Pattern of relapse in patients with osteosarcoma of the extremities treated with neoadjuvant chemotherapy. Eur J Cancer. 2001;37(1):32–8. doi: 10.1016/s0959-8049(00)00361-0. [DOI] [PubMed] [Google Scholar]