Abstract
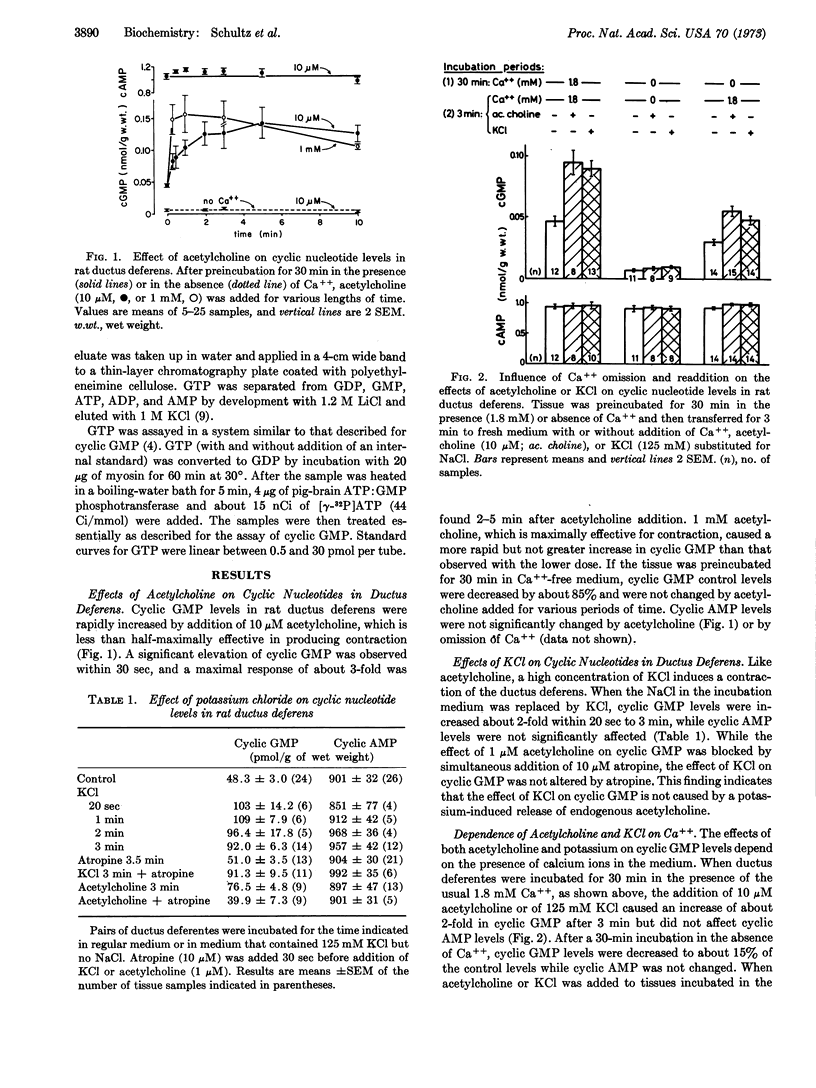
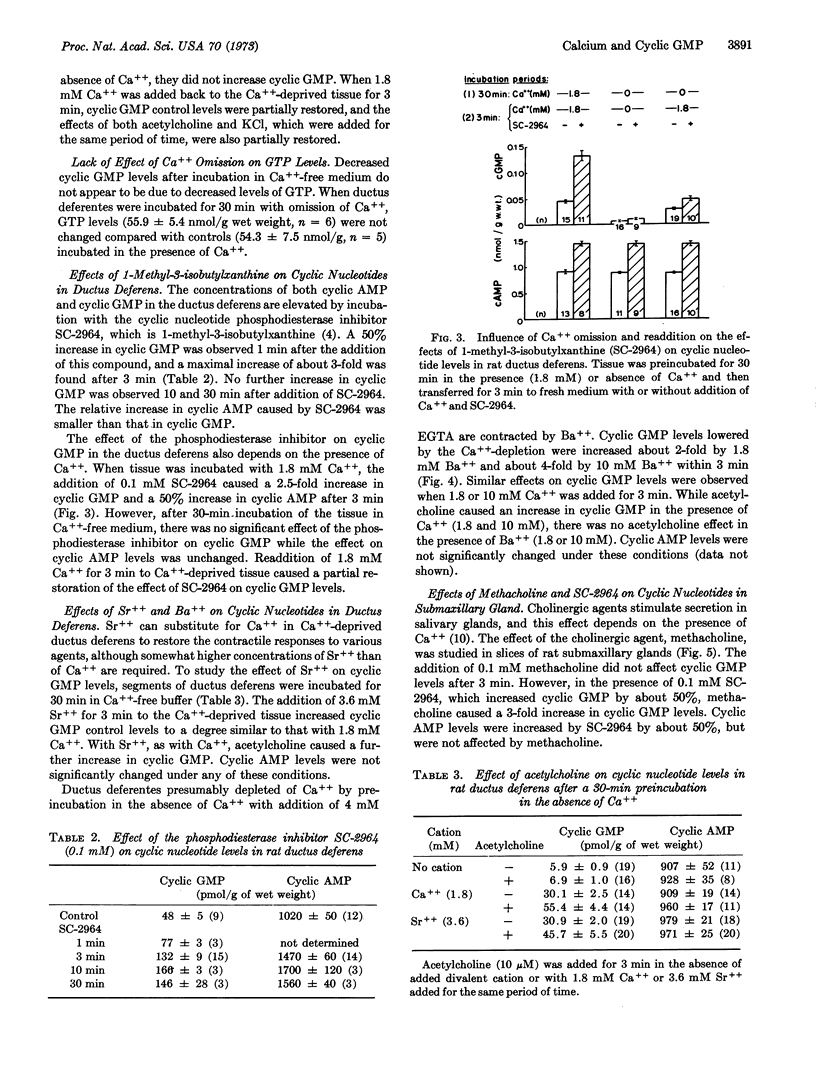
Guanosine 3′:5′-cyclic monosphosphate (cyclic GMP) levels in the ductus deferens of the rat were increased 2- to 3-fold by acetylcholine (10-1000 μM) or by 125 mM KCl, while adenosine 3′:5′-cyclic monophosphate (cyclic AMP) levels were not changed. After incubation for 30 min in the absence of Ca++, cyclic GMP control levels were decreased by 85% and were not affected by acetylcholine or KCl. The readdition of Ca++ (1.8 mM) for 3 min to Ca++-deprived tissue partially restored basal cyclic GMP levels and the effects of acetylcholine and KCl. The addition of Sr++ (3.6 mM) or of Ba++ (1.8 or 10 mM) also caused an increase in basal cyclic GMP in Ca++-deprived tissue. Cyclic AMP levels were not significantly changed under any of these conditions. The addition of the phosphodiesterase inhibitor, 1-methyl-3-isobutylxanthine (0.1 mM), to ductus deferentes increased the amount of cyclic AMP about 50% and that of cyclic GMP about 2-fold. The later effect also depended on the presence of Ca++. 1-Methyl-3-isobutylxanthine (0.1 mM) increased cyclic GMP and cyclic AMP levels in slices of rat submaxillary glands. Methacholine increased cyclic GMP if added in the presence of methyl isobutylxanthine. Cyclic GMP control levels and the effect of methyl isobutylxanthine were unchanged by Ca++ omission, but the effect of methacholine was abolished.
These findings indicate that calcium ions are important for the control of cyclic GMP levels in these tissues.
Keywords: cyclic nucleotides, cholinergic agents, phosphodiesterase inhibitors, smooth muscle, salivary gland
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman M., Oka H., Field J. B. Effect of TSH, acetylcholine, epinephrine, serotonin and synkavite on 32-P incorporation into phospholipids in dog-thyroid slices. Biochim Biophys Acta. 1966 Jun 1;116(3):586–588. doi: 10.1016/0005-2760(66)90131-7. [DOI] [PubMed] [Google Scholar]
- Ball J. H., Kaminsky N. I., Hardman J. G., Broadus A. E., Sutherland E. W., Liddle G. W. Effects of catecholamines and adrenergic-blocking agents on plasma and urinary cyclic nucleotides in man. J Clin Invest. 1972 Aug;51(8):2124–2129. doi: 10.1172/JCI107019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr I. M., Taft H. P., Stauffacher W., Renold A. E. On the role of cyclic AMP in insulin release: II. Dynamic aspects and relations to adrenergic receptors in the perfused pancreas of adult rats. Ann N Y Acad Sci. 1971 Dec 30;185:245–262. doi: 10.1111/j.1749-6632.1971.tb45253.x. [DOI] [PubMed] [Google Scholar]
- DOUGLAS W. W., POISNER A. M. On the mode of action of acetylcholine in evoking adrenal medullary secretion: increased uptake of calcium during the secretory response. J Physiol. 1962 Aug;162:385–392. doi: 10.1113/jphysiol.1962.sp006940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrendelli J. A., Kinscherf D. A., Chang M. M. Regulation of levels of guanosine cyclic 3',5'-monophosphate in the central nervous system: effects of depolarizing agents. Mol Pharmacol. 1973 Jul;9(4):445–454. [PubMed] [Google Scholar]
- Goldberg N. D., O'Dea R. F., Haddox M. K. Cyclic GMP. Adv Cyclic Nucleotide Res. 1973;3:155–223. [PubMed] [Google Scholar]
- Hadden J. W., Hadden E. M., Haddox M. K., Goldberg N. D. Guanosine 3':5'-cyclic monophosphate: a possible intracellular mediator of mitogenic influences in lymphocytes. Proc Natl Acad Sci U S A. 1972 Oct;69(10):3024–3027. doi: 10.1073/pnas.69.10.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardman J. G., Beavo J. A., Gray J. P., Chrisman T. D., Patterson W. D., Sutherland E. W. The formation and metabolism of cyclic GMP. Ann N Y Acad Sci. 1971 Dec 30;185:27–35. doi: 10.1111/j.1749-6632.1971.tb45232.x. [DOI] [PubMed] [Google Scholar]
- Hardman J. G., Sutherland E. W. Guanyl cyclase, an enzyme catalyzing the formation of guanosine 3',5'-monophosphate from guanosine trihosphate. J Biol Chem. 1969 Dec 10;244(23):6363–6370. [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Hurwitz L., Joiner P. D. Mobilization of cellular calcium for contraction in intestinal smooth muscle. Am J Physiol. 1970 Jan;218(1):12–19. doi: 10.1152/ajplegacy.1970.218.1.12. [DOI] [PubMed] [Google Scholar]
- Hurwitz L., Suria A. The link between agonist action and response in smooth muscle. Annu Rev Pharmacol. 1971;11:303–326. doi: 10.1146/annurev.pa.11.040171.001511. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Colombo C. Enzyme release from polymorphonuclear leukocyte lysosomes: regulation by autonomic drugs and cyclic nucleotides. Science. 1973 Jun 15;180(4091):1181–1183. doi: 10.1126/science.180.4091.1181. [DOI] [PubMed] [Google Scholar]
- Kaliner M., Orange R. P., Austen K. F. Immunological release of histamine and slow reacting substance of anaphylaxis from human lung. J Exp Med. 1972 Sep 1;136(3):556–567. doi: 10.1084/jem.136.3.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo J. F., Wyatt G. R., Greengard P. Cyclic nucleotide-dependent protein kinases. IX. Partial purification and some properties of guanosine 3',5'-monophosphate-dependent and adenosine 3',5'-monophosphate-dependent protein kinases from various tissues and species of Arthropoda. J Biol Chem. 1971 Dec 10;246(23):7159–7167. [PubMed] [Google Scholar]
- Mayhew D. A., Wright P. H., Ashmore J. Regulation of insulin secretion. Pharmacol Rev. 1969 Sep;21(3):183–212. [PubMed] [Google Scholar]
- PASTAN I., HERRING B., JOHNSON P., FIELD J. B. Stimulation in vitro of glucose oxidation in thyroid by acetylcholine. J Biol Chem. 1961 Feb;236:340–342. [PubMed] [Google Scholar]
- Schultz G., Hardman J. G., Schultz K., Davis J. W., Sutherland E. W. A new enzymatic assay for guanosine 3':5'-cyclic monophosphate and its application to the ductus deferens of the rat. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1721–1725. doi: 10.1073/pnas.70.6.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUBOI K. K., PRICE T. D. Isolation, detection and measure of microgram quantities of labeled tissue nucleotides. Arch Biochem Biophys. 1959 Mar;81(1):223–237. doi: 10.1016/0003-9861(59)90192-4. [DOI] [PubMed] [Google Scholar]
- Whitney R. B., Sutherland R. M. Enhanced uptake of calcium by transforming lymphocytes. Cell Immunol. 1972 Sep;5(1):137–147. doi: 10.1016/0008-8749(72)90091-3. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Field J. B. Elevation of cyclic guanosine 3',5'-monophosphate levels in dog thyroid slices caused by acetylcholine and sodium fluoride. J Biol Chem. 1972 Nov 10;247(21):7062–7066. [PubMed] [Google Scholar]


