Abstract
Background:
Exposure to polycyclic aromatic hydrocarbons (PAHs) has been linked to bladder cancer.
Objective:
To evaluate the role of PAHs in bladder cancer, PAHs serum levels were measured in patients and controls from a case-control study.
Methods:
A total of 140 bladder cancer patients and 206 healthy controls were included in the study. Sixteen PAHs were analyzed from the serum of subjects by gas chromatography–mass spectrometry.
Results:
Serum PAHs did not appear to be related to bladder cancer risk, although the profile of contamination by PAHs was different between patients and controls: pyrene (Pyr) was solely detected in controls and chrysene (Chry) was exclusively detected in the cases. Phenanthrene (Phe) serum levels were inversely associated with bladder cancer (OR = 0·79, 95%CI = 0·64–0·99, P = 0·030), although this effect disappeared when the allelic distribution of glutathione-S-transferase polymorphisms of the population was introduced into the model (multinomial logistic regression test, P = 0·933). Smoking (OR = 3·62, 95%CI = 1·93–6·79, P<0·0001) and coffee consumption (OR = 1·73, 95%CI = 1·04–2·86, P = 0·033) were relevant risk factors for bladder cancer.
Conclusions:
Specific PAH mixtures may play a relevant role in bladder cancer, although such effect seems to be highly modulated by polymorphisms in genes encoding xenobiotic-metabolizing enzymes.
Keywords: Polycyclic aromatic hydrocarbons, Bladder cancer, Risk profession, Gene-environment interaction
INTRODUCTION
Polycyclic aromatic hydrocarbons (PAHs) are environmental pollutants of great concern due to their persistence in the environment, bioaccumulation in biota, and carcinogenicity.1 Human beings are constantly exposed to PAHs through food and air as they are produced by natural or anthropogenic pyrogenic processes, such as the incomplete burning of coal, oil, gas, wood, garbage, tobacco, and other organic substances.2 Polycyclic aromatic hydrocarbons generally occur in complex mixtures, consisting of dozens or even hundreds of compounds. Among them, sixteen PAHs have been identified by the United States Environmental Protection Agency (USEPA)3 as a priority because they show clear evidence of mutagenicity and genotoxicity in vivo and in animal experiments. Although it is difficult to evaluate PAH exposure in human beings, measurement of parental PAHs and/or their metabolites has been proposed as biomarkers to assess individual exposure to PAHs.4,5
Recent epidemiological evidence links occupational and environmental chemical exposures to nearly 30 types of cancer,6 including bladder cancer. Bladder cancer is the most common urinary tract cancer and the fourth most common cancer in developed countries.7 Since the 1950s, the incidence of bladder cancer in Western countries, including Spain, has increased by 50%.8 Smoking constitutes the major risk factor for bladder cancer, although PAH exposure is also a risk factor.9–12 Polycyclic aromatic hydrocarbons exposure occurs through many occupations or mixtures [such as diesel-engine (classified as group 1) or gasoline-engine (classified into group 2B)], as evaluated by IARC.13 However, there is limited evidence from epidemiological studies (assessing PAHs exposure indirectly via surveys) of the association between diesel exposure and bladder cancer.14 Despite this, the IARC has concluded that there is ‘sufficient evidence’ of a relationship between PAHs and bladder cancer in human beings. Similarly, multiple occupations have been associated with PAH exposure (including painters, machinists, mechanics, and drivers), and occupation appears to be another risk factor for bladder cancer.15 Certain foods (charcoal broiled meats and other smoked foods and coffee) may also be a source of PAH exposure,4 and dietary habits (such as coffee and water intake) are relevant in bladder cancer tumorigenesis.16–20
Genetic polymorphisms may be important in determining individual susceptibility to bladder cancer because genetic differences in detoxification capabilities can modulate PAH-induced damage and carcinogenesis.21 Thus, the alterations of the function of detoxifying enzymes, such as glutathione-S-transferases (GST-T1, GST-M1), which facilitate the cellular removal of bioactivated forms of certain environmental contaminants (such as PAHs and metabolites), are likely involved in bladder cancer susceptibility.22 However, this is controversial because GST enzymes metabolize many substrates in addition to PAHs. Although it has been demonstrated that both reactive PAH metabolites and arylamines are detoxified by cytosolic members of GST family of enzymes, the association with bladder cancer is conditioned by the intensity and duration of smoking habits.23 Moreover, a gene-smoking interaction was previously established regarding GST in the context of bladder cancer.23 We previously found that patients with the GST-T1-null genotype may be at increased risk of bladder cancer compared to subjects with the GST-T1-positive variant.24
Based on this finding and because the incidence of bladder cancer in the Canary Islands has increased dramatically in the last decades, we conducted a hospital-based case-control study to assess the potential role played by PAH exposure on bladder cancer. Unlike previous studies, we measured participant serum PAH levels directly in order to study their role in bladder cancer.
METHODS
Study population and selection of cases and controls
The study population included the reference area of the Complejo Hospitalario Universitario Insular – Materno Infantil de Gran Canaria (CHUIMI), which includes 395 155 individuals according to the 2007 census (baseline date). We recruited 140 patients diagnosed with bladder cancer admitted for surgical treatment in CHUIMI between February 2007 and August 2009. The inclusion criteria for the study were incident bladder cancer cases and at least 18 years old. In all the cases, the disease was confirmed by histological diagnosis. Cases were excluded if bladder cancer was secondary to another tumor; cases resided outside the CHUIMI reference area; or individuals had difficulty in understanding the questionnaire due to cognitive problems. Study controls were patients from different hospital surgical services. The inclusion criteria for controls were admission diagnosis unrelated to known risk factors for bladder cancer or oncological diseases (i.e., orthopedic diseases, minor surgery, hernias or other abdominal surgery, skin problems, and ophthalmologic diseases), admission diagnosis unrelated to known risk factors for exposure to chemical contaminants (e.g., acute or chronic poisoning by chemicals), and over 18 years old. The exclusion criteria included previous diagnosis of any cancer, history of hematuria, and difficulty in understanding the questionnaire. A total of 206 controls were included in the study. Before surgery, cases and controls were contacted to participate in the study and complete a questionnaire developed ad hoc (based on questions of well-known PAH-related lifestyle and occupational risk factors for bladder cancer). The questionnaire collected data on the consumption of beverages (including coffee), the duration and intensity of smoking, and professional activities, following the model of other studies such as the SBC/EPICURO study carried out in Spain.25 All patients provided written informed consent before sample collection, and the Research and Ethics Committee of our hospital approved the study. The characteristics of the study subjects are shown in Table 1.
Table 1. Distribution of bladder cancer cases (n = 140) and controls (n = 206) by demographical indicators, smoking habits, and risk professions for PAHs exposure.
| Cases (%) | Controls (%) | P | |
| Gender | 0.379 | ||
| Male | 120 (85.7) | 168 (81.6) | |
| Female | 20 (14.3) | 38 (18.4) | |
| Age (years old) | 0.067 | ||
| <50 | 13 (9.3) | 40 (19.4) | |
| 51–60 | 30 (21.4) | 29 (14.1) | |
| 61–70 | 42 (30.0) | 64 (31.1) | |
| 71–80 | 36 (25.7) | 45 (21.8) | |
| >80 | 19 (13.6) | 28 (13.6) | |
| Drinking habits | |||
| Non-carbonated soft drinks | 8 (5.8) | 17 (8.3) | 0.380 |
| Carbonated soft drinks | 28 (20.0) | 59 (28.6) | 0.069 |
| Coffee with caffeine | 102 (72.7) | 126 (61.2) | 0.027 |
| Coffee without caffeine | 50 (35.7) | 85 (41.3) | 0.299 |
| Tea | 10 (7.1) | 7 (3.4) | 0.114 |
| Beer | 58 (41.4) | 78 (37.9) | 0.505 |
| Wine | 45 (32.1) | 58 (28.2) | 0.426 |
| Other alcoholic beverages | 26 (18.6) | 33 (16.0) | 0.536 |
| Tap water | 36 (25.7) | 42 (20.4) | 0.245 |
| Bottled water without gas | 96 (68.6) | 138 (67.0) | 0.758 |
| Bottled water with gas | 36 (25.7) | 64 (31.1) | 0.281 |
| Smoking habit | <0.001 | ||
| Non-smokers | 22 (15.7) | 72 (35.0) | |
| Ex-smokers | 54 (38.6) | 25 (12.1) | |
| Smokers | 64 (45.7) | 109 (52.9) | |
| Packyears* | 0.001 | ||
| 0 | 22 (15.7) | 72 (35.0) | |
| 1–24.9 | 37 (26.4) | 40 (19.4) | |
| 25–49.9 | 23 (16.4) | 48 (23.3) | |
| ≧50 | 18 (12.9) | 14 (6.8) | |
| Age of onset of smoking | 0.023 | ||
| <15 years old | 18 (12.9) | 8 (3.9) | |
| ≧15 years old | 99 (70.7) | 121 (58.7) | |
| Risk profession** | 0.014 | ||
| Yes | 66 (47.1) | 70 (34.0) | |
| No | 74 (52.9) | 136 (66.0) | |
| Type of risk profession | |||
| Farmer | 26 (18.6) | 28 (13.6) | 0.210 |
| Painter | 8 (5.7) | 3 (1.5) | 0.050 |
| Driver | 25 (17.9) | 20 (9.7) | 0.027 |
| Chemical industry | 5 (3.6) | 6 (2.9) | 0.732 |
| Tourism | 9 (6.4) | 14 (6.8) | 0.893 |
P values obtained by chi-square-test.
* Packyears = (number of cigarettes per years smoking)/20.
** Farmer, painter, driver, chemical industry and tourism were considered as risk professions, grouped into one variable.
Sample preparation and analytical procedures
All the samples were analyzed for PAHs within two months of diagnosis. A volume of 2 ml of serum was applied to 200-mg (3-ml) Chromabond® C18 cartridges (Macherey-Nagel, Germany) mounted on a vacuum manifold (Waters Corporation, USA). Before the application of the samples, the cartridges were cleaned and conditioned as indicated by the manufacturer. The samples were then passed through the cartridge by gravity flow. The sorbed chemicals were eluted with 2×2 ml of dichloromethane. The solvent of the extracts was then evaporated under a gentle nitrogen stream. The extracted analytes were solubilized in 200 μl of cyclohexane and used for chromatographic analysis.26 The analytes included in this study were the 16 most environmentally relevant PAHs: naphthalene (Naph), acenaphthylene (Acy), acenaphthene (Ace), fluorene, anthracene (Ant), phenanthrene (Phe), fluoranthene, pyrene (Pyr), benz[a]anthracene (BaA), chrysene (Chry), benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[a]pyrene (BaP), indeno[1,2,3,-cd]pyrene, dibenzo[a,h]anthracene, and benzo[ghi]perylene. The recovery rates were approximately 89–107%. All the standard analytes for the study were purchased from Dr. Ehrenstorfer (Riedel-de-Haën, Sigma-Aldrich Laborchemikalien GmbH, Germany).
The gas chromatography separations were performed on a Thermo Trace GC Ultra equipped with a TriPlus autosampler and a split/splitless injector with electronic pressure control (Thermo Fisher Scientific Inc., Waltham, MA, USA). A fused silica BPX5 capillary column (crosslinked 5% phenyl methylpolysiloxane, SGE Inc., USA) with a length of 30 m, an internal diameter of 0.25 mm, and a film thickness of 0.25 μm was used as the stationary phase. Helium (99.999%) at a constant flow rate of 1.0 ml minute−1 was used as the carrier gas. The temperature was programed as follows: the oven temperature was initially set to 60°C, maintained for 1 minute, increased at a rate of 12°C/minute to 210°C, increased at a rate of 8°C/minute to 320°C, and maintained at this final temperature for 6 minutes. The total run time was 61 minutes. The temperatures of the injector and transfer lines were set to 270 and 310°C, respectively. The standards and samples were injected (1 μl) in the splitless mode. Acenaphthylene D8 was used as the surrogate and BaP D12 was used as the internal standard. We considered the limit of quantification (LOQ) as 10-fold the standard deviation (SD) of the blank. The LOQ was 0.05 ng/ml.
The GC was interfaced with a TSQ Quantum Max QqQ mass spectrometer (mass range, m/z, from 10 to 1050) for the detection of the PAHs included in this study. The instrument data system also contained an electron ionization (EI)-MS/MS library, which was specially created for the target analytes under our experimental conditions. The mass spectrometer scale was calibrated weekly with perfluorotributylamine. ThermoFisher Xcalibur Software (Ver. 2.0.1) was used for instrument control, data acquisition, and data analysis.
A timed selected reaction monitoring (SRM) method was developed to analyze the 16 target compounds in addition to one surrogate and one internal standard within a single run. A calibration curve was constructed from 0.05 to 100 ng ml−1 with all the compounds, with the exception of the surrogates and internal standards, contained in each calibration standard mixture. Argon (99.99%) was used as the collision gas and the collision cell pressure was set to 0.2 Pa. The QqQ mass spectrometer was operated under the following conditions: ionization with electron impact at 70 eV in MRM with an emission current of 50 μA. The ionization source temperature was set to 220°C. A filament multiplier delay of 5 minutes was established to prevent instrument damage. The electron multiplier voltage was set to 1500 V. The scan width was 0.15 and the scan time was 0.05 s. Peak widths of m/z 0.7 Da were set for both the first (Q1) and third quadrupoles (Q3).
Compounds were identified as the target analytes when their chromatographic peaks satisfied the following criteria: (1) the retention time (tR) of the candidate was within three SD of the average tR (tR±3SD) obtained when six blank samples spiked at the second level of calibration were injected; (2) the ion ratios matched those of the standard with a tolerance of ±30% in absolute ion abundances; and (3) the S/N ratio of the target analytes was >10 for the sample extract.
Statistical analysis
Statistical analysis of the data was conducted using the Statistical Package for Social Sciences software (SPSS, version 17.0, SPSS, Chicago, IL, USA). Continuous variables were expressed as the means (and SD) if normally distributed or by the medians (and 5th and 95th percentiles) if skewed. Differences in means were analyzed using Student's t-test (t-test) or ANOVA. Continuous variables that were not normally distributed were analyzed using Mann–Whitney and Kruskal–Wallis tests. Categorical variables were expressed as percentages and differences were assessed using the chi-squared test. The magnitude of association was assessed using odds ratios (OR), and the corresponding 95% confidence interval was calculated through multinomial logistic regression, which is a statistical model used to predict the probabilities of the different possible outcomes of a categorically distributed dependent variable (such as bladder cancer), given a set of independent variables (that is, adjusting for appropriate covariates previously associated to the dependent variable in univariate analysis).
RESULTS
As expected, the percentage of non-smokers was higher in the control population than in patients with bladder cancer (35.0 vs 15.7%, respectively). The percentage of ex-smokers was higher in the bladder cancer group (P<0.001). In addition, as shown in Table 1, the intensity of smoking (number of cigarettes smoked multiplied by the number of smoking years, measured as packyears) was higher (P<0.001) and the age of onset for smoking was earlier among bladder cancer cases (16.8±3.6 in bladder cancer cases and 18.2±2.6 years in controls, P = 0.006; data not shown). Consequently, smoking was a clear risk factor for bladder cancer, as determined through a multivariate analysis adjusted by age and gender (OR = 3.62, 95%CI = 1.93–6.79, P<0.0001).
Coffee consumption was significantly higher in cases than controls (72.7 vs 61.2; P = 0.027; Table 1). Furthermore, the median values of coffee consumption were higher among the cases (90 ml/day for the cases and 45 ml/day for the controls; P = 0.005; data not shown). Because coffee consumption is associated with smoking, we performed multivariate logistic regression analysis to determine the risk of bladder cancer for subjects who consumed coffee adjusting for gender, age, and smoking. Our results showed that coffee consumption, independent of smoking, increased the risk of bladder cancer by 1.73-fold (95%CI = 1.04–2.86, P = 0.033).
Although there were no statistically significant differences in the PAH serum levels by occupation (data not shown), we observed differences in the distribution of high-risk occupations among the cases and the controls (Table 1). For example, 47.1% of bladder cancer cases were employed in jobs associated with increased risk for PAH exposure compared with 34.0% of the control population (P = 0.014). Being employed as a ‘driver’ (P = 0.034) or ‘painter’ (P = 0.050) was more frequent among bladder cancer cases. However, because certain professions are associated with smoking, multivariate logistic regression analysis adjusting for gender, age, and smoking revealed that being employed as a driver or painter did not increase the risk for bladder cancer (OR = 1.64, 95%CI = 0.85–3.17, P = 0.136).
Approximately 70% of studied individuals showed measurable PAH serum levels. As shown in Table 2, the low-molecular-weight PAHs (di- and tricyclic PAHs) were most common. The most frequently detected PAH was Phe (Table 2). Six compounds (fluoranthene, benzo[b]fluoranthene, benzo[k]fluoranthene, indenopyrene, benzoperylene, and dibenzo[a,h]anthracene) were not detected in any of the samples. The total burden of PAHs (∑PAHs) was similar (median values close to 1 ng/ml) for cases and controls. Total burden of PAHs were independent of smoking (data not shown). Similarly, there were no differences in PAH serum levels by diet.
Table 2. Distribution of polycyclic aromatic hydrocarbons (PAHs) among cases and controls.
| Compound | Cases | Controls | P* | P# | |||
| IARC group† | % | Median (p5–p95) | % | Median (p5–p95) | |||
| Naphthalene | 2B | 2.1 | 0.0 (0.0–0.0) | 2.4 | 0.0 (0.0–0.0) | 1.000 | 0.807 |
| Acenaphthylene | – | 0.0 | <LOD | 1.0 | 0.0 (0.0–0.0) | 0.513 | 0.233 |
| Acenaphthene | 3 | 27.1 | 0.0 (0.0–1.6) | 27.2 | 0.0 (0.0–1.0) | 0.901 | 0.967 |
| Fluorene | 3 | 0.0 | <LOD | 0.0 | <LOD | NA | NA |
| Anthracene | 3 | 0.7 | 0.0 (0.0–0.0) | 0.5 | 0.0 (0.0–0.0) | 1.000 | 0.811 |
| Phenanthrene | 3 | 49.3 | 0.5 (0.0–3.3) | 62.6 | 0.9 (0.0–4.0) | 0.002 | 0.003 |
| Fluoranthene | 3 | 0.0 | <LOD | 0.0 | <LOD | NA | NA |
| Pyrene | 3 | 3.6 | 0.0 (0.0–0.0) | 8.3 | 0.0 (0.0–0.03) | 0.074 | 0.066 |
| Benz[a]anthracene | 2B | 11.4 | 0.0 (0.0–0.02) | 14.6 | 0.0 (0.0–0.02) | 0.337 | 0.355 |
| Chrysene | 2B | 5.7 | 0.0 (0.0–0.01) | 4.9 | 0.0 (0.0–0.01) | 0.810 | 0.802 |
| Benzo[b]fluoranthene | 2B | 0.0 | <LOD | 0.0 | <LOD | NA | NA |
| Benzo[k]fluoranthene | 2B | 0.0 | <LOD | 0.0 | <LOD | NA | NA |
| Benzo[a]pyrene | 3 | 0.0 | <LOD | 0.5 | 0.0 (0.0–0.0) | 1.000 | 0.400 |
| Indeno pyrene | 2B | 0.0 | <LOD | 0.0 | <LOD | NA | NA |
| Dibenz[a,h]anthracene | 2A | 0.0 | <LOD | 0.0 | <LOD | NA | NA |
| Benzo[g,h,i]perylene | 3 | 0.0 | <LOD | 0.0 | <LOD | NA | NA |
| ΣPAHs | 63.6 | 0.6 (0.0–4.7) | 71.4 | 1.0 (0.0–5.8) | 0.027 | 0.010 | |
IARC, International Agency for Research on Cancer; p5–p95, percentiles 5 and 95 of the distribution; LOD, limit of detection; NA, not applicable.
†Carcinogenicity of PAHs according to the IARC classification: 2A, probably carcinogenic to humans; 2B, possibly carcinogenic to human beings; 3, not classifiable as carcinogenic to human beings.
*Kruskal–Wallis test comparing the percentage of detection among cases and controls.
#Kruskal–Wallis test comparing the level of detection among cases and controls.
The profile of serum PAHs measured in bladder cancer patients was qualitatively different to the profile of the control group. Acenaphthylene and BaP were measured only in the serum samples from the controls (Table 2). Moreover, all of the samples in which Pyr was the only compound detected belonged to the control group, and all of the samples in which Chry was the only compound detected belonged to the bladder cancer group (Fig. 1). Analysis of the individual contaminants revealed that only Phe showed a different distribution between cases and controls. Approximately, 63% of the controls had detectable levels of Phe with a median concentration of 0.9 ng/ml of serum, whereas approximately 49% of the cases had measurable levels of Phe with a median concentration of 0.5 ng/ml of serum (P = 0.002 and 0.003, respectively) (Table 2). Phe appeared to be negatively associated with bladder cancer, as determined through multivariate analysis adjusting for gender, age, and smoking (OR = 0.79, 95%CI = 0.64–0.99, P = 0.030). Including the distribution of the GST polymorphism (GST null vs heterozygotes+homozygote wild-type) into the multivariate analyses removed the negative association between Phe and bladder cancer (Table 3).
Figure 1.
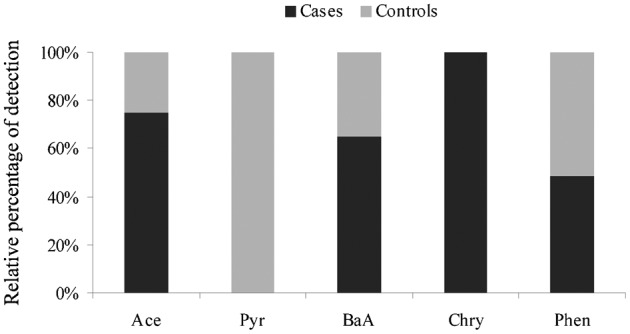
Frequency of detection of PAHs among bladder cancer patients and controls. Ace, acenaphthene; Pyr, pyrene; BaA, benz[a]anthracene; Chry, chrysene; Phe, phenanthrene.
Table 3. Independent risk factors for bladder cancer in multivariate analysis*.
| Variable | OR | CI95% | P |
| Age** | 1.02 | 0.99–1.05 | 0.294 |
| Gender | 0.61 | 0.18–2.03 | 0.417 |
| Smoking | 3.31 | 1.32–8.33 | 0.011 |
| Coffee drinking | 2.64 | 1.12–6.26 | 0.027 |
| Phenantrene** | 0.98 | 0.70–1.37 | 0.922 |
| GST-T1 | 1.39 | 0.56–3.42 | 0.474 |
OR, odds ratio; CI, confidence interval; GST-M1, glutathione-S-transferase mu1.
* Multivariate analysis including age, gender, smoking, coffee drinking, phenantrene, and GST-T1 as co-variables.
** Included in the model as continuous variables.
DISCUSSION
Epidemiological studies have suggested an association between exposure to PAHs and bladder cancer.1,10 To our knowledge, this is the first study evaluating the role of PAH exposure (as PAH serum levels) in bladder cancer.
Our results indicate that most of the subjects had measurable PAH serum levels. Furthermore, the distribution of PAHs was similar between cases and controls and between the different occupations and did not appear to be related with coffee consumption or smoking status. These findings indicate that exposure to PAHs is difficult to avoid given their ubiquitous presence in fossil fuels, plastic and rubber containers, food, and tobacco smoke. This is concerning because no dose–response relationship exists for PAHs and therefore there is no safe PAH exposure level.27 This study detected the most volatile PAHs (Naph, Acy, Ace, Phe, and Ant), suggesting that inhalation of PAHs was the most relevant source of PAH exposure in our sample. This does not necessarily imply the existence of a relevant source of contamination in this region because low-molecular-weight PAHs travel long distances and originate from distant sources of pollution.28,29 Diesel vehicle emissions are a source of the lightest PAHs.30 The widespread exposure to environmental tobacco smoke and traffic-related air pollution makes it difficult to find differences in the PAH serum levels in a study with a limited sample size and may explain the absence of statistically significant differences in the PAH serum levels between the groups (i.e., cases and controls, smokers and non-smokers, etc.). In addition, our study may be biased because PAHs measurements were taken at one point in time and therefore may only reflect recent exposure and not lifetime exposure. In this sense, although serum PAH levels are proposed biomarkers of PAH exposure,4,31 our results indicate that blood does not appear to be the most adequate biological sample to assess quantitative differences in the PAH burden between populations because the mechanisms underlying the differences in PAH serum levels may be impacted by several factors, including genetics that may modulate the metabolism of these compounds. In any case, the measurement of parental PAHs and/or their metabolites in body fluids may be useful to assess exposure to these chemicals, although, as a consequence of genetic polymorphism in enzyme-detoxifying genes, PAH serum levels may vary between individuals with a similar exposure.
A number of studies suggest the existence of complex gene–environment interactions in bladder carcinogenesis. Thus, polymorphic low-penetrance genes, including GST, in association with environmental exposure to chemicals may contribute significantly to bladder tumorigenesis.21,32 Our previous results demonstrating that subjects with the GST-T1-null genotype may be at increased risk of bladder cancer,24 reinforce the hypotheses that the gene–environment interaction may be a determinant factor in the carcinogenic processes of the bladder. In this context, our findings that the PAH serum levels were not associated to bladder cancer in our population should be evaluated from a gene–environment perspective. Thus, the introduction of the distribution of the GST polymorphism in our population24 into the multivariate analyses resulted in the disappearance of the negative association found for Phe and bladder cancer. This suggests that genetic differences in detoxification capabilities may highly modulate the PAH-induced carcinogenicity on the bladder and reinforce the possibility that a potential interaction of this polymorphism or other susceptible genetic variants with environmental chemicals may be relevant in the carcinogenic processes induced by these chemicals.
In addition, although our findings do not demonstrate a relationship between PAH serum levels and bladder cancer risk, a potential PAH source may also be a simultaneously relevant source for other environmental pollutants; consequently, the biological effect may be due to a mixture of chemicals, making it difficult to demonstrate whether individual environmental PAHs are risk factors for bladder cancer without confounding from other environmental carcinogens. In fact, PAHs usually coexist as mixtures in the environment, and the toxicity of the organic chemicals in these mixtures is receiving increasing attention.33 However, research on the toxic effects of mixtures of environmental pollutants is lacking, specially taking into account that the biological effects exerted by mixtures of environmental pollutants are different to those exerted by single chemicals.34–36 It has been suggested that different PAHs in complex mixtures have a larger influence on carcinogenic potency than the most carcinogenic PAH (BaP) alone.37 Bearing in mind that the potential carcinogenicity of PAH mixtures has been scarcely explored in epidemiological studies involving serum samples, our results that the profiles of PAHs and their mixtures appear to be different between cases and controls raise the possibility that certain combinations of PAHs influence the carcinogenic activity of these chemicals. Future studies are needed to evaluate the biological consequences derived from exposure to different PAH mixtures.
With respect to bladder cancer risk, our results are consistent with previously published results finding that smoking is the main risk factor for bladder cancer.9–11 In addition, our findings reinforce the role played by the duration, intensity, and age of onset of smoking.38,39 Although a number of occupations with PAH exposure have been associated with bladder cancer risk,40 strong evidence of increased risk is apparent for very few professions, including painters and professional drivers.40,41 Multiple studies have reported a positive association between driver employment and bladder cancer.42,43 However, workers from this professional sector (especially truckers and taxi drivers) often smoke; thus, this relationship is influenced by smoking,44 as appears to be the case in our sample, because this profession was not a risk factor for bladder cancer when smoking status was included in the statistical model. The analysis of dietary habits revealed that coffee may be a relevant PAH source,45 and coffee consumption has also been previously associated with bladder cancer.46,47 However, this association is controversial19,20,48 because coffee drinkers tend to smoke more than non-coffee drinkers; thus, the potential role played by coffee on bladder cancer risk may be attributed to the presence of smoking as a confounder.49,50 In our population, coffee consumption increased the risk of bladder cancer independent of smoking. These results agree with other studies and suggest a positive association between coffee consumption and the risk of bladder cancer, particularly for males consuming more than seven cups of coffee per day.20
In conclusion, our study does not provide evidence for a relationship between PAH exposure (as measured by the PAH serum levels) and bladder cancer, but does suggest that the burden of specific PAH mixtures may be related to bladder cancer. It also confirmed that certain lifestyle factors presumably related to increased PAH exposure, including smoking and coffee consumption, appear to be relevant risk factors for bladder cancer in this population. In addition, the new finding stemming from this study, namely, that GST polymorphisms are modifiers of the effect of certain PAHs, reinforces the hypothesis that genetic characteristics contribute to an individual’s susceptibility to bladder cancer. However, our results must be interpreted with caution. First, the limited size of our sample may limit the accuracy of the statistical analyses. Additionally, it is possible that unknown risk factors for bladder cancer were not considered and that as a consequence, unmeasured or residual confounding factors were not included in the analyses.
DISCLAIMER STATEMENTS
Contributors LDB and LAHH have contributed equally to this work. Thus, they must be considered as first authors indistinctly.
Funding FUNCIS (Gobierno de Canarias) and ICIC (Instituto Canario de Investigación del Cáncer)
Conflicts of interest The authors declare no conflict of interest.
Ethics approval Not applicable.
Acknowledgments
This work was supported by FUNCIS PI 35/08 and FUNCIS PI 55/07 of the Gobierno de Canarias (Spain) and by PI 19/08 of the Instituto Canario de Investigación del Cáncer (ICIC).
REFERENCES
- 1.Georgiadis P, Topinka J, Stoikidou M, Kaila S, Gioka M, Katsouyanni K, et al. Biomarkers of genotoxicity of air pollution (the AULIS project): bulky DNA adducts in subjects with moderate to low exposures to airborne polycyclic aromatic hydrocarbons and their relationship to environmental tobacco smoke and other parameters. Carcinogenesis. 2001;22((9)):1447–57. doi: 10.1093/carcin/22.9.1447. [DOI] [PubMed] [Google Scholar]
- 2.Gini M, Lianou M, Chalbot MC, Kotronarou A, Kavouras IG, Helmis CG. Quantification of environmental tobacco smoke contribution on outdoor particulate aliphatic and polycyclic aromatic hydrocarbons. Arch Environ Contam Toxicol. 2013;64((3)):347–56. doi: 10.1007/s00244-012-9844-6. [DOI] [PubMed] [Google Scholar]
- 3.EPA. EPA method 8100 polynuclear aromatic hydrocarbons, 1986. Available from: http://wwwepagov/osw/hazard/testmethods/sw846/pdfs/8100pdf [Google Scholar]
- 4.Al-Daghri NM, Alokail MS, Abd-Alrahman SH, Draz HM, Yakout SM, Clerici M. Polycyclic aromatic hydrocarbon exposure and pediatric asthma in children: a case-control study. Environ Health. 2013;12:1. doi: 10.1186/1476-069X-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li Z, Sandau CD, Romanoff LC, Caudill SP, Sjodin A, Needham LL, et al. Concentration and profile of 22 urinary polycyclic aromatic hydrocarbon metabolites in the US population. Environ Res. 2008;107((3)):320–31. doi: 10.1016/j.envres.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Cogliano VJ, Baan R, Straif K, Grosse Y, Lauby-Secretan B, El Ghissassi F, et al. Preventable exposures associated with human cancers. J Natl Cancer Inst. 2011;103((24)):1827–39. doi: 10.1093/jnci/djr483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murta-Nascimento C, Schmitz-Drager BJ, Zeegers MP, Steineck G, Kogevinas M, Real FX, et al. Epidemiology of urinary bladder cancer: from tumor development to patient's death. World J Urol. 2007;25((3)):285–95. doi: 10.1007/s00345-007-0168-5. [DOI] [PubMed] [Google Scholar]
- 8.Silverberg E. Statistical and epidemiologic data on urologic cancer. Cancer. 1987;60((3 Suppl)):692–717. doi: 10.1002/1097-0142(19870801)60:3+<692::aid-cncr2820601541>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 9.Burger M, Catto JW, Dalbagni G, Grossman HB, Herr H, Karakiewicz P, et al. Epidemiology and risk factors of urothelial bladder cancer. Eur Urol. 2013;63((2)):234–41. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 10.Kellen E, Zeegers M, Paulussen A, Vlietinck R, Vlem EV, Veulemans H, et al. Does occupational exposure to PAHs, diesel and aromatic amines interact with smoking and metabolic genetic polymorphisms to increase the risk on bladder cancer? The Belgian case control study on bladder cancer risk. Cancer Lett. 2007;245((1–2)):51–60. doi: 10.1016/j.canlet.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 11.Okada Y, Hanada M, Sugiura Y. Bladder cancer and smoking. Br Med J. 1964;1((5384)):649–50. [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffman D, Masuda Y, Wynder EL. Alpha-naphthylamine and beta-naphthylamine in cigarette smoke. Nature. 1969;221((5177)):255–6. doi: 10.1038/221254a0. [DOI] [PubMed] [Google Scholar]
- 13.Benbrahim-Tallaa L, Baan RA, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of diesel-engine and gasoline-engine exhausts and some nitroarenes. Lancet Oncol. 2012;13((7)):663–4. doi: 10.1016/s1470-2045(12)70280-2. [DOI] [PubMed] [Google Scholar]
- 14.Loomis D, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, et al. The carcinogenicity of outdoor air pollution. Lancet Oncol. 2013;14((13)):1262–3. doi: 10.1016/s1470-2045(13)70487-x. [DOI] [PubMed] [Google Scholar]
- 15.la Vecchia C, Negri E, D'Avanzo B, Franceschi S. Occupation and the risk of bladder cancer. Int J Epidemiol. 1990;19((2)):264–8. doi: 10.1093/ije/19.2.264. [DOI] [PubMed] [Google Scholar]
- 16.Cantor KP, Villanueva CM, Silverman DT, Figueroa JD, Real FX, Garcia-Closas M, et al. Polymorphisms in GSTT1, GSTZ1, and CYP2E1, disinfection by-products, and risk of bladder cancer in Spain. Environ Health Perspect. 2010;118((11)):1545–50. doi: 10.1289/ehp.1002206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nomura A, Heilbrun LK, Stemmermann GN. Prospective study of coffee consumption and the risk of cancer. J Natl Cancer Inst. 1986;76((4)):587–90. doi: 10.1093/jnci/76.4.587. [DOI] [PubMed] [Google Scholar]
- 18.Ros MM, Bas Bueno-de-Mesquita HB, Buchner FL, Aben KK, Kampman E, Egevad L, et al. Fluid intake and the risk of urothelial cell carcinomas in the European Prospective Investigation into Cancer and Nutrition (EPIC). Int J Cancer. 2011;128((11)):2695–708. doi: 10.1002/ijc.25592. [DOI] [PubMed] [Google Scholar]
- 19.Sala M, Cordier S, Chang-Claude J, Donato F, Escolar-Pujolar A, Fernandez F, et al. Coffee consumption and bladder cancer in nonsmokers: a pooled analysis of case-control studies in European countries. Cancer Causes Control. 2000;11((10)):925–31. doi: 10.1023/a:1026524014954. [DOI] [PubMed] [Google Scholar]
- 20.Zeegers MP, Dorant E, Goldbohm RA, van den Brandt PA. Are coffee, tea, and total fluid consumption associated with bladder cancer risk? Results from the Netherlands Cohort Study. Cancer Causes Control. 2001;12((3)):231–8. doi: 10.1023/a:1011245627593. [DOI] [PubMed] [Google Scholar]
- 21.Whyatt RM, Perera FP, Jedrychowski W, Santella RM, Garte S, Bell DA. Association between polycyclic aromatic hydrocarbon-DNA adduct levels in maternal and newborn white blood cells and glutathione S-transferase P1 and CYP1A1 polymorphisms. Cancer Epidemiol Biomarkers Prev. 2000;9((2)):207–12. [PubMed] [Google Scholar]
- 22.Tang W, Fu YP, Figueroa JD, Malats N, Garcia-Closas M, Chatterjee N, et al. Mapping of the UGT1A locus identifies an uncommon coding variant that affects mRNA expression and protects from bladder cancer. Hum Mol Genet. 2012;21((8)):1918–30. doi: 10.1093/hmg/ddr619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matic M, Pekmezovic T, Djukic T, Mimic-Oka J, Dragicevic D, Krivic B, et al. GSTA1, GSTM1, GSTP1, and GSTT1 polymorphisms and susceptibility to smoking-related bladder cancer: a case-control study. Urol Oncol. 2013;31((7)):1184–92. doi: 10.1016/j.urolonc.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 24.Henriquez-Hernandez LA, Navarro P, Luzardo OP, Alvarez-Leon EE, Boada LD, Zumbado M, et al. Polymorphisms of glutathione S-transferase mu and theta, MDR1 and VEGF genes as risk factors of bladder cancer: a case-control study. Urol Oncol. 2012;30((5)):660–5. doi: 10.1016/j.urolonc.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 25.Samanic C, Kogevinas M, Dosemeci M, Malats N, Real FX, Garcia-Closas M, et al. Smoking and bladder cancer in Spain: effects of tobacco type, timing, environmental tobacco smoke, and gender. Cancer Epidemiol Biomarkers Prev. 2006;15((7)):1348–54. doi: 10.1158/1055-9965.EPI-06-0021. [DOI] [PubMed] [Google Scholar]
- 26.Camacho M, Boada LD, Oros J, Calabuig P, Zumbado M, Luzardo OP. Comparative study of polycyclic aromatic hydrocarbons (PAHs) in plasma of Eastern Atlantic juvenile and adult nesting loggerhead sea turtles (Caretta caretta). Mar Pollut Bull. 2012;64((9)):1974–80. doi: 10.1016/j.marpolbul.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Gaga EO, Ari A, Dogeroglu T, Cakirca EE, Machin NE. Atmospheric polycyclic aromatic hydrocarbons in an industrialized city, Kocaeli, Turkey: study of seasonal variations, influence of meteorological parameters and health risk estimation. J Environ Monit. 2012;14((8)):2219–29. doi: 10.1039/c2em30118k. [DOI] [PubMed] [Google Scholar]
- 28.Callen MS, Lopez JM, Iturmendi A, Mastral AM. Nature and sources of particle associated polycyclic aromatic hydrocarbons (PAH) in the atmospheric environment of an urban area. Environ Pollut. 2012;183:166–74. doi: 10.1016/j.envpol.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Chang KF, Fang GC, Chen JC, Wu YS. Atmospheric polycyclic aromatic hydrocarbons (PAHs) in Asia: a review from 1999 to 2004. Environ Pollut. 2006;142((3)):388–96. doi: 10.1016/j.envpol.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 30.Guo H, Lee SC, Ho KF, Wang MX, Zou SC. Particle-associated polycyclic aromatic hydrocarbons in urban air of Hong Kong. Atmos Environ. 2003;37:5307–17. [Google Scholar]
- 31.Al-Saleh I, Alsabbahen A, Shinwari N, Billedo G, Mashhour A, Al-Sarraj Y, et al. Polycyclic aromatic hydrocarbons (PAHs) as determinants of various anthropometric measures of birth outcome. Sci Total Environ. 2013;444:565–78. doi: 10.1016/j.scitotenv.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 32.Pavanello S, Mastrangelo G, Placidi D, Campagna M, Pulliero A, Carta A, et al. CYP1A2 polymorphisms, occupational and environmental exposures and risk of bladder cancer. Eur J Epidemiol. 2010;25((7)):491–500. doi: 10.1007/s10654-010-9479-8. [DOI] [PubMed] [Google Scholar]
- 33.Jarvis IW, Dreij K, Mattsson A, Jernstrom B, Stenius U. Interactions between polycyclic aromatic hydrocarbons in complex mixtures and implications for cancer risk assessment. Toxicology. 2014;321:27–39. doi: 10.1016/j.tox.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Boada LD, Lara PC, Alvarez-Leon EE, Losada A, Zumbado ML, Liminana-Canal JM, et al. Serum levels of insulin-like growth factor-I in relation to organochlorine pesticides exposure. Growth Horm IGF Res. 2007;17((6)):506–11. doi: 10.1016/j.ghir.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Boada LD, Zumbado M, Henriquez-Hernandez LA, Almeida-Gonzalez M, Alvarez-Leon EE, Serra-Majem L, et al. Complex organochlorine pesticide mixtures as determinant factor for breast cancer risk: a population-based case-control study in the Canary Islands (Spain). Environ Health. 2012;11:28. doi: 10.1186/1476-069X-11-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kortenkamp A. Breast cancer, oestrogens and environmental pollutants: a re-evaluation from a mixture perspective. Int J Androl. 2006;29((1)):193–8. doi: 10.1111/j.1365-2605.2005.00613.x. [DOI] [PubMed] [Google Scholar]
- 37.Jarvis IW, Bergvall C, Bottai M, Westerholm R, Stenius U, Dreij K. Persistent activation of DNA damage signaling in response to complex mixtures of PAHs in air particulate matter. Toxicol Appl Pharmacol. 2013;266((3)):408–18. doi: 10.1016/j.taap.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 38.Brennan P, Bogillot O, Cordier S, Greiser E, Schill W, Vineis P, et al. Cigarette smoking and bladder cancer in men: a pooled analysis of 11 case-control studies. Int J Cancer. 2000;86((2)):289–94. doi: 10.1002/(sici)1097-0215(20000415)86:2<289::aid-ijc21>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 39.Doll R, Peto R, Wheatley K, Gray R, Sutherland I. Mortality in relation to smoking: 40 years' observations on male British doctors. BMJ. 1994;309((6959)):901–11. doi: 10.1136/bmj.309.6959.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samanic CM, Kogevinas M, Silverman DT, Tardon A, Serra C, Malats N, et al. Occupation and bladder cancer in a hospital-based case-control study in Spain. Occup Environ Med. 2008;65((5)):347–53. doi: 10.1136/oem.2007.035816. [DOI] [PubMed] [Google Scholar]
- 41.IARC. Overall Evaluations of Carcinogenicity: An Updating of Selected IARC Monographs, Volumes 1 to 42. International Agency for Research on Cancer Monographs; 1987 Lyon, France. Supplement 7. [Google Scholar]
- 42.Manju L, George PS, Mathew A. Urinary bladder cancer risk 3among motor vehicle drivers: a meta-analysis of the evidence, 1977–2008. Asian Pac J Cancer Prev. 2009;10((2)):287–94. [PubMed] [Google Scholar]
- 43.Reulen RC, Kellen E, Buntinx F, Brinkman M, Zeegers MP. A meta-analysis on the association between bladder cancer and occupation. Scand J Urol Nephrol Suppl. 2008;218:64–78. doi: 10.1080/03008880802325192. [DOI] [PubMed] [Google Scholar]
- 44.Vineis P, Simonato L. Proportion of lung and bladder cancers in males resulting from occupation: a systematic approach. Arch Environ Health. 1991;46((1)):6–15. doi: 10.1080/00039896.1991.9937423. [DOI] [PubMed] [Google Scholar]
- 45.Grover IS, Sharma R, Singh S, Pal B. Polycyclic aromatic hydrocarbons in some grounded coffee brands. Environ Monit Assess. 2012;185:6459–63. doi: 10.1007/s10661-012-3037-7. [DOI] [PubMed] [Google Scholar]
- 46.D'Avanzo B, La Vecchia C, Franceschi S, Negri E, Talamini R, Buttino I. Coffee consumption and bladder cancer risk. Eur J Cancer. 1992;28A((8–9)):1480–4. doi: 10.1016/0959-8049(92)90548-g. [DOI] [PubMed] [Google Scholar]
- 47.Stensvold I, Jacobsen BK. Coffee and cancer: a prospective study of 43,000 Norwegian men and women. Cancer Causes Control. 1994;5((5)):401–8. doi: 10.1007/BF01694753. [DOI] [PubMed] [Google Scholar]
- 48.Yu X, Bao Z, Zou J, Dong J. Coffee consumption and risk of cancers: a meta-analysis of cohort studies. BMC Cancer. 2011;11:96. doi: 10.1186/1471-2407-11-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pelucchi C, La Vecchia C. Alcohol, coffee, and bladder cancer risk: a review of epidemiological studies. Eur J Cancer Prev. 2009;18((1)):62–8. doi: 10.1097/CEJ.0b013e32830c8d44. [DOI] [PubMed] [Google Scholar]
- 50.Villanueva CM, Silverman DT, Murta-Nascimento C, Malats N, Garcia-Closas M, Castro F, et al. Coffee consumption, genetic susceptibility and bladder cancer risk. Cancer Causes Control. 2009;20((1)):121–7. doi: 10.1007/s10552-008-9226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


