Abstract
Background:
There is a large and consistent body of evidence showing that research sponsored by for-profit industries tends to have pro-industry conclusions in comparison with similar research or re-analyses not funded by industry. Disclosure of financial conflicts via statements is presently the standard method for notification of potential biases. However, many journals are not consistent in publishing financial conflicts of interest (FCoI) statements. Furthermore, even when divulged, disclosure merely shifts the burden of evaluating conflicts to readers and the general public. Moreover, there has been an absence of a means of quantifying FCoI.
Objectives:
To propose a solution for the question: What are we doing about FCoI that continue to compromise the integrity of the scientific enterprise?
Methods:
The FCoI Scale was developed for scoring and comparing FCoI and describing potential biases.
Results:
The FCoI Scale consists of a score that may be expressed in whole numbers and decimal fractions, correlated to descriptive terms for potential biases and examples of financial conflicts at 11 levels.
Conclusions:
The FCoI score (FCoIS) provides a means for a more uniform and concise method of disclosure compared to statements, while at the same time permitting flexibility. It encourages the disclosure of relevant information and transparency in the reporting of financial conflicts. The FCoI Scale has the potential to become the standard basis for measuring, reporting, and comparing financial conflicts, suitable for disciplines in science, medicine, and beyond.
Keywords: Campaign contributions, Ethics, FCoIS, Industry or corporate funding, Instrument or scale, International organizations, Public health and policy, Scientific and medical journals and publishing, Scientific societies and professional associations
INTRODUCTION
Financial conflicts of interest (FCoI) are increasingly relevant and have far-reaching implications in scientific, medical, and public realms. They threaten the integrity of the scientific and medical literature, work that is used to shape key decisions in public health and policy and form the evidence base for patient care. Although other conflicts exist, there is a large and consistent body of evidence showing that research sponsored by for-profit companies tends to have pro-industry conclusions compared to similar research or re-analyses not funded by industry.1–13 Disclosure of financial conflicts via statements is presently the standard method for notification of potential biases. However, this raises the question of how statements should be interpreted – particularly by the general public, who has limited ability to evaluate technical information presented in peer-reviewed journal articles. In an effort to advance a better-informed public and alleviate the burden of interpreting disclosure, I developed an instrument for scoring and comparing FCoI and describing potential biases. Designed to be easy to use and generalizable, the proposed FCoI Scale may be of value across science and medical disciplines and to the general public.
THE SCALE
The FCoI Scale produces a numerical score that is correlated to descriptive terms for potential biases, including financial conflicts, at 11 levels (Table 1). A present or anticipated financial interest of any dollar amount indicates an inherent conflict of interest, and specifically a FCoI, with the potential for bias reasonably increasing as the FCoI level increases. The term “present” refers to the time frame of the study or project, including its full duration up to submission of the work. The “examples” column in Table 1 illustrates activities and/or received benefits including indirect and direct support from all entities that stand (or potentially stand) to gain or lose financially in reference to the subject matter at hand. Indirect benefits include benefits from a third party funded via the vested interest party.
Table 1. Financial conflict of interest scale (FCoI Scale).
| Financial conflict of interest score or level | Potential bias | Examples |
| 10 | Maximum | Employment or consultancies; Paid authorship; Study intended for use in litigation |
| 9 | Extreme | Study or presentation (design, analysis, interpretation, or writing) reviewed, approved, or modified by sponsor; Expert testimony |
| 8 | High | Stock share ownership or options, bonds |
| 7 | Substantial | Funding, grants, patents, royalties, copyrights, trademarks; Sponsor income for service on advisory boards, board of directors, review panels; Large gifts/products, e.g. vacations or (any) research-related |
| 6 | Significant | Honoraria; Payment for attendance at lectures/meetings or enrolling patients in trials; Non-sponsor income for services and service on advisory committees, review panels |
| 5 | Considerable | Lodging, travel, transportation; Meeting registration fees; Off-site meals; Medium-large gifts/products, e.g. sporting event tickets; Campaign or charitable contributions and donations |
| 4 | Moderate | Medium gifts/products, e.g. bottle of wine; Moderate entertainment activities |
| 3 | Minimal | On-site meals; Use of samples (personal/family or by staff) |
| 2 | Low | Small-medium gifts/products, e.g. books |
| 1 | Insignificant | Small gifts/products, e.g. pens, notepads, calendars, candy |
| 0 | Nonexistent | Nothing to declare |
The examples in Table 1 highlight typical financial conflicts, listed according to the nature and magnitude of the conflict. “Paid authorship” (Level 10: maximum) does not refer to ghost authorship (which is unacceptable), but to support for writing commentaries and letters to the editor (often written by financially conflicted authors to cast doubt on previously published work with unfavorable results to their sponsors) or writing manuscripts initiated by the sponsor (e.g. under contract). These types of work do not fall under grant-supported research. “Stock share ownership or options, bonds” (Level 8: high) refer to where the buying or selling is controlled by the purchaser/recipient, and does not include mutual funds, pensions, or investment trusts, where the purchaser/recipient is not in control of the buying or selling of assets. “Non-sponsor income for services and service on advisory committees, review panels” (Level 6: significant) is defined as income other than from the sponsor, but where one has a FCoI on the topic. An example of a “non-sponsor income for services” is a physician who received payment as a surgeon in the previous 5 years for implanting a particular medical device and then submits a manuscript on the device. An example of a “non-sponsor income for service” would be income derived from service on an advisory committee from a public or nonprofit entity not associated with the vested interest party, but where the issue being evaluated is one where the individual has a financial conflict. Costs (reimbursed or subsidized by a vested interest party) associated with continuing medical education are included in FCoI Level 5.
In cases where multiple FCoI levels apply, the highest applicable level determines the FCoI score (FCoIS), except when averages (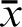 ) are provided (see below). An individual’s FCoIS must also reflect the financial conflicts of his or her spouse and dependent children. Averages should not be used to avoid citing the appropriate (higher) FCoIS and should never be used by individuals, as unaveraged individual FCoI scores are a needed source of primary data. Decimal fractions to the tenths place should be reported for standardization. The FCoIS is equally applicable if accepted gifts/products or remunerations are transferred to another party at a later date, including as donations to nonprofit organizations. For activities or support not covered in Table 1, the reporting entity (see below) should determine the conflict level using the most similar example from the table.
) are provided (see below). An individual’s FCoIS must also reflect the financial conflicts of his or her spouse and dependent children. Averages should not be used to avoid citing the appropriate (higher) FCoIS and should never be used by individuals, as unaveraged individual FCoI scores are a needed source of primary data. Decimal fractions to the tenths place should be reported for standardization. The FCoIS is equally applicable if accepted gifts/products or remunerations are transferred to another party at a later date, including as donations to nonprofit organizations. For activities or support not covered in Table 1, the reporting entity (see below) should determine the conflict level using the most similar example from the table.
THE FCOIS
A FCoIS can be reported by any entity, including individuals, departments, and organizations (e.g. medical center, research institute, university). Since journals, professional associations, and nonprofits may also be in-service to or sponsored by industry,14–16 there is a need for these entities to also report their FCoI scores.
In addition, FCoI scores may be reported for scientific manuscripts, supplemental issues of journals, and even in politics (for example ad hoc committees), by citing the respective average scores of the authors, papers, and committee members. A hypothetical example would be considering all sources of support to a surgeon over a five-year period. This would be reported as FCoIS = 6.0 (Surgeon AB, medical device name, 2000–2005). The FCoIS for a journal’s publications may be calculated by taking the average author scores for an issue, and averaging the issues over a certain period, e.g. FCoIS = 1.8 (Journal Name, issues (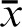 ), 2013). A researcher or editor may report his or her FCoIS for a specified subject over time as follows: FCoIS = 10.0 (Researcher AB, name of subject, 2004–2014). A chairperson in a medical school may calculate the FCoIS of his or her department by averaging scores from faculty papers published in a given year, as in FCoIS = 7.5 (Department Name, faculty articles (
), 2013). A researcher or editor may report his or her FCoIS for a specified subject over time as follows: FCoIS = 10.0 (Researcher AB, name of subject, 2004–2014). A chairperson in a medical school may calculate the FCoIS of his or her department by averaging scores from faculty papers published in a given year, as in FCoIS = 7.5 (Department Name, faculty articles (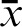 ), 2013).
), 2013).
Thus, an editor may ask for an author’s FCoIS on a given subject for the last 3, 5, or 10 years, or a patient may request his or her doctor’s score. Three real-world examples of the FCoIS are provided in the Acknowledgements section of this paper.
GUIDELINES
Disclosure statements are not required by all journals, and those that collect the information do not always publish it, particularly when there are no financial conflicts to report. Journals may be more willing to publish FCoI scores, as they use less space than statements, yet typically contain more information. Similarly, organizations that refuse to disclose funders may be willing to provide FCoI scores instead, as no names are necessary to report conflict levels (see note below for further discussion).
Journals should require FCoI scores for all manuscript authors, disclose scores to reviewers, and publish scores. The peer-review process would benefit from reviewers reporting their scores on the topic for the number of years as determined by the journal. In addition, stricter standards are needed for financially conflicted authors. For example, as reviews involve selection and interpretation of the literature, and editorials are often influential, such works by high FCoI scoring authors (for the 10-year period prior to submission) should be held to a stricter review process and no review or editorial submissions should be accepted from authors with the maximum FCoIS.
In addition, reporting of FCoI scores for the most recent 10-year period should be mandatory for candidates of public scientific advisory and review panels, and for leadership positions at government agencies (e.g. U.S. FDA, EPA) charged with protecting the public, with candidates with lower FCoI scores being favored. Also, in the interest of openness and clarity, all marketing (for instance, promotional brochures) and educational efforts produced by industry (especially indirectly via academic researchers acting as contractors) that are direct-to-consumer should report a FCoIS.
Politics in the United States has reached an egregious state, with not only campaign contributions by industry being permitted, but political action committees are allowed to anonymously raise billions of dollars. Any individual or group responsible for making decisions that impact the public should have no FCoI (keep in mind: performing activities or receiving support as listed in Table 1 does not, in and of itself, constitute a FCoI; see definitions). However, if a politician has a considerable (or greater) FCoI (defined as a FCoI level of ≧5 for the previous 10-years) then he or she should recuse himself or herself from decisions regarding that particular issue. This guideline provides a means to contend with the inadequacies of the current law, while allowing for industry campaign contributions. It also better ensures that the public is served as opposed to the industry that contributed to the campaign.
Where there is an inordinate amount of individual sources of support to track, as may be the case for politicians receiving campaign contributions or nonprofit organizations receiving donations, only sources of funding equal to and greater than a certain dollar amount (i.e. $1,000) should be investigated as a potential financial conflict.
Furthermore, it is in the public’s interest for additional sectors to use the FCoI Scale. For example, journalists should report their own scores and exclude the use of experts at and above the FCoI level where the potential for bias is considerable (Table 1).
Finally, scores from all sectors should be compiled and made available to the public and researchers studying FCoI via a free, publically accessible website where financially conflicted individuals and associations are required to list sources of industry support by name and dollar amount for public scrutiny and spot-checking by journals.
Failure to report or accurately report FCoI scores or policy breaches (e.g. a financially conflicted elected official found to have knowingly voted when he or she should not have) should be considered misconduct and sanctioned. For instance, an author who deliberately under-reported his or her FCoIS should be barred from publishing in journals of the relevant publisher for a 10-year period.
LIMITATIONS
A limitation of this system is that the financial conflict rankings were determined by one scholar. However, financial conflicts were ranked in a logical manner: “large gifts” or “off-site meals” should naturally constitute greater FCoI scores than “small gifts” or “on-site meals.” Perhaps less obvious, “honoraria” and “payment for attendance at lectures/meetings” should be ranked at a higher conflict level than “lodging, travel, transportation,” and “meeting registration fees,” because the former is likely to be more influential than the latter, which does not impart a direct personal gain.
Thus, I believe that this instrument is an intuitive construct with high face validity and that the guidelines represent needed solutions.
Another limitation of this work is that FCoI scores are self-reported. However, the current method of disclosure statements is also dependent on the individual to report financial conflicts. Verification of FCoI scores and ethical breaches (including in reporting of scores) can be handled using existing methods, those recommended above, and others yet to be developed. A further limitation of this method is that the source of funding is not provided by name. Nevertheless, the purpose of naming sources is for the reader or general public to try to determine whether a FCoI exists. Such guesswork is eliminated by use of the FCoI Scale (providing greater transparency), which places the burden for determining, scoring, and reporting on the financially conflicted entity.
CONCLUSIONS
In summary, use of the FCoI Scale has the potential to enhance the decision-making processes in a myriad of situations common to science, medicine, politics, and beyond. The FCoI score provides a uniform and concise method of disclosure in comparison to statements, while at the same time providing flexibility, additional information, and transparency in the reporting of financial conflicts. It is hoped that the Scale, definitions, and guidelines herewith will be used to help (1) in reducing FCoI by motivating individuals to show higher ethical standards; (2) in increasing the integrity of the scientific and medical literature, which will in turn; (3) minimize the collateral damage of potentially biased results informing regulatory decisions; (4) mitigate the increasing political trend of prioritizing private profit over public and patient health;17 and (5) empower everyday citizens with a basis for redress whenever financial interests conflict with that of the public good.
DISCLAIMER STATEMENTS
Contributors There is only one author, and I did all work.
Funding None.
Conflicts of interest None.
Ethics approval Not applicable.
Acknowledgments
FCoIS = 0.0 (Maharaj SVM, this study, 2014); FCoIS = 0.0 (Maharaj SVM, financial conflicts of interest, 2004–2014); FCoIS = 0.0 [Center for Research on Environmental Medicine, all programs (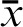 ), 2005 (inception)–2014].
), 2005 (inception)–2014].
REFERENCES
- 1.Djulbegovic B, Lacevic M, Cantor A, Fields KK, Bennett CL, Adams JR, et al. The uncertainty principle and industry-sponsored research. Lancet. 2000;356:635–8. doi: 10.1016/S0140-6736(00)02605-2. [DOI] [PubMed] [Google Scholar]
- 2.Yaphe J, Edman R, Knishkowy B, Herman J. The association between funding by commercial interests and study outcome in randomized controlled drug trials. Fam Pract. 2001;18:565–8. doi: 10.1093/fampra/18.6.565. [DOI] [PubMed] [Google Scholar]
- 3.Als-Nielsen B, Chen W, Gluud C, Kjaergard LL. Association of funding and conclusions in randomized drug trials. JAMA. 2003;290:921–8. doi: 10.1001/jama.290.7.921. [DOI] [PubMed] [Google Scholar]
- 4.Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ. 2003;326:1167–70. doi: 10.1136/bmj.326.7400.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhandari M, Busse JW, Jackowski D, Montori VM, Schunemann H, Sprague S, et al. Association between industry funding and statistically significant pro-industry findings in medical and surgical randomized trials. CMAJ. 2004;170:477–80. [PMC free article] [PubMed] [Google Scholar]
- 6.Perlis RH, Perlis CS, Wu Y, Hwang C, Joseph M, Nierenberg AA. Industry sponsorship and financial conflict of interest in the reporting of clinical trials in psychiatry. Am J Psychiatry. 2005;162:1957–60. doi: 10.1176/appi.ajp.162.10.1957. [DOI] [PubMed] [Google Scholar]
- 7.Jorgensen AW, Hilden J, Gotzsche PC. Cochrane reviews compared with industry supported meta-analyses and other meta-analyses of the same drugs: systematic review. BMJ. 2006;333:782. doi: 10.1136/bmj.38973.444699.0B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bero L, Oostvogel F, Bacchetti P, Lee K. Factors associated with findings of published trials of drug–drug comparisons: why some statins appear more efficacious than others. PLoS Med. 2007;4:e184. doi: 10.1371/journal.pmed.0040184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huss A, Egger M, Hug K, Huwiler-Muntener K, Roosli M. Source of funding and results of studies of health effects of mobile phone use: Systematic review of experimental studies. Environ Health Perspect. 2007;115:1–4. doi: 10.1289/ehp.9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nieto A, Mazon A, Pamies R, Linana JJ, Lanuza A, Jimenez FO, et al. Adverse effects of inhaled corticosteroids in funded and nonfunded studies. Arch Intern Med. 2007;167:2047–53. doi: 10.1001/archinte.167.19.2047. [DOI] [PubMed] [Google Scholar]
- 11.Turner EH, Matthews AM, Linardatos E, Tell RA, Rosenthal R. Selective publication of antidepressant trials and its influence on apparent efficacy. N Engl J Med. 2008;358:252–60. doi: 10.1056/NEJMsa065779. [DOI] [PubMed] [Google Scholar]
- 12.Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database Syst Rev. 2012;12:MR000033. doi: 10.1002/14651858.MR000033.pub2. [DOI] [PubMed] [Google Scholar]
- 13.Egilman D, Scout, Kol L, Hegg LA, Bohme SR. Manipulated data in Shell’s benzene historical exposure study. Int J Occup Environ Health. 2007;13:222–32. doi: 10.1179/oeh.2007.13.2.222. [DOI] [PubMed] [Google Scholar]
- 14.Gallagher R. For shame, Merck and Elsevier. The Scientist. June. 2009;23:13. [Google Scholar]
- 15.Ladou J, Teitelbaum DT, Egilman DS, Frank AL, Kramer SN, Huff J. American College of Occupational and Environmental Medicine (ACOEM): a professional association in service to industry. Int J Occup Environ Health. 2007;13:404–26. doi: 10.1179/oeh.2007.13.4.404. [DOI] [PubMed] [Google Scholar]
- 16.Jacobson M. Lifting the veil of secrecy from industry funding of nonprofit health organizations. Int J Occup Environ Health. 2005;11:349–55. doi: 10.1179/oeh.2005.11.4.349. [DOI] [PubMed] [Google Scholar]
- 17.Huff J. Industry influence on occupational and environmental public health. Int J Occup Environ Health. 2007;13:107–17. doi: 10.1179/107735207800244929. [DOI] [PubMed] [Google Scholar]


