Abstract
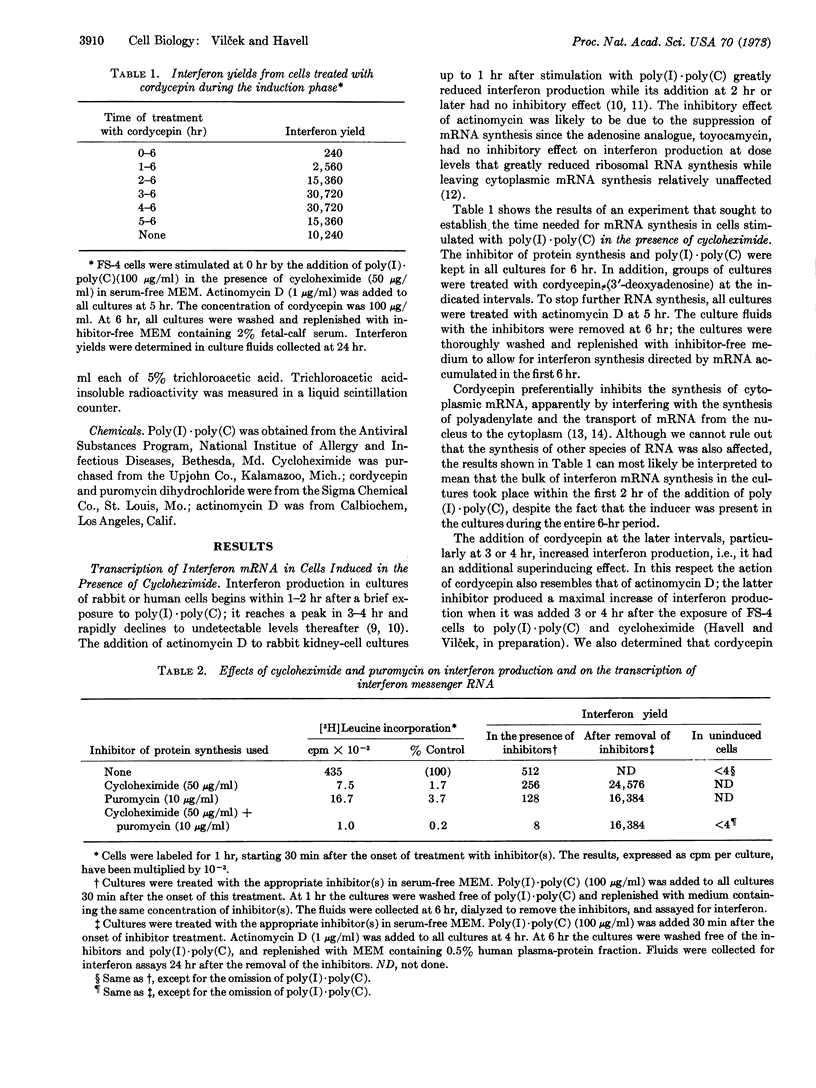
Interferon production was induced in a strain of human diploid foreskin cells with poly(I)·poly(C). Cycloheximide was included in the culture medium at the time of addition of the inducer. Actinomycin D was added to the cultures 4 or 5 hr later, before the inhibition of protein synthesis was reversed at 6 hr. Thus, subsequent interferon synthesis had to be directed by messenger RNA synthesized before addition of actinomycin D. The amount of interferon produced after such treatment was about 50-fold greater than in cells induced with poly(I)·poly(C) but not treated with inhibitors. Experiments using cordycepin suggested that in spite of the continued presence of the inducer the synthesis of the bulk of interferon messenger RNA was completed within the first 2 hr of exposure of cells to poly(I)·poly(C) and cycloheximide. Transcription of interferon messenger RNA was apparently not affected when interferon synthesis was suppressed to various degrees by different inhibitors of protein synthesis, indicating the independence of transcription and translation. The high rate of interferon synthesis after the reversal of cycloheximide action was more sustained at 32° than at 37°. The rate of decrease of overall protein synthesis in cells treated with actinomycin D and then incubated either at 32° or 37° showed a similar dependence on incubation temperature, suggesting that the stability of messenger RNA (or of another actinomycin D-sensitive component required for protein synthesis) was greater at the lower temperature.
Keywords: human cells, poly(I)·poly(C), induction and superinduction, translation control
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Salditt M., Thomas W., Darnell J. E. Evidence that all messenger RNA molecules (except histone messenger RNA) contain Poly (A) sequences and that the Poly(A) has a nuclear function. J Mol Biol. 1972 Oct 28;71(1):21–30. doi: 10.1016/0022-2836(72)90397-x. [DOI] [PubMed] [Google Scholar]
- Billiau A., Joniau M., De Somer P. Mass production of human interferon in diploid cells stimulated by poly-I:C. J Gen Virol. 1973 Apr;19(1):1–8. doi: 10.1099/0022-1317-19-1-1. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Philipson L., Wall R., Adesnik M. Polyadenylic acid sequences: role in conversion of nuclear RNA into messenger RNA. Science. 1971 Oct 29;174(4008):507–510. doi: 10.1126/science.174.4008.507. [DOI] [PubMed] [Google Scholar]
- Finkelstein M. S., Bausek G. H., Merigan T. C. Interferon inducers in vitro: difference in sensitivity to inhbitiros of RNA and protein synthesis. Science. 1968 Aug 2;161(3840):465–468. doi: 10.1126/science.161.3840.465. [DOI] [PubMed] [Google Scholar]
- GARREN L. D., HOWELL R. R., TOMKINS G. M., CROCCO R. M. A PARADOXICAL EFFECT OF ACTINOMYCIN D: THE MECHANISM OF REGULATION OF ENZYME SYNTHESIS BY HYDROCORTISONE. Proc Natl Acad Sci U S A. 1964 Oct;52:1121–1129. doi: 10.1073/pnas.52.4.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A., Vilcek J. Production of high-titered interferon in cultures of human diploid cells. Antimicrob Agents Chemother. 1972 Dec;2(6):476–484. doi: 10.1128/aac.2.6.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennell D., Bicknell I. Decay of messenger ribonucleic acid from the lactose operon of Escherichia coli as a function of growth temperature. J Mol Biol. 1973 Feb 15;74(1):21–31. doi: 10.1016/0022-2836(73)90351-3. [DOI] [PubMed] [Google Scholar]
- Myers M. W., Friedman R. M. Potentiation of human interferon production by superinduction. J Natl Cancer Inst. 1971 Oct;47(4):757–764. [PubMed] [Google Scholar]
- Ng M. H., Vilcek J. Temperature-sensitive interferon production in rabbit kidney cell cultures treated with toyocamycin. Biochim Biophys Acta. 1973 Jan 19;294(2):284–291. [PubMed] [Google Scholar]
- Nu M. H., Berman B., Vilcek J. Membrane-bound intracellular interferon in rabbit kidney cell cultures. Virology. 1972 Jul;49(1):322–325. doi: 10.1016/s0042-6822(72)80037-0. [DOI] [PubMed] [Google Scholar]
- Reel J. R., Kenney F. T. "Superinduction" of tyrosine transaminase in hepatoma cell cultures: differential inhibition of synthesis and turnover by actionomycin D. Proc Natl Acad Sci U S A. 1968 Sep;61(1):200–206. doi: 10.1073/pnas.61.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T., Palacios R., Palmiter R. D., Rhoads R. E. Hormonal regulation of ovalbumin synthesis in chick oviduct. Basic Life Sci. 1973;1:123–135. doi: 10.1007/978-1-4684-0877-5_10. [DOI] [PubMed] [Google Scholar]
- Tan Y. H., Armstrong J. A., Ho M. Accentuation of interferon production by metabolic inhibitors and its dependence on protein synthesis. Virology. 1971 Jun;44(3):503–509. doi: 10.1016/0042-6822(71)90363-1. [DOI] [PubMed] [Google Scholar]
- Tan Y. H., Armstrong J. A., Ho M. Intracellular interferon: kinetics of formation and release. Virology. 1971 Sep;45(3):837–840. doi: 10.1016/0042-6822(71)90209-1. [DOI] [PubMed] [Google Scholar]
- Tan Y. H., Armstrong J. A., Ke Y. H., Ho M. Regulation of cellular interferon production: enhancement by antimetabolites. Proc Natl Acad Sci U S A. 1970 Sep;67(1):464–471. doi: 10.1073/pnas.67.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson E. B., Tomkins G. M., Curran J. F. Induction of tyrosine alpha-ketoglutarate transaminase by steroid hormones in a newly established tissue culture cell line. Proc Natl Acad Sci U S A. 1966 Jul;56(1):296–303. doi: 10.1073/pnas.56.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkins G. M., Gelehrter T. D., Granner D., Martin D., Jr, Samuels H. H., Thompson E. B. Control of specific gene expression in higher organisms. Expression of mammalian genes may be controlled by repressors acting on the translation of messenger RNA. Science. 1969 Dec 19;166(3912):1474–1480. doi: 10.1126/science.166.3912.1474. [DOI] [PubMed] [Google Scholar]
- Tomkins G. M., Levinson B. B., Baxter J. D., Dethlefsen L. Further evidence for posttranscriptional control of inducible tyrosine aminotransferase synthesis in cultured hepatoma cells. Nat New Biol. 1972 Sep 6;239(88):9–14. doi: 10.1038/newbio239009a0. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Ng M. H. Post-transcriptional control of interferon synthesis. J Virol. 1971 May;7(5):588–594. doi: 10.1128/jvi.7.5.588-594.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilcek J., Rossman T. G., Varacalli F. Differential effects of actinomycin D and puromycin on the release of interferon induced by double stranded RNA. Nature. 1969 May 17;222(5194):682–683. doi: 10.1038/222682a0. [DOI] [PubMed] [Google Scholar]



