Abstract
Background
ST2 is a member of the interleukin-1 receptor family with a soluble form that is markedly upregulated on application of biomechanical strain to cardiac myocytes. Circulating ST2 levels are elevated in the setting of acute myocardial infarction, but the predictive value of ST2 independent of traditional clinical factors and of an established biomarker of biomechanical strain, N-terminal prohormone B-type natriuretic peptide (NT-proBNP), has not been established.
Methods and Results
We measured ST2 at baseline in 1239 patients with ST-elevation myocardial infarction from the CLopidogrel as Adjunctive ReperfusIon TherapY–Thrombolysis in Myocardial Infarction 28 (CLARITY-TIMI 28) trial. Per trial protocol, patients were to undergo coronary angiography after 2 to 8 days and were followed up for 30 days for clinical events. In contrast to NT-proBNP, ST2 levels were independent of clinical factors potentially related to chronic increased left ventricular wall stress, including age, hypertension, prior myocardial infarction, and prior heart failure; levels also were only modestly correlated with NT-proBNP (r=0.14). After adjustment for baseline characteristics and NT-proBNP levels, an ST2 level above the median was associated with a significantly greater risk of cardiovascular death or heart failure (third quartile: adjusted odds ratio, 1.42; 95% confidence interval, 0.68 to 3.57; fourth quartile: adjusted odds ratio, 3.57; 95% confidence interval, 1.87 to 6.81; P<0.0001 for trend). When both ST2 and NT-proBNP were added to a model containing traditional clinical predictors, the c statistic significantly improved from 0.82 (95% confidence interval, 0.77 to 0.87) to 0.86 (95% confidence interval, 0.81 to 0.90) (P=0.017).
Conclusions
In ST-elevation myocardial infarction, high baseline ST2 levels are a significant predictor of cardiovascular death and heart failure independently of baseline characteristics and NT-proBNP, and the combination of ST2 and NT-proBNP significantly improves risk stratification. These data highlight the prognostic value of multiple, complementary biomarkers of biomechanical strain in ST-elevation myocardial infarction.
Keywords: myocardial infarction, natriuretic peptides, prognosis
Biomarkers currently used in cardiovascular disease for diagnosis and risk assessment have emerged largely from targeted analyses of known myocardial proteins such as creatine kinase (CK)-MB isoenzyme, cardiac troponin, heart-type fatty-acid binding protein, and B-type natriuretic peptide.1–6 More recently, unbiased genome- or proteome-wide approaches have been harnessed for novel biomarker discovery. Applying the most mature of the genomics technologies, transcript profiling, Weinberg et al7 discovered that gene expression of ST2, a member of the interleukin (IL)-1 receptor family, is markedly upregulated on the application of mechanical strain to cardiac myocytes. We subsequently showed that levels of the soluble ST2 receptor were elevated in the serum of patients after myocardial infarction (MI).8
However, the predictive value of ST2 levels independent of traditional clinical factors and of an established biomarker of left ventricular (LV) wall stress, namely N-terminal prohormone B-type natriuretic peptide (NT-proBNP), has not been established. Specifically, whether these 2 biomarkers offer complementary insights into cardiovascular pathophysiology or are redundant is unclear. For that reason, we conducted a comprehensive evaluation of ST2 and NT-proBNP in a large cohort of patients with ST-elevation myocardial infarction (STEMI). Our goals were to investigate the relation between ST2 and NT-proBNP levels and baseline clinical characteristics, to determine the association between angiographic parameters and serial ST2 and NT-proBNP levels, to examine the correlation between ST2 and NT-proBNP levels, to determine the predictive ability of ST2 levels for clinical outcomes independent of traditional clinical risk factors and NT-proBNP levels, and to ascertain the incremental value of adding biomechanical biomarkers to established clinical risk scores.
Methods
Patient Population
The design and primary results of CLopidogrel as Adjunctive ReperfusIon TherapY–Thrombolysis in Myocardial Infarction 28 (CLARITY-TIMI 28) have been published.9,10 In brief, 3491 patients with STEMI who presented within 12 hours of symptom onset were to receive aspirin, a fibrinolytic, and heparin (required if they were to receive a fibrin-specific lytic) and were randomized to clopidogrel or placebo. Exclusion criteria included age >75 years, prior coronary artery bypass graft surgery, serum creatinine >2.5 mg/dL, or evidence of cardiogenic shock. The protocol was approved by the relevant institutional review boards, and written informed consent was obtained from all patients. Twelve-lead ECGs were performed at baseline and at 90 minutes. As part of the trial protocol, patients were scheduled to undergo coronary angiography 2 to 8 days after initiation of therapy to assess late patency of the infarct-related artery. Angiography was permitted before 48 hours only if clinically indicated. Patients were followed up for clinical outcomes and adverse events through 30 days after randomization.
Biomarkers
A sample of blood was obtained at the time of enrollment in 1277 subjects and at angiography in 914 subjects. The baseline characteristics and clinical outcomes were similar to those of the overall trial cohort. Serum was isolated within 1 hour of collection, frozen at ≤−20°C, and batch shipped on dry ice to the TIMI Biomarker Laboratory (Boston, Mass), where samples were stored at ≤−80°C until thawed for determination of biomarkers. NT-proBNP was measured in 1179 patients with the Elecsys 2010 (Roche Diagnostics, Indianapolis, Ind). The Elecsys proBNP Immunoassay has a lower limit of detection of 5 pg/mL and a coefficient of variation of 3.2% at 175 pg/mL. The upper reference limit reported by the manufacturer for patients <75 years of age is 125 pg/mL. ST2 was measured in 1239 patients with the Medical and Biological Labs immunoassay (Woburn, Mass), which has a lower limit of detection of 32 pg/mL and a coefficient of variation of 5.2% at 2.5 ng/mL. In individuals with stable coronary artery disease, levels are undetectable (R.T.L., unpublished observations, 2005). Levels of both biomarkers were obtained in 1167 patients. We measured only these 2 biomarkers for this experiment. All testing was performed by personnel blinded to patient characteristics, clinical outcomes, and treatment allocation.
Outcomes
Clinical outcomes for this analysis included cardiovascular death, congestive heart failure (CHF), recurrent MI, and stroke through 30 days of follow-up. Outcomes were defined according to previously reported criteria.9 Based on prior data for these 2 biomarkers, our primary hypothesis was that ST2 and NT-proBNP levels would be associated with cardiovascular death and heart failure.6,8 All ischemic events were adjudicated by a Clinical Events Committee that was blinded to assigned treatment arm. Information on the development of new or worsening CHF was collected from the case report forms. Angiographic outcomes, including TIMI flow grade and TIMI myocardial perfusion grade, were assessed in a blinded manner as previously defined at the TIMI Angiographic Core Laboratory.11,12 On ECG, complete ST-segment resolution was defined as ≥70% resolution after 90 minutes.13 All ECGs were interpreted at the TIMI Electrocardiography Core Laboratory by investigators who were blinded to treatment assignment and outcomes.
Statistical Analyses
ST2 and NT-proBNP levels are reported as median values with interquartile ranges (IQRs) because of their nonnormal distribution. Biomarkers were modeled as continuous variables and divided into quartiles and at the median. ANOVA and χ2 tests for trend were used to compare baseline characteristics across biomarker quartiles. Spearman correlation coefficient was used to assess the correlation between biomarker values and other continuous variables. Comparison of serial biomarker values was conducted with the Wilcoxon signed-rank test. Wilcoxon rank-sum tests were used to compare biomarker levels between patients with and without specific angiographic and clinical outcomes. We used χ2 tests for trend to compare the incidence of clinical outcomes across biomarker quartiles. Logistic regression was used to model the relationship between biomarker levels and outcomes with adjustment for age, sex, hypertension, diabetes mellitus, prior MI, prior CHF, creatinine clearance, infarct location (anterior versus nonanterior), Killip class, time from symptom onset to initiation of fibrinolytic therapy, type of lytic (fibrin-specific versus non–fibrin-specific lytic), and peak CK. The TIMI score for STEMI was calculated as previously described.14 Biomarkers were modeled as log-transformed continuous variables (with 1 added to all ST2 values because of 0 values) and as quartiles. The discriminative ability of multivariable models was assessed by computing the c statistic and was compared by use of a nonparametric test.15 Analyses were done with SAS version 9.1.3 (SAS Institute Inc, Cary, NC).
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Baseline Biomarkers and Baseline Characteristics
Baseline measurements of ST2 were available in 1239 patients. The median concentration of ST2 was 80 pg/mL, and the 25th and 75th percentiles were 0 and 325 pg/mL, respectively. Table 1 shows patient characteristics at enrollment by quartile of baseline ST2 level. ST2 levels were significantly associated with diabetes mellitus and creatinine clearance (although the difference in creatinine clearance between the extreme ST2 quartiles was <6 mL/min); ST2 levels were not associated with age, sex, prior hypertension, smoking, prior MI, or prior CHF. ST2 levels were associated with time from symptom onset to initiation of fibrinolytic therapy and peak CK (P≤0.0002 for both) and were modestly associated with infarct location and Killip class (P<0.10 for both).
Table 1.
Baseline Characteristics by ST2 Quartile
| Quartiles of Baseline ST2, pg/mL | |||||
|---|---|---|---|---|---|
| Baseline Characteristics | 0 (n=367) | 3–79 (n=250) | 80–325 (n=312) | >325 (n=310) | P for Trend |
| Age, y | 57.1±10.2 | 58.0±10.7 | 58.8±9.9 | 58.3±10.4 | 0.08 |
| Female, n (%) | 74 (20.2) | 46 (18.4) | 63 (20.2) | 71 (22.9) | 0.36 |
| BMI, kg/m2 | 27.6±4.0 | 27.7±4.2 | 27.5±4.2 | 27.7±5.3 | 0.98 |
| Hypertension, n (%) | 131 (35.9) | 93 (37.5) | 133 (43.3) | 126 (40.9) | 0.08 |
| Current smoker, n (%) | 185 (50.4) | 112 (44.8) | 145 (46.9) | 143 (46.1) | 0.33 |
| Diabetes mellitus, n (%) | 36 (9.9) | 49 (19.6) | 61 (19.7) | 67 (22.0) | <0.0001 |
| Hyperlipidemia, n (%) | 127 (39.3) | 99 (45.6) | 116 (41.4) | 103 (38.4) | 0.72 |
| Prior MI, n (%) | 31 (8.5) | 24 (9.6) | 28 (9.0) | 21 (6.8) | 0.46 |
| Prior CHF, n (%) | 6 (1.7) | 4 (1.6) | 2 (0.6) | 3 (1.0) | 0.27 |
| Creatinine clearance, mL/min | 93.5±30.1 | 93.5±32.2 | 89.1±30.1 | 87.7±33.0 | 0.008 |
| Index presentation | |||||
| Time from symptom onset to lytic, h | 2.7±1.8 | 2.8±1.8 | 3.3±2.1 | 3.5±2.7 | <0.0001 |
| Anterior MI, n (%) | 125 (34.1) | 95 (38.0) | 113 (36.2) | 133 (42.9) | 0.04 |
| Killip class II–IV, n (%) | 23 (6.3) | 10 (4.0) | 27 (8.7) | 27 (8.7) | 0.08 |
| Fibrin-specific lytic, n (%) | 334 (91.0) | 216 (86.4) | 263 (84.3) | 260 (83.9) | 0.004 |
| Peak CK, IU/L | 1694±1535 | 1895±2058 | 1986±1754 | 2293±2503 | 0.0002 |
BMI indicates body mass index. Data are presented as mean±SD for continuous variables and number (%) for dichotomous variables.
Baseline measurements of NT-proBNP were available in 1179 patients. The median concentration of NT-proBNP was 104.4 pg/mL, and the 25th and 75th percentiles were 42.3 and 308.0 pg/mL, respectively. In contrast to the findings for ST2, higher baseline NT-proBNP levels were significantly associated with older age, female sex, prior hypertension, not being a current smoker, prior MI, prior CHF, and lower creatinine clearance (P≤0.01 for all; Table 2). In terms of index presentation, higher baseline NT-proBNP levels were significantly associated with increasing time from symptom onset to initiation of fibrinolytic therapy, anterior location of the MI, and Killip class II to IV (P<0.001 for all) but not peak CK (P=0.91). The correlation between baseline NT-proBNP and ST2 levels was slight (r=0.14), albeit statistically significant (P<0.001).
Table 2.
Baseline Characteristics by NT-proBNP Quartile
| Quartiles of Baseline NT-proBNP, pg/mL | |||||
|---|---|---|---|---|---|
| Baseline Characteristics | <42.3 (n=294) | 42.3–104.3 (n=295) | 104.4–308.0 (n=295) | >308.0 (n=295) | P for Trend |
| Age, y | 52.8±9.2 | 57.2±9.5 | 61.3±9.0 | 62.6±9.9 | <0.0001 |
| Female, n (%) | 30 (10.2) | 54 (18.3) | 69 (23.4) | 95 (32.2) | <0.0001 |
| BMI, kg/m2 | 27.4±4.8 | 27.6±4.3 | 27.6±4.2 | 27.9±4.9 | 0.27 |
| Hypertension, n (%) | 95 (32.8) | 92 (31.5) | 131 (44.7) | 152 (51.9) | <0.0001 |
| Current smoker, n (%) | 163 (55.6) | 149 (50.5) | 125 (42.7) | 120 (40.7) | <0.0001 |
| Diabetes mellitus, n (%) | 38 (13.1) | 46 (15.8) | 48 (16.3) | 71 (24.5) | 0.0005 |
| Hyperlipidemia, n (%) | 115 (43.2) | 113 (42.6) | 107 (42.0) | 81 (32.7) | 0.02 |
| Prior MI, n (%) | 20 (6.8) | 18 (6.1) | 30 (10.2) | 34 (11.6) | 0.01 |
| Prior CHF, n (%) | 0 (0.0) | 1 (0.3) | 3 (1.0) | 12 (4.1) | <0.0001 |
| Creatinine clearance, mL/min | 98.1±32.1 | 94.4±29.8 | 87.0±26.8 | 82.3±33.1 | <0.0001 |
| Index presentation | |||||
| Time from symptom onset to lytic, h | 2.4±1.3 | 2.6±1.5 | 3.2±2.0 | 4.2±3.0 | <0.0001 |
| Anterior MI, n (%) | 96 (32.7) | 100 (33.9) | 113 (38.3) | 141 (47.8) | <0.0001 |
| Killip class II–IV, n (%) | 12 (4.1) | 17 (5.8) | 19 (6.5) | 37 (12.6) | 0.0001 |
| Fibrin-specific lytic, n (%) | 263 (89.5) | 255 (86.4) | 262 (88.8) | 240 (81.4) | 0.01 |
| Peak CK, IU/L | 1867±1649 | 2047±1995 | 1940±1884 | 1923±1872 | 0.91 |
BMI indicates body mass index. Data are presented as mean±SD for continuous variables and number (%) for dichotomous variables.
Biomarkers at the Time of Angiography
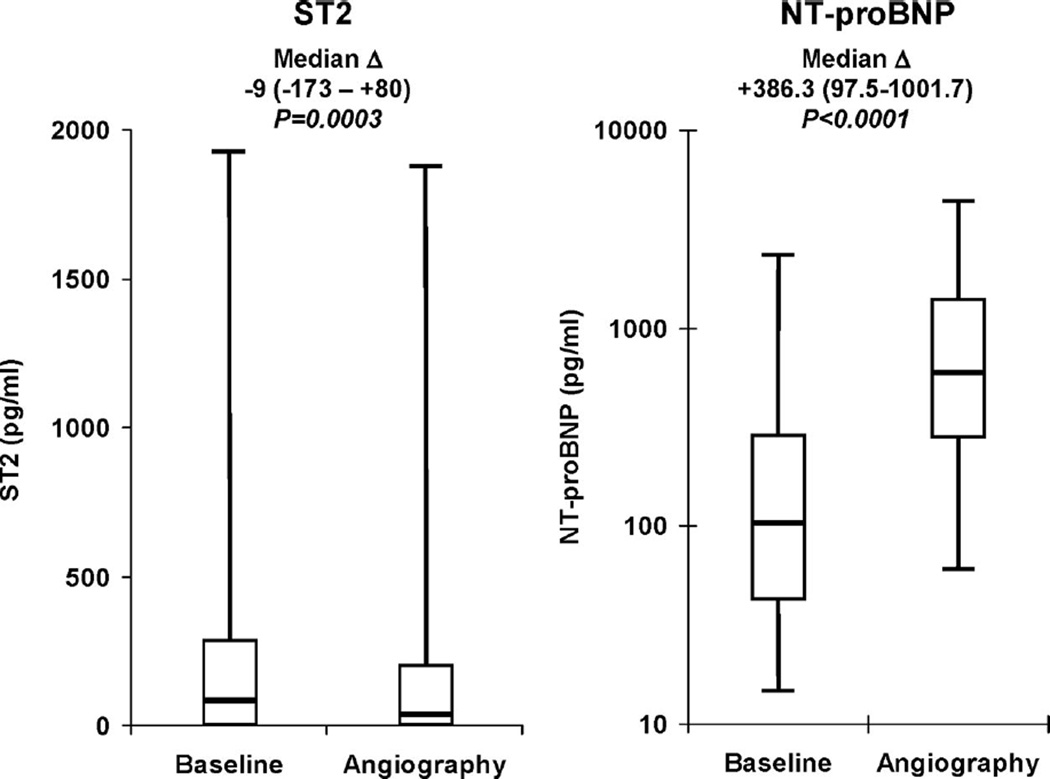
ST2 and NT-proBNP levels also were determined at the time of angiography (mean, 96±51 hours after randomization) in 898 and 890 patients, respectively. The median concentration of ST2 at the time of angiography was 30 pg/mL, and the 25th and 75th percentiles were 0 and 194 pg/mL, respectively. ST2 values had modestly but statistically significantly decreased by the time of angiography (median difference, −9 pg/mL; IQR, −173 to 80 pg/mL; P=0.0003; Figure 1A). The median concentration of NT-proBNP at angiography was 591.4 pg/mL, and the 25th and 75th percentiles were 284.1 and 1380.0 pg/mL, respectively. In contrast to ST2, NT-proBNP values at the time of angiography were significantly higher than at baseline (Figure 1B), with the median difference being 386.3 pg/mL (IQR, 97.5 to 1001.7; P<0.0001). Factors associated with a significant increase in NT-proBNP from baseline to angiography included MI location (anterior: 464.4 pg/mL; IQR, 63.4 to 1651.8 pg/mL; nonanterior: 348.4 pg/mL; IQR, 107.8 to 782.2 pg/mL; P=0.01) and ST-segment resolution at 90 minutes (complete: 355.1 pg/mL; IQR, 135.1 to 756.3 pg/mL; incomplete: 505.2 pg/mL; IQR, 129.7 to 1313.3 pg/mL; P=0.06). No correlation was found between the time to angiography and the change in either ST2 or NT-proBNP levels from baseline to angiography (|r|<0.02 and P=NS for both).
Figure 1.
Baseline and angiography levels of ST2 and NT-proBNP. Analyses were restricted to patients in whom measurements were available at both time points. NT-proBNP levels are plotted on a logarithmic scale. The median difference (Δ) and IQR for levels of each biomarker between the 2 time points are shown above the boxes. The bottom and top whiskers indicate the 5th and 95th percentile levels; the lower and upper boundaries of the boxes, the 25th and 75th percentile levels; and the horizontal line within the box, the median level.
The associations between angiographic parameters and ST2 and NT-proBNP levels at angiography are shown in Table 3. Impaired epicardial flow and lack of myocardial perfusion were each strongly associated with ≈4-fold-higher ST2 levels (P<0.002). Both angiographic parameters also were associated with nearly 2-fold-higher NT-proBNP levels, as was having a left anterior descending culprit artery. In patients in whom LV ejection fraction was measured (575 of whom had ST2 measured and 551 of whom had NT-proBNP measured), ST2 was only weakly correlated with ejection fraction (r=−0.17, P<0.001), whereas NT-proBNP was moderately correlated with ejection fraction (r=−0.45, P<0.0001).
Table 3.
Angiographic Findings, ST2 Levels, and NT-proBNP Levels
| Angiographic Finding | Present | Absent | P |
|---|---|---|---|
| ST2 at angiography, pg/mL | |||
| TFG 0/1 | 93 (0–500) | 22 (0–184) | 0.0007 |
| TMPG 0 | 80 (0–550) | 24 (0–163) | 0.0015 |
| LAD culprit vessel | 36 (0–255) | 21 (0–192) | 0.19 |
| NT-proBNP at angiography, pg/mL | |||
| TFG 0/1 | 889.3 (379.3–2113.0) | 570.7 (271.6–1317) | 0.017 |
| TMPG 0 | 978.1 (462.4–2644.0) | 557.8 (258.6–1231.5) | <0.0001 |
| LAD culprit vessel | 833.1 (343.1–2337.0) | 532.6 (258.9–1090.0) | <0.0001 |
TFG indicates TIMI flow grade; TMPG, TIMI myocardial perfusion grade; and LAD, left anterior descending artery. NT-proBNP and ST2 levels are presented as median (25th to 75th percentiles).
Cardiovascular Outcomes
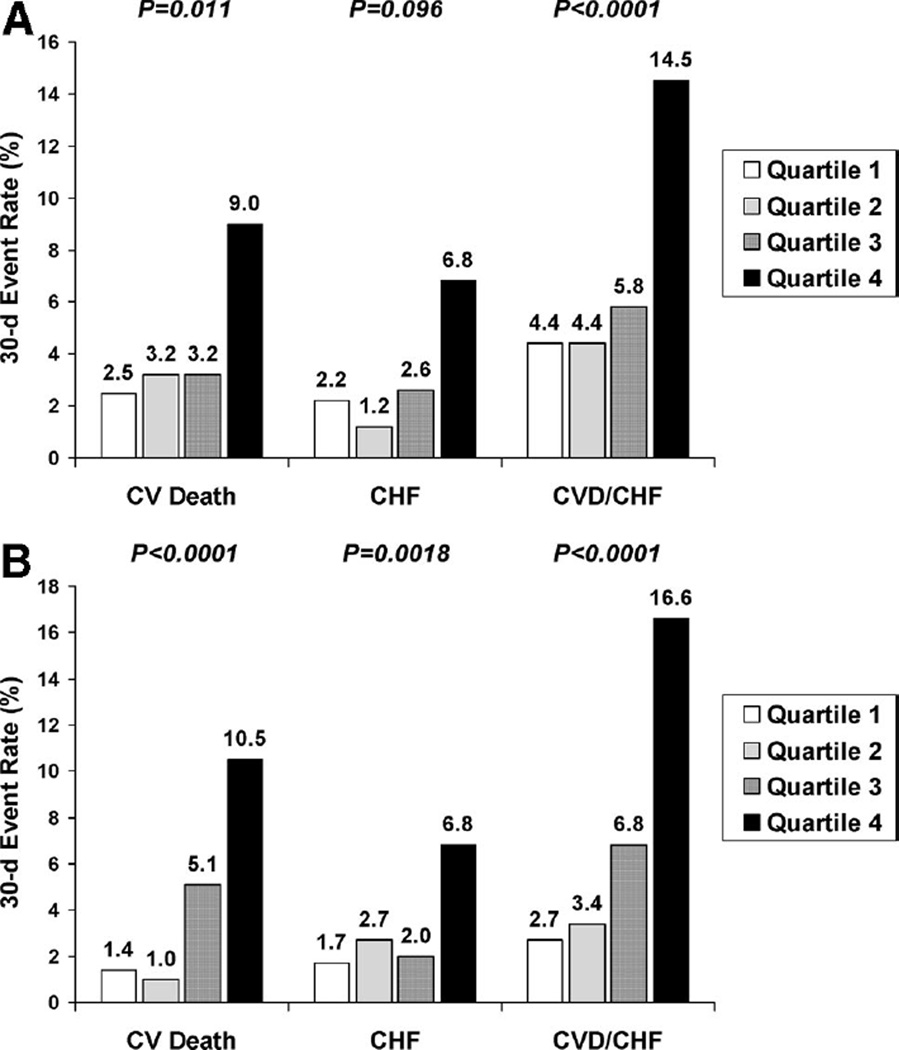
Baseline levels of both ST2 and NT-proBNP were significantly higher in patients who suffered cardiovascular death and in patients who developed CHF than in those who did not (Table 4). Levels of NT-proBNP also were higher in patients who suffered a stroke than in those who did not. The reverse pattern was seen with recurrent MI, with lower levels in patients who had recurrent MI than in those who did not. A 1-SD increase in log-transformed ST2+1 was associated with a 2.43-fold increase in the odds of cardiovascular death or heart failure over 30 days (95% confidence interval [CI], 1.67 to 3.53; P<0.001). Similarly, a 1-SD increase in log-transformed NT-proBNP was associated with a 1.67-fold increase in the odds of cardiovascular death or heart failure over 30 days (95% CI, 1.47 to 1.89; P<0.001). In quartile analysis, the risk of cardiovascular death or heart failure over 30 days increased significantly with increasing quartiles of ST2 (P<0.0001; Figure 2A) and with increasing quartiles of NT-proBNP (P<0.0001; Figure 2B), especially in the third and fourth quartiles. ST2 and NT-proBNP were significant predictors of cardiovascular death or heart failure both in patients who did (P=0.007 for trend across ST2 quartiles, P<0.001 for trend across NT-proBNP quartiles) and in those who did not (P<0.001 for both biomarkers) undergo coronary revascularization.
Table 4.
Clinical Outcomes, Baseline ST2 Levels, and Baseline NT-proBNP Levels
| 30-Day Outcomes | Had Outcome | Did Not Have Outcome | P |
|---|---|---|---|
| Baseline ST2, pg/mL | |||
| CV death | 325 (42–907) | 76 (0–281) | <0.0001 |
| CHF | 385 (67–1127) | 76 (0–294) | 0.0003 |
| CV death or CHF | 316 (44–1000) | 74 (0–264) | <0.0001 |
| Recurrent MI | 74 (0–716) | 79 (0–307) | 0.56 |
| Stroke | 280 (0–778) | 78 (0–312) | 0.11 |
| NT-proBNP, pg/mL | |||
| CV death | 455.2 (153–2071) | 98.4 (41.2–279.0) | <0.0001 |
| CHF | 312.6 (68.7–1765.0) | 102.7 (41.5–289.5) | 0.0007 |
| CV death or CHF | 411.8 (120.0–2071.0) | 96.7 (40.6–263.5) | <0.0001 |
| Recurrent MI | 70.1 (27.7–131.0) | 107.7 (44.1–321.9) | 0.0013 |
| Stroke | 248.2 (108.1–969.7) | 103.1 (42.0–303.2) | 0.033 |
CV indicates cardiovascular. ST2 and NT-proBNP levels are presented as median (25th to 75th percentiles).
Figure 2.
A, Association between baseline ST2 levels and cardiovascular (CV) death (CVD) and heart failure. P values are for trends across ST2 quartiles. B, Association between baseline NT-proBNP levels and cardiovascular (CV) death (CVD) and heart failure. P values are for trends across NT-proBNP quartiles.
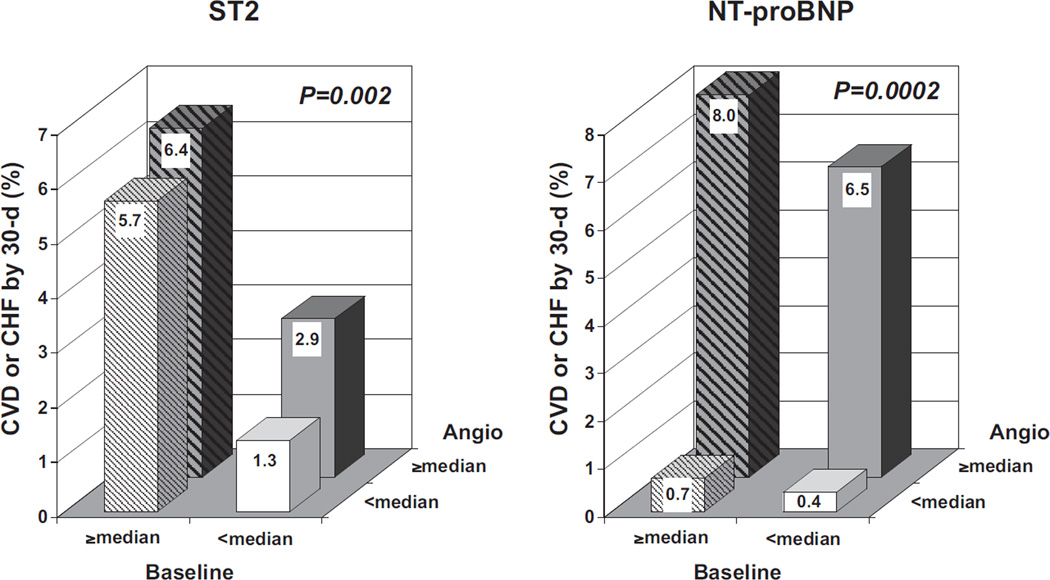
When patients were categorized on the basis of both baseline and angiography biomarker values (dichotomized as below versus above the median), 2 different patterns were apparent for the 2 biomarkers (Figure 3). For ST2, elevated levels at baseline (odds ratio [OR], 3.02; 95% CI 1.40 to 6.51; P=0.005) were a better predictor of risk than elevated levels at angiography (OR, 1.33; 95% CI, 0.68 to 2.63; P=0.41), whereas for NT-proBNP, elevated levels at angiography (14.94; 95% CI, 3.50 to 63.79; P<0.001) were a better predictor of risk than elevated levels at baseline (OR, 1.30; 95% CI, 0.61 to 2.74; P=0.50).
Figure 3.
Association between serial biomarker levels and cardiovascular death (CVD) and heart failure. Patients in whom measurements were available at both time points were categorized into 4 groups on the basis of their biomarker levels (left, ST2; right, NT-proBNP) at baseline and at angiography (Angio). Solid bars indicate baseline values below the median; striped bars, baseline values above the median. White background indicates angiography values below the median; gray background, angiography values above the median. The number of patients in each group is as follows (from left to right and from back to front): 235, 207, 209, and 228 for ST2 and 276, 155, 138, and 266 for NT-proBNP. P values are for global χ2 tests.
Multivariable and Multimarker Analyses
In multivariable analysis, after adjustment for traditional risk factors, including age, sex, hypertension, diabetes mellitus, prior MI, prior CHF, creatinine clearance, infarct location, Killip class, time from symptom onset to initiation of fibrinolytic therapy, type of lytic, and peak CK, a 1-SD increase in log-transformed ST2+1 was associated with a 1.94-fold increase in the odds of cardiovascular death or CHF over 30 days (95% CI, 1.25 to 3.03; P=0.003). Analogously, in quartile analysis, patients with higher baseline ST2 levels were at significantly increased risk for cardiovascular death or CHF, with adjusted ORs of 1.44 (95% CI, 0.71 to 2.94) for those in the third quartile and 3.41 (95% CI, 1.84 to 6.31) for those in the fourth quartile compared with those below the median (P=0.006 for trend). CK-MB was available in a smaller subset of the population; nonetheless, substitution of CK-MB for CK in the multivariable model did not change the results substantially (adjusted OR for risk in the fourth quartile, 3.28). Adding ST-segment resolution of 90 minutes to the model did not attenuate the effect estimates (OR, 1.40; 95% CI, 0.57 to 3.46 for those in the third quartile of ST2; and OR, 3.83; 95% CI, 1.80 to 8.16 for those in the fourth quartile). Although ejection fraction was available in only a subset of patients (n=575) and was determined on average 4 days after presentation, adding ejection fraction to the multivariable model did not attenuate the prognostic significance of ST2 (OR, 1.50; 95% CI, 0.36 to 6.24 for those in the third quartile of ST2; and OR, 4.49; 95% CI, 1.34 to 15.02 for those in the fourth quartile).
Similarly, after multivariable adjustment for traditional risk factors, a 1-SD increase in log-transformed NT-proBNP was associated with a 1.46-fold increase in the odds of cardiovascular death or CHF over 30 days (95% CI, 1.22 to 1.76; P<0.001). In quartile analysis, patients with higher baseline levels of NT-proBNP were at significantly increased risk for cardiovascular death or CHF, with adjusted ORs of 1.43 (95% CI, 0.66 to 3.06) for those in the third quartile and 2.86 (95% CI, 1.41 to 5.77) for those in the fourth quartile compared with those below the median (P=0.004 for trend). Again, substitution of CK-MB for CK in the multivariable model diminished the risk associated with an elevated NTproBNP (adjusted OR for risk in fourth quartile, 3.62).
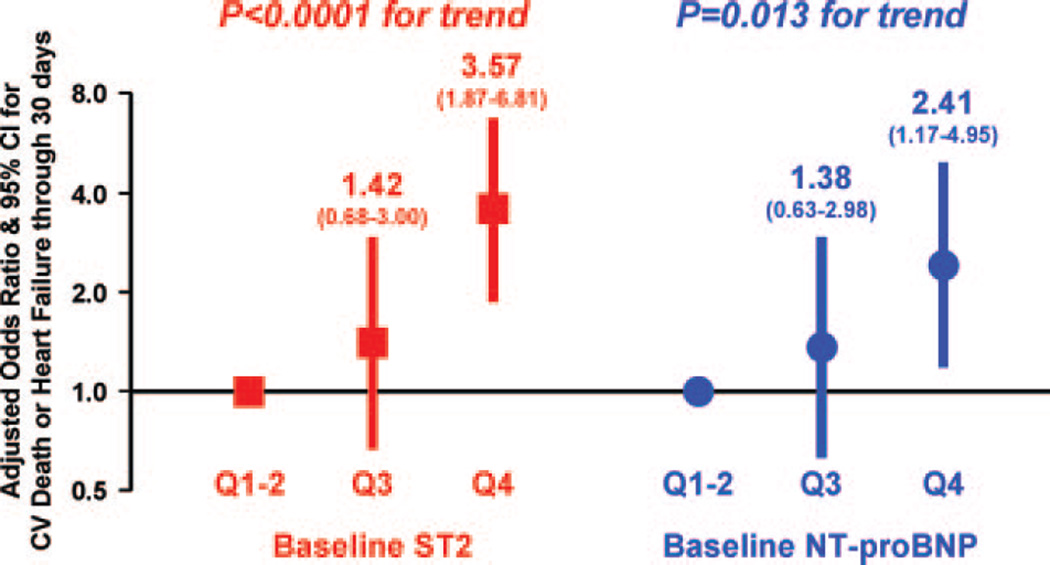
In a multimarker model that included traditional risk factors and both ST2 and NT-proBNP, both biomarkers remained independent predictors of cardiovascular death or CHF, with an OR per 1-SD increase in log-transformed ST2+1 of 1.88 (95% CI, 1.17 to 3.03; P=0.009) and per 1-SD increase in log-transformed NT-proBNP of 1.41 (95% CI, 1.17 to 1.69; P<0.001) and with analogously significant trends in quartile analysis (P<0.0001 for ST2, P=0.013 for NT-proBNP; Figure 4). When the biomarkers were added to a model containing the aforementioned clinical covariates, the c statistic significantly improved from 0.82 (95% CI, 0.77 to 0.87) to 0.86 (95% CI, 0.81 to 0.90; P=0.017).
Figure 4.
Multivariable-adjusted multimarker analysis of the association between baseline biomarker levels and cardiovascular (CV) death and heart failure. Adjusted ORs for cardiovascular death or heart failure through 30 days are depicted with red squares for ST2 quartiles and blue circles for NT-proBNP quartiles; vertical bars denote 95% CIs. The regression model included both biomarkers and age, sex, hypertension, diabetes mellitus, prior MI, prior CHF, creatinine clearance, infarct location, Killip class, time from symptom onset to lytic, type of lytic, and peak CK.
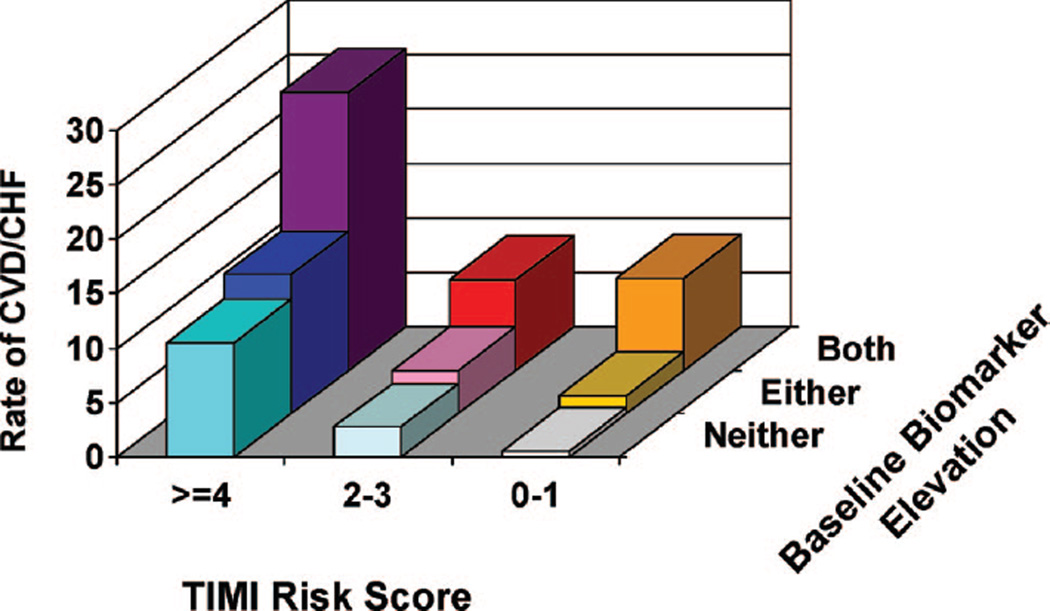
The adjusted ORs for the risk of cardiovascular death or CHF for various combinations of elevations of each biomarker (Table 5) show a stepwise increase in risk with both biomarkers, particularly when at least one of them is in the fourth quartile. When added to the TIMI Risk Score for STEMI, ascertainment of whether either or both ST2 and NT-proBNP were above the median provided additional, significant risk stratification (P=0.001; Figure 5). This was especially apparent in patients with a TIMI Risk Score of <4, in whom further classification by biomarker status allowed an ≈6-fold gradient of risk stratification (OR, 6.2; 95% CI, 2.0 to 18.9). The addition of biomarker status to the TIMI Risk Score significantly improved the c statistic from 0.73 (95% CI, 0.68 to 0.78) to 0.78 (95% CI, 0.74 to 0.83; P=0.0025).
Table 5.
Risk Groups Defined by Combinations of ST2 and NT-proBNP Elevation: Adjusted ORs (95% CIs) for Cardiovascular Death or Heart Failure Through 30 Days
| ST2 | |||
|---|---|---|---|
| NT-proBNP | Quartiles 1 and 2 | Quartile 3 | Quartile 4 |
| Quartiles 1 and 2 | 1.0 (Reference) | 1.14 (0.28–4.66) | 2.24 (0.68–7.38) |
| Quartile 3 | 0.72 (0.18–2.97) | 1.32 (0.35–4.90) | 5.62 (1.86–16.96) |
| Quartile 4 | 1.99 (0.66–5.97) | 2.98 (0.95–9.38) | 6.58 (2.43–17.84) |
ORs were adjusted for age, sex, hypertension, diabetes mellitus, prior MI, prior CHF, creatinine clearance, infarct location, Killip class, time from symptom onset to lytic, type of lytic, and peak CK.
Figure 5.
Combined risk stratification with the TIMI Risk Score for STEMI and NT-proBNP and ST2. Rate of cardiovascular death or heart failure in patients was categorized into 9 groups on the basis of the TIMI Risk Score for STEMI and biomarker status; the latter was categorized as neither ST2 and NT-proBNP above the median, 1 of the 2 above the median, or both above the median. The number of patients with events/ patients in each group is as follows (from left to right and from back to front): 36/141, 10/121, 5/59, 16/125, 9/226, 3/181, 4/38, 3/109, and 1/167.
Discussion
We found that in patients with STEMI, an elevated level of ST2 was a strong predictor of cardiovascular death or heart failure through 30 days. ST2 was a predictor of adverse cardiovascular outcomes independently of traditional risk factors, including the TIMI Risk Score for STEMI, and provided prognostic information that was independent of and complementary to NT-proBNP.
In prior studies, we have shown that circulating levels of ST2 could be detected in the serum of patients after acute MI and that those levels were associated with an increased risk of death and heart failure.7,8 Now, by using a large cohort of patients with STEMI, we have been able to advance our understanding of the significance of ST2 and its relation to an established biomarker of LV wall stress, NT-proBNP, in this setting. First, in terms of baseline variables, we found that ST2 levels on presentation were not associated with clinical characteristics potentially related to chronic increased LV wall stress such as age, sex, hypertension, prior MI, and prior CHF. This independence was in sharp contrast to NT-proBNP levels, which were strongly related to all of the aforementioned characteristics. Furthermore, ST2 levels were strongly associated with peak CK, whereas NT-proBNP levels were strongly associated with infarct location and Killip class. These fundamental differences help to explain why we observed only a weak correlation between ST2 and NT-proBNP levels.
Second, we were able to examine the changes in both ST2 and NT-proBNP levels over time. ST2 levels modestly but significantly decreased from baseline to angiography (an average of 4 days later). In contrast, NT-proBNP levels increased nearly 6-fold over the same time period. Accordingly, for ST2, levels at baseline rather than subsequent values appeared more predictive of risk of cardiovascular death or heart failure, whereas for NT-proBNP, subsequent values appeared more predictive. These observations likely stem from the differences in the kinetics of the 2 biomarkers. Ex vivo data from cardiac myocytes subjected to biomechanical strain showed that maximal induction of ST2 transcription occurred by 2 hours, is sustained for 9 hours, and then declines by 15 hours, whereas maximal induction of BNP transcription remains elevated through at least 48 hours.7,16 We observed greater increases in NT-proBNP in patients with anterior MI and lack of ST-segment resolution at 90 minutes. Moreover, we demonstrated that the magnitude of elevation of both ST2 and NT-proBNP after fibrinolytic therapy is directly related to both impaired epicardial flow in and impaired myocardial perfusion downstream of the culprit artery. However, it should be noted that the range of values in those with and without reperfusion overlap; thus, neither biomarker is ideally suited to discriminate reperfusion status. Whereas NT-proBNP was moderately strongly correlated with LV ejection fraction at the time of angiography, ST2 was not.
Third, we demonstrated that an elevated ST2 level at baseline is a significant predictor of cardiovascular death or heart failure through 30 days. Importantly, ST2 added to traditional risk factors and provided prognostic information that was independent of and complementary to NT-proBNP levels. Moreover, adding ST2 and NT-proBNP either to a comprehensive multivariable model or to the TIMI Risk Score for STEMI resulted in significantly improved risk stratification and discrimination, as evidenced by a significantly higher c statistic. In practical terms, a patient with a low TIMI Risk Score for STEMI (0 to 1) but elevated ST2 and NTproBNP levels is at a risk of cardiovascular death or CHF that is similar to that of a patient with a high TIMI Risk Score for STEMI (≥4) but low levels of ST2 and NT-proBNP.
As we better define the epidemiological characteristics and prognostic significance of ST2, the fundamental biology of ST2 in the heart also is emerging. ST2, a member of the IL-1 receptor family, also is known as IL-1 receptor-like-1. Soluble and membrane receptor forms are produced by alternative promoter usage. We have previously shown that mechanical strain induces the expression of soluble ST2 by both cardiomyocytes and cardiac fibroblasts. After an intense search for more than a decade, the functional ligand for this orphan receptor was recently found to be IL-33.17 We have recently demonstrated that IL-33 also is a biomechanically induced protein that is synthesized primarily by cardiac fibroblasts.18 Furthermore, we have shown that IL-33 protects the myocardium during experimental pressure overload and that soluble ST2 can block the antihypertrophic effects of IL-33. In mice subjected to pressure overload, recombinant IL-33 reduces fibrosis and improves survival in wild-type but not in ST2 knockout mice. Thus, IL-33/ST2 signaling may serve as a crucial cardioprotective response to biomechanical strain.
Potential limitations of the study merit consideration. Because CLARITY-TIMI 28 excluded patients >75 years of age, those with serum creatinine >2.5 mg/dL, and those in cardiogenic shock, we cannot comment on the utility of ST2 in such individuals, who are at very high risk for death and heart failure. It will be important to assess the utility of ST2 in cohorts that contain such patients. Furthermore, the predictive ability of ST2 in patients undergoing primary PCI remains unknown. Levels of ST2 were below the limit of detection in 30% of the population. Although we saw no gradient of risk within the bottom half of ST2 values in this population, the development of more sensitive assays may be useful in risk stratification in patients with less severe myocardial injury. Heart failure was not adjudicated by a clinical events committee. However, any misclassification should be random with respect to biomarker levels and thus typically would bias only toward the null hypothesis.19 Finally, ejection fraction was not necessarily determined with 3-dimensional imaging.
With regard to future directions, the protective effect of the IL-33/ST2 system observed in animal models raises the possibility that soluble ST2 may be maladaptive. Consequently, the higher levels of ST2 we observed in patients with STEMI who ultimately experienced death or heart failure may have directly contributed to those outcomes. Further studies are needed to elucidate whether soluble ST2 is a risk marker or a direct risk factor. Moreover, when a robust assay for IL-33 is available, determination of circulating levels in our patient cohort should shed additional light on the prognostic relevance of the IL-33/ST2 system.
Conclusions
In patients with STEMI, ST2, a novel biomarker of biomechanical strain, is a strong predictor of cardiovascular death and heart failure. ST2 levels provide prognostic information that is not only independent of traditional risk factors but also complementary to NT-proBNP, and the combination offers improved risk stratification and discrimination compared with the TIMI Risk Score alone. These data demonstrate the prognostic utility of multiple, complementary biomarkers of biochemical strain in STEMI and highlight the importance of the IL-33/ST2 system as a target of further study and potential therapeutic intervention.
CLINICAL PERSPECTIVE.
ST2 is a member of the interleukin (IL)-1 receptor family with a soluble form that is markedly upregulated on application of biomechanical strain to cardiac myocytes. The ligand for this receptor is IL-33; we have recently shown that IL-33 protects the myocardium during experimental pressure overload and that soluble ST2 can block the antihypertrophic effects of IL-33. In this study, we measured levels of ST2 and an established biomarker of ventricular wall stress, N-terminal prohormone B-type natriuretic peptide, at baseline in 1239 patients with ST-elevation myocardial infarction from the Clopidogrel as Adjunctive Reperfusion Therapy–Thrombolysis in Myocardial Infarction 28 trial. We found that ST2 levels on presentation were not associated with clinical characteristics potentially related to chronic increased left ventricular wall stress such as age, sex, hypertension, prior myocardial infarction, or prior heart failure. This independence was in sharp contrast to N-terminal prohormone B-type natriuretic peptide levels, which were strongly related to all of the aforementioned characteristics. Accordingly, levels of the 2 biomarkers were only weakly correlated. In a multivariable model that contained both biomarkers and adjusted for baseline characteristics, elevations in ST2 levels and in N-terminal prohormone B-type natriuretic peptide levels each were significant independent predictors of the risk of cardiovascular death or heart failure through 30 days. When both ST2 and N-terminal prohormone B-type natriuretic peptide were added to a model containing traditional clinical predictors, the c statistic significantly improved. These data highlight the prognostic value of multiple, complementary biomarkers of biomechanical strain in ST-elevation myocardial infarction and highlight the importance of the IL-33/ST2 system as a target of further study and potential therapeutic intervention.
Acknowledgments
Sources of Funding
Drs Sabatine, Morrow, and Gerszten are supported in part by a grant from the National Institutes of Health (U01 HL083-1341). The reagent for NT-proBNP testing was generously supplied by Roche Diagnostics. The parent clinical trial, CLARITY-TIMI 28, was supported in part by the pharmaceutical partnership of Sanofi-Aventis and Bristol-Myers Squibb.
Dr Sabatine reports having received research grant support from diaDexus and Roche and honoraria from diaDexus. Dr Morrow reports having received research grants from Bayer, Beckman-Coulter, Biosite, GlaxoSmithKline, Ortho-Clinical Diagnostics, and Roche; having received honoraria from Bayer, Beckman-Coulter, Dade-Behring, and Roche; and having served on advisory boards for Critical Diagnostics, GlaxoSmithKline, Beckman-Coulter, and Ortho-Clinical Diagnostics. Brigham and Women’s Hospital has filed for patents on ST2, listing Dr Lee as the inventor.
Footnotes
Disclosures
The other authors report no conflicts.
References
- 1.Wagner GS, Roe CR, Limbird LE, Rosati RA, Wallace AG. The importance of identification of the myocardial-specific isoenzyme of creatine phosphokinase (MB form) in the diagnosis of acute myocardial infarction. Circulation. 1973;47:263–269. doi: 10.1161/01.cir.47.2.263. [DOI] [PubMed] [Google Scholar]
- 2.Hamm CW, Ravkilde J, Gerhardt W, Jorgensen P, Peheim E, Ljungdahl L, Goldmann B, Katus HA. The prognostic value of serum troponin T in unstable angina. N Engl J Med. 1992;327:146–150. doi: 10.1056/NEJM199207163270302. [DOI] [PubMed] [Google Scholar]
- 3.Antman EM, Tanasijevic MJ, Thompson B, Schactman M, McCabe CH, Cannon CP, Fischer GA, Fung AY, Thompson C, Wybenga D, Braunwald E. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med. 1996;335:1342–1349. doi: 10.1056/NEJM199610313351802. [DOI] [PubMed] [Google Scholar]
- 4.O’Donoghue M, de Lemos JA, Morrow DA, Murphy SA, Buros JL, Cannon CP, Sabatine MS. Prognostic utility of heart-type fatty acid binding protein in patients with acute coronary syndromes. Circulation. 2006;114:550–557. doi: 10.1161/CIRCULATIONAHA.106.641936. [DOI] [PubMed] [Google Scholar]
- 5.de Lemos JA, Morrow DA, Bentley JH, Omland T, Sabatine MS, McCabe CH, Hall C, Cannon CP, Braunwald E. The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001;345:1014–1021. doi: 10.1056/NEJMoa011053. [DOI] [PubMed] [Google Scholar]
- 6.Mega JL, Morrow DA, De Lemos JA, Sabatine MS, Murphy SA, Rifai N, Gibson CM, Antman EM, Braunwald E. B-type natriuretic peptide at presentation and prognosis in patients with ST-segment elevation myocardial infarction: an ENTIRE-TIMI-23 substudy. J Am Coll Cardiol. 2004;44:335–339. doi: 10.1016/j.jacc.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 7.Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, Rouleau JL, Lee RT. Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106:2961–2966. doi: 10.1161/01.CIR.0000038705.69871.D9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimpo M, Morrow DA, Weinberg EO, Sabatine MS, Murphy SA, Antman EM, Lee RT. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. 2004;109:2186–2190. doi: 10.1161/01.CIR.0000127958.21003.5A. [DOI] [PubMed] [Google Scholar]
- 9.Sabatine MS, McCabe CH, Gibson CM, Cannon CP. Design and rationale of Clopidogrel as Adjunctive Reperfusion Therapy (CLARITY)-Thrombolysis in Myocardial Infarction (TIMI) 28 trial. Am Heart J. 2005;149:227–233. doi: 10.1016/j.ahj.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 10.Sabatine MS, Cannon CP, Gibson CM, Lopez-Sendon JL, Montalescot G, Theroux P, Claeys MJ, Cools F, Hill KA, Skene AM, McCabe CH, Braunwald E. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevation. N Engl J Med. 2005;352:1179–1189. doi: 10.1056/NEJMoa050522. [DOI] [PubMed] [Google Scholar]
- 11.TIMI Study Group. The Thrombolysis in Myocardial Infarction (TIMI) trial: phase I findings. N Engl J Med. 1985;312:932–936. doi: 10.1056/NEJM198504043121437. [DOI] [PubMed] [Google Scholar]
- 12.Gibson CM, Cannon CP, Murphy SA, Ryan KA, Mesley R, Marble SJ, McCabe CH, Van De Werf F, Braunwald E. Relationship of TIMI myocardial perfusion grade to mortality after administration of thrombolytic drugs. Circulation. 2000;101:125–130. doi: 10.1161/01.cir.101.2.125. [DOI] [PubMed] [Google Scholar]
- 13.Schroder R, Dissmann R, Bruggemann T, Wegscheider K, Linderer T, Tebbe U, Neuhaus KL. Extent of early ST segment elevation resolution: a simple but strong predictor of outcome in patients with acute myocardial infarction. J Am Coll Cardiol. 1994;24:384–391. doi: 10.1016/0735-1097(94)90292-5. [DOI] [PubMed] [Google Scholar]
- 14.Morrow DA, Antman EM, Charlesworth A, Cairns R, Murphy SA, de Lemos JA, Giugliano RP, McCabe CH, Braunwald E. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an Intravenous nPA for Treatment of Infarcting Myocardium Early II Trial substudy. Circulation. 2000;102:2031–2037. doi: 10.1161/01.cir.102.17.2031. [DOI] [PubMed] [Google Scholar]
- 15.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 16.Liang F, Wu J, Garami M, Gardner DG. Mechanical strain increases expression of the brain natriuretic peptide gene in rat cardiac myocytes. J Biol Chem. 1997;272:28050–28056. doi: 10.1074/jbc.272.44.28050. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT. IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007;117:1538–1549. doi: 10.1172/JCI30634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hennekens CH, Buring JE. Epidemiology in Medicine. Boston, Mass: Little, Brown and Co; 1987. [Google Scholar]