Abstract
Lung transplantation through controlled donation after circulatory death (cDCD) has slowly gained universal acceptance with reports of equivalent outcomes to those through donation after brain death. In contrast, uncontrolled DCD (uDCD) lung use is controversial and requires ethical, legal and medical complexities to be addressed in a limited time. Consequently, uDCD lung use has not previously been reported in the United States. Despite these potential barriers, we present a case of a patient with multiple gunshot wounds to the head and the body who was unsuccessfully resuscitated and ultimately became an uDCD donor. A cytomegalovirus positive recipient who had previously consented for CDC high-risk, DCD and participation in the NOVEL trial was transplanted from this uDCD donor, following 3 hours of ex vivo lung perfusion. The postoperative course was uneventful and the recipient was discharged home on day 9. While this case represents a “best-case scenario,” it illustrates a method for potential expansion of the lung allograft pool through uDCD after unsuccessful resuscitation in hospitalized patients.
Keywords: Cardiac arrest, cardiac death donors, ex vivo lung perfusion, intensive care, lung transplantation, resuscitation
Introduction
Lung transplantation is the only effective therapy for end-stage lung diseases, but the clinical success of lung transplantation has been tempered by a significant shortage of available lung allografts. Many strategies have attempted to overcome this limitation including public education campaigns, legislative action, civic engagement (1), improved donor management (2), use of extended criteria donor lungs, lobar lung transplantation (3), donation after circulatory death (DCD) (4, 5) and ex vivo lung perfusion (EVLP) (6–8). Lung transplantation through controlled DCD (cDCD) has gained widespread adoption in the United States with evidence suggesting equivalent outcomes to those through donation after brain death (DBD) (9–12). The hesitation for use of DCD lungs is in part due to concern over ethical, legal, and allocation issues, limited pre-procurement assessment, increased logistical requirements, concern over secondary injury and relatively high frequency of failed procurement (12, 13). Reported experience worldwide primarily consists of cDCDs, with only two groups in Europe previously reporting experience with uDCDs (14). In the current era, with technologies available to safely prolong the evaluation of organs (8), as well as allow time for donor studies to be obtained, we believe that lungs from uDCDs should be reconsidered for use in the United States. To accelerate this discussion, we report our clinical experience in a case of uDCDs with unsuccessful resuscitation in the intensive care unit (ICU), which resulted in successful lung transplantation using EVLP.
Case
A 26 year-old male suffered multiple gunshot wounds to the head, right chest, right trunk and right leg.
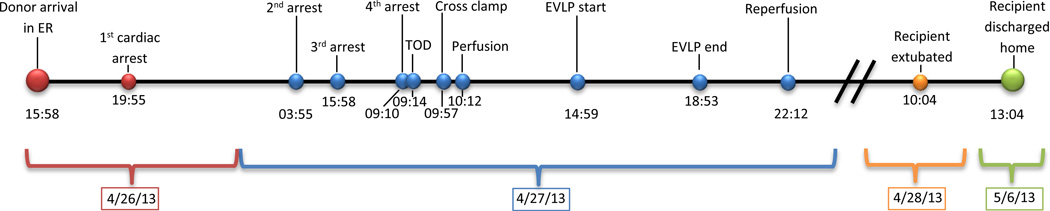
On presentation, the patient had agonal respirations, GCS 3 and four wounds to the head and face with extrusion of brain tissue. The patient was intubated, assessed, and scanned. The head CT demonstrated numerous areas of comminuted blow-out and blow-in fractures of the calvarium involving the right temporal, left frontal and right parietal regions. Multifocal intracerebral hemorrhagic areas were identified in bilateral frontal and left occipital lobes with loss of grey-white differentiation, partial effacement of the supracellar cistern and mild uncal herniation. Vascular injury of the superior sagittal sinus was suspected. In the ER, the patient experienced hypovolemic shock and experienced a pulseless electrical activity (PEA) arrest prompting initiation of massive transfusion protocol with successful resuscitation. The patient was emergently taken to the operating room. A craniotomy with ventricular drain placement and sagittal sinus repair was performed. Laparotomy demonstrated irreparable ballistic injuries to the liver. The abdomen was packed, a right chest tube was placed and the patient was subsequently transferred to the ICU. It was determined that the patient had suffered a non-recoverable neurologic injury and met the hospital’s potential organ donor referral trigger. The local organ procurement organization (OPO) was notified and a coordinator was dispatched to the ICU for evaluation. In the ICU, the patient continued to experience coagulopathy and remained hemodynamically unstable despite aggressive vasopressor and volume replacement therapy (9,563 ml packed red blood cells; 3,445 ml plasma; 3,049 ml platelets; 5,000 ml saline). The patient suffered the second PEA arrest due to ongoing hemorrhage with successful resuscitation. The first clinical assessment for brain death was performed given the extent of the neurologic injury sustained (Figure 1). The legal next of kin (LNOK) was apprised of first brain death exam and the plan to conduct a second exam. Following this discussion, the patient suffered a third PEA arrest with successful resuscitation. Given the family’s understanding of the patient’s condition and the possibility of impending death, a decision was made for the OPO coordinator to approach the family regarding donation. The coordinator consented the LNOK to both DBD and DCD. Prior to a second brain death exam, the patient experienced an asystolic arrest, went through three rounds of ACLS protocol with no return of vital signs or electrical activity and was pronounced dead based on cardiocirculatory criteria (Figure 1). LNOK was notified of patient’s death. Following the pronouncement of death 2 minutes elapsed without intervention (“hands-off” period) prior to an initiation of DCD protocol resuming chest compressions and manual bagging. These maneuvers continued as the donor was transferred to the operating room for DCD organ recovery, throughout which the underlying rhythm remained asystole. An abdominal recovery team had been mobilized on site earlier in the day following donation authorization in preparation for impending DCD recovery.
Figure 1.
Summary of the time points of events.
CIT: cold ischemic time, ER: emergency room, EVLP: ex vivo lung perfusion, IT: ischemic time, OR: operating room, PEA: pulseless electrical activity, TOD: time of death, WIT: warm ischemic time
A call was made from the OPO to our center prior to the fourth arrest for potential expedited DBD or DCD. At time of offer, the donor was known to have a 10 pack-year smoking history, traumatic thoracic injury with consequent massive transfusion and was considered high-risk by the Centers for Disease Control and Prevention (CDC) due to hemodilution. Chest computed tomography demonstrated right hemopneumothorax and middle lobe contusion. The arterial blood gas demonstrated a pH of 7.00, PaCO2 of 31 mmHg and PaO2 of 194 mmHg (admission PaO2 525 mmHg). Final serologic testing and donor HLA type were pending. Standard organ allocation procedures were followed and our center had the first four candidates on the UNOS match run, and the decision was made to accept the left lung for the second candidate. This candidate was chosen because he had consented to receive lungs from CDC high-risk donors, was cytomegalovirus positive with no panel reactive antibody reactivity and had consented for participation in the NOVEL lung trial (NCT01365429). He was a 62 year-old with emphysema (FEV1 14% predicted; PaCO2 64mmHg).
In the operating room chest compressions and bagging continued, heparin was administered, and surgical incision was made 46 minutes after asystolic arrest. The timeline and procedural conduct is demonstrated in Figure 1. The lungs were packed with saline slush by the abdominal surgeon 5 minutes after aortic cross-clamping and ventilation of the lung was resumed with room air. Total warm ischemic time from cardiac asystole until topical cooling was 60 minutes. On arrival, the lung procurement surgeon identified contusions without consolidation in the left lung. Lungs were perfused with 5500 ml of antegrade and 1000 ml of retrograde cold Perfadex® (XVIVO Perfusion, Göteborg, Sweden) 120 minutes after asystolic arrest. No pulmonary emboli were identified. Lungs were inflated with room air, stored and transported using standard methods.
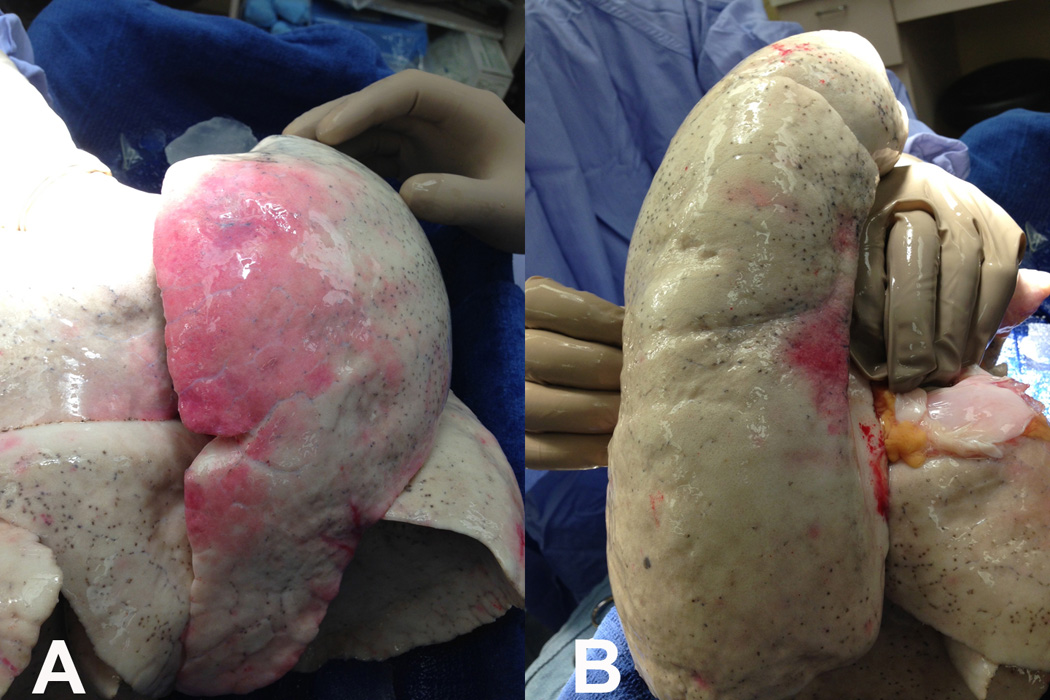
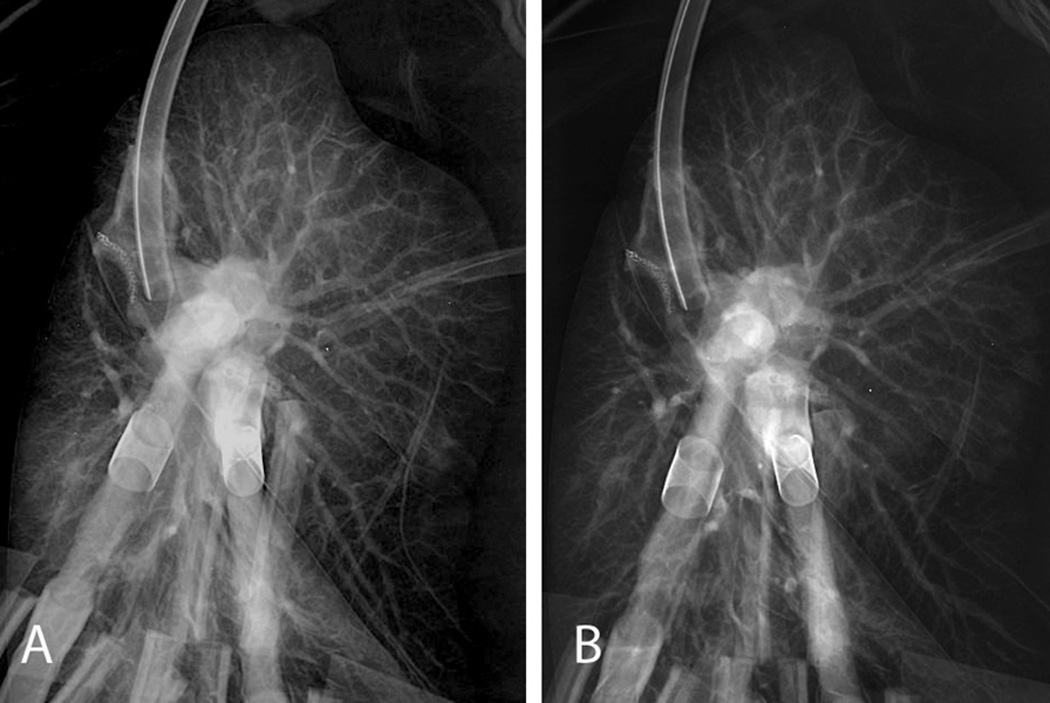
On arrival, the allograft was taken to our EVLP suite in the main operating room core. A 15×10 cm contusion on the anterior aspect of the left upper lobe and a 5×2 cm contusion on the posterior portion of the left lower lobe were identified (Figure 2). The left lung was separated, cannulated and placed on EVLP 357 minutes after asystolic arrest. The lung was evaluated over 3 hours, with decreasing peak airway pressures (18 to 16 cmH2O), decreasing pulmonary vascular resistance (494 to 296 dyn×s/cm5), improving static compliance (35 to 38 cmH2O) and excellent gas exchange (PaO2 of 450–530 mmHg). Sequential chest radiographs demonstrated decreased pulmonary edema (Figure 3). Bronchoscopy demonstrated no significant pathology. During evaluation, serologies and HLA results from the donor became available, and were compatible with the recipient. The decision was made to proceed and the recipient underwent single left lung transplantation. Total ischemic time was 790 minutes. The postoperative course was uneventful; the recipient was extubated within 12 hours of reperfusion and transferred to the ward on day 2. Primary graft dysfunction ISHLT grades at 24, 48 and 72 hours were 1, 2 and 1, respectively. The recipient was discharged home on day 9 and has subsequently done well without airway complications or episodes of rejection as of this report (31 weeks).
Figure 2.
Images showing areas of lung contusion in the anterior portion of the donor left upper lobe (A) and in the posterior portion of the donor left lower lobe (B).
Figure 3.
Chest X-ray films 1 hour after ex vivo lung perfusion (A) and 3 hours after ex vivo lung perfusion (B) showing that pulmonary edema of the donor left lung slightly improved on ex vivo lung perfusion.
Discussion
In the United States, expansion of the lung allograft pool through uDCD is perceived to be associated with non-quantifiable risks (9). Although lung transplantation through cDCD is gaining universal acceptance, logistic and medical complexities are harder to overcome in uDCD. Despite these barriers, we present this case to initiate discussion for potential lung allograft pool expansion through uDCDs emphasizing three key areas for consideration - logistical, legal and ethical.
Logistical considerations
The hesitation for use of lungs through uDCDs stems mainly from medical concerns over secondary injury from prolonged warm ischemia (12, 13), despite the lungs unique physiology with a low metabolic rate and dual oxygen supply from alveolar diffusion (4). Animal studies have demonstrated that lungs tolerate warm ischemia up to an hour (4, 15) and that topical cooling and lung inflation are effective preservation methods for up to 6 hours without cold perfusion (6, 16, 17). These studies are the foundation for the use of cDCD lungs (4, 10). In the case presented, there were several logistical complexities. The first involved resource use and medical responsibility. The trauma team committed significant resources after determining survival was unlikely and continued allocating resources after death to make organ donation possible. For organ donation from uDCD to be routinely possible, centers will have to allocate resources specifically for these purposes. The second logistical hurdle entailed timing of notification. Because the OPO was notified early after the patient’s initial assessment, early brain death evaluation, discussion with the family, creating a match run and mobilization of an abdominal procurement team was possible. Timely identification of potential uDCDs is critical to allow a period of time for discussion with LNOK, evaluation and documentation, and coordination with transplant centers. Ideally, stabilization would be preferable to uDCD but this may not always be possible. The third complexity involved center coordination. Though we anticipated an expedited cDCD recovery, we did not have sufficient time prior to final arrest to mobilize our teams and our candidate. In order to avoid further delay, our lung transplant surgeon traveled directly to the donor hospital from home and equipment and supplies were sent from our center to the donor hospital by ambulance, saving 60 minutes. While the lung surgeon was in transit, the abdominal procurement surgeon gained access to the chest and established expedited topical cooling after aortic cross clamp and visceral organ perfusion. This maneuver, with resumption of lung ventilation, was a critically important adjunct to restrict warm ischemic time. Additionally, serologies and HLA typing were not available; therefore donor-recipient compatibility had not been definitively established. Choosing a recipient with the least chance for incompatibility was critical. Having the ability to use EVLP to allow for extended evaluation of the lung allograft, collecting critical laboratory information and mobilizing the recipient and surgical team made organ use possible. For these reasons, we believe EVLP will be critical in determining the success of lung transplantation through uDCD (6–10, 14), especially in light of additional high-risk factors compared to cDCD and DBD (14).
Legal considerations
Unlike the Spanish group which has a national policy and ICU infrastructure to support uDCD lung transplantation (14), in the United States there is no opt-out system and only 43% of Americans are designated organ donors. The Spanish model and law allows for the organs to be preserved following death but no surgical incision for removal is performed until the family has been contacted. Authorizing uDCD donation at the time of death poses additional challenges in timely notification of LNOK and a compressed time period to provide families information about donation. uDCD from donors who are registered donors at the time of death may help facilitate this process; however, this would limit the pool of potential donors and families may be mistrustful of the health care team if they are notified of the patients death after the organs have already been retrieved. Additionally, though this case did not experience any barriers from the medical examiner, it is important that medical examiners and coroners are educated regarding uDCD and protocols are put in place for the preservation of any needed forensic evidence. These are only some potential issues and more are likely to be identified.
Ethical considerations
uDCD has the potential to create ethical dilemmas and as such will require careful consideration. Current clinical practice requires adherence to the “Dead Donor Rule” which stipulates the donor must be dead prior to the recovery of organs. This principle was established to ensure all patients are given the opportunity to recover from life threatening injuries and patient families feel confident that there is no conflict of interest. In cDCD transplants, a 2–5 minute hands-off period has been recommended by the Institute of Medicine and Society of Critical Care Medicine to be a period of circulatory failure from which spontaneous recovery of heart function was exceedingly unlikely and has been adopted by most OPOs and centers for cDCD. Guidelines for uDCDs in the US are beginning to be offered, but lack consensus (18). Because it is logically and mathematically impossible to prove that autoresuscitation cannot occur beyond a specific time point, some authorities have advocated allowing donation to proceed so long as patients are near death and donation is clearly consistent with the patient’s desires, even if the moment of death cannot be unambiguously defined (19). Greater ethical consensus on how and when death may be declared may be needed before the pool of potential uDCD can be utilized in the U.S. In the case presented, a 2 minute “hands-off” observation period was utilized, though asystole was present throughout the entirety of the last resuscitation attempt, the “hands-off” period and the donor management period until aortic cross-clamping (total of 55 minutes). Within this OPO, 74 Maastricht Category II uDCDs have been coordinated over the last 17 years with cardiac compressions beginning after 2 minutes and lasting as long as 211 minutes without a single instance of autoresuscitation (20).
The need for complex ethical decisions to be made by multiple practitioners in a short period of time with great potential for uncertainty should prompt open discussion and creation of general guidelines. Though this case represents a “best-case scenario” for lung donation from an uDCD, national guidelines to address some of the logistical, ethical and legal issues raised are needed to ensure public trust and safe implementation of this practice.
To our knowledge, this is the first report, in the United States, of successful lung transplantation through uDCD using EVLP. Although our recipient did well, enthusiasm should be tempered. We present this case to facilitate further discussion. Several EVLP clinical trials (NCT01615484 and NCT01365429) will soon further define the safety and feasibility of this novel strategy. With the increasing acceptance of cDCD and the advent of EVLP, we believe lung transplantation through uDCD should be considered in the United States.
Acknowledgements
The authors would like to acknowledge the contributions of Scott D. Halpern MD, PhD, M. Bioethics for his insight and review of the ethical considerations discussed. This work was supported by NIH HL116656 and RWJ AMFDP11642.
Abbreviations
- ACLS
advanced cardiovascular life support
- CDC
centers for disease control and prevention
- DBD
donation after brain death
- DCD
donation after circulatory death
- EVLP
ex vivo lung perfusion
- FEV1
forced expiratory volume in 1 second
- ICU
intensive care unit
- ISHLT
International Society for Heart and Lung Transplantation
- LNOK
legal next of kin
- OPO
organ procurement organization
- PEA
pulseless electrical activity
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Shemie SD. Brain arrest to neurological determination of death to organ utilization: the evolution of hospital-based organ donation strategies in Canada. Can J Anaesth. 2006;53(8):747–752. doi: 10.1007/BF03022789. [DOI] [PubMed] [Google Scholar]
- 2.Angel LF, Levine DJ, Restrepo MI, Johnson S, Sako E, Carpenter A, et al. Impact of a lung transplantation donor-management protocol on lung donation and recipient outcomes. Am J Respir Crit Care Med. 2006;174(6):710–716. doi: 10.1164/rccm.200603-432OC. [DOI] [PubMed] [Google Scholar]
- 3.Bisson A, Bonnette P, Perruchoud A, Leroy M, Colchen A, Raffin L, et al. Left lower lobe transplantation during a bilateral single lung transplantation (pulmonary lobe transplantation) Eur J Cardiothorac Surg. 1992;6(10):568–570. doi: 10.1016/1010-7940(92)90011-l. [DOI] [PubMed] [Google Scholar]
- 4.Egan TM, Lambert CJ, Jr, Reddick R, Ulicny KS, Jr, Keagy BA, Wilcox BR. A strategy to increase the donor pool: use of cadaver lungs for transplantation. Ann Thorac Surg. 1991;52(5):1113–1120. doi: 10.1016/0003-4975(91)91290-c. discussion 1120-1111. [DOI] [PubMed] [Google Scholar]
- 5.D'Alessandro AM, Hoffmann RM, Knechtle SJ, Eckhoff DE, Love RB, Kalayoglu M, et al. Successful extrarenal transplantation from non-heart-beating donors. Transplantation. 1995;59(7):977–982. doi: 10.1097/00007890-199504150-00009. [DOI] [PubMed] [Google Scholar]
- 6.Steen S, Liao Q, Wierup PN, Bolys R, Pierre L, Sjoberg T. Transplantation of lungs from non-heart-beating donors after functional assessment ex vivo. Ann Thorac Surg. 2003;76(1):244–252. doi: 10.1016/s0003-4975(03)00191-7. discussion 252. [DOI] [PubMed] [Google Scholar]
- 7.Egan TM, Haithcock JA, Nicotra WA, Koukoulis G, Inokawa H, Sevala M, et al. Ex vivo evaluation of human lungs for transplant suitability. Ann Thorac Surg. 2006;81(4):1205–1213. doi: 10.1016/j.athoracsur.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 8.Cypel M, Yeung JC, Machuca T, Chen M, Singer LG, Yasufuku K, et al. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J Thorac Cardiovasc Surg. 2012;144(5):1200–1206. doi: 10.1016/j.jtcvs.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Wigfield CH, Love RB. Donation after cardiac death lung transplantation outcomes. Curr Opin Organ Transplant. 2011;16(5):462–468. doi: 10.1097/MOT.0b013e32834a99ac. [DOI] [PubMed] [Google Scholar]
- 10.Love RB. Perspectives on lung transplantation and donation-after-determination-of-cardiac-death donors. Am J Transplant. 2012;12(9):2271–2272. doi: 10.1111/j.1600-6143.2012.04199.x. [DOI] [PubMed] [Google Scholar]
- 11.Mason DP, Thuita L, Alster JM, Murthy SC, Budev MM, Mehta AC, et al. Should lung transplantation be performed using donation after cardiac death? The United States experience. J Thorac Cardiovasc Surg. 2008;136(4):1061–1066. doi: 10.1016/j.jtcvs.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 12.De Oliveira NC, Osaki S, Maloney JD, Meyer KC, Kohmoto T, D'Alessandro AM, et al. Lung transplantation with donation after cardiac death donors: long-term follow-up in a single center. J Thorac Cardiovasc Surg. 2010;139(5):1306–1315. doi: 10.1016/j.jtcvs.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Puri V, Scavuzzo M, Guthrie T, Hachem R, Krupnick AS, Kreisel D, et al. Lung transplantation and donation after cardiac death: a single center experience. Ann Thorac Surg. 2009;88(5):1609–1614. doi: 10.1016/j.athoracsur.2009.06.039. discussion 1614-1605. [DOI] [PubMed] [Google Scholar]
- 14.Gomez-de-Antonio D, Campo-Canaveral JL, Crowley S, Valdivia D, Cordoba M, Moradiellos J, et al. Clinical lung transplantation from uncontrolled non-heart-beating donors revisited. J Heart Lung Transplant. 2012;31(4):349–353. doi: 10.1016/j.healun.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Rega FR, Neyrinck AP, Verleden GM, Lerut TE, Van Raemdonck DE. How long can we preserve the pulmonary graft inside the nonheart-beating donor? Ann Thorac Surg. 2004;77(2):438–444. doi: 10.1016/S0003-4975(03)01343-2. discussion 444. [DOI] [PubMed] [Google Scholar]
- 16.Steen S, Ingemansson R, Budrikis A, Bolys R, Roscher R, Sjoberg T. Successful transplantation of lungs topically cooled in the non-heart-beating donor for 6 hours. Ann Thorac Surg. 1997;63(2):345–351. doi: 10.1016/s0003-4975(96)01101-0. [DOI] [PubMed] [Google Scholar]
- 17.Van Raemdonck DE, Jannis NC, Rega FR, De Leyn PR, Flameng WJ, Lerut TE. Extended preservation of ischemic pulmonary graft by postmortem alveolar expansion. Ann Thorac Surg. 1997;64(3):801–808. doi: 10.1016/s0003-4975(97)00627-9. [DOI] [PubMed] [Google Scholar]
- 18.Bernat JL, Bleck TP, Blosser SA, Bratton SL, Capron AM, Cornell D, et al. Circulatory Death Determination in Uncontrolled Organ Donors: A Panel Viewpoint. Ann Emerg Med. 2013 doi: 10.1016/j.annemergmed.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Truog RD, Miller FG, Halpern SD. The Dead-Donor Rule and the Future of Organ Donation. New England Journal of Medicine. 2013;369(14):1287–1289. doi: 10.1056/NEJMp1307220. [DOI] [PubMed] [Google Scholar]
- 20.Hasz RD, Nathan HM, Abrams JD, West SM, Moritz MJ. One OPO's 17 Year Experience with Uncontrolled DCD Donation. Transplantation. 2013;96(10S):S217–S217. [Google Scholar]