Abstract
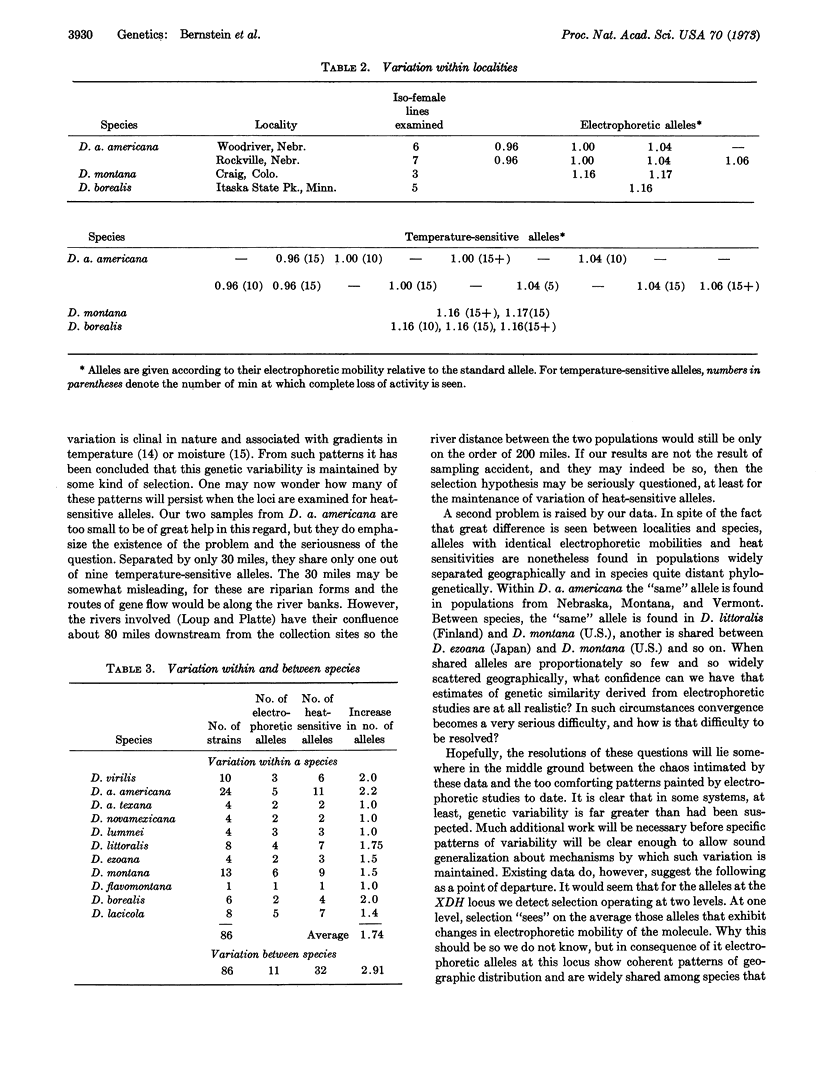
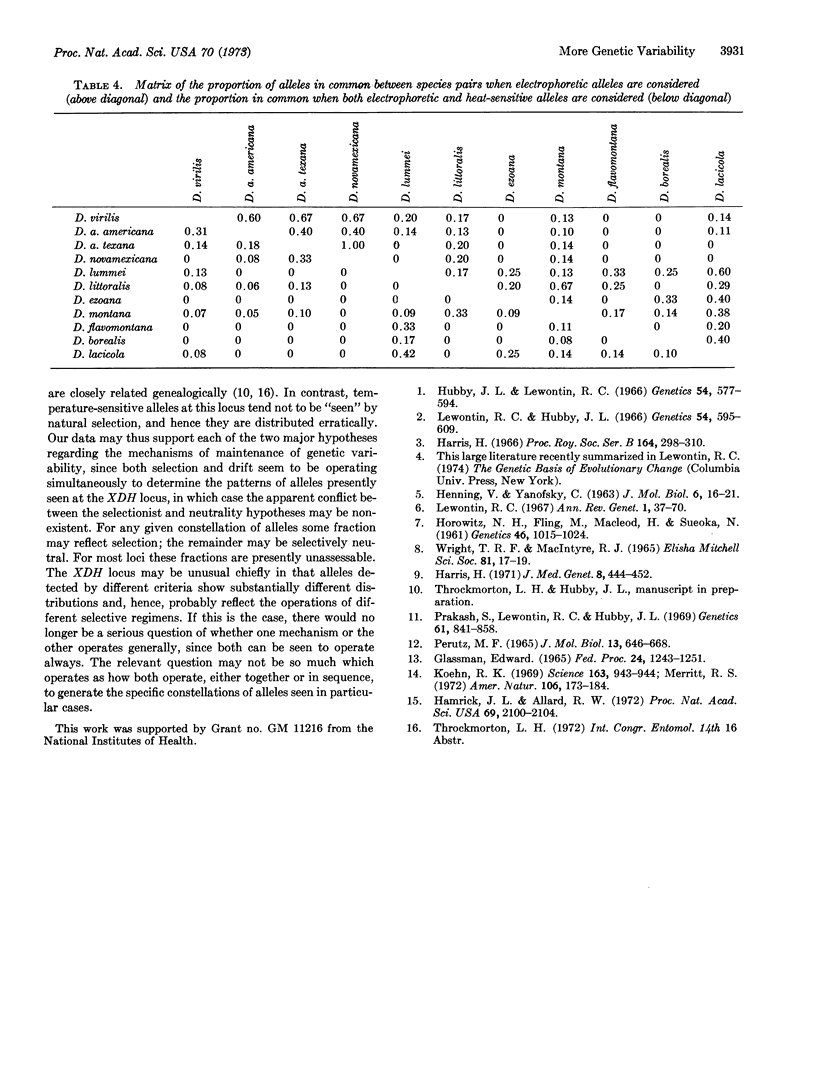
Heat-denaturation studies of xanthine dehydrogenase have revealed many more additional alleles at the locus controlling this enzyme than are revealed by electrophoretic studies. In natural populations of species in the virilis group of the genus Drosophila, heat-denaturation studies of flies from the same locality revealed 1.74 times as many alleles as did electrophoretic studies. Similarly, studies of several species over their geographic range also revealed 1.74 times as many alleles. In addition, for the nine species studied, electrophoretic analysis had revealed only 11 alleles within the group, whereas heat-denaturation studies revealed a total of 32 alleles. These findings are discussed in the light of the continuing controversy over Darwinian and non-Darwinian theories of evolution.
Keywords: heat-sensitive allozymes, gel electrophoresis, neutral and selection hypotheses
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Glassman E. Genetic regulation of xanthine dehydrogenase in Drosophila melanogaster. Fed Proc. 1965 Sep-Oct;24(5):1243–1251. [PubMed] [Google Scholar]
- HENNING U., YANOFSKY C. An electrophoretic study of mutationally altered A proteins of the tryptophan synthetase of Escherichia coli. J Mol Biol. 1963 Jan;6:16–21. doi: 10.1016/s0022-2836(63)80077-7. [DOI] [PubMed] [Google Scholar]
- HOROWITZ N. H., FLING M., MACLEOD H., SUEOKA N. A genetic study of two new structural forms of tyrosinase in Neurospora. Genetics. 1961 Aug;46:1015–1024. doi: 10.1093/genetics/46.8.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrick J. L., Allard R. W. Microgeographical Variation in Allozyme Frequencies in Avena barbata. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2100–2104. doi: 10.1073/pnas.69.8.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris H. Enzyme polymorphisms in man. Proc R Soc Lond B Biol Sci. 1966 Mar 22;164(995):298–310. doi: 10.1098/rspb.1966.0032. [DOI] [PubMed] [Google Scholar]
- Harris H. Polymorphism and protein evolution. The neutral mutation-random drift hypothesis. J Med Genet. 1971 Dec;8(4):444–452. doi: 10.1136/jmg.8.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubby J. L., Lewontin R. C. A molecular approach to the study of genic heterozygosity in natural populations. I. The number of alleles at different loci in Drosophila pseudoobscura. Genetics. 1966 Aug;54(2):577–594. doi: 10.1093/genetics/54.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehn R. K. Esterase heterogeneity: dynamics of a polymorphism. Science. 1969 Feb 28;163(3870):943–944. doi: 10.1126/science.163.3870.943. [DOI] [PubMed] [Google Scholar]
- Lewontin R. C., Hubby J. L. A molecular approach to the study of genic heterozygosity in natural populations. II. Amount of variation and degree of heterozygosity in natural populations of Drosophila pseudoobscura. Genetics. 1966 Aug;54(2):595–609. doi: 10.1093/genetics/54.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S., Lewontin R. C., Hubby J. L. A molecular approach to the study of genic heterozygosity in natural populations. IV. Patterns of genic variation in central, marginal and isolated populations of Drosophila pseudoobscura. Genetics. 1969 Apr;61(4):841–858. doi: 10.1093/genetics/61.4.841. [DOI] [PMC free article] [PubMed] [Google Scholar]


