Abstract
Cytokines are key regulatory mediators involved in the host response to immunological challenges, but also play a critical role in the communication between the immune and the central nervous system. For this, their expression in both systems is under a tight regulatory control. However, pathological conditions may lead to an overproduction of pro-inflammatory cytokines that may have a detrimental impact on central nervous system. In particular, they may damage neuronal structure and function leading to deficits of neuroplasticity, the ability of nervous system to perceive, respond and adapt to external or internal stimuli. In search of the mechanisms by which pro-inflammatory cytokines may affect this crucial brain capability, we will discuss one of the most interesting hypotheses: the involvement of the neurotrophin brain-derived neurotrophic factor (BDNF), which represents one of the major mediators of neuroplasticity.
Keywords: BDNF, neurogenesis, lipopolysaccaride, pro-inflammatory cytokines, depression
Neuroplasticity and brain-derived neurotrophic factor
For many years the medical field held the belief that the brain did not make major changes after a certain point in time. It was fixed or set on a specific path. Today, in contrast, we know that the brain is actually capable of changing and developing throughout a lifetime. It is plastic or malleable, and the term neuroplasticity is used to describe this tendency for the brain to keep developing, changing, and potentially healing itself.
Specifically, neuroplasticity or neuronal plasticity refers to the ability of the nervous system to respond and adapt to environmental challenges and encompasses a series of functional and structural mechanisms that may lead to neuronal remodeling, formation of novel synapses and birth of new neurons. Neuronal plasticity is intimately linked to cellular responsiveness and may therefore be considered an index of the neuronal capability to adapt its function to a different demand. Failure of such mechanisms might enhance the susceptibility to environmental challenges, such as stress, and ultimately lead to psychopathology.
Among the genes responsive to neuronal activity, neurotrophic factors (NTFs), and in particular the neurotrophin family, play an important role. In fact, besides their classical role in supporting neuronal survival, NTFs finely modulate all the crucial steps of network construction, from neuronal migration to experience-dependent refinement of local connections (Poo, 2001). These functions were first reported based on the observation that, during the development of the nervous system, neuron survival depends on the limited amount of specific NTFs secreted by target cells (Huang and Reichardt, 2001). However, it is now well established that NTFs are important mediators of neuronal plasticity also in adulthood where they modulate axonal and dendritic growth and remodeling, membrane receptor trafficking, neurotransmitter release, synapse formation and function (Lu et al., 2005). The neurotrophin brain-derived neurotrophic factor (BDNF) has emerged as crucial mediator of neuronal plasticity, since it is abundant in brain regions particularly relevant for plasticity, but also because it shows a remarkable activity-dependent regulation of expression and secretion (Bramham and Messaoudi, 2005), suggesting that it might indeed bridge experience with enduring change in neuronal function. BDNF has a complex genomic structure, which results into a sophisticated organization in terms of transcriptional, translational and post-translational regulatory mechanisms (Aid et al., 2007). In particular, the rat BDNF gene—that is similar to the human gene—can generate nine distinct transcripts through the alternative splicing of 5’ un-translated exons to a common 3’ exon (IX), which encodes the BDNF protein (Aid et al., 2007). These transcripts have different distribution and/or translation efficacy and, more importantly, may sub-serve different functions. For example, transcripts that are primarily localized or targeted to dendrites may sustain local neurotrophin production, thus providing an effective mechanism to regulate synaptic structure and function (An et al., 2008; Wu et al., 2011). Since the transcription of the different isoforms is regulated by specific signaling pathways (Pruunsild et al., 2011), their investigation may provide useful information on the up-stream mechanisms contributing to the changes of BDNF gene expression.
The mechanisms that lie downstream from NTFs and contribute to the maintenance of neuroplasticity are different i.e., adult neurogenesis and neuronal remodeling, but on the purpose of this mini-review we will focus only on adult neurogenesis, the process by which neurons are generated. Neurogenesis occurs under precise spatial and temporal control, but it can be modulated by both internal and external stimuli. Among these, several sources of data indicate the positive impact of BDNF on adult neurogenesis (Lee et al., 2002; Sairanen et al., 2005; Scharfman et al., 2005; Gass and Riva, 2007; Bergami et al., 2008; Chan et al., 2008; Li et al., 2008; Waterhouse et al., 2012), however in this review we will focus our attention on the effects of pro-inflammatory cytokines.
Neurogenesis and inflammatory state
Neurogenesis has been defined as the process in which newborn neurons are generated from progenitors to functionally integrate in the neuronal network (Ming and Song, 2005; Balu and Lucki, 2009; Aimone et al., 2014). Actually, active neurogenesis take place, in the healthy central nervous system, only in two specific regions: neurons are continuously generated in the sub-ventricular zone (SVZ) and migrate into the olfactory bulb to become interneurons and, in parallel, neurogenesis occurs also in the sub-granular zone (SGZ) of the dentate gyrus of the hippocampus, where new granule neurons are continually generated. Depending on different stimuli, neural stem cells, located in so-called stem cell niches, could divide symmetrically, leading to the generation of two identical cells to maintain the pool of undifferentiated progenitors or, on the other hand, they can divide asymmetrically in order to generate an identical daughter cell and a second cell that starts to differentiate. The de novo formation and integration of new neurons into the existing circuitry is one of the various plastic changes that allow the adult brain to adapt to exogenous stimuli (Amrein et al., 2011). In particular, adult neurogenesis within the hippocampus could contribute to enhanced neural plasticity, a process that is fundamental for specific brain functions such as spatial learning, pattern discrimination, contextual memory and mood regulation (Clelland et al., 2009; Sahay et al., 2011; Denny et al., 2014). The important role of hippocampal neurogenesis is underlined by the fact that this system is altered after various types of negative stimuli such as stress, one of the major risk factors for psychiatric diseases. Specifically, repeated restraint and inescapable foot shock, two examples of physical stressors, inhibit one or more steps of adult neurogenesis in the dentate gyrus (Malberg and Duman, 2003; Pham et al., 2003); the social defeat paradigm leads to an inhibitory effect on cell proliferation and survival of newborn granule neurons in rodents (Czéh et al., 2002; Jun et al., 2012); and social isolation, which is associated with decreased neurogenesis and behavioral alterations in rodents, has been recently proven to be deleterious also for hippocampal neurogenesis and behavior in non human primates (Cinini et al., 2014).
As previously mentioned, neurogenesis is conditioned by a very complex microenvironment constituted by the vascular net, different growth and NTFs, changes in electrical and chemical environment and support by glial cells (Kohman and Rhodes, 2013). In this scenario, neuroinflammation is emerging as one of the main actors. In fact the immune system, through cells within the brain (e.g., microglia) and the detrimental or the beneficial action of signaling molecules (pro-inflammatory or anti-inflammatory cytokines) could participate in the response to different exogenous and endogenous stimuli. The negative effects of neuroinflammation on neurogenesis could lead to impaired survival and proliferation of new neurons. For example, intracortical or intraperitoneal administration of lipopolysaccaride (LPS) from E. coli, an agent able to induce a strong immune response, decreases new neurons survival and the differentiation of new cells into neurons (Ekdahl et al., 2003; Monje et al., 2003). The consequences of inflammation on neurogenesis could have also functional implications for cognition. In fact, the impact of neuroinflammation could affect also the correct integration of newborn neurons into pre-existing circuits, through changes in cellular morphology and in electrophysiological properties (Jakubs et al., 2008) and reduction in recruitment into hippocampal networks encoding spatial information (Belarbi et al., 2012).
The impact of pro-inflammatory cytokines on neurogenesis
Neuroinflammation has an important role in the pathophysiology of different acute or chronic CNS disorders such as cerebral ischemia, multiple sclerosis, Alzheimer’s disease, Parkinson’s disease and major depression (Wang and Jin, 2014). These diseases are characterized by the modulation of different mediators of inflammation and among them pro-inflammatory cytokines seem to play a key role. It is important to note that the same cytokines that in a physiological state are involved in the maintenance of neuronal integrity, may instead have detrimental effects under pathological conditions. Accordingly, the impact of the pro-inflammatory cytokines on neurogenesis depends on their concentration, on the specific cells activated (astrocytes and microglia) and on the presence of other factors secreted in the neurogenic niche (Eyre and Baune, 2012). The increase of pro-inflammatory cytokines is not only due to a direct inflammatory stimulus (infection or trauma), but it could be caused by environmental stimuli such as stress (García-Bueno et al., 2008). The main consequence of a dysregulation of cytokine levels within the brain is the production of inflammatory, oxidative and nitrosative molecules that could affect neurogenesis and the neural homeostasis (Kubera et al., 2011; Stepanichev et al., 2014).
The most common pro-inflammatory cytokines are IL-1β, IL-6, TNF-α and IFN-γ and here we will present some examples of the involvement of these molecules in the modulation of neurogenesis.
The main actions of IL-1β are the stimulation of immune cells to produce pro-inflammatory cytokines, the activation of microglia, and the regulation of growth factors activity (Audet and Anisman, 2013). Recently, IL-1β has been proven to influence hippocampal cytogenesis and neurogenesis in different ways: by direct interaction to its receptor (IL-1R1) and the consequent activation of the nuclear factor-kappa B (NFkB; Koo and Duman, 2008) or through the promotion of glucocorticoids secretion after the exposure to environmental stressors (Goshen et al., 2008). Moreover this cytokine has been proposed as the central mediator of antineurogenic effect of stress (Ben Menachem-Zidon et al., 2008). In fact the blockade of IL-1β signaling, using knockout mice for its receptor or administrating a IL-1R1 antagonist (IL-1Ra), prevents the decrease in neurogenesis observed after acute stressors such as footshock and immobilization in rats (Koo and Duman, 2008). Another relevant cytokine is IL-6 that is involved in a multitude of neuroprotective functions. In physiological conditions IL-6 is able to activate pathways related to neural plasticity, neurogenesis, Long Term Potentiation, and memory (Eyre and Baune, 2012). On the other hand, this cytokine is also responsible of mediating synthesis of acute phase proteins, growth and differentiation of immune cells and regulation of pro-inflammatory factors (Audet and Anisman, 2013). Monje et al. demonstrated that the incubation of hippocampal progenitor cells with recombinant IL-6 decreases neurogenesis by half and reduces neuronal differentiation in favor of astrocytogenesis (Monje et al., 2003; Taga and Fukuda, 2005), an effect mediated by the activation of the JAK/STAT3 pathway via gp130 (Namihira and Nakashima, 2013). Tumor necrosis factor—alpha (TNF-α) is a potent inductor of inflammation and has been linked to decreased neural stem cell proliferation, decreased neurogenesis, neurodegenerative processes, apoptosis and excitotoxicity (Dantzer et al., 2008; Belarbi et al., 2012), but also to the modulation of synaptic strength and synaptic preservation through the increase of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (Khairova et al., 2009). The negative action of TNF-α on neurogenesis is mediated by the activation of its receptor TNF-R1, conversely the interaction with TNF-R2 increases proliferation and survival of newborn neurons, as demonstrated by using transgenic animals with deletion of TNF-R1 or TNF-R2 (Iosif et al., 2006). A similar result on the beneficial role of TNF-R2 activation after irradiation injury has been recently reported (Chen and Palmer, 2013). Moreover, the up-regulation of TNF-α observed in the hippocampus of adult rats pre-exposed to maternal deprivation has been associated with impaired memory consolidation (Pinheiro et al., 2014). IFN-γ is a pro-inflammatory cytokine with in vitro anti-neurogenic effect able to reduce the number of neural stem cells. The negative action of IFN-γ on neurogenesis may be exerted by the activation of the caspase 3/7, the upregulation of sonic hedgehog (SHH) pathway and promotion of an abnormal marker profile of neural stem cells, expressing both GFAP and βIII tubulin (Walter et al., 2011). Nevertheless, IFN-γ may also exerts positive action on neurogenesis. For example, it enhances neurogenesis in dentate gyrus of adult mice and ameliorates spatial learning and memory performance (Baron et al., 2008). These observations suggest that IFN-γ has different effects depending on tissues involved and on the neurogenic process involved.
Taken together, all these studies indicate that a dysregulation of pro-inflammatory cytokines may have a detrimental effect on neurogenesis and point out the importance of neuroinflammation in the microenvironment around neural stem cell development. On this context, the identification and characterization of the mechanisms by which pro-inflammatory cytokines affect neurogenesis are crucial to develop new strategies to maintain the proper function of stem cell niches within the brain.
The impact of pro-inflammatory cytokines on BDNF
Given the role of BDNF as an important mediator of neuroplasticity and on the basis of its positive contribution on neurogenesis in contrast to the detrimental effect of pro-inflammatory cytokines, we may hypothesize that one of the mechanisms by which inflammation may affect brain function could involve BDNF modulation.
Several in vivo studies demonstrated that inflammation clearly affects the expression of BDNF within the brain. In particular, it has been reported that the administration of pro-inflammatory cytokines or of the cytokine-inducer lipopolysaccharide, (LPS; Raetz and Whitfield, 2002) causes a significant reduction of BDNF gene expression. For example, the mRNA levels of BDNF were significantly decreased in the rat hippocampus 4 h after intraperitoneal injection of IL-1β or LPS (Lapchak et al., 1993) and a similar reduction was also observed in several cortical regions and at protein level (Guan and Fang, 2006; Schnydrig et al., 2007). Interestingly, the effect of the systemic inflammatory challenge was not restricted to BDNF: other neurotrophins such as nerve growth factor (NGF) and neurotrophin-3 (NT-3) were similarly reduced although with different magnitude (Guan and Fang, 2006).
Recently, it has also been evaluated the effect of peripheral immune challenge on the different BDNF transcripts, finding that the expression of exons I, II, and IV in the dentate gyrus was reduced in the CA1 and in the dentate gyrus of rats acutely treated with E. coli (Chapman et al., 2012), indicating that inflammation may affect specific isoforms of the neurotrophin. Nevertheless, there is a critical lack of information about the effects of inflammation on the expression of specific BDNF transcripts and further studies are demanded in order to clarify the mechanisms involved in the modulation of the neurotrophin by the immune/inflammatory system.
The negative impact of inflammation on BDNF has important implications for a number of pathological conditions. For example, it is known that pro-inflammatory cytokines compromise hippocampus-dependent memory (Pugh et al., 1998), spatial memory (Arai et al., 2001) and increase apoptosis in the brain (Nolan et al., 2003), features that are involved in many aging-associated pathologies and neurodegenerative diseases. In addition, it is well-know that the activation of the immune/inflammatory system may contribute to the development of different psychiatric diseases such as schizophrenia and major depression (Dantzer et al., 2008; Miller et al., 2009; Leonard and Maes, 2012; Zunszain et al., 2013). Regarding depression, there are three main supportive evidences: first, depressed subjects exhibit increased levels of inflammatory markers both in the periphery and in brain (Howren et al., 2009; Dowlati et al., 2010); second: several pathologies associated with moderate inflammatory grade present high depression comorbidity (Benton et al., 2007); third: a high percentage of patients with cancer or hepatitis C treated with interferon-alpha develop major depression (Valentine and Meyers, 2005; Udina et al., 2012). In addition, it has to be noted that animals exposed to immune challenges display depressive-like behaviors (Yirmiya, 1996; Frenois et al., 2007) that can be normalized, or at least limited, by antidepressant treatment (Yirmiya et al., 2001). In contrast, mice that lack IL-6 are stress resistant and have a reduced disposition for depressive-like behaviors (Chourbaji et al., 2006). The mechanism underlying the anti-inflammatory properties of antidepressant is still unknown and is beyond the aim of our mini-review. However, the results of several in vitro and in vivo studies indicate that these drugs are able to modulate cytokine functioning through their effects on intracellular cyclic adenosyl monophosphate, serotonin metabolism, the hypothalamo-pituitary-adrenocortical axis (Janssen et al., 2010; Walker, 2013; Leonard, 2014).
It is important to consider that the immune/inflammatory alterations previously described are actually in parallel with changes on BDNF expression and function (Figure 1). Indeed, BDNF has a well recognized role in the etiology as well as in the treatment response of patients affected by different psychiatric disorders including major depression (Pezet and Malcangio, 2004; Duman and Monteggia, 2006). For example, decreased expression of the neurotrophin has been found in the hippocampus and prefrontal cortex of postmortem brains from depressed and suicide victims (Dwivedi et al., 2003). Moreover, BDNF mRNA levels are reduced in the brain of genetic animal models of depression (Ridder et al., 2005; Calabrese et al., 2010; Molteni et al., 2010a,c) as well as in animal models based on the environmental component of the disease (Duman and Monteggia, 2006; Tsankova et al., 2006; Chourbaji et al., 2012).
Figure 1.
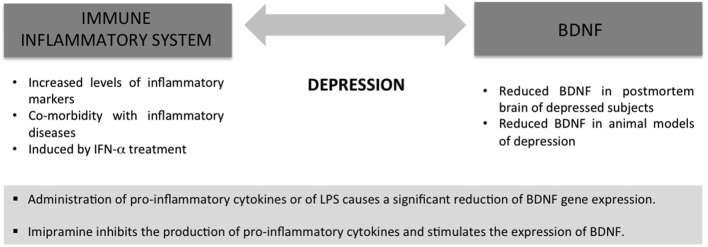
Immune/inflammatory alterations and changes in BDNF expression and function are characteristic of the same pathologies, for example major depression, suggesting a cross-talk between the two systems.
All these findings support the possibility that inflammation contributes to the development of depression by compromising neuroplasticity via reduction of BDNF. In agreement with this line of thinking, it has been recently reported that intranigral LPS infusion induced an anxious and depressive phenotype in the rat that was associated with decreased hippocampal expression of BDNF (Hritcu and Gorgan, 2014).
In order to have a unequivocal proof for causality, inflammation-dependent decrease of BDNF should be normalized or at least attenuated by antidepressant treatment, as occurs in experimental models where BDNF expression is up-regulated in response to prolonged treatment with different antidepressant drugs (Schmidt and Duman, 2007; Calabrese et al., 2010; Molteni et al., 2010b; Park et al., 2011).
Although there are only few data on this issue, it has been demonstrated that the incubation of rat neural stem cells with the antidepressant imipramine inhibits the production of pro-inflammatory cytokines, whereas stimulates the expression of BDNF (Peng et al., 2008), nevertheless, further studies are demanded to clarify this issue in order to provide unequivocal proof for causality.
Concluding remarks
In conclusion, we attempted to provide evidence on the possibility that one of the mechanisms underlying the negative impact of pro-inflammatory cytokines on neuroplasticity is the reduction of BDNF expression and function (Figure 2).
Figure 2.
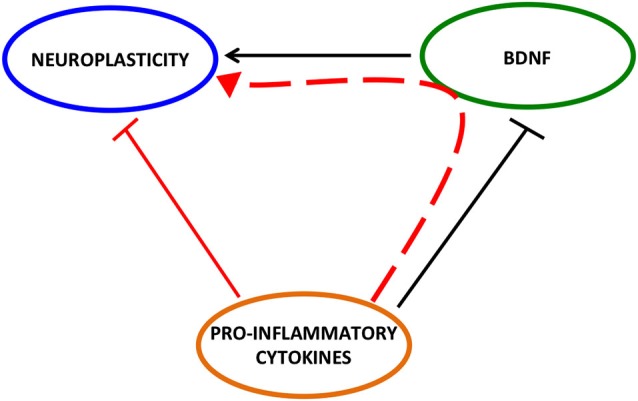
Detrimental effect of pro-inflammatory cytokines on neuroplasticity may be mediated by BDNF.
Although several data support this hypothesis, further studies are demanded to better clarify how it occurs. A number of result points out a key role for the pro-inflammatory cytokine IL-1β as it has been shown that the inhibitory effect of stress paradigms on cerebral BDNF expression may be attenuated by intracerebroventricular injection of IL-1 receptor antagonist (Barrientos et al., 2003). However, how this -or others- pro-inflammatory cytokine affects the neurotrophin is still not well understood. Since in vitro and in vivo studies indicate that glucocorticoids decrease the neurotrophin (Hansson et al., 2003; Gubba et al., 2004; Hansson and Fuxe, 2008), one possibility is the involvement of the Hypotalamus-Pituitary-Axis (HPA), which is strongly stimulated by pro-inflammatory cytokines (Rivest, 2010). However, we have to be aware that pro-inflammatory cytokines act on a plethora of different targets, for example the neurotransmitters glutamate (Viviani et al., 2007; Di Filippo et al., 2013) and GABA (Galic et al., 2012), both able to modulate BDNF. In this context, it is feasible that the effect of the immune/inflammatory system on BDNF results from the integration of multiple mechanisms. A better knowledge of these events may be useful to develop new therapeutic strategies aimed to normalize, or at least ameliorate, the pathological consequences of the negative impact of inflammation on brain structure and function.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Aid T., Kazantseva A., Piirsoo M., Palm K., Timmusk T. (2007). Mouse and rat BDNF gene structure and expression revisited. J. Neurosci. Res. 85, 525–535. 10.1002/jnr.21139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone J. B., Li Y., Lee S. W., Clemenson G. D., Deng W., Gage F. H. (2014). Regulation and function of adult neurogenesis: from genes to cognition. Physiol. Rev. 94, 991–1026. 10.1152/physrev.00004.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrein I., Isler K., Lipp H. P. (2011). Comparing adult hippocampal neurogenesis in mammalian species and orders: influence of chronological age and life history stage. Eur. J. Neurosci. 34, 978–987. 10.1111/j.1460-9568.2011.07804.x [DOI] [PubMed] [Google Scholar]
- An J. J., Gharami K., Liao G. Y., Woo N. H., Lau A. G., Vanevski F., et al. (2008). Distinct role of long 3′ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell 134, 175–187. 10.1016/j.cell.2008.05.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K., Matsuki N., Ikegaya Y., Nishiyama N. (2001). Deterioration of spatial learning performances in lipopolysaccharide-treated mice. Jpn. J. Pharmacol. 87, 195–201. 10.1254/jjp.87.195 [DOI] [PubMed] [Google Scholar]
- Audet M. C., Anisman H. (2013). Interplay between pro-inflammatory cytokines and growth factors in depressive illnesses. Front. Cell. Neurosci. 7:68. 10.3389/fncel.2013.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu D. T., Lucki I. (2009). Adult hippocampal neurogenesis: regulation, functional implications and contribution to disease pathology. Neurosci. Biobehav. Rev. 33, 232–252. 10.1016/j.neubiorev.2008.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R., Nemirovsky A., Harpaz I., Cohen H., Owens T., Monsonego A. (2008). IFN-gamma enhances neurogenesis in wild-type mice and in a mouse model of Alzheimer’s disease. FASEB J. 22, 2843–2852. 10.1096/fj.08-105866 [DOI] [PubMed] [Google Scholar]
- Barrientos R. M., Sprunger D. B., Campeau S., Higgins E. A., Watkins L. R., Rudy J. W., et al. (2003). Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience 121, 847–853. 10.1016/s0306-4522(03)00564-5 [DOI] [PubMed] [Google Scholar]
- Belarbi K., Arellano C., Ferguson R., Jopson T., Rosi S. (2012). Chronic neuroinflammation impacts the recruitment of adult-born neurons into behaviorally relevant hippocampal networks. Brain Behav. Immun. 26, 18–23. 10.1016/j.bbi.2011.07.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Menachem-Zidon O., Goshen I., Kreisel T., Ben Menahem Y., Reinhartz E., Ben Hur T., et al. (2008). Intrahippocampal transplantation of transgenic neural precursor cells overexpressing interleukin-1 receptor antagonist blocks chronic isolation-induced impairment in memory and neurogenesis. Neuropsychopharmacology 33, 2251–2262. 10.1038/sj.npp.1301606 [DOI] [PubMed] [Google Scholar]
- Benton T., Staab J., Evans D. L. (2007). Medical co-morbidity in depressive disorders. Ann. Clin. Psychiatry 19, 289–303. 10.1080/10401230701653542 [DOI] [PubMed] [Google Scholar]
- Bergami M., Rimondini R., Santi S., Blum R., Götz M., Canossa M. (2008). Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc. Natl. Acad. Sci. U S A 105, 15570–15575. 10.1073/pnas.0803702105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham C. R., Messaoudi E. (2005). BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog. Neurobiol. 76, 99–125. 10.1016/j.pneurobio.2005.06.003 [DOI] [PubMed] [Google Scholar]
- Calabrese F., Molteni R., Cattaneo A., Macchi F., Racagni G., Gennarelli M., et al. (2010). Long-Term duloxetine treatment normalizes altered brain-derived neurotrophic factor expression in serotonin transporter knockout rats through the modulation of specific neurotrophin isoforms. Mol. Pharmacol. 77, 846–853. 10.1124/mol.109.063081 [DOI] [PubMed] [Google Scholar]
- Chan J. P., Cordeira J., Calderon G. A., Iyer L. K., Rios M. (2008). Depletion of central BDNF in mice impedes terminal differentiation of new granule neurons in the adult hippocampus. Mol. Cell. Neurosci 39, 372–383. 10.1016/j.mcn.2008.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman T. R., Barrientos R. M., Ahrendsen J. T., Hoover J. M., Maier S. F., Patterson S. L. (2012). Aging and infection reduce expression of specific brain-derived neurotrophic factor mRNAs in hippocampus. Neurobiol. Aging 33, 832.e1–832.e14. 10.1016/j.neurobiolaging.2011.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Palmer T. D. (2013). Differential roles of TNFR1 and TNFR2 signaling in adult hippocampal neurogenesis. Brain Behav. Immun. 30, 45–53. 10.1016/j.bbi.2013.01.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourbaji S., Hörtnagl H., Molteni R., Riva M. A., Gass P., Hellweg R. (2012). The impact of environmental enrichment on sex-specific neurochemical circuitries—effects on brain-derived neurotrophic factor and the serotonergic system. Neuroscience 220, 267–276. 10.1016/j.neuroscience.2012.06.016 [DOI] [PubMed] [Google Scholar]
- Chourbaji S., Urani A., Inta I., Sanchis-Segura C., Brandwein C., Zink M., et al. (2006). IL-6 knockout mice exhibit resistance to stress-induced development of depression-like behaviors. Neurobiol. Dis. 23, 587–594. 10.1016/j.nbd.2006.05.001 [DOI] [PubMed] [Google Scholar]
- Cinini S. M., Barnabe G. F., Galvão-Coelho N., de Medeiros M. A., Perez-Mendes P., Sousa M. B., et al. (2014). Social isolation disrupts hippocampal neurogenesis in young non-human primates. Front. Neurosci. 8:45. 10.3389/fnins.2014.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland C. D., Choi M., Romberg C., Clemenson G. D., Jr., Fragniere A., Tyers P., et al. (2009). A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–213. 10.1126/science.1173215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czéh B., Welt T., Fischer A. K., Erhardt A., Schmitt W., Müller M. B., et al. (2002). Chronic psychosocial stress and concomitant repetitive transcranial magnetic stimulation: effects on stress hormone levels and adult hippocampal neurogenesis. Biol. Psychiatry 52, 1057–1065. 10.1016/s0006-3223(02)01457-9 [DOI] [PubMed] [Google Scholar]
- Dantzer R., O’connor J. C., Freund G. G., Johnson R. W., Kelley K. W. (2008). From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46–56. 10.1038/nrn2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny C. A., Kheirbek M. A., Alba E. L., Tanaka K. F., Brachman R. A., Laughman K. B., et al. (2014). Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron 83, 189–201. 10.1016/j.neuron.2014.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Filippo M., Chiasserini D., Gardoni F., Viviani B., Tozzi A., Giampà C., et al. (2013). Effects of central and peripheral inflammation on hippocampal synaptic plasticity. Neurobiol. Dis. 52, 229–236. 10.1016/j.nbd.2012.12.009 [DOI] [PubMed] [Google Scholar]
- Dowlati Y., Herrmann N., Swardfager W., Liu H., Sham L., Reim E. K., et al. (2010). A meta-analysis of cytokines in major depression. Biol. Psychiatry 67, 446–457. 10.1016/j.biopsych.2009.09.033 [DOI] [PubMed] [Google Scholar]
- Duman R. S., Monteggia L. M. (2006). A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 59, 1116–1127. 10.1016/j.biopsych.2006.02.013 [DOI] [PubMed] [Google Scholar]
- Dwivedi Y., Rizavi H. S., Conley R. R., Roberts R. C., Tamminga C. A., Pandey G. N. (2003). Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch. Gen. Psychiatry 60, 804–815. 10.1001/archpsyc.60.8.804 [DOI] [PubMed] [Google Scholar]
- Ekdahl C. T., Claasen J. H., Bonde S., Kokaia Z., Lindvall O. (2003). Inflammation is detrimental for neurogenesis in adult brain. Proc. Natl. Acad. Sci. U S A 100, 13632–13637. 10.1073/pnas.2234031100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre H., Baune B. T. (2012). Neuroplastic changes in depression: a role for the immune system. Psychoneuroendocrinology 37, 1397–1416. 10.1016/j.psyneuen.2012.03.019 [DOI] [PubMed] [Google Scholar]
- Frenois F., Moreau M., O’connor J., Lawson M., Micon C., Lestage J., et al. (2007). Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology 32, 516–531. 10.1016/j.psyneuen.2007.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galic M. A., Riazi K., Pittman Q. J. (2012). Cytokines and brain excitability. Front. Neuroendocrinol. 33, 116–125. 10.1016/j.yfrne.2011.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Bueno B., Caso J. R., Leza J. C. (2008). Stress as a neuroinflammatory condition in brain: damaging and protective mechanisms. Neurosci. Biobehav. Rev. 32, 1136–1151. 10.1016/j.neubiorev.2008.04.001 [DOI] [PubMed] [Google Scholar]
- Gass P., Riva M. A. (2007). CREB, neurogenesis and depression. Bioessays 29, 957–961. 10.1002/bies.20658 [DOI] [PubMed] [Google Scholar]
- Goshen I., Kreisel T., Ben-Menachem-Zidon O., Licht T., Weidenfeld J., Ben-Hur T., et al. (2008). Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol. Psychiatry 13, 717–728. 10.1038/sj.mp.4002055 [DOI] [PubMed] [Google Scholar]
- Guan Z., Fang J. (2006). Peripheral immune activation by lipopolysaccharide decreases neurotrophins in the cortex and hippocampus in rats. Brain Behav. Immun. 20, 64–71. 10.1016/j.bbi.2005.04.005 [DOI] [PubMed] [Google Scholar]
- Gubba E. M., Fawcett J. W., Herbert J. (2004). The effects of corticosterone and dehydroepiandrosterone on neurotrophic factor mRNA expression in primary hippocampal and astrocyte cultures. Brain Res. Mol. Brain Res. 127, 48–59. 10.1016/j.molbrainres.2004.05.004 [DOI] [PubMed] [Google Scholar]
- Hansson A. C., Fuxe K. (2008). Time-course of immediate early gene expression in hippocampal subregions of adrenalectomized rats after acute corticosterone challenge. Brain Res. 1215, 1–10. 10.1016/j.brainres.2008.03.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson A. C., Sommer W., Rimondini R., Andbjer B., Strömberg I., Fuxe K. (2003). c-fos reduces corticosterone-mediated effects on neurotrophic factor expression in the rat hippocampal CA1 region. J. Neurosci. 23, 6013–6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren M. B., Lamkin D. M., Suls J. (2009). Associations of depression with C-reactive protein, IL-1 and IL-6: a meta-analysis. Psychosom. Med. 71, 171–186. 10.1097/psy.0b013e3181907c1b [DOI] [PubMed] [Google Scholar]
- Hritcu L., Gorgan L. D. (2014). Intranigral lipopolysaccharide induced anxiety and depression by altered BDNF mRNA expression in rat hippocampus. Prog. Neuropsychopharmacol. Biol. Psychiatry 51, 126–132. 10.1016/j.pnpbp.2014.01.016 [DOI] [PubMed] [Google Scholar]
- Huang E. J., Reichardt L. F. (2001). Neurotrophins: roles in neuronal development and function. Annu. Rev. Neurosci. 24, 677–736. 10.1146/annurev.neuro.24.1.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iosif R. E., Ekdahl C. T., Ahlenius H., Pronk C. J., Bonde S., Kokaia Z., et al. (2006). Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J. Neurosci. 26, 9703–9712. 10.1523/jneurosci.2723-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubs K., Bonde S., Iosif R. E., Ekdahl C. T., Kokaia Z., Kokaia M., et al. (2008). Inflammation regulates functional integration of neurons born in adult brain. J. Neurosci. 28, 12477–12488. 10.1523/jneurosci.3240-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen D. G., Caniato R. N., Verster J. C., Baune B. T. (2010). A psychoneuroimmunological review on cytokines involved in antidepressant treatment response. Hum. Psychopharmacol. 25, 201–215. 10.1002/hup.1103 [DOI] [PubMed] [Google Scholar]
- Jun H., Mohammed Qbsim Hussaini S., Rigby M. J., Jang M. H. (2012). Functional role of adult hippocampal neurogenesis as a therapeutic strategy for mental disorders. Neural Plast. 2012:854285. 10.1155/2012/854285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khairova R. A., Machado-Vieira R., Du J., Manji H. K. (2009). A potential role for pro-inflammatory cytokines in regulating synaptic plasticity in major depressive disorder. Int. J. Neuropsychopharmacol. 12, 561–578. 10.1017/s1461145709009924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman R. A., Rhodes J. S. (2013). Neurogenesis, inflammation and behavior. Brain Behav. Immun. 27, 22–32. 10.1016/j.bbi.2012.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo J. W., Duman R. S. (2008). IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc. Natl. Acad. Sci. U S A 105, 751–756. 10.1073/pnas.0708092105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubera M., Obuchowicz E., Goehler L., Brzeszcz J., Maes M. (2011). In animal models, psychosocial stress-induced (neuro)inflammation, apoptosis and reduced neurogenesis are associated to the onset of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 744–759. 10.1016/j.pnpbp.2010.08.026 [DOI] [PubMed] [Google Scholar]
- Lapchak P. A., Araujo D. M., Hefti F. (1993). Systemic interleukin-1 beta decreases brain-derived neurotrophic factor messenger RNA expression in the rat hippocampal formation. Neuroscience 53, 297–301. 10.1016/0306-4522(93)90196-m [DOI] [PubMed] [Google Scholar]
- Lee J., Duan W., Mattson M. P. (2002). Evidence that brain-derived neurotrophic factor is required for basal neurogenesis and mediates, in part, the enhancement of neurogenesis by dietary restriction in the hippocampus of adult mice. J. Neurochem. 82, 1367–1375. 10.1046/j.1471-4159.2002.01085.x [DOI] [PubMed] [Google Scholar]
- Leonard B. E. (2014). Impact of inflammation on neurotransmitter changes in major depression: an insight into the action of antidepressants. Prog. Neuropsychopharmacol. Biol. Psychiatry 48, 261–267. 10.1016/j.pnpbp.2013.10.018 [DOI] [PubMed] [Google Scholar]
- Leonard B., Maes M. (2012). Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci. Biobehav. Rev. 36, 764–785. 10.1016/j.neubiorev.2011.12.005 [DOI] [PubMed] [Google Scholar]
- Li Y., Luikart B. W., Birnbaum S., Chen J., Kwon C. H., Kernie S. G., et al. (2008). TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron 59, 399–412. 10.1016/j.neuron.2008.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B., Pang P. T., Woo N. H. (2005). The yin and yang of neurotrophin action. Nat. Rev. Neurosci. 6, 603–614. 10.1038/nrn1726 [DOI] [PubMed] [Google Scholar]
- Malberg J. E., Duman R. S. (2003). Cell proliferation in adult hippocampus is decreased by inescapable stress: reversal by fluoxetine treatment. Neuropsychopharmacology 28, 1562–1571. 10.1038/sj.npp.1300234 [DOI] [PubMed] [Google Scholar]
- Miller A. H., Maletic V., Raison C. L. (2009). Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 65, 732–741. 10.1016/j.biopsych.2008.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming G. L., Song H. (2005). Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 28, 223–250. 10.1146/annurev.neuro.28.051804.101459 [DOI] [PubMed] [Google Scholar]
- Molteni R., Calabrese F., Chourbaji S., Brandwein C., Racagni G., Gass P., et al. (2010a). Depression-prone mice with reduced glucocorticoid receptor expression display an altered stress-dependent regulation of brain-derived neurotrophic factor and activity-regulated cytoskeleton-associated protein. J. Psychopharmacol. 24, 595–603. 10.1177/0269881108099815 [DOI] [PubMed] [Google Scholar]
- Molteni R., Calabrese F., Pisoni S., Gabriel C., Mocaer E., Racagni G., et al. (2010b). Synergistic mechanisms in the modulation of the neurotrophin BDNF in the rat prefrontal cortex following acute agomelatine administration. World J. Biol. Psychiatry 11, 148–153. 10.3109/15622970903447659 [DOI] [PubMed] [Google Scholar]
- Molteni R., Cattaneo A., Calabrese F., Macchi F., Olivier J. D., Racagni G., et al. (2010c). Reduced function of the serotonin transporter is associated with decreased expression of BDNF in rodents as well as in humans. Neurobiol. Dis. 37, 747–755. 10.1016/j.nbd.2009.12.014 [DOI] [PubMed] [Google Scholar]
- Monje M. L., Toda H., Palmer T. D. (2003). Inflammatory blockade restores adult hippocampal neurogenesis. Science 302, 1760–1765. 10.1126/science.1088417 [DOI] [PubMed] [Google Scholar]
- Namihira M., Nakashima K. (2013). Mechanisms of astrocytogenesis in the mammalian brain. Curr. Opin. Neurobiol. 23, 921–927. 10.1016/j.conb.2013.06.002 [DOI] [PubMed] [Google Scholar]
- Nolan Y., Vereker E., Lynch A. M., Lynch M. A. (2003). Evidence that lipopolysaccharide-induced cell death is mediated by accumulation of reactive oxygen species and activation of p38 in rat cortex and hippocampus. Exp. Neurol. 184, 794–804. 10.1016/s0014-4886(03)00301-7 [DOI] [PubMed] [Google Scholar]
- Park S. W., Phuong V. T., Lee C. H., Lee J. G., Seo M. K., Cho H. Y., et al. (2011). Effects of antipsychotic drugs on BDNF, GSK-3beta and beta-catenin expression in rats subjected to immobilization stress. Neurosci. Res. 71, 335–340. 10.1016/j.neures.2011.08.010 [DOI] [PubMed] [Google Scholar]
- Peng C. H., Chiou S. H., Chen S. J., Chou Y. C., Ku H. H., Cheng C. K., et al. (2008). Neuroprotection by Imipramine against lipopolysaccharide-induced apoptosis in hippocampus-derived neural stem cells mediated by activation of BDNF and the MAPK pathway. Eur. Neuropsychopharmacol. 18, 128–140. 10.1016/j.euroneuro.2007.05.002 [DOI] [PubMed] [Google Scholar]
- Pezet S., Malcangio M. (2004). Brain-derived neurotrophic factor as a drug target for CNS disorders. Expert Opin. Ther. Targets 8, 391–399. 10.1517/14728222.8.5.391 [DOI] [PubMed] [Google Scholar]
- Pham K., Nacher J., Hof P. R., Mcewen B. S. (2003). Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur. J. Neurosci. 17, 879–886. 10.1046/j.1460-9568.2003.02513.x [DOI] [PubMed] [Google Scholar]
- Pinheiro R. M., de Lima M. N., Portal B. C., Busato S. B., Falavigna L., Ferreira R. D., et al. (2014). Long-lasting recognition memory impairment and alterations in brain levels of cytokines and BDNF induced by maternal deprivation: effects of valproic acid and topiramate. J. Neural Transm. [Epub ahead of print]. 10.1007/s00702-014-1303-2 [DOI] [PubMed] [Google Scholar]
- Poo M. M. (2001). Neurotrophins as synaptic modulators. Nat. Rev. Neurosci. 2, 24–32. 10.1038/35049004 [DOI] [PubMed] [Google Scholar]
- Pruunsild P., Sepp M., Orav E., Koppel I., Timmusk T. (2011). Identification of cis-elements and transcription factors regulating neuronal activity-dependent transcription of human BDNF gene. J. Neurosci. 31, 3295–3308. 10.1523/jneurosci.4540-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh C. R., Kumagawa K., Fleshner M., Watkins L. R., Maier S. F., Rudy J. W. (1998). Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav. Immun. 12, 212–229. 10.1006/brbi.1998.0524 [DOI] [PubMed] [Google Scholar]
- Raetz C. R., Whitfield C. (2002). Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71, 635–700. 10.1146/annurev.biochem.71.110601.135414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridder S., Chourbaji S., Hellweg R., Urani A., Zacher C., Schmid W., et al. (2005). Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. J. Neurosci. 25, 6243–6250. 10.1523/jneurosci.0736-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S. (2010). Interactions between the immune and neuroendocrine systems. Prog. Brain Res. 181, 43–53. 10.1016/S0079-6123(08)81004-7 [DOI] [PubMed] [Google Scholar]
- Sahay A., Scobie K. N., Hill A. S., O’carroll C. M., Kheirbek M. A., Burghardt N. S., et al. (2011). Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472, 466–470. 10.1038/nature09817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sairanen M., Lucas G., Ernfors P., Castrén M., Castrén E. (2005). Brain-derived neurotrophic factor and antidepressant drugs have different but coordinated effects on neuronal turnover, proliferation and survival in the adult dentate gyrus. J. Neurosci. 25, 1089–1094. 10.1523/jneurosci.3741-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman H., Goodman J., Macleod A., Phani S., Antonelli C., Croll S. (2005). Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp. Neurol. 192, 348–356. 10.1016/j.expneurol.2004.11.016 [DOI] [PubMed] [Google Scholar]
- Schmidt H. D., Duman R. S. (2007). The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav. Pharmacol. 18, 391–418. 10.1097/fbp.0b013e3282ee2aa8 [DOI] [PubMed] [Google Scholar]
- Schnydrig S., Korner L., Landweer S., Ernst B., Walker G., Otten U., et al. (2007). Peripheral lipopolysaccharide administration transiently affects expression of brain-derived neurotrophic factor, corticotropin and proopiomelanocortin in mouse brain. Neurosci. Lett. 429, 69–73. 10.1016/j.neulet.2007.09.067 [DOI] [PubMed] [Google Scholar]
- Stepanichev M., Dygalo N. N., Grigoryan G., Shishkina G. T., Gulyaeva N. (2014). Rodent models of depression: neurotrophic and neuroinflammatory biomarkers. Biomed Res. Int. 2014:932757. 10.1155/2014/932757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga T., Fukuda S. (2005). Role of IL-6 in the neural stem cell differentiation. Clin. Rev. Allergy Immunol. 28, 249–256. 10.1385/criai:28:3:249 [DOI] [PubMed] [Google Scholar]
- Tsankova N. M., Berton O., Renthal W., Kumar A., Neve R. L., Nestler E. J. (2006). Sustained hippocampal chromatin regulation in a mouse model of depression and antidepressant action. Nat. Neurosci. 9, 519–525. 10.1038/nn1659 [DOI] [PubMed] [Google Scholar]
- Udina M., Castellví P., Moreno-España J., Navinés R., Valdés M., Forns X., et al. (2012). Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. J. Clin. Psychiatry 73, 1128–1138. 10.4088/jcp.12r07694 [DOI] [PubMed] [Google Scholar]
- Valentine A. D., Meyers C. A. (2005). Neurobehavioral effects of interferon therapy. Curr. Psychiatry Rep. 7, 391–395. 10.1007/s11920-005-0042-3 [DOI] [PubMed] [Google Scholar]
- Viviani B., Gardoni F., Marinovich M. (2007). Cytokines and neuronal ion channels in health and disease. Int. Rev. Neurobiol. 82, 247–263. 10.1016/s0074-7742(07)82013-7 [DOI] [PubMed] [Google Scholar]
- Walker F. R. (2013). A critical review of the mechanism of action for the selective serotonin reuptake inhibitors: do these drugs possess anti-inflammatory properties and how relevant is this in the treatment of depression? Neuropharmacology 67, 304–317. 10.1016/j.neuropharm.2012.10.002 [DOI] [PubMed] [Google Scholar]
- Walter J., Honsek S. D., Illes S., Wellen J. M., Hartung H. P., Rose C. R., et al. (2011). A new role for interferon gamma in neural stem/precursor cell dysregulation. Mol. Neurodegener. 6:18. 10.1186/1750-1326-6-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Jin K. (2014). Current perspectives on the link between neuroinflammation and neurogenesis. Metab. Brain Dis. [Epub ahead of print]. 10.1007/s11011-014-9523-6 [DOI] [PubMed] [Google Scholar]
- Waterhouse E. G., An J. J., Orefice L. L., Baydyuk M., Liao G. Y., Zheng K., et al. (2012). BDNF promotes differentiation and maturation of adult-born neurons through GABAergic transmission. J. Neurosci. 32, 14318–14330. 10.1523/jneurosci.0709-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. C., Williamson R., Li Z., Vicario A., Xu J., Kasai M., et al. (2011). Dendritic trafficking of brain-derived neurotrophic factor mRNA: regulation by translin-dependent and -independent mechanisms. J. Neurochem. 116, 1112–1121. 10.1111/j.1471-4159.2010.07166.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R. (1996). Endotoxin produces a depressive-like episode in rats. Brain Res. 711, 163–174. 10.1016/0006-8993(95)01415-2 [DOI] [PubMed] [Google Scholar]
- Yirmiya R., Pollak Y., Barak O., Avitsur R., Ovadia H., Bette M., et al. (2001). Effects of antidepressant drugs on the behavioral and physiological responses to lipopolysaccharide (LPS) in rodents. Neuropsychopharmacology 24, 531–544. 10.1016/s0893-133x(00)00226-8 [DOI] [PubMed] [Google Scholar]
- Zunszain P. A., Hepgul N., Pariante C. M. (2013). Inflammation and depression. Curr. Top. Behav. Neurosci. 14, 135–151. 10.1007/7854_2012_211 [DOI] [PubMed] [Google Scholar]


