Abstract
Purpose
Vision and hearing impairments are known to increase in middle age. In this study we describe the prevalence of vision impairment and dual sensory impairment in UK adults aged 40 to 69 years in a very large and recently ascertained data set. The associations between vision impairment, age, sex, socioeconomic status, and ethnicity are reported.
Methods
This research was conducted using the UK Biobank Resource, with subsets of UK Biobank data analysed with respect to self-report of eye problems and glasses use. Better-eye visual acuity with habitually worn refractive correction was assessed with a logMAR chart (n = 116,682). Better-ear speech reception threshold was measured with an adaptive speech in noise test, the Digit Triplet Test (n = 164,770). Prevalence estimates were weighted with respect to UK 2001 Census data.
Results
Prevalence of mild visual impairment and low vision was estimated at 15.2% (95% CI 14.9–15.5%) and 0.9% (95% CI 0.8–1.0%), respectively. Use of glasses was 88.0% (95% CI 87.9–88.1%). The prevalence of dual sensory impairment was 3.1% (95% CI 3.0–3.2%) and there was a nine-fold increase in the prevalence of dual sensory problems between the youngest and oldest age groups. Older adults, those from low socioeconomic and ethnic minority backgrounds were most at risk for vision problems.
Conclusions
Mild vision impairment is common in middle aged UK adults, despite widespread use of spectacles. Possible barriers to optometric care for those from low socioeconomic and ethnic minority backgrounds may require attention. A higher than expected prevalence of dual impairment suggests that hearing and vision problems share common causes. Optometrists should consider screening for hearing problems, particularly among older adults.
Keywords: vision impairment, dual sensory problems
Introduction
The primary aim of this study was to provide an objective current estimate of prevalence visual impairment and dual-sensory impairment among UK adults aged 40 to 69 years. Secondary aims were to document associated demographics and prevalence of spectacle use. Definitions of visual impairment have been recommended by the International Council of Ophthalmology (Table 1); where such detailed reporting is not possible, the WHO categories are used. “Low vision” is considered to mean that the individual may require access to vision rehabilitation in order to prevent activity limitation resulting from that impairment. Mild visual impairment represents a level of visual acuity (VA) which is beyond the 99% confidence limits of the visual performance of the normal population. (1) Although described as ‘mild’, this level of impairment may still have an adverse impact on visual function and quality of life. (2) One particular task where good vision is essential is driving, where the legal VA limit is 6/12. For this reason some studies consider low vision as <6/12 rather than <6/18. When considering functional ability it is important to measure habitual acuity (presenting acuity), rather than best corrected. (3) Any difference between the habitual and best corrected acuity is due to uncorrected refractive error (i.e. the lack of up-to-date distance spectacles). However, perhaps due to an interest in impairment rather than disability, most population studies tend to assess best-corrected vision. (4) Studies that have assessed both presenting/habitual and best-corrected acuity suggest that there is potential for significant improvement, especially for lower levels of impairment (prevalence of VA<6/12 fell from 2.6% to 0.61% with correction). (5) Two recent studies have suggested an even higher prevalence of habitual mild impairment. (6, 7) Besides an emphasis on best corrected acuity, most previous studies focused on older age groups, because of higher levels of impairment among older adults, and on low vision rather than mild visual impairment, because of its functional significance. (8)
Table 1.
Definitions of visual impairment
| International Council of Ophthalmology | Maximum VA | Minimum VA | WHO | Maximum VA | Minimum VA | Acceptable driving standard (Europe) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Snellen | logMAR | Snellen | LogMAR | Snellen | logMAR | Snellen | logMAR | Snellen | logMAR | |||||||
| Normal | 0.8 | 6/7.5 | 0.1 | |||||||||||||
| Mild vision loss | <0.8 | 6/7.5 | 0.1 | 0.3 | 6/18 | 0.48 | ≥0.5 | 6/12 | 0.3 | |||||||
| Moderate vision loss | <0.3 | 6/18 | 0.48 | 0.125 | 6/48 | 0.9 | Low vision category 1 | <0.3 | 6/18 | 0.48 | 0.1 | 6/60 | 1.0 | |||
| Severe vision loss | <0.125 | 6/48 | 0.9 | 0.05 | 3/60 | 1.3 | Low vision category 2 | <0.1 | 6/60 | 1.0 | 0.05 | 3/60 | 1.3 | |||
| Profound vision loss | <0.05 | 3/60 | 1.3 | 0.02 | 6/300 | 1.7 | Blindness category 3 | <0.05 | 3/60 | 1.3 | 0.0167 | 6/360 | 1.78 | |||
| Near total vision loss (near blindness) | <0.02 | 6/300 | 1.7 | NLP (sic) | Blindness category 4 | <0.0167 | 6/360 | 1.78 | LP | |||||||
| Total vision loss (total blindness) | NLP | Blindness category 5 | NLP | |||||||||||||
In previous studies in the UK, the prevalence of low vision (visual acuity <6/18) in those aged between 65 and 74 years has been estimated at 6%, increasing to 32% in those aged over 85, based on habitual visual acuity. (9) Mild visual impairment (visual acuity <6/12) was estimated at 16% in 65 to 74 year-olds, rising to 54% in those aged over 85 years.
Comparable results were obtained in a study of those aged 75 years and older (10). A summary concluded that visual impairment affects ~10% of those aged 65–75 and ~20% of those aged over 75 years. (11) Estimates of the prevalence of visual impairment in younger age groups are summarised in Table 2, and show a considerably lower prevalence. Two US, one Danish and one Icelandic study show that prevalence of best corrected VA <6/12 rises by a factor of at least 30x between the ages of 40 and 80 years.
Table 2.
Estimates of the prevalence of visual impairment
| Study | Setting | N | Age | VA | Testing conditions | Prevalence |
|---|---|---|---|---|---|---|
| Klein et al, 1991 | Beaver Dam, USA | 4926 | 43–54 | ≤6/12->6/60 | Best corrected better eye | 0.7% |
| 55–64 | 0.7% | |||||
| 65–74 | 4.7% | |||||
| 75+ | 19.1% | |||||
| Gunnlaugsdottir et al 2008 | Reykjavik, Iceland | 1045 | >50–80+ | <6/18 | Best corrected, better eye | 0.96% |
| <6/12 | 2.01% | |||||
| 50–59 | <6/18 | 0% | ||||
| <6/12 | 0.28% | |||||
| >80 | <6/18 | 7.9% | ||||
| <6/12 | 11.80% | |||||
| Buch et al 2004 | Copenhagen, Denmark | 9980 | 20–39 | <6/12->6/60 | Best corrected, better eye | 0.13% |
| 20–64 | 0.25% | |||||
| 65–84 | 2.24% | |||||
| 80–84 | 8.29% | |||||
| Tielsch et al1990 | Baltimore, US | 2490 | 40–59 | <6/18–0.5/60 | Best corrected, better eye | 0.17% Caucasians |
| 0.83% Blacks | ||||||
| Taylor et al, 1997 | Melbourne, Australia | 3268 | 40–90+ | <6/12-≥6/18 | Habitual, better eye | 2.6% |
| <6/18-≥6/60 | 0.92% | |||||
| <6/60-≥3/60 | 0.21% | |||||
| <6/12-≥6/18 | Best corrected, better eye | 0.61% | ||||
| <6/18-≥6/60 | 0.43% | |||||
| <6/60-≥3/60 | 0.15% | |||||
| Robinson et al 2013 | Ontario, Canada | 768 | 39–94 | <6/7.5 (0.1 logMAR) | Habitual, better eye (weighted prevalence) | 15.2% |
| <6/12 (0.3 logMAR) | 2.7% | |||||
| Khawaja et al 2013 | Norwich, UK | 8563 | 48–92 | <6/10 (0.22 logMAR) | Habitual, better eye (weighted prevalence) | 5.65% |
| <6/18 (0.48 logMAR) | 0.55% |
A dual sensory problem refers to the co-existence of both vision and hearing difficulties. Some studies suggest increased difficulty with activities of daily living, (12, 13) increased risk of depression, (14) lower quality of life (15) and higher risk of mortality (16) for those with dual sensory problems compared to those with either hearing or visual impairment alone. Estimates of the prevalence of dual sensory problems are rarer than those for either vision or hearing impairment, as studies have usually focused on one or the other. As there is no accepted definition of dual sensory problems, estimates of prevalence also vary widely depending on definition and study population. (17) Based on self-report, 1.3% of US adults aged over 18 years (16) and 21% of those aged over 70 years (12) were reported to have a dual sensory problem. Based on visual acuity and audiometric measures (best-corrected better-eye visual acuity <20/40 and better ear threshold > 25dB HL across 0.5 to 4 kHz), 1.5% of those aged over 20 years had dual sensory problems. (18)
The present study provides a snapshot of vision impairment and dual sensory impairment experienced by UK adults aged 40 to 69 years based on a large and inclusive sample. Vision assessment was based on presenting/habitual visual acuity. Relations between vision impairment, age, sex, socioeconomic status and ethnicity are also described.
Methods
The UK Biobank is a resource for the investigation of the genetic, environmental and lifestyle causes of diseases in middle and older age. Participants were recruited via the UK National Health Service and aimed to be as inclusive and representative as possible of the UK population with reference to the 2001 UK Census. (19) Over the course of 2006–2010, 503,325 participants were recruited with a response rate of 5.47%. All participants responded to questions on sex and ethnicity based on 2001 UK Census categories. Townsend deprivation score of the area of residence was recorded for each participant. The Townsend index is a proxy measure of socioeconomic status widely used in health studies. (20) It is comprised of four input variables on unemployment, non-home ownership, non-car ownership and household overcrowding. Each variable is standardised with respect to national level and summed to give a single deprivation score for each area. Lower scores represent less deprived socioeconomic status. Table 3 shows the demographic profile for the UK Biobank sample and for the corresponding section of the UK population. The UK Biobank contained a marginally higher proportion of females, ethnically White people and people living in less deprived areas than the general population. During the course of data collection, additional measures were added to the test protocol such that some measures were completed by a subset of participants. In the present study, prevalence estimates are based on the subset of participants that completed each measure. For visual acuity data and dual sensory impairment, data for 116,682 participants were obtained. Different numbers of participants also completed self-report questions on glasses use and eye problems dependent on when the question was included in the protocol. The sample size for each question is reported below.
Table 3.
Participants in the UK Biobank versus 2001 UK Census data for sex, age, ethnicity and socioeconomic status. Sex and ethnicity are shown as percentages while socioeconomic status is reported as average Townsend deprivation index score (with standard deviation).
| UK Biobank | UK Census 2001 | |
|---|---|---|
| Sex | ||
| Male | 45.6 | 49.2 |
| Age group (years) | ||
| 40–44 | 10.4 | 20.1 |
| 45–49 | 13.2 | 18.0 |
| 50–54 | 15.3 | 19.3 |
| 55–59 | 18.2 | 16.3 |
| 60–64 | 24.3 | 13.8 |
| 65–69 | 18.7 | 12.5 |
| Ethnicity | ||
| White | 94.1 | 91.3 |
| Mixed | 0.6 | 1.3 |
| Asian or Asian British | 2.0 | 4.4 |
| Black or Black British | 1.6 | 2.2 |
| Chinese | 0.3 | 0.4 |
| Other ethnic group | 0.9 | 0.4 |
| Prefer not to answer | 0.3 | - |
| Missing data | 0.2 | - |
| Socioeconomic status | ||
| Mean Townsend score* (SD) | −1.3 (3.1) | 0.7 (4.2) |
Lower Townsend scores indicate less deprivation
Participants attended a UK Biobank assessment centre and provided informed written consent. They then completed a 90 minute assessment which included questionnaire and physical measures. Questionnaire measures involved lifestyle, environmental and medical factors, with responses collected via a touchscreen computer. Detailed information on the protocol and other data collected may be found elsewhere (http://www.ukbiobank.ac.uk/).
Vision self-report questions
Participants responded to questions on use of glasses or contact lenses, eye problems and reason for using glasses presented via the computerised touchscreen interface. They included
“Do you wear glasses or contact lenses to correct your vision? (Yes; No; Prefer not to answer)” (N=499,365);
“Why were you prescribed glasses/contacts? (You can select more than one answer) (For short-sightedness, i.e. only or mainly for distance viewing such as driving, cinema etc (called ‘myopia’); For long-sightedness, i.e. for distance and near, but particularly for near tasks like reading (called ‘hypermetropia’); For just reading/near work as you are getting older (called ‘presbyopia’); For ‘astigmatism’; For a ‘squint’ or ‘turn’ in an eye since childhood (called ‘strabismus’); For a ‘lazy’ eye or an eye with poor vision since childhood (called ‘amblyopia’); Other eye condition; Do not know; Prefer not to answer)” (N=106,043);
“Do you have any other problems with your eyes or eyesight? (Yes; No; Prefer not to answer)” (N=499,365);
“Has a doctor told you that you have any of the following problems with your eyes? (You can select more than one answer) (Diabetes related eye disease; Glaucoma; Injury or trauma resulting in loss of vision; Cataract; Macular degeneration; Other serious eye condition; None of the above; Prefer not to answer; Do not know)” (N=173,671).
Visual acuity test
Visual acuity (VA) testing was based on reading high contrast letters, with the participant seated at a distance of 4 metres. Visual correction was worn for those participants that normally wore glasses or contact lenses for distance, and visual acuity measures were completed monocularly on both eyes. The score was determined as the logMAR size at which 3 out of the 5 letters presented were read correctly. Normal vision was defined as visual acuity (decimal Snellen) ≥0.8, mild impairment as <0.8 and ≥ 0.3 and low vision <0.3 and ≥ 0.05. Blindness (<0.05) was not a focus of the current study. Those with visual acuity within the blindness range (n = 4) were excluded from analysis. We are not aware of any standard criteria for dual sensory impairment. In this study, dual sensory impairment was identified based on ‘insufficient’ or ‘poor’ performance on the DTT hearing test combined with visual acuity in the ‘mild impairment’ or ‘low vision’ range.
Digit Triplet Test
The Digit Triplet Test is a speech-in-noise test originally developed in Dutch by Smits and colleagues (21) which provides an objective and ecologically relevant measure of hearing disability and correlates highly with audiometric thresholds (r = 0.77 (21)). The DTT is described elsewhere (http://biobank.ctsu.ox.ac.uk/crystal/label.cgi?id=100049). The signal to noise ratio (dB SNR) for the 50% correct speech recognition threshold is estimated via an adaptive tracking method for each ear. Lower scores correspond to better performance. DTT performance was categorised with respect to a normative sample of young normally hearing listeners. (22) Performance cut-offs were based on previously recommended standards, (21, 23) such that insufficient or poor performance corresponds to performance lower (worse) than −2 standard deviations with respect to the normative group, or a 50% correct recognition threshold higher than −5.5 dB. (22)
Data analysis
Analyses were performed with Stata version 12.1. In each subsample, iterative proportional fitting (IPF, or raking; ipfweight command in Stata) was applied in each age category to adjust the subsample margins to known population margins of sex, ethnicity and socioeconomic status from the 2001 UK Census. For socioeconomic status, deciles of deprivation weighted for each five year age-group using 2001 UK Census data were linked to each participant. This allowed for the Biobank sample being selective of people living in slightly less deprived circumstances and that the distribution of people across differently deprived areas varies by age. The 2001 UK Census was selected as the reference population because Biobank recruitment aimed for comparability with this census. Because different subsets of participants completed each measure, the weights were calculated separately within subsamples based on whether the respective outcome variable was observed. It was assumed that any missing data may be ignored because the reason for missing data is not systematically related to the outcome variable nor any other variable. Missing data were largely accounted for by the addition of measures at different points over the course of data collection, and this was unrelated to the hearing or vision status of participants. The iterative proportional fitting procedure involves a stepwise adjustment of sampling weights until the difference between the observed subsample margins and the known population margins across sex, ethnicity and socioeconomic status is less than a specified tolerance, set at 0.2%. Convergence of the fitting procedure was achieved in less than 10 iterations for all subsamples and age categories. All subsamples were weighted and cross tabulations performed to generate the population prevalence estimates. Multinomial logistic regression was used to model the association of age, sex, ethnicity and socioeconomic status with vision impairment.
Results
Vision
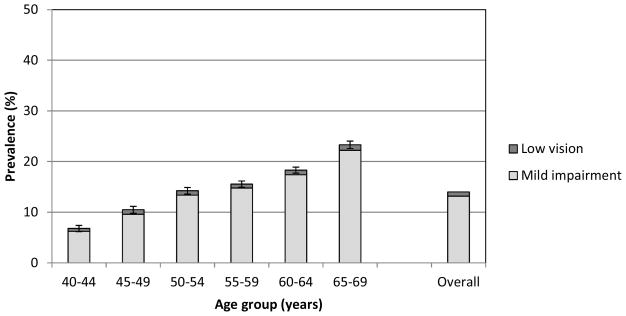
The prevalence of both mild impairment and low vision with habitually worn refractive correction for distance viewing increased with age (Figure 1), with proportional increases of 3.6x and 2x between the youngest and oldest age groups for mild impairment and low vision, respectively.
Figure 1.
Prevalence (%) of visual impairment by age group. Error bars show the 95% confidence interval for performance outside the normal range (Mild impairment/Low vision).
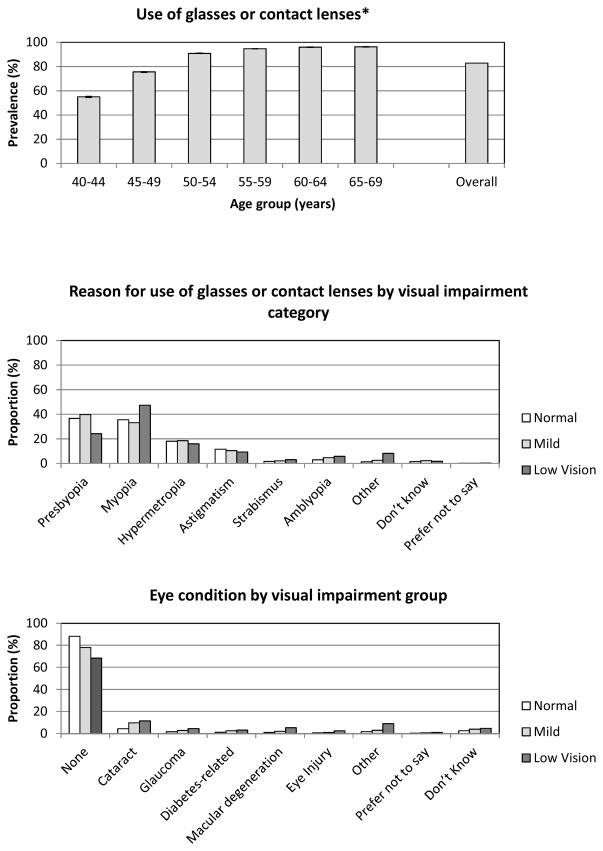
Self-reported use of glasses was common (Figure 2); by age 50 and over 90% of participants reported using glasses or contact lenses. Use of glasses or contact lenses was similar among all categories of visual impairment; 88.1%, 91.0% and 91.3% for normal vision, mild impairment and low vision, respectively. For those with normal vision or mild impairment, the commonest reason for use of glasses was presbyopia (use of glasses for reading or close viewing). Myopia, hypermetropia and astigmatism were next most common. Myopia was a particularly common reason for use of glasses in the low vision group. Eye conditions were more common among those with mild impairment or low vision. The overall rate of eye conditions in the impaired categories was 18.2%. For those in the impaired categories who reported no eye condition, 46.6% used glasses for distance viewinga while 36.8% reported neither an eye condition nor using glasses for distance viewing. Cataracts were the most commonly reported eye condition (~10% of those with mild impairment or low vision), followed by macular degeneration, glaucoma and diabetes-related eye disease.
Figure 2.
Prevalence (%) of use of glasses or contact lenses by age group, reason for use of glasses or contact lenses and eye conditions by visual impairment category. *Error bars show the 95% confidence interval.
The main effects of age, sex, socioeconomic status, and ethnicity were tested in a logistic regression model for the prevalence of visual impairment (‘mild impairment’ and ‘low vision’; Table 4). Increasing age was associated with the largest risk for visual impairment. Low socioeconomic background and Non-white ethnicity were associated with higher risk for vision impairment. Although Non-white ethnicity was associated with higher risk of vision impairment than White ethnicity, the proportion of non-Whites who reported an eye problem was significantly lower than the number of Whites (19.7% versus 21.4%; Χ2(1)= 6.58, p = 0.01). Use of glasses was also lower among non-Whites compared to Whites (79.1% versus 88.9%; Χ2(1)= 162.3, p < 0.01). Logistic models were re-run to provide risk estimates for ethnic sub-groups compared to White British for vision impairment (mild or low; see Supplemental Tables). Ethnicities at highest risk were Black Other, ‘Don’t know’, Bangladeshi, Black African and Pakistani (ORs 2.0 to 3.5, p < 0.001). Female sex was a small risk for mild visual impairment. The odds ratio for sex was the same for low vision as for mild impairment, although the association was not significant for low vision (perhaps due to a smaller number of participants and reduced statistical power).
Table 4.
The odds ratios from the logistic models fitted to the prevalence of better-eye vision impairment.
| Factor | Odds ratio | |
|---|---|---|
| Mild VI | Low Vision | |
| Age | ||
| 40–44 | - | - |
| 45–49 | 1.6*** | 1.5*** |
| 50–54 | 2.4*** | 1.7*** |
| 55–59 | 2.9*** | 1.6** |
| 60–64 | 3.5*** | 1.9*** |
| 65–69 | 4.8*** | 2.4*** |
| Sex | ||
| Female | - | - |
| Male | 0.9*** | 0.9 |
| Ethnicity | ||
| White | - | - |
| Non-white | 1.7*** | 1.4*** |
| Socioeconomic status | ||
| Medium-high socioeconomic status (>-1SD) | - | - |
| Low socioeconomic status (<-1SD)† | 1.5*** | 2.0*** |
p < 0.001
p < 0.01
p < 0.05
Low socioeconomic status was defined as a Townsend deprivation index score lower than 1 standard deviation (SD) below the mean with reference to the general population of 40 to 69 year-olds; i.e. the most deprived 15% of the population.
Dual sensory problems
Prevalence of dual sensory problems (Figure 3) has a higher proportional increase with age than for vision impairment alone; a 9x increase between youngest and oldest age groups. Overall, 2.4% of participants had a dual sensory problem. The occurrence of dual sensory problems was significantly greater than if hearing and vision problems occur independently (expected proportion of 1.5%; Χ2(1)= 584, p < 0.01). Average speech in noise recognition thresholds were significantly worse for those with mild or low vision compared to those with normal vision (by 0.5 and 0.6 dB respectively, p<0.01). Risk factors for dual sensory problems followed a similar pattern to those of hearing and vision (not shown here).
Figure 3.
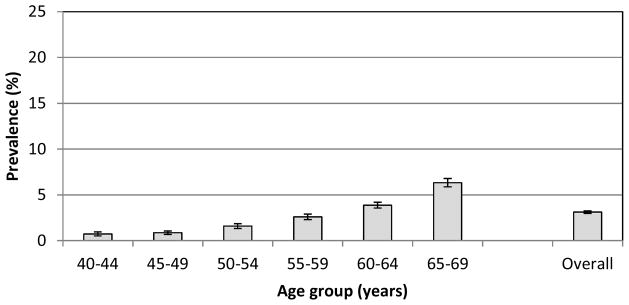
Prevalence (%) of dual sensory impairment by age group. Error bars show the 95% confidence interval.
Discussion
Vision
The overall prevalence of vision impairment (mild and low vision) was 14.0%, based on visual acuity measures with participants wearing the spectacles or contact lenses habitually worn for distance viewing. With the available data, we cannot distinguish the proportion of impairment due to refractive error or to an eye condition. The overall rate of self-reported eye conditions among those with impaired vision was 16.1%. Previous studies concluded that the majority of cases of impairment in western countries were due to imperfectly corrected or uncorrected refractive errors. (24–26) In a population (age 30 years and above) in Finland, of those with VA <0.5, 37% had no documented eye disease: of those only 25% reported using spectacles, 61% had not had an eye examination for 5 years and 35% had never had an eye examination. (27) In a recent UK study, of those with VA <6/18, 28% had uncorrected refractive error. (7) We therefore suspect that refractive error is likely to be the most common cause of visual impairment in this UK sample. Despite the suspicion of high rates of un- or imperfectly corrected refraction, use of glasses was very common, and matches the pattern reported in earlier studies. In an Australian study described earlier, (5) participants aged 40–98 years reported that 56% wore distance spectacles and 87% women and 85% men wore near spectacles. Distance spectacle wear progressively increased throughout age span, but near spectacles increased notably between the 40–49 (approx. 60% wearing) and 50–59 (95%+ wearing) age groups (5). High levels of spectacle use have been reported in the UK with older age groups (aged 65+ years); 60.6% had distance specs and 89% had near spectacles. (9)
It was surprising that refractive error is likely to be the most common cause of visual impairment in this sample, despite the use of spectacles being so common. It may be that spectacles were originally optimally prescribed by an optometrist but the prescription has become outdated. A recent study of 768 Canadians aged over 40 years found the prevalence of presenting acuity <20/40 in the better eye was 2.7%, with over 70% being correctable by refraction, despite the fact that 68% of participants in that study already wore distance correction, and 82.6% near spectacles. (6) The numbers with correctable vision impairment decreased as age increased, but increased in those not tested for >2 years. It is recommended that adults under 70 years have an eye examination every 2 years, with an annual examination for those aged over 70 years. Evans and Rowlands (11) felt that additional publicity to raise awareness of the need for regular checks may be necessary, and reviewed other reasons for the high prevalence of correctable visual impairment. They include cost (or perceived cost) of spectaclesb, inadequate service provision, lack of a screening programme, poor recognition of the treatability of vision problems and avoidance of healthcare services. For older adults, mobility and cognition problems may limit access to services. Those from ethnic minority backgrounds may perceive language or cultural barriers and tend to be under-represented in ophthalmology case-loads (28, 29) despite being at greater risk of certain eye diseases.
In the present study, older age was the factor most prominently associated with poor vision. Low socioeconomic status was also associated with higher odds of vision impairment. Male sex was associated with slightly less risk for mild vision impairment, in agreement with previous studies. (25, 30) Non-white ethnicities were associated with increased risk of vision impairment. Examination of risks associated with ethnic subgroups suggested that this association is driven by ethnic subgroups that are at particular risk for vision problems; Black Other, Bangladeshi, Black African, and Pakistani in particular. This is in line with findings of poorer general health within particular ethnic minorities in the UK. (31) Suggested reasons for health inequality centre around culture and lifestyle, socioeconomic factors, reduced uptake of services and biological susceptibility. (32) Despite higher odds of visual impairment, use of glasses and self-reported eye problems were significantly lower in Non-whites versus White ethnic groups in the present study. Taken together with previous research (28, 29), this suggests that uptake of vision services may be lower among ethnic minorities.
Cataracts were the most commonly reported eye condition in mild and low vision categories, while macular degeneration, diabetic retinopathy and glaucoma were proportionally more prominent in the low vision category compared to the mild category. . The pattern of self-reported eye conditions agrees with previous studies. (25, 33–35) The average waiting time for cataract surgery in England (estimated in 2011) was 60 days.(36) It therefore seems unlikely that a long delay for surgery is the reason that cataracts were the most commonly reported condition.
Dual sensory problems
The prevalence of dual sensory problems was statistically significantly greater than expected if vision and hearing problems occur independently. The proportional increase with age in the prevalence of dual sensory problems was high compared to increases vision impairment and previously reported (22) increases in hearing impairment alone (9x versus 3.3x and 3.9x for vision and hearing impairment, respectively), suggesting that risks for sensory impairment are not simply additive. The tendency for vision and hearing impairments to co-occur has been noted previously, with a suggestion that they may share common risk factors. (37–39) Factors associated with vision impairment in the present study were similar to those previously observed for hearing impairment. (22) The consequences of the dual sensory loss may be greater than predicted on the basis of the severity of the hearing and vision losses when considered in isolation. For example, Dickinson and Taylor simulated hearing and vision impairments in healthy volunteers, and found that even minor visual defects significantly compromised speech-reading abilityc when there was a concurrent hearing loss. (40)
Given the tendency for hearing and vision problems to occur together and the impact of dual sensory problems on quality of life, it may be helpful for audiologists and optometrists to screen for impairments in both hearing and vision. (30) Audiologists could ask patients for the date of their latest eye examination and if more than two years (or one year in those individuals aged over 70 years, according to National Health Service guidelines; http://www.nhs.uk/chq/Pages/1093.aspx?CategoryID=68&SubCategoryID=157), then advise them to go to their local optometrist. Optometrists could also advise patients to request a hearing test. This is not currently readily available in the UK, as this would require a referral to a National Health Service audiology clinic from a general medical practitioner. However, this is set to change with moves in England to open hearing aid provision to commercial competition (the ‘any qualified provider’ scheme).
Limitations
Potentially the most significant limitation of the current study is that, despite the large number of participants, the low response rate of 5.47% may have introduced unknown biases into prevalence estimates that may not be accounted for by the statistical weighting procedures used in this study. The UK Biobank argued that despite the low response rate, the size and coverage of the sample allows generalisable associations between relevant risk factors and health outcomes. (41) The size and coverage of the UK Biobank sample may also give confidence in the reliability of prevalence estimates reported here. An additional limitation is that UK Biobank recruitment and testing was not designed to cater for those with vision impairment. This may have excluded those with vision problems, and so prevalence figures reported here may be an underestimate.
The current gold standard acuity test in research is the Early Treatment Diabetic Retinopathy Study (ETDRS) chart. The letter presentation and the testing conditions in the Biobank assessment differ from this; it is unclear how the “crowding” of the letter targets is arranged, and acuity measurements are conducted in a darkened room (http://biobank.ctsu.ox.ac.uk/crystal/field.cgi?id=5201). The procedure to derive the VA score is also unclear, but appears to be similar to the “ETDRS-FAST” used by Camparini and colleagues. (42) Visual acuity was categorised according to ‘better eye’ performance based on recommended cut-offs for mild impairment and low vision. (43, 44) It has been suggested that binocular vision would be a more accurate representation of how the individual functions in everyday life. (45) However binocular VA is determined by the VA in the better eye (http://www.ski.org/Colenbrander/Images/Visual_Impairmnt_Guide.pdf), and two population studies (46, 47) both identified the mean population difference in binocular and better eye VA to be 0.02 logMAR, which Rubin and colleagues (46) noted to be an insignificant difference. Better eye visual acuity estimates are therefore a reasonable approximation of binocular performance in a population-based study.
Conclusions
Older people, those from low socioeconomic and ethnic minority backgrounds are particularly at risk for vision problems. Vision impairment is rather prevalent despite widespread use of spectacles and contact lenses. A high proportion of those who would benefit from correction may not receive effective intervention. Possible reasons for low uptake may include lack of recognition of difficulties or lack of awareness of treatment options. Cost may be a particular barrier for vision services. Hearing and vision problems tend to occur together, and the proportional increase with age in those with both hearing and vision problems was higher than for hearing or vision problems separately. This suggests that causes of hearing and vision problems are not merely additive. Audiologists and optometrists should test for dual sensory problems, as these persons are at a much greater disadvantage in daily life.
Supplementary Material
Acknowledgments
This research was facilitated by Manchester Biomedical Research Centre and ulitised the UK Biobank resource. The Nottingham Hearing Biomedical Research Unit is funded by the National Institute for Health Research. DRM was supported by the Intramural Programme of the Medical Research Council [Grant U135097130]. KJC was supported by R37AG11099, R01AG021917 and an Unrestricted Grant from Research to Prevent Blindness
This paper presents independent research funded in part by the National Institute for Health Research (NIHR). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Footnotes
Distance viewing was estimated as the sum of those who report using glasses or contact lenses for either myopia, hypermetropia or astigmatism.
In the UK, eligibility for free eye tests is currently means tested for individuals between the ages of 16 and 60 (except for a few specific exceptions), and vouchers towards glasses are provided on a means tested basis for adults of all ages. However for those entitled to an NHS eye examination, this can be carried out in the home for those who have limited mobility. Optical low vision aids (magnifiers) are however provided free of charge (by hospital clinics). In contrast, audiological services and hearing aids are freely provided.
Speech-reading refers to the ability to recognise speech sounds visually, using movements of the speaker’s mouth or other sources of visual information.
Disclosure
The authors report no conflicts of interest and have no proprietary interest in any of the materials mentioned in this article.
References
- 1.Leat SJ, Legge GE, Bullimore MA. What is low vision? A re-evaluation of definitions. Optometry & Vision Science. 1999;76(4):198–211. doi: 10.1097/00006324-199904000-00023. [DOI] [PubMed] [Google Scholar]
- 2.West SK, Rubin G, Broman AT, Munoz B, Bandeen–Roche K, Turano K. How does visual impairment affect performance on tasks of everyday life? The SEE Project Archives of ophthalmology. 2002;120(6):774–80. doi: 10.1001/archopht.120.6.774. [DOI] [PubMed] [Google Scholar]
- 3.Dandona L, Dandona R. Revision of visual impairment definitions in the International Statistical Classification of Diseases. BMC medicine. 2006;4(1):7. doi: 10.1186/1741-7015-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Resnikoff S, Pascolini D, Mariotti SP, Pokharel GP. Global magnitude of visual impairment caused by uncorrected refractive errors in 2004. Bulletin of the World Health Organization. 2008;86(1):63–70. doi: 10.2471/BLT.07.041210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor HR, Liningson PM, Stanislavsky YL, McCarty CA. Visual impairment in Australia: Distance visual acuity, near vision, and visual field findings of the Melbourne Visual Impairment Project. American Journal of Opthalmology. 1997;123(3):328–37. doi: 10.1016/s0002-9394(14)70128-x. [DOI] [PubMed] [Google Scholar]
- 6.Robinson BE, Feng Y, Fonn D, Woods CA, Gordon KD, Gold D. Risk Factors For Visual Impairment-Report From A Population-based Study (CURES) Investigative Ophtalmology and Visual Science. 2011;52(6):4217. [Google Scholar]
- 7.Khawaja AP, Chan MP, Hayat S, Broadway DC, Luben R, Garway-Heath DF, et al. The EPIC-Norfolk Eye Study: rationale, methods and a cross-sectional analysis of visual impairment in a population-based cohort. BMJ open. 2013;3(3) doi: 10.1136/bmjopen-2013-002684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tate R, Smeeth L, Evans J, Fletcher A, Owen C, Rudnicka A. A review of the literature. London: Royal National Institute for the Blind; 2005. The prevalence of visual impairment in the UK. [Google Scholar]
- 9.van der Pols JC, Bates CJ, McGraw PV, Thompson JR, Reacher M, Prentice A, et al. Visual acuity measurements in a national sample of British elderly people. British Journal of Opthalmology. 2000;84:165–70. doi: 10.1136/bjo.84.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans JR, Fletcher AE, Wormald RPL, Siu-Woon Ng E, Stirling L, Smeeth L, et al. Prevalence of visual impairment in people aged 75 years and older in Britain: Results from the MRC trial of assessment and management of older people in the community. British Journal of Opthalmology. 2002;86:795–800. doi: 10.1136/bjo.86.7.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans BJW, Rowlands G. Correctable visual impairment in older people: A major unmet need. Ophthalmic and Physiological Optics. 2004;24:161–80. doi: 10.1111/j.1475-1313.2004.00197.x. [DOI] [PubMed] [Google Scholar]
- 12.Brennan M, Horowitz A, Su Y. Dual sensory loss and its impact on everyday competence. The Gerontologist. 2005;45(3):337–46. doi: 10.1093/geront/45.3.337. [DOI] [PubMed] [Google Scholar]
- 13.Crews JE, Campbell VA. Vision impairment and hearing loss among community-dwelling older Americans: Implications for health and functioning. American Journal of Public Health. 2004;94(5):823–9. doi: 10.2105/ajph.94.5.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Capella-McDonnall ME. The effects of single and dual sensory loss on symptoms of depression in the elderly. Int J Geriatr Psychiatry. 2005;20(9):855–61. International Journal of Geriatric Pyschiatry. 2005;20(9):855–61. doi: 10.1002/gps.1368. [DOI] [PubMed] [Google Scholar]
- 15.Fisher ME, Cruickshanks KJ, Klein B, Klein R, Schubert CR, Wiley TL. Multiple sensory impairment and quality of life. Opthalmic Epidemiology. 2009;16(6):346–53. doi: 10.3109/09286580903312236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lam BL, Lee DJ, Gomez-Marin O, Zheng DD, Caban AJ. Concurrent visual and hearing impairment and risk of mortality: The National Health Interview Survey. Archives of Opthalmology. 2006;124(1):95–101. doi: 10.1001/archopht.124.1.95. [DOI] [PubMed] [Google Scholar]
- 17.Smith SL, Bennett LW, Wilson RH. Prevalence and characteristics of dual sensory impairment (hearing and vision) in a veteran population. Journal of Rehabilitation Research & Development. 2008;45(4):597–610. doi: 10.1682/jrrd.2007.02.0023. [DOI] [PubMed] [Google Scholar]
- 18.Swenor BK, Ramulu PY, Willis JR, Friedman D, Lin FR. The Prevalence of Concurrent Hearing and Vision Impairment in the United States. JAMA internal medicine. 2013;173(4):312–3. doi: 10.1001/jamainternmed.2013.1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Office for National Statistics. Census 2001: General report for England and Wales. 2005. [Google Scholar]
- 20.Norman P. Identifying change over time in small area socio-economic deprivation. Applied Spatial Analysis and Policy. 2010;3(2):107–38. [Google Scholar]
- 21.Smits C, Kapteyn TS, Houtgast T. Development and validation of an automatic speech-in-noise screening test by telephone. International Journal of Audiology. 2004;43:15–28. doi: 10.1080/14992020400050004. [DOI] [PubMed] [Google Scholar]
- 22.Dawes P, Fortnum H, Moore DR, Emsley R, Norman P, Cruickshanks KJ, et al. Hearing in middle age: a population snapshot of 40–69 year olds in the UK. Ear and hearing. doi: 10.1097/AUD.0000000000000010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smits C, Houtgast T. Results from the Dutch speech-in-noise screening test by telephone. Ear & Hearing. 2005;26:89–95. doi: 10.1097/00003446-200502000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Weih LM, VanNewkirk MR, McCarty CA, Taylor HR. Age-specific causes of bilateral visual impairment. Archives of ophthalmology. 2000;118(2):264. doi: 10.1001/archopht.118.2.264. [DOI] [PubMed] [Google Scholar]
- 25.Attebo K, Mitchell P, Smith W. Visual acuity and the causes of visual loss in Australia. The Blue Mountains Eye Study. Ophthalmology. 1996;103(3):357. doi: 10.1016/s0161-6420(96)30684-2. [DOI] [PubMed] [Google Scholar]
- 26.VanNewkirk MR, Weih L, McCarty CA, Taylor HR. Cause-specific prevalence of bilateral visual impairment in Victoria, Australia: the Visual Impairment Project. Ophthalmology. 2001;108(5):960. doi: 10.1016/s0161-6420(01)00554-1. [DOI] [PubMed] [Google Scholar]
- 27.Laitinen A, Laatikainen L, Harkanen T, Koskinen S, Reunanen A, Aromaa A. Prevalence of major eye diseases and causes of visual impairment in the adult Finnish population: a nationwide population-based survey. Acta Ophthalmologica. 2010;88(4):463–71. doi: 10.1111/j.1755-3768.2009.01566.x. [DOI] [PubMed] [Google Scholar]
- 28.Lindesay J, Jagger C, Hibbett MJ, Peet SM, Moledina F. Knowledge, uptake and availability of health and social services among Asian Gujarati and white elderly persons. Ethnicity & health. 1997;2(1–2):59–69. doi: 10.1080/13557858.1997.9961815. [DOI] [PubMed] [Google Scholar]
- 29.Pardhan S, Mahomed I. The clinical characteristics of Asian and Caucasian patients on Bradford’s Low Vision Register. Eye. 2002;16(5):572–6. doi: 10.1038/sj.eye.6700164. [DOI] [PubMed] [Google Scholar]
- 30.Klein R, Klein B, Linton K, De Mets D. The Beaver Dam Eye Study: visual acuity. Ophthalmology. 1991;98(8):1310. doi: 10.1016/s0161-6420(91)32137-7. [DOI] [PubMed] [Google Scholar]
- 31.Department of Health. Health Survey for England 1999: The health of minority ethnic groups. London: 2001. Available from: http://webarchive.nationalarchives.gov.uk/+/www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsStatistics/DH_4009393. [Google Scholar]
- 32.Smith GD, Chaturvedi N, Harding S, Nazroo J, Williams R. Ethnic inequalities in health: a review of UK epidemiological evidence. Critical Public Health. 2000;10(4):375–408. [Google Scholar]
- 33.Rahmani B, Tielsch JM, Katz J, Gottsch J, Quigley H, Javitt J, et al. The cause-specific prevalence of visual impairment in an urban population. The Baltimore Eye Survey. Ophthalmology. 1996;103(11):1721. doi: 10.1016/s0161-6420(96)30435-1. [DOI] [PubMed] [Google Scholar]
- 34.Wang JJ, Foran S, Mitchell P. Age-specific prevalence and causes of bilateral and unilateral visual impairment in older Australians: the Blue Mountains Eye Study. Clinical & experimental ophthalmology. 2001;28(4):268–73. doi: 10.1046/j.1442-9071.2000.00315.x. [DOI] [PubMed] [Google Scholar]
- 35.Klein R, Wang Q, Klein B, Moss SE, Meuer SM. The relationship of age-related maculopathy, cataract, and glaucoma to visual acuity. Investigative ophthalmology & visual science. 1995;36(1):182–91. [PubMed] [Google Scholar]
- 36.OECD Health Statistics. Waiting times for elective surgery. 2013 [cited 2014 27 February 2014]; Available from: http://dx.doi.org/10.1787/health-data-en.
- 37.Chia EM, Mitchell P, Rochtchina E, Golding M, Foran S, Golding M, et al. Association between vision and hearing impairments and their combined effects on quality of life. Archives of Opthalmology. 2006;124(10):1465–70. doi: 10.1001/archopht.124.10.1465. [DOI] [PubMed] [Google Scholar]
- 38.Schneck ME, Lott LA, Haegerstrom-Portnoy G, Brabyn JA. Association between hearing and vision impairments in older adults. Ophthalmic and Physiological Optics. 2012 doi: 10.1111/j.1475-1313.2011.00876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cruickshanks KJ, Wichmann MA, editors. Seminars in Hearing. Thieme Medical Publishers; 2012. Hearing Impairment and Other Health Conditions in Older Adults: Chance Associations or Opportunities for Prevention? [Google Scholar]
- 40.Dickinson CM, Taylor J. The effect of simulated visual impairment on speech-reading ability. Ophthalmic and Physiological Optics. 2011;31(3):249–57. doi: 10.1111/j.1475-1313.2010.00810.x. [DOI] [PubMed] [Google Scholar]
- 41.Allen N, Sudlow C, Downey P, Peakman T, Danesh J, Elliott P, et al. UK Biobank: Current status and what it means for epidemiology. Health Policy and Technology. 2012;1:123–126. [Google Scholar]
- 42.Camparini M, Cassinari P, Ferrigno L, Macaluso C. ETDRS-fast: implementing psychophysical adaptive methods to standardized visual acuity measurement with ETDRS charts. Investigative ophthalmology & visual science. 2001;42(6):1226–31. [PubMed] [Google Scholar]
- 43.World Health Organisation. Chapter VII Diseases of the eye and adnexa. 1994 [cited 2013 January]; Available from: http://apps.who.int/classifications/apps/icd/icd10online2004/fr-icd.htm?gh53.htm+
- 44.International Council of Ophthalmology. Aspects and ranges of vision loss with emphasis on population surveys. San Francisco, California: 2002. Visual standards. [Google Scholar]
- 45.Brown MM, Brown GC, Sharma S, Busbee B, Brown H. Quality of life associated with unilateral and bilateral good vision. Opthalmology. 2001;108(4):643–7. doi: 10.1016/s0161-6420(00)00635-7. [DOI] [PubMed] [Google Scholar]
- 46.Rubin GS, Munoz B, Bandeen–Roche K, West SK. Monocular versus binocular visual acuity as measures of vision impairment and predictors of visual disability. Investigative ophthalmology & visual science. 2000;41(11):3327–34. [PubMed] [Google Scholar]
- 47.Azen SP, Varma R, Preston-Martin S, Ying-Lai M, Globe D, Hahn S. Binocular visual acuity summation and inhibition in an ocular epidemiological study: the Los Angeles Latino Eye Study. Investigative ophthalmology & visual science. 2002;43(6):1742–8. [PubMed] [Google Scholar]
- 48.Nissen LR, Sjolie AK, Jensen H, Borch-Johnson K, Rosenberg T. The prevalence and incidence of visual impairment in people of age 20–59 years in industrialised countries: A review. Opthalmic Epidemiology. 2003;10(4):279–91. doi: 10.1076/opep.10.4.279.15909. [DOI] [PubMed] [Google Scholar]
- 49.Klein R, Klein BE, Linton KL, De Mets DL. The prevalence and incidence of visual impairment in people of age 20–59 years in industrialized countries: A review. Opthalmic Epidemiology. 1991;10(4):279–91. doi: 10.1076/opep.10.4.279.15909. [DOI] [PubMed] [Google Scholar]
- 50.Gunnlaugsdottir E, Arnarsson A, Jonasson F. Prevalence and causes of visual impairment and blindness in Icelanders aged 50 years and older: the Reykjavik Eye Study. Acta ophthalmologica. 2008;86(7):778–85. doi: 10.1111/j.1755-3768.2008.01191.x. [DOI] [PubMed] [Google Scholar]
- 51.Buch H, Vinding T, la Cour M, Appleyard M, Jensen GB, Vesti Nielsen N. Prevalence and causes of visual impairment and blindness among 9980 Scandinavian adults: the Copenhagen City Eye Study. Ophthalmology. 2004;111(1):53–61. doi: 10.1016/j.ophtha.2003.05.010. [DOI] [PubMed] [Google Scholar]
- 52.Tielsch JM, Sommer A, Witt K, Katz J, Royall RM. Blindness and visual impairment in an American urban population: The Baltimore Eye Survey. Archives of Opthalmology. 1990;108(2):286–90. doi: 10.1001/archopht.1990.01070040138048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.