Abstract
Despite being regarded as a hippie science for decades, cannabinoid research has finally found its well-deserved position in mainstream neuroscience. A series of groundbreaking discoveries revealed that endocannabinoid molecules are as widespread and important as conventional neurotransmitters like glutamate or GABA, yet act in profoundly unconventional ways. We aim to illustrate how uncovering the molecular, anatomical and physiological characteristics of endocannabinoid signaling revealed new mechanistic insights into several fundamental phenomena in synaptic physiology. First, we summarize unexpected advances in the molecular complexity of biogenesis and inactivation of the two endocannabinoids, anandamide and 2-arachidonoylglycerol. Then we show how these new metabolic routes are integrated into well-known intracellular signaling pathways. These endocannabinoid-producing signalosomes operate in phasic and tonic modes thereby differentially governing homeostatic, short-term and long-term synaptic plasticity throughout the brain. Finally, we discuss how cell type- and synapse-specific refinement of endocannabinoid signaling may explain the characteristic behavioral effects of cannabinoids.
Keywords: retrograde signaling, feed-back inhibition, synaptic plasticity, G-protein-coupled receptors, diacylglycerol lipase
The “Grass Route” to the Discovery of the Endocannabinoid System
Predator-prey competition is a major driving force behind evolution. For example, most plants developed a dedicated repertoire of chemical molecules to distract consumption. These allelochemicals often mimic or perturb endogenous signaling pathways in the nervous system, thereby becoming behaviorally effective. It seems that the underlying evolutionary processes were robust enough to invent receptor agonists or antagonists and even allosteric activators or inhibitors of enzymes with excellent potency and affinity. This natural treasure trove served traditional medicine for several thousand years and still remains a major frontier for drug discovery (Li and Vederas, 2009). Moreover, neuroscience research has also greatly profited from deciphering the mechanisms by which these plant products are behaviorally active, paving the way for the discovery of several endogenous signaling systems primarily active in the brain (Prisinzano, 2009).
A prime example is the cannabis plant (Cannabis sativa L.) and the discovery of the endocannabinoid system in animals. Cannabis plants produce a unique mixture of chemical constituents, the most famous products being the C21 terpenophenolic compounds, which are collectively called phytocannabinoids. Detailed chemical analysis have identified about 70 molecular species of phytocannabinoids (ElSohly and Slade, 2005), but unquestionably, one molecule and its discovery stands out. Intriguingly, this aromatic terpenoid has been more famous (or infamous, as one wishes) among the public than among neuroscientists until very recently. This compound called (−)-Δ9-tetrahydrocannabinol (Δ9-THC) was isolated from confiscated hashish by Yechiel Gaoni and Raphael Mechoulam (Fig. 1A-B) (Gaoni and Mechoulam, 1964), and was shown to account for the psychotropic effects of cannabis preparations in rhesus monkeys (Mechoulam et al., 1970). This seminal discovery transformed cannabinoid research from an anecdote-based practice into an evidence-based modern research field. The use of the chemically defined Δ9-THC molecule made it possible to obtain qualitatively and quantitatively reproducible pharmacological, physiological or behavioral data, which then helped to uncover the neurobiological substrates of psychoactive effects of cannabis.
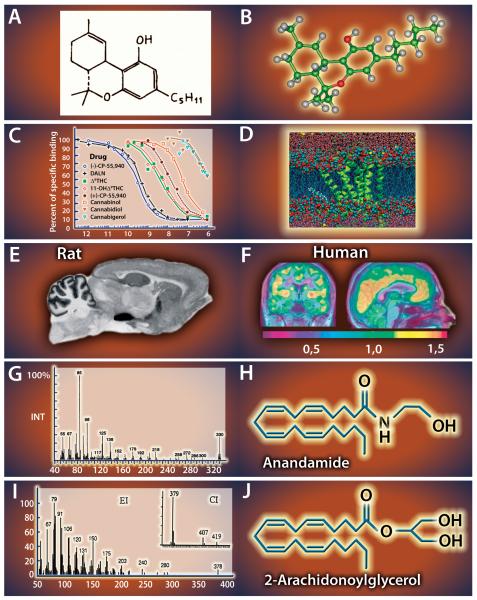
Figure 1. A tribute to the discoveries unraveling the endocannabinoid system.
A,B) First identification of the chemical structure with its absolute configuration of the psychoactive compound in marijuana, Δ9-THC (Gaoni and Mechoulam 1967). C) First demonstration by competitive inhibition of the existence of a high affinity, stereoselective, pharmacologically distinct cannabinoid receptor in brain tissue (Devane et al., 1988). D) The 3D molecular model of the 7-transmembrane CB1 receptor (Shim, 2009). E) Localization of CB1 by high affinity receptor binding and autoradiography in the rat (Herkenham et al 1990), and F) by PET in human brain (Burns et al 2007). G) Mass spectra of anandamide (Devane 1992) and H-J) 2-AG, together with the chemical structures of the two major endocannabinoids (Mechoulam et al., 1995). The individual figures have been modified from the originals with permission from the authors.
The second major breakthrough in cannabinoid research provided answer to the conceptual question of why our brain reacts to cannabis. Using [3H]-CP55,940, a potent radioactively-labeled synthetic cannabinoid, Bill Devane, Allyn Howlett and their colleagues obtained the first unequivocal evidence for the presence of a specific cannabinoid receptor, which inhibits adenylate cyclase via Gi-protein signaling in the brain (Fig. 1C-D) (Devane et al., 1988; Bidaut-Russell et al., 1990). This discovery is also considered as the first direct evidence for existence of the endocannabinoid system. The subsequent qualitative and quantitative radioligand binding studies quickly revealed the distribution of cannabinoid receptors in the brain (Fig 1E) (Herkenham et al., 1990). First, lesion experiments showed that the vast majority of cannabinoid binding sites in the brain are on neurons, and most likely on their axonal bundles (Herkenham et al., 1991). Second, the quantitative distribution pattern fitted well with the brain regions underlying the behavioral effects of cannabis. Third, this pattern was remarkably similar across species indicating a conserved physiological function for cannabinoid receptors. Finally, and most importantly, the density of cannabinoid receptors in the brain was comparable to the levels of glutamate, GABA or striatal dopamine receptors (Herkenham et al., 1990). Thus, these observations collectively predicted in advance that cannabinoid receptors are as ubiquitous components of chemical synapses as conventional neurotransmitter receptors.
This period was the golden age for the cloning of G-protein-coupled receptors, thus, the molecular identification of the first cannabinoid receptor has followed very soon (Matsuda et al. 1990). The CB1 cannabinoid receptor indeed turned out to be a class A G-protein-coupled receptor, and has a notably similar sequence (97-99% amino acid sequence identity) across mammalian species, supporting once again a phylogenetically conserved function for CB1. In situ hybridization confirmed neuronal expression and revealed a heterogeneous distribution pattern largely corresponding to the ligand binding sites (Matsuda et al. 1990). With the help of significant homology (44% at the amino acid level), a second cannabinoid receptor was also discovered thereafter (Munro et al., 1993). These two receptors originated from a common ancestor, and it is now fairly safe to conclude that a third, phylogenetically closely related third cannabinoid receptor is unlikely to be found (Pertwee et al., 2010).
Compelling evidence shows that CB1 receptors are the major neurobiological substrates for Δ9-THC effects on the human brain. The acute psychological consequences of marijuana smoking such as the subjective “high” experience was efficiently blocked by pretreatment with the CB1 antagonist, rimonabant in healthy human subjects (Huestis et al., 2001). Moreover, the development of novel inverse agonist radioligands for positron emission tomography now allows the monitoring of CB1 receptor availability in the living human brain (Burns et al., 2007) (Fig. 1F). The tremendous potential of this new approach is reflected by the emerging data showing robust changes in CB1 receptor availability in patients with Huntington disease, or temporal lobe epilepsy (Van Laere et al., 2010; Goffin et al., 2011), or by the demonstration of cortex-specific downregulation of CB1 availability in chronic cannabis smokers, which is a long-suspected mechanism of cannabis tolerance (Hirvonen et al., 2011).
While CB1 receptors are considered as primarily neuronal receptors, CB2 receptors are highly expressed in the spleen and regarded as the predominant cannabinoid receptor of the immune system. This somewhat simplified concept has been ideal to provide a framework for the potential therapeutic exploitation of the endocannabinoid system; one particularly exciting example is that the beneficial effects of Δ9-THC in multiple sclerosis is mediated by neuronal CB1 receptors and by CB2 receptors on autoreactive T cells in tandem (Maresz et al., 2007). However, accumulating data also support important physiological and pathophysiological functions for peripheral CB1 receptors (Kunos et al., 2009), whereas central effects of CB2 receptors are well-documented in emesis regulation or in rewarding effects of cocaine (Van Sickle et al., 2005; Xi et al., 2011).
The discovery of cannabinoid receptors initiated an immediate quest for their endogenous ligands, the so-called endocannabinoids. Because of the lipophilic nature of phytocannabinoids, Devane, Mechoulam and their colleagues argued that lipid-soluble fractions of the brain should contain the putative endocannabinoid molecule, which finally led them to its chemical identification (Fig. 1G-H) (Devane et al., 1992). This compound, N-arachidonoylethanolamide was termed anandamide based on the Sanskrit word “ananda” meaning “inner bliss”. This name reflects a good foresight, because anandamide plasma levels are significantly reduced in patients with major depression (Hill et al., 2009), and blockade of anandamide hydrolysis exerts robust anti-depressant-like effects (Gobbi et al., 2005). Anandamide turned out to be a partial agonist of the two cannabinoid receptors, which is unusual for an endogenous natural ligand, and it is found at low concentrations (pmol/g tissue) in the brain (Pertwee et al., 2010). Because its pharmacological activity did not fully recapitulate the behavioral effects of Δ9-THC (Smith et al., 1994), the existence of a second endocannabinoid was postulated, and soon identified as 2-arachidonoylglycerol (2-AG) (Fig. 1I-J) (Mechoulam et al., 1995; Sugiura et al., 1995). 2-AG is a full agonist at both CB1 and CB2 receptors and it is present at much higher levels (nmol/g tissue) in the brain (Stella et al., 1997). With the discovery of anandamide, 2-AG and the cannabinoid receptors, the “grass route” has gloriously ended after 3 decades, and gave the green light to neuroscientists to continue with hypothesis-driven questions on the function of endocannabinoid signaling, which has culminated in a paradigm shift in neuroscience.
The “Retrograde Route” of Endocannabinoids in the Brain
While anterograde synaptic transmission has been extensively studied for decades, much less information was known about retrograde signaling pathways at chemical synapses. Several candidates such as gases (e.g. nitric oxide), peptides (e.g. dynorphin), growth factors (e.g. brain-derived neurotrophic factor) or even conventional amino acid transmitters such as glutamate or GABA were shown to be released by the somatodendritic domain of postsynaptic neurons and then to act on the axon terminals of presynaptic neurons (Regehr et al., 2009). However, most of these retrograde messengers operate at specific synapses or restricted to a few cell types, and none of them could fully explain most common forms of retrograde synaptic communication. Based on high anandamide synthase activity in the hippocampus and the then prevailing view that the chemically related lipid molecule arachidonic acid is a retrograde messenger in hippocampal long-term potentiation (Williams et al., 1989), Devane and Axelrod proposed first that anandamide may also play a retrograde messenger role on axonal CB1 receptors (Devane and Axelrod, 1994). In accordance, the first immunohistochemical studies visualizing CB1 protein localization reported dense meshwork of fibers throughout the brain at the light microscopic level (Egertova et al., 1998; Tsou et al., 1998). These fibers proved to be axons in both the rat and human brain, as revealed by high-resolution immunogold staining and electron microscopy (Katona et al., 1999; Katona et al., 2000). In fact, the majority of CB1 receptors accumulated presynaptically on axon terminals of GABAergic interneurons in the hippocampus (Fig. 2A). Furthermore, CB1 receptor agonists reduced electrically-evoked GABA release (Fig. 2B), in accordance with a proposed retrograde messenger function of endocannabinoid signaling (Katona et al., 1999). The presynaptic localization of CB1 receptors and its inhibitory effect on neurotransmitter release have proved to be a general feature of most axon terminals in the central (Kano et al., 2009), and peripheral nervous system (Vizi et al., 2001).
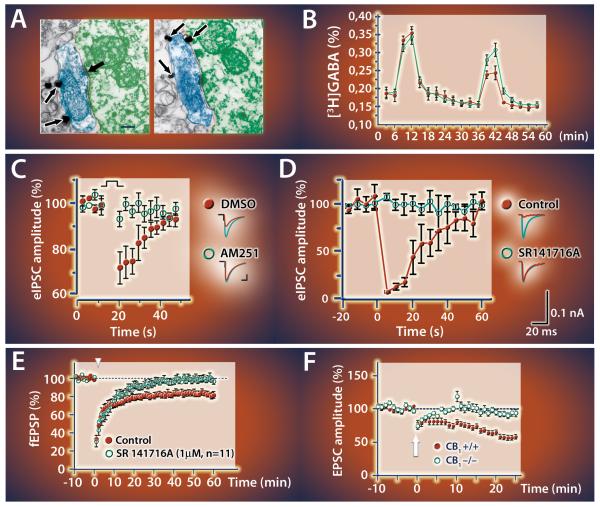
Figure 2. The retrograde route of endocannabinoid signaling in the brain.
A) Selective localization of CB1 receptors on axon terminals suggesting a retrograde direction of endocannabinoid action (Katona et al 1999). B) Activation of presynaptic CB1 receptors reduces 3H-GABA release from hippocampal slices (Katona et al 1999). C,D) Endocannabinoids mediate depolarization-induced suppression of inhibition (DSI) in hippocampus (C: Wilson and Nicoll 2001; D: Ohno-Shosaku et al., 2001). E) Long-term depression of glutamatergic transmission is mediated by endocannabinoids via CB1 receptors in the nucleus accumbens (Robbe et al., 2002), and F) the striatum (Gerdeman et al., 2002). The individual figures have been modified from the originals with permission from the authors.
The most important support for the retrograde scenario came subsequently from the study of an electrophysiological paradigm, in which selective depolarization of a postsynaptic neuron induces short-term depression of GABA release from axon terminals innervating the same postsynaptic neuron (DSI) (Llano et al., 1991; Pitler and Alger, 1992). This robust phenomenon premised the existence of a bona fide retrograde messenger, and thus, the demonstration that three independent antagonists of CB1 receptors block DSI at hippocampal GABAergic synapses (Fig. 2C-D) (Ohno-Shosaku et al., 2001; Wilson and Nicoll, 2001), together with the presynaptic localization of CB1 receptors (Katona et al., 1999), were in agreement with the plausible scenario that endocannabinoids may be the long-awaited retrograde messengers. Importantly, depolarization-induced suppression of excitation (DSE) was also inhibited by a CB1 antagonist at cerebellar excitatory synapses (Kreitzer and Regehr, 2001). Several other forms of synaptic plasticity were subsequently reported to be CB1-dependent including e.g. long-term depression (Fig 2E-F) (Gerdeman et al., 2002; Marsicano et al., 2002; Robbe et al., 2002). Collectively, these findings indicated that endocannabinoid signaling plays a conceptually similar role at distinct types of synapses throughout the brain, and represent the turning point, when endocannabinoid research finally became a major focus for mainstream neuroscience.
Although the most parsimonious scenario is the retrograde route for endocannabinoids, the manner of how anandamide and/or 2-AG get through the extracellular space is still an unresolved issue considering that these lipid messengers are fairly hydrophobic with logP values of 5.1 and 5.39 for anandamide and 2-AG, respectively (Maccarrone, 2008). Nevertheless, converging evidence from the combination of biochemical, anatomical, pharmacological and physiological experiments fully supports the retrograde scenario. As the first step, molecular identification of enzymes involved in endocannabinoid metabolism helped to define which endocannabinoid molecule is responsible for given forms of endocannabinoid-mediated plasticity. For example, the inactivation of anandamide is carried out by a serine hydrolase called fatty acid amide hydrolase (FAAH) (Cravatt et al., 1996). Thus, the lack of effect of FAAH inhibitors in a given paradigm indicates that anandamide is not involved in that particular phenomenon as it was stated for example for hippocampal DSI (Kim and Alger, 2004). The second candidate 2-AG is synthesized by two isoforms of diacylglycerol lipase, α and β, (Bisogno et al., 2003), whereas a monoacylglycerol lipase (MGL) was found to degrade the majority (85%) of 2-AG in the brain (Dinh et al., 2002; Blankman et al., 2007). Genetic inhibition of MGL consistently enhanced short-term synaptic depression (Pan et al., 2011; Straiker et al., 2011), whereas genetic inactivation of DGL-α fully eliminated all forms of endocannabinoid-mediated synaptic plasticity in the prefrontal cortex (Yoshino et al., 2011), hippocampus (Gao et al., 2010; Tanimura et al., 2010), striatum and cerebellum (Tanimura et al., 2010). Thus, the indispensable involvement of DGL-α and the regulatory role of MGL in short-term synaptic depression clearly supports the view that 2-AG is the enigmatic synaptic endocannabinoid.
However, these pharmacological and genetic perturbations would not exclude a scenario that 2-AG plays an autocrine role on axon terminals, where CB1 receptors are located. The additional support derives from anatomical observations, which revealed that the subcellular segregation of endocannabinoid-metabolizing enzymes paves a retrograde way for 2-AG throughout the brain. DGL-α was found postsynaptically at several synapse types in the spinal cord (Nyilas et al., 2009), cerebellum (Yoshida et al., 2006), ventral tegmental area (Mátyás et al., 2008), striatum (Uchigashima et al., 2007), basolateral amygdala (Yoshida et al., 2011), hippocampus (Katona et al., 2006; Yoshida et al., 2006), prefrontal cortex (Lafourcade et al., 2007) and even in the human hippocampus (Ludányi et al., 2011). Conversely, MGL was observed presynaptically in axon terminals throughout the brain (Gulyás et al., 2004; Ludányi et al., 2011; Uchigashima et al., 2011 Yoshida et al., 2011). Taken together, these anatomical and physiological experiments outlined that the most parsimonious scenario for retrograde synaptic signaling involves 2-AG, which is synthesized and released postsynaptically and then acts on CB1 receptors located on nearby presynaptic axon terminals.
Common Principles of Anterograde Amino Acid Transmission and Retrograde Endocannabinoid Transmission
In their influential review, Sudhof and Malenka recently argued that one of the most important advances in our understanding of synaptic transmission in the last 20 years was the discovery that endocannabinoids are the principal mediators of retrograde synaptic communication (Sudhof and Malenka, 2008). Since the breakthrough discoveries ten years ago, several hundred studies have dealt with the role of endocannabinoids in synaptic transmission and the retrograde scenario has become widely accepted. It is vital in most biological signaling systems that information flow is precisely controlled by feed-back mechanisms. Thus, it is not surprising that synaptic transmission in chemical synapses requires a similar feed-back mechanism, although it was clearly unforeseen that the consensus molecule for this function would be an endocannabinoid. Also unexpected was that the basic operational principles of retrograde endocannabinoid signaling have so many features in common with conventional anterograde synaptic transmission. In the following sections, we aim to highlight the striking conceptual similarities between classical amino acid transmitter-mediated neurotransmission and retrograde endocannabinoid signaling arguing that the endocannabinoid system is a component of chemical synapses as basic as the conventional neurotransmitter systems (Table 1). It is impossible in a single review to detail these biological phenomena, hence, we picked a few notable examples to summarize key features of endocannabinoid signaling together with some physiological and pathophysiological implications focusing on burning questions in endocannabinoid research.
Table 1.
Analogies can be observed in many respects between anterograde transmission mediated by classical amino acid transmitters and endocannabinoid-mediated retrograde signaling. See main list for abbreviations.
| Anterograde transmission Classical amino acid transmitters |
Retrograde transmission Endocannabinoids |
|
|---|---|---|
| Multiple transmitters | Glu and GABA | AEA and 2-AG |
| Basic biomolecules | Amino acids are also used as protein building blocks. | Glycerol, arachidonic acid, ethanolamine are widely used for e.g. energy produc- tion, and as metabolic intermediers. |
| Can be metabolized to each other | Decarboxylation of Glu leads to GABA. | 2-AG is degraded to arachidonic acid, which is conjugated with ethanolamine to form AEA. AEA level is reduced upon perturbation of 2-AG biosynthesis. |
| Multiple synthesizing enzymes | Glu synthesized by AAT or glutaminase in neurons. GABA can be synthesized either by GAD65 or GAD67. | 5 biosynthetic routes were postulated for AEA. DAG as precursor for 2-AG is hydrolyzed by DGL-α or DGL-β. Lyso-PI may also be a precursor for 2-AG. |
| More than one receptor families | Glu: 3 ionotropic receptor families, 8 mGluRs. GABA: GABAA, GABAB, GABAC. | AEA: full agonist on TRPV1, partial on CB1 and CB2. Acts on potassium channels, NMDA or Gly receptors. 2-AG: full agonist on CB1 and CB2, desensitizes Gly receptors. |
| Several receptor compositions | Subunit composition of iGluRs define e.g. Ca2+-permeability. GABAA subunits underlie tonic or phasic GABA signaling. | Hetero-dimerization of CB1, e.g. with angiotensin, orexin, GABAB, dopamine or opiate receptors. |
| Additional receptor regulation | Glycine, D-serine are endogenous allosteric regulators of NMDA receptors, neurosteroids act on GABAA receptors. | Endogenous antagonist of CB1: virodhamine (but an agonist of CB2). Hemopressin: inverse agonist of CB1. |
| Activity-dependent receptor regulation | Lateral trafficking or internalization of AMPA subunits in LTP and LTD are well-characterized examples | Lateral movement and internalization regulate CB1 availability on axons. Decoupling of Gq/11-coupled GPCRs from DGL-α by short Homer isoform. |
| Tonic regulation of neuronal activity | Ambient GABA targets specific GABAA receptors and evokes tonic inhibition of target cells. | Tonic endocannabinoid levels regulate transmitter release probability in several brain areas and cell types. |
| Homosynaptic effects and its plasticity | Glu and GABA transmission is involved and undergo homosynaptic plasticity. | 2-AG is a consensus mediator of homosynaptic short-term depression. |
| Heterosynaptic effects and its plasticity | Both glutamateric and GABAergic transmission are known to mediate several forms of heterosynaptic plasticity. | 2-AG has been shown to mediate heterosynaptic depression between glutamatergic and GABAergic synapses in several brain areas. |
| Synaptic homeostasis | Tonic GABAA replaces 1(h) in synaptic homeostasis. Increased mEPSC frequency is a hallmark of synaptic gain. | Anandamide and 2-AG both mediate synaptic homeostasis. |
| Autocrine effects | Glu affects its own release via group II and III mGluRs. GABA via GABA(B). | Slow-self inhibition characterizes the autocrine effects of 2-AG. |
| Neuron-glia communication | Glu and GABA are accepted gliotransmitters, glial cell types express several Glu and GABA receptors. | Glial cells release both AEA and 2-AG. Neuronal 2-AG controls Ca2+-signaling via CB1 receptors on astrocytes. |
Molecular Complexity of Endocannabinoid Mobilization and Degradation
Why the brain needs - at least - two endocannabinoid molecules? Neurons exploit several messengers to operate anterograde synaptic transmission in the brain, predominantly the classical amino acids glutamate and GABA, but glycine and the aromatic amino acid derivative monoamines such as dopamine, serotonin or noradrenaline have also important functions. As retrograde messenger, 2-AG is the key player in most forms of homo- and heterosynaptic short-term depression and in some forms of long-term depression (Heifets and Castillo, 2009; Kano et al., 2009). It is clear however that anandamide also acts through CB1 receptors in several neurobiological paradigms (Kinsey et al., 2009; Clapper et al., 2010; Straiker et al., 2011), where it may function as a retrograde synaptic messenger and mediate certain forms of synaptic homeostasis and plasticity in presynaptic CB1 receptor-dependent manner (Gerdeman et al., 2002, Kim and Alger, 2010). Alternatively, anandamide’s synaptic function can also be the activation of postsynaptic TRPV1 receptors (Chavez et al., 2010; Grueter et al., 2010). The division of labor between anandamide and 2-AG may be reflected in spatial segregation, if anandamide and 2-AG serve as messengers at different synapses, in distinct microcircuits, or in separate brain regions. This is supported by observations that specific FAAH and MGL inhibitors recapitulate only subsets of behavioral components of cannabinoid effects in vivo (Long et al., 2009a). These behaviors are regulated by CB1 receptors located in distinct cell types and brain circuits (Monory et al., 2007), and spatial isolation also occurs at the subcellular level, because MGL and FAAH are segregated into the presynaptic and postsynaptic domains of neurons, respectively (Gulyás et al., 2004). The division of labor may also happen at different time scales. Phasic endocannabinoid signaling, such as DSI is mediated by 2-AG (Kim and Alger, 2004, Tanimura et al., 2010), whereas tonic endocannabinoid signaling involves the mobilization of both 2-AG (Hashimotodani et al., 2007) and anandamide (Kim and Alger, 2010) at hippocampal GABAergic synapses, though likely under different physiological conditions. Finally, the two signaling systems may even interplay in certain behavioral processes. Neither FAAH, nor MAGL inhibitors alone could recapitulate catalepsy or drug discrimination, typical CB1 agonist-evoked behavioral effects. However, a dual FAAH/MGL inhibitor produces catalepsy and also Δ9-THC-like drug discrimination response (Long et al., 2009b). Thus, the combined action of 2-AG and anandamide may be required to fully engage CB1 receptors at specific synapses, and if the mobilization of two messenger molecules require different physiological signals, then presynaptic CB1 receptors may operate as coincidence detectors to underlie synaptic plasticity analogously as postsynaptic NMDA receptors.
The level of complexity in the operational principles and functional significance of retrograde endocannabinoid signaling is further increased by the emerging concept that, in contrast to the primarily amino acid-based anterograde transmission, a multifaceted lipid signaling system evolved to fulfill the complex physiological tasks reliant on retrograde signaling. For example, both anandamide and 2-AG are oxygenated by cyclooxygenase-2 (COX-2) at postsynaptic sites (Yu et al., 1997; Kozak et al., 2000; Kim and Alger, 2004; Straiker et al., 2011). The resulting prostanoids, e.g. prostaglandin E2 glycerol ester (PGE2-G) increase neurotransmitter release from axon terminals (Yang et al., 2008), which is the opposite effect to that of 2-AG. Thus, COX-2, which is transported to synapses in an activity-dependent manner may be an important molecular switch to change the direction of synaptic plasticity. Therapeutically important in vivo manifestation of this phenomenon occurs in supraspinal and spinal pain circuitries, where 2-AG has an overall anti-nociceptive effect (Hohmann et al., 2005; Nyilas et al., 2009), whereas 2-AG-derived PGE2-G causes hyperalgesia (Hu et al., 2008). Remarkably, the highly potent analgesic effects of nonsteroidal anti-inflammatory drugs (NSAIDs) like paracetamol (acetaminophen), a selective COX-2 inhibitor (Hinz et al., 2008), or ibuprofen, a potent inhibitor of 2-AG oxidation by COX-2 (Prusakiewicz et al., 2009) require the activation of CB1 receptors (Ahn et al., 2007; Telleria-Diaz et al., 2010). Thus, these NSAIDs may partially exhibit their analgesic effects via vetoing the metabolism from anti-nociceptive 2-AG to hyperalgesic prostaglandin caused by hyperalgesia-induced elevations in COX-2 activity.
The discovery of 2-epoxyeicosatrienoylglycerols (2-EGs) also revealed a link to lipoxygenase and cytochrome P450 pathways. These novel lipids are present in the brain (especially 2-11,12-EG) and surprisingly, they are potent endogenous ligands of both cannabinoid receptors in vivo (Chen et al., 2008). Anandamide can be transformed to 5′,6′-epoxyeicosatrienoic acid by CP450 epoxygenases, and activates TRPV4 channels at submicromolar concentrations (Watanabe et al., 2003a), whereas the related eicosanoid 12-(S)-hydroperoxyeicosatetraenoic acid is the retrograde mediator of TRPV1-dependent long-term depression at hippocampal Schaffer collateral-interneuron synapses (Gibson et al., 2008). Although the puzzling question, why lipids are utilized so extensively as retrograde messengers in contrast to the predominantly amino acid-based anterograde transmitters may not be easy to answer, this perplexing diversity of lipid signaling pathways should definitely be in focus for neuroscience.
A striking example of parsimony in biology is the way in which neurons exploit amino acids (like glutamate or glycine) as neurotransmitters, which are otherwise basic building blocks of proteins. These amino acids can even be metabolized to each other to produce additional messengers, as GABA is synthesized from glutamate by decarboxylation. This parsimony is also shared by the endocannabinoid system. Glycerol, ethanolamine and arachidonic acid are at the crossroads of several metabolic pathways, included in several major components of biological membranes or provide energy for cellular functions. Arachidonic acid, the common constituent of 2-AG and anandamide is a conditionally essential n-6 polyunsaturated fatty acid (n-6 PUFA) and makes up a significant fraction of brain lipids (Rapoport, 2008). It is present in the diet together with its precursor linoleic acid, and consequently, when their dietary concentrations are increased, anandamide and 2-AG levels also increase (Watanabe et al., 2003b). Remarkably, this has an impact on synaptic endocannabinoid signaling, because changing the dietary n-6/n-3 PUFA ratio abolishes endocannabinoid-mediated LTD in the prefrontal cortex and nucleus accumbens (Lafourcade et al., 2011). Although the underlying mechanistic process is not fully understood, chronic elevation of 2-AG levels also disrupts CB1-mediated signaling highlighting the importance of precise metabolic regulation of 2-AG levels in the brain (Chanda et al., 2010; Schlosburg et al., 2010). Anandamide and 2-AG can also be metabolized to each other like amino acid transmitters and this metabolic pathway can even be region- and subcellular domain-specific. First, anandamide levels are decreased in the hippocampus, but not in the prefrontal cortex of DGL-α knockout animals (Gao et al., 2010, Tanimura et al., 2010, Yoshino et al., 2011). Second, free arachidonic acid can be released from 2-AG by at least three serine hydrolases (Blankman et al., 2007), and one of these, α/β-hydrolase-6 (ABHD6) regulates synaptic endocannabinoid signaling in long-term depression (Marrs et al., 2010). Moreover, ABHD6 colocalizes postsynaptically in dendrites with FAAH, which conjugates ethanolamine with arachidonic acid to form anandamide (Mukhopadhyay et al., 2011).
The biogenesis of endocannabinoids is also highly complex. Anandamide and related N-acylethanolamines (NAEs) were postulated to be synthesized by at least 5 metabolic pathways. Using N-acyl-phosphatidylethanolamines (NAPEs) as precursors, the PLA route involves phospholipase A2 and lysophospholipase D (Sun et al., 2004), the PLB route includes α/β-hydrolase 4 and glycerophosphodiesterase 1 (Simon and Cravatt, 2006; Simon and Cravatt, 2008), the PLC route is mediated by a phospholipase C and protein tyrosine phosphatase type-22 (Liu et al., 2006), whereas the PLD route exploits NAPE-hydrolyzing phospholipase D (NAPE-PLD) (Okamoto et al., 2004). In addition, anandamide can be formed by conjugation of arachidonic acid and ethanolamine in brain synaptosomes (Devane and Axelrod, 1994). A most exciting question for endocannabinoid research is to understand the functional significance of this metabolic diversity. Spatial segregation of distinct anandamide- and NAE-synthesizing pathways may underlie division of labor. Indeed, while FAAH is distributed in the somatodendritic domain of neurons (Egertova et al., 1998, Gulyas et al., 2004), NAPE-PLD was found inside glutamatergic axon terminals (Nyilas et al., 2008). Another indication of functional diversity is the different kinetics of anandamide synthesis, which suggests that the PLC route may operate at a different time scale than the PLB route (Liu et al., 2008).
The biosynthesis of 2-AG may seem to be more simple, but this can be misleading. While the role of DGL-α in synaptic plasticity is unequivocal (Tanimura et al., 2010), the precursor DAG can be synthesized in several ways. The canonical pathway includes phospholipase C-βs, which hydrolyze phophatidylinositol 4,5-bisphosphate (PIP2) to inositol 1,4,5-triphosphate (IP3) and DAG (Stella et al., 1997). Most receptor-driven 2-AG synthesis may go through this route. However, PLCβ1 is not required for depolarization-induced forms of endocannabinoid-mediated synaptic plasticity such as hippocampal DSI (Hashimotodani et al., 2005). DAG-independent 2-AG biosynthesis may occur via PLA1 and lyso-PI-specific PLC activity, the latter of which is found in brain synaptosome preparations (Tsutsumi et al., 1994; Sugiura et al., 1995). Finally, brain homogenates can also synthesize 2-AG from 2-arachidonoyl-lysophosphatidic acid by an unidentified phosphatase (Nakane et al., 2002). Thus, another important task for endocannabinoid research is to dissect which biochemical pathways are responsible for 2-AG mobilization at given locations in neuronal networks and to identify which physiological or pathophysiological stimuli activate these pathways.
A spatial segregation indicating functional division of labor has already been reported for the two isoforms of DGL (Bisogno et al., 2003). While DGL-α is found in the plasma membrane (Katona et al., 2006, Yoshida et al., 2006; Jung et al., 2011), DGL-β is restricted to intracellular membrane segments, including peri-nuclear lipid droplets (Jung et al., 2011). This spatial segregation suggests that DGL-α may play a role in intercellular 2-AG signaling, whereas DGL-β has an intracellular function. In parallel, basal 2-AG levels in the brain of DGL-α knockout mice were dropped by 80%, but remained unaffected or only partially reduced in DGL-β knockout mice (Gao et al., 2010, Tanimura et al., 2010). In contrast to DGL-α knockout mice, synaptic endocannabinoid signaling was not impaired by genetic inactivation of DGL-β (Gao et al., 2010; Tanimura et al., 2010). It is interesting to note that neurite outgrowth triggered by overexpression of DGL-α could be blocked by a CB1 receptor antagonist, whereas neuritogenesis induced by overexpression of DGL-β was CB1 receptor-independent (Jung et al., 2011). Similarly, adult neurogenesis is impaired in DGL-α, but not in DGL-β knockout mice (Gao et al., 2010).
Endocannabinoid Signalosomes
The convincing demonstration that DGL-α is indispensable for various forms of retrograde synaptic signaling (Tanimura et al., 2010) calls for investigations of how this important enzyme is integrated into neuronal operations. At hippocampal glutamatergic synapses, DGL-α accumulates around the postsynaptic density at the edge of synapses (Katona et al., 2006; Yoshida et al., 2006). Group I mGlu receptors are located within the same perisynaptic annulus (Lujan et al., 1996) and their agonists evoke 2-AG release through the canonical Gq/11 and PLC-β pathway (Jung et al., 2005). Because both mGlu receptors and DGL-α contain binding motifs for the synaptic scaffold protein Homer (Jung et al., 2007), we proposed that they form a macromolecular complex around the postsynaptic density, the so-called perisynaptic signaling machinery (PSM), which evolved to translate excess presynaptic activity - glutamate spillover in this case - into a negative feed-back signal (Katona and Freund, 2008). Since then, new findings confirmed and extended the PSM concept. PLC-β1, another molecular constituent of this pathway was also found in the perisynaptic annulus (Fukaya et al., 2008). Moreover, the long-isoform Homer2b turned out to be necessary for mGlu-triggered 2-AG release (Won et al., 2009, Roloff et al., 2010), whereas the activity-dependent short isoform (Homer1a) dismantled the PSM and ablated this process (Roloff et al., 2010).
Importantly, DGL-α may not only function as a 2-AG-synthesizing enzyme, but could play another role in regulating DAG levels. This function may even be phylogenetically more ancient, because insects lack cannabinoid receptors, but express a DGL-α ortholog encoded by the inaE gene, which is necessary for the opening of TRP channels by DAG (Leung et al., 2008). In mammals, TRP channels like TRPC1 or TRPC3 are also known to be anchored by Homer, concentrated perisynaptically, and stimulated by DAG (Kim et al., 2003, Yuan et al., 2003). In addition, activation of TRPC channels accounts for group I mGlu receptor-triggered feed-forward enhancement of excitability of postsynaptic neurons (Kim et al., 2003; Hartmann et al., 2008). Taken together, some molecular players within the PSM are primarily responsible for feed-forward excitation. However this signal is controlled by DGL-α, which may act as a molecular switch by transforming a feed-forward excitatory DAG signal into a negative feed-back signal via 2-AG (Fig. 3). Perisynaptic metabotropic glutamate receptors cannot be activated by single synaptic volleys as intrasynaptic ionotropic glutamate receptors, but require elevated, usually bursting presynaptic population activity (Tempia et al., 1998; Fan et al., 2010). Therefore, we propose that the essential physiological function of the PSM domain of excitatory synapses is to monitor the magnitude of presynaptic activity. Increased presynaptic activity may need to be transformed to a feed-forward excitatory response to increase the excitability of the postsynaptic neuron and to support synaptic potentiation. However, excess presynaptic activity can also become pathological, hence efficient negative feed-back mechanisms should kick in. We suggest that DGL-α may be involved in this mechanism in a strikingly parsimonious manner by terminating feed-forward excitation and initating feed-back inhibition, in other words, by eliminating the cause and the consequence of the excess presynaptic activity at the same time (Fig. 3A-B).
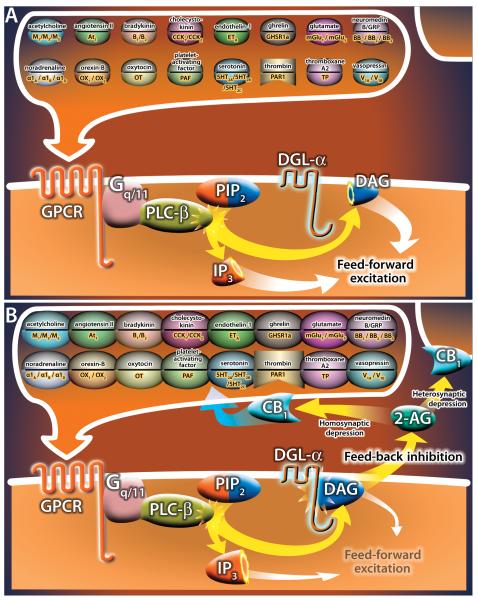
Figure 3. DGL-α as a molecular switch between GPCR-mediated feed-forward excitation and feed-back inhibition.
A) The cascade of events triggered by a multitude of ligands via the activation of GPCRs coupled to Gq/11 and PLC-β at low agonist concentrations: PLC-β will split PIP2 into IP3 and DAG, both of which enhance excitability of the target cell e.g. by stimulating calcium release from intracellular stores or via TRP channels, respectively. B) The cascade of events triggered by a multitude of ligands via the activation of GPCRs coupled to Gq/11 and PLC-β at high agonist concentrations: larger amount of DAG is produced, in which case DGL-α will step in, and convert DAG to 2-AG that will mediate feed-back inhibition in the form of homo- or heterosynaptic depression of transmitter release.
We recently termed the second, 2-AG leg of the pathway a “synaptic circuit-breaker” to indicate that this process may not only happen at single synapses in a homosynaptic manner, but may be a general network mechanism that has a pivotal role in regulating the overall level of network excitability under pathophysiological conditions with an excess glutamatergic tone (Katona and Freund, 2008). Neuronal insults e.g. convulsions or closed-head injury evoke 2-AG release (Panikashvili et al., 2001; Wettschureck et al., 2006), whereas perturbations of the synaptic circuit-breaker leads to reduced seizure thresholds and increased incidence of epileptic seizures. Double Gq/11 and PLC-β1 knockout animals die at a young age as a result of spontaneous seizures (Kim et al., 1997; Wettschureck et al., 2006), whereas glutamatergic cell-specific overexpression or deletion of CB1 receptors are protective or convulsive, respectively (Marsicano et al., 2003; Monory et al., 2006; Guggenhuber et al., 2010). Finally, breakdown of the circuit-breaker also occurs in human patients with chronic, intractable temporal lobe epilepsy, whose hippocampus has reduced levels of DGL-α and CB1 receptors (Ludányi et al., 2008). Taken together, the significance of the PSM and retrograde 2-AG signaling is also reflected at the pathophysiological level and may be exploited for therapy in the future.
A salient emerging concept is that the PSM at glutamatergic synapses may only be a specialized case of a much more fundamental cell physiological mechanism involving the same macromolecular complex and retrograde endocannabinoid signaling. Following the initial observations that Gq/11-coupled, postsynaptic mGlu1 and mGlu5 receptors can elicit synaptic endocannabinoid signaling in the cerebellum and hippocampus (Maejima et al., 2001; Varma et al., 2001), at least 16 other neurotransmitter molecules were shown to trigger 2-AG release and CB1 receptor activation via Gq/11-coupled receptors, PLC-β and DGL-α (Fig 3). Notably, besides the homosynaptic feed-back processes, upstream activation of this macromolecular complex can often lead to heterosynaptic depression of the release of another neurotransmitter as is the case for serotonin and 5HT2A/5HT2B/5HT2C receptors in the inferior olive and elsewhere (Parrish and Nicolls, 2006; Best and Regehr, 2008). These signalosomes may not always be restricted to the perisynaptic zone of synapses, but their subcellular location reflects the source and chemical nature of the given transmitter. For example acetylcholine, which primarily reaches its receptors by volume transmission can evoke endocannabinoid signaling in several brain regions through M1 receptors, which are distributed throughout the somatodendritic surface of postsynaptic neurons (Kim et al., 2002; Uchigashima et al., 2007; Yamasaki et al., 2010). Some of these signaling mechanisms can be surprisingly cell type-specific as has been shown for the neuropeptide cholecystokinin and CCK2 receptors in the hippocampus (Lee and Soltesz, 2011; Lee et al., 2011). Some may convey information about the general physiological or metabolic state of the animal like the wake-promoting peptide orexin-B and its Gq/11-coupled receptors OX1 and OX2 (Haj-Dahmane and Shen, 2005), endothelin-1 and its ETA receptor (Zampronio et al., 2010), oxytocin and its OT receptor (Oliet et al., 2007) or ghrelin and its receptor (Kola et al., 2008), and then regulate feed-back hormonal responses via the modification of synaptic weights in subcortical and cortical circuits. Particularly interesting is how these signalosomes may be involved in pathophysiological processes upon CNS injury. Thrombin-induced arachidonic acid release led to the original discovery of a PLC-DGL pathway (Bell et al., 1979), and thrombin regulates GABAergic synaptic currents through PAR-1 receptors and retrograde 2-AG signaling in the hippocampus (Hashimotodani et al., 2011). Other functionally related pathways also stimulate endocannabinoid signaling as has been demonstrated for thromboxane A2 via the prostanoid receptor TP (Rademacher et al., 2005), and for the platelet-activating factor through its receptor PAF (Berdyshev et al., 2001). Some other GPCR-endocannabinoid signalosomes are also expected to be found in the CNS, as they are widely distributed throughout the body such as angiotensin II and its AT1 receptor (Turu et al., 2009), the bombesin’s neuromedin B, gastrin-releasing peptide and its BB1/BB2/BB3 receptors (Shimizu et al, 2011), bradykinin and its B1 and B2 receptors (Turu et al., 2009), noradrenaline and the adrenoceptors α1A α1B α1D (Turu et al., 2009) and vasopressin and its V1A and V1B receptors (Turu et al., 2009). It may be too early to claim that the DGL-α - 2-AG - CB1 (and maybe also CB2) endocannabinoid signaling pathway is a built-in feature of the downstream signaling pathway upon Gq/11-coupled receptor activation, nevertheless, when present, it can efficiently translate a feed-forward signal into a feed-back signal to regulate cellular functions.
Molecular Complexity of Endocannabinoid-targeted Receptors
As a conceptual similarity again with classical amino acid transmitters, which act on several ionotropic (AMPA, NMDA, kainate or GABAA, GABAC and Gly) and metabotropic (mGlu or GABAB) receptors to accomplish their diverse responsibilities, these lipid messengers can also interact with several other molecular targets besides CB1 and CB2 cannabinoid receptors. Among these potential targets are ligand-gated ion channels like 5-HT3, glycine and nicotinic acetylcholine receptors, non-selective cation channels like TRPV1, TRPA1 or TRPM8, voltage-gated ion channels like T-type calcium channels or the TASK potassium channels, and metabotropic receptors like GPR55 (for review see Pertwee et al., 2010). An especially exciting research direction is to delineate the neurobiological significance of these interactions, which, despite some promising progress (Chavez et al., 2010; Grueter et al., 2010), is largely unknown (Pertwee et al., 2010).
An additional level of signaling complexity for anterograde transmission comes from the variable subunit compositions of amino acid receptors, some well-known examples of which are the synapse-specific segregation of calcium-permeable AMPA receptors determined by absence of the GluR2 subunit (Tóth and McBain, 1998), or the observation that tonic and phasic modes of GABA signaling are mediated by GABAA receptors with different subunit compositions (Glykys and Mody, 2007). A potential mechanism to increase the complexity of endocannabinoid signaling may be the phenomenon of receptor heteromerization. Heterodimers of CB1 receptors have been observed for example with D2 dopamine receptors (Kearn et al., 2005), μ-opioid receptors (Rios et al., 2006) or OX1 orexin receptors (Ellis et al., 2006). Heteromerization may impact ligand sensitivity, downstream signaling and even compartmentalization of a given receptor, which all contribute to the ultimate physiological role of the receptor complex. Thus, it will be a very important task to exploit the latest available microscopy techniques offering appropriate spatial resolution like super-resolution microscopy to characterize the cell type- and synapse type-specific distributions of given cannabinoid receptor heterodimers in brain circuits.
It is widely accepted that besides their primary ligands, the activity of most receptors is controlled by endogenous allosteric modulators. Some famous examples for glutamate receptors include the role of glycine or D-serine in the regulation of NMDA receptors (Johnson and Ascher, 1987; Kleckner and Dingledine, 1988), or the profound impact of neurosteroids on δ-subunit-containing GABAA receptors (Stell et al., 2003). Similar endogenous modulators for cannabinoid receptors are just starting to appear. The lipid virodhamine was the first reported endogenous antagonist of CB1 receptors, which surprisingly acts as an agonist on CB2 receptors (Porter et al., 2002). An unexpected new family of CB1 receptor modulators comprises the hemoglobin-derived nonapeptide hemopressin and its longer congeners, which act as an inverse agonist or agonist, respectively (Heimann et al., 2007; Gomes et al., 2009). The potential presence of allosteric binding sites on CB1 receptors and the design of selective agents targeting these sites would be especially advantageous, because full agonists evoke robust internalization of cannabinoid receptors (Jin et al., 1999), which lead to in vivo tolerance (Tappe-Theodor et al., 2007), and renders pharmacological exploitation difficult. On the other hand, internalization of presynaptic CB1 receptors on axon terminals offers a new level of physiological control (Coutts et al., 2001), whereby the efficacy of endocannabinoid-mediated synaptic plasticity can be dynamically adjusted in an activity-dependent manner. Besides internalization, lateral movement of CB1 receptors on the surface of axon terminals is also well-suited to regulate CB1 receptor availability and desensitization (Mikasova et al., 2008). Notably, lateral mobility and internalization of postsynaptic AMPA receptors are also key underlying mechanisms for experience-dependent plasticity of anterograde excitatory synaptic transmission (Newpher and Ehlers, 2009).
Functional Complexity of Endocannabinoid-mediated Synaptic Plasticity
Probably the most spectacular evidence for the profound neurobiological significance of endocannabinoid signaling is the wide repertoire of synaptic physiological processes which are mediated by endocannabinoids. It is again conceivable to suppose that nature followed the same rule of parsimony just as for glutamatergic and GABAergic neurotransmission by adapting the activity of the same conserved molecular players throughout the central and peripheral nervous system to accomplish so many different synaptic physiological tasks for the proper operation at the brain circuit levels. It is out of the scope of this work to summarize the hundreds of studies describing the specific function of retrograde endocannabinoid signaling from the spinal cord to the neocortex, but we recommend two excellent reviews for further reading (Heifets and Castillo, 2009; Kano et al., 2009). Instead, we aim to illustrate with a few select examples the conceptual similarities in which the brain exploits endocannabinoids for retrograde signaling processes, whereas classical amino acid transmitters for anterograde communication.
Regarding the two major modes of endocannabinoid signaling, one has to differentiate between tonic and phasic actions. Just as ambient GABA has a crucial role in establishing the excitability of postsynaptic neurons through extrasynaptic GABAA receptors (Glykys and Mody, 2007), the pivotal role of ambient extracellular endocannabinoid concentrations in the determination of neurotransmitter release probability has just begun to unfold. Paired recordings from a special subtype of GABAergic interneuron and postsynaptic CA3 pyramidal neurons in the hippocampus revealed that tonic endocannabinoid signaling can mute GABA release from axon terminals through CB1 receptor activation (Losonczy et al., 2004). Subsequent research has shown that this phenomenon is not due to the constitutive activity of CB1 receptors per se, but depends on the constitutive release of endocannabinoids from the postsynaptic neuron, as postsynaptic BAPTA chelation of intracellular Ca2+ signals abolished the tonic endocannabinoid signaling (Hentges et al., 2005; Neu et al., 2007). Remarkably, tonic endocannabinoid signaling is also cell type-specific. It can depend on the type of the postsynaptic neuron, e.g. proopiomelanocortin (POMC) neurons, but not the neighboring non-POMC neurons, release endocannabinoids constitutively and regulate their incoming GABAergic inputs in the arcuate nucleus of the hypothalamus, although both cell populations could produce endocannabinoids in a stimulation-dependent manner (Hentges et al., 2005). Alternatively, the same postsynaptic neuron can also regulate GABAergic inputs in a different manner as has been elegantly demonstrated in the hippocampus, where CA1 pyramidal neurons regulate perisomatic inhibition via endocannabinoids both in a tonic and phasic manner, whereas only phasic endocannabinoid signaling was found at dendritic inhibitory inputs (Lee et al., 2010). This latter observation at unitary connections also confirms that even constitutive endocannabinoid release can act in a homosynaptic and subcellularly restricted manner (Neu et al., 2007; Lee et al., 2010). Tonic endocannabinoid signaling may involve both anandamide and 2-AG, although probably at different time scales (Hashimotodani et al., 2007; Kim and Alger, 2010). The presence of tonic endocannabinoid control of neurotransmitter release indicates that physiological signals must exist to override it whenever necessary. On the other hand, this phenomenon can also serve to integrate the efficacy of a dedicated unitary connection into network activity, because high-frequency firing of the presynaptic neuron can eliminate the tonic endocannabinoid blockade (Losonczy et al., 2004; Foldy et al., 2006).
Salient features of synaptic endocannabinoid signaling ideally support the induction of changes in synaptic strength in a phasic, activity-dependent manner. The governing rules for these retrograde forms of synaptic plasticity also follow similar logic, and these mechanisms are nicely integrated into several well-known forms of anterograde synaptic plasticity mediated by glutamate or GABA. Homosynaptic short-term synaptic depression of excitation and inhibition was the first described form of endocannabinoid-mediated synaptic plasticity (Kreitzer and Regehr, 2001; Ohno-Shosaku et al., 2001; Wilson and Nicoll, 2001), and supposed to be mediated predominantly by 2-AG (Hashimotodani et al., 2007; Gao et al., 2010; Tanimura et al., 2010; Yoshino et al., 2011). Although the extracellular spread of endocannabinoids is limited (Wilson and Nicoll, 2001), endocannabinoid-mediated short-term depression is also involved indirectly in heterosynaptic forms of plasticity (Lourenco et al., 2010). Endocannabinoid-mediated forms of long-term depression were first described in the dorsal and ventral striatum and in the amygdala, but are also present at most synapses in the brain (Gerdeman et al., 2002; Marsicano et al., 2002; Robbe et al., 2002, Heifets and Castillo, 2009; Kano et al., 2009). A special, potentially homosynaptic form is the so-called spike timing-dependent long-term depression, which was described first in the neocortex and requires presynaptic NMDA or postsynaptic mGlu receptors (Sjostrom et al., 2003; Nevian and Sakmann, 2006). Heterosynaptic forms of long-term depression are also known to exploit endocannabinoids (Chevaleyre et al., 2003).
Another special form of synaptic plasticity serves to re-adjust synaptic gain in response to persistent changes of neuronal activity. This phenomenon of synaptic homeostasis involves increase in the action-potential independent release probability of glutamate and in the density of postsynaptic glutamate receptors, but also a decrease in the number of postsynaptic GABA receptors to restore circuit activity (Turrigiano et al., 2007). There are likely to be multiple forms of synaptic scaling and correspondingly, both anandamide and 2-AG was reported to contribute to homeostatic regulation of GABAergic synapses in the hippocampus (Zhang et al., 2009, Kim and Alger, 2010). In addition, just as glutamate and GABA are known to act also in an autocrine manner to regulate their own release, compelling evidence supports that both endocannabinoids may also have autocrine functions. The postsynaptic role of anandamide in long-term depression was postulated in the hippocampus and striatum (Chavez et al., 2010; Grueter et al., 2010), whereas 2-AG is the mediator of the autocrine phenomenon of slow-self inhibition in neocortical interneurons (Marinelli et al., 2008). Although it took some time for neuroscientists to accept that glutamate can also be a gliotransmitter (there are multiple types of glutamate and GABA receptors on glial cells), the idea that molecules can be utilized for multiple physiological functions gained wider recognition. In parallel, exciting new discoveries revealed that endocannabinoid signaling not only depresses, but also potentiates glutamatergic transmission via a novel form of neuron-glia crosstalk (Navarrete and Araque, 2010).
Cell Type- and Synapse-specific Differences in Endocannabinoid Signaling underlying Circuit-dependent Behaviors
In the previous section, we aimed to illustrate the perplexing chemical and functional diversity of the endocannabinoid system in the brain and to highlight that the same neurobiological principles may govern anterograde synaptic transmission and retrograde endocannabinoid signaling. Finally, we consider the idea that subtle, but important refinements in the logic of endocannabinoid signaling were evolved to provide the most optimal contribution of retrograde communication to the functional operation of microcircuits. Probably all brain circuits require detailed studies to fully delineate how given endocannabinoid signalosomes in particular subcellular compartments mediate certain forms of synaptic plasticity and thereby regulate network activity and behavioral processes. Here we describe a few striking examples from the hippocampus (Fig. 4).
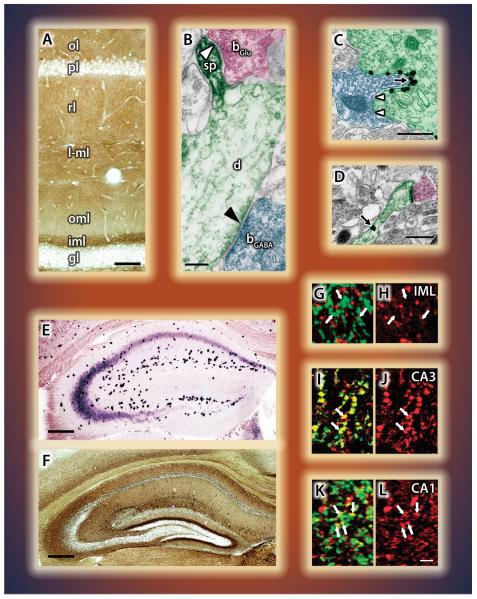
Figure 4. Quantitatively differential distribution of the molecular players of retrograde 2-AG signaling in cortical areas.
A) Laminar distribution of DGL-α in association with glutamatergic synapses in the hippocampus (Katona et al., 2006) and B) in the neocortex (courtesy of Barna Dudok). C,D) In the amygdala, high density of DGL-α occurs at invaginating - but not at flat - GABAergic synapses (C) as well as at glutamatergic (D) axospinous contacts (Yoshida et al., 2011). E) In situ hybridization in the hippocampus reveals high levels of CB1 mRNA in interneurons, lower levels in CA3, and even lower in CA1 pyramidal cells. Dentate granule cells are devoid of labeling. F) Immunostaining for the CB1 receptor protein reveals a striking laminar pattern associated with both GABAergic and glutamatergic terminals (Katona et al., 2006). G-L) Great variability in MGL content between glutamatergic pathways is revealed by double-labeling for vGluT1 (green) and MGL (red). No co-localization in the dentate inner molecular layer (G,H), but a high degree of overlap in recurrent axon terminals in CA3 (I,J), and moderate co-localization in Schaffer collateral terminals in CA1 (K,L) (Uchigashima et al., 2011). The individual figures have been modified from the originals with permission from the authors.
In contrast to the qualitative uniformity of synaptic 2-AG signaling, each glutamatergic and GABAergic synapse is quantitatively different regarding the density of the molecular components of the 2-AG pathway. DGL-α has the highest density in the inner molecular layer at mossy cell-granule cell synapses, is abundant at the Schaffer collateral-CA1 pyramidal neuron synapses, and is localized at other glutamatergic synapses throughout the hippocampal formation (Katona et al., 2006) (Fig. 4A). The functional consequence of this input-specific pattern is reflected in the distinct thresholds necessary to evoke 2-AG mediated DSE at different glutamatergic synapses (Uchigashima et al., 2011). This complexity is further increased at the ultrastructural level, and may underlie the contribution of 2-AG to different homo- or heterosynaptic forms of synaptic plasticity. DGL-α is concentrated in a perisynaptic annulus in the head of dendritic spines in the CA1 subfield (Katona et al., 2006; Yoshida et al., 2006), conversely, it is accumulated around the necks of spines of dentate gyrus granule cells (Uchigashima et al., 2011). In contrast to glutamatergic synapses, only a very small amount of DGL-α is present at hippocampal or neocortical GABAergic synapses (I. Katona pers. comm.), as clearly reflected by the lack of DGL-α immunostaining in the cell body layers (Fig. 4A-B). On the other hand, GABAergic synapses in the basolateral amygdala show an extremely high density of DGL-α (Fig. 4C) (Yoshida et al., 2011).
CB1 receptors also exhibit a characteristic expression pattern and layer-specific distribution (Fig. 4E-F). In contrast to DGL-α, CB1 receptors have the highest density on GABAergic synapses derived from the CCK-positive class of interneurons in the rodent and human hippocampus (Katona et al., 1999; Katona et al., 2000). However, even distinct types of CCK-positive interneurons, the perisomatic basket cells and the Schaffer collateral-associated dendritic inhibitory cells, differ in their CB1 content, and this matches the distinct efficacy of endocannabinoid-mediated synaptic plasticity at these synapses (Lee et al., 2010). CB1 receptors are also present at glutamatergic synapses, albeit at much lower levels (Katona et al., 2006; Kawamura et al., 2006). The distribution of the degrading enzyme MGL displays an even more surprising pattern. While axon terminals of GABAergic interneurons and recurrent collaterals of CA3 pyramidal neurons bear a high density of MGL (Fig. 4I-J), Schaffer collaterals derived from the same CA3 pyramidal cells may contain less MGL (Fig. K-L) (Gulyás et al., 2004; Uchigashima et al., 2011). Moreover, MGL density is strikingly low in the inner molecular layer on axon terminals of mossy cells (Fig. 4G-H) (Ludányi et al., 2011; Uchigashima et al., 2011). Although the physiological consequences of these quantitative differences are not yet fully understood, it suggests that the contribution of 2-AG signaling to distinct behavioral phenomena and pathophysiological processes may be qualitatively different at specific synapses and microcircuits.
The development of cell type-specific CB1 receptor knockout models represent the key innovation to elucidate how endocannabinoid signaling at specific microcircuit locations contributes to network activity and behavior (Marsicano et al., 2003; Monory et al., 2006, Monory et al., 2007). Although GABAergic axon terminals carry much more CB1 receptors than their glutamatergic counterparts, they are not involved in seizure susceptibility (Monory et al., 2006). Instead, these receptors play a pivotal role in Δ9-THC-induced long-term memory deficits (Puighermanal et al., 2009), and protect against age-related cognitive decline (Albayram et al., 2011). A similar cell type-specific functional dichotomy was observed in the regulation of feeding and energy balance, where CB1 receptors on striatal GABAergic neurons reduce food intake, whereas those on forebrain glutamatergic axons convey an orexigenic signal (Bellochio et al., 2010). Opposite function of CB1 receptors on different cell types is also exemplified in the pain transmission circuitry, where deletion of CB1 from primary nociceptive neurons in the dorsal root ganglia proved to be pro-nociceptive, whereas removal from GABA/glycinergic terminals protects from central hyperalgesia (Agarwal et al., 2007; Pernia-Andrade et al., 2009). Collectively, these findings demonstrate that endocannabinoid signaling at different synapses contributes to distinct behavioral components controlled by certain neuronal circuits.
Closing remarks
The ultimate mission of life sciences in the post-genomic era of biology is to provide a full understanding of the function of all molecular players encoded in our genome together with all small-molecule metabolites comprising our metabolome. In neuroscience, we will even need to integrate these emerging data with the cell type-catalog of the brain and functional connectomics. One admitted expectation is that these enormous datasets generated by large-scale community efforts will support systems biology approaches to uncover conceptually new principles of biology, whereas another major force fuelling these efforts is the tremendous potential for evidence-based novel therapeutics. The unfolding of the molecular, anatomical, physiological and behavioral features of endocannabinoid signaling in the last decade fully justifies this expectation, because it has led to our appreciation of the fundamental role of retrograde communication in the brain. Besides its multiple functions, molecular complexity and selective impairment in distinct brain disorders also offer hopes that the endocannabinoid system may be exploited therapeutically.
Acknowledgements
The authors are grateful M. Herkenham, R. Hargraeves, A. Howlett, M. Kano, D. Lovinger, O. Manzoni, R. Mechoulam, R. Nicoll, Y. Shim, K. Van Laere and M. Watanabe for permission to modify figures from their original work. We thank Balázs Baksa for the artwork, Drs. Chris Henstridge, Ewen Legg, Barna Dudok, Eszter Horváth, Balázs Pintér for help with the preparation of the manuscript, and to members of the Katona and Freund labs for discussions. The authors were supported by grants from the Swiss Contribution (SH7/2/18), the Hungarian Scientific Research Fund (NK77793), the Norwegian Financial Mechanism Joint Program (NNF 78918), the European Research Council (No: 243153), and NIH (MH 54671 and NS30549). I.K. is the recipient of a Wellcome Trust International Senior Research Fellowship.
Abbreviations
- 2-AG
2-arachidonoylglycerol
- 2-EG
2-epoxyeicosatrienoylglycerol
- Δ9-THC
(−)-Δ9-tetrahydrocannabinol
- AA
arachidonic acid
- AAT
aspartate aminotransferase
- ABHD6
α/β hydrolase 6
- AEA
anandamide
- Ang
angiotensin
- CNS
central nervous system
- COX-2
cyclooxygenase-2
- DAG
diacylglycerol
- DGL
diacyglycerol lipase
- DSE
depolarization-induced suppression of excitation
- DSI
depolarization-induced suppression of inhibition
- EA
ethanolamine
- FAAH
fatty acid amide hydrolase
- GABA
gamma-aminobutyric acid
- GAD
glutamic acid decarboxylase
- Glu
glutamate
- Gly
glycine
- GPCR
G protein-coupled receptor
- IP3
inositol 1,4,5-triphosphate
- IUPHAR
International Union of Basic and Clinical Pharmacology
- LTD
long-term depression
- lyso-PI
lyso-phosphatidylinositol
- MGL
monoacylglycerol lipase
- nAChR
nicotinic acetylcholine receptor
- NAE
N-acylethanolamine
- NAPE
N-acyl-phosphatidylethanolamine
- NAPE-PLD
N-acyl-phosphatidylethanolamine-hydrolyzing phospholipase D
- NSAID
nonsteroidal anti-inflammatory drug
- PGE2-G
prostaglandin E2 glycerol ester
- PIP2
phophatidylinositol 4,5-bisphosphate
- PLA
phospholipase A
- PLB
phospholipase B
- PLC
phospholipase C
- PLD
phospholipase D
- POMC
proopiomelanocortin
- PSM
perisynaptic signaling machinery
- PUFA
polyunsaturated fatty acid
Terms for mini-glossary
- Retrograde signaling
Retrograde messengers are released from the somatodendritic domain of neurons and then modify release properties of afferent axon terminals or regulate activity in nearby glial processes.
- Depolarization-induced suppression of inhibition or excitation (DSI or DSE)
Depolarized neurons release endocannabinoids that transiently inhibit GABA or glutamate release, respectively from their afferent synaptic terminals.
References
- Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, et al. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci. 2007;10:870–9. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn DK, Choi HS, Yeo SP, Woo YW, Lee MK, et al. Blockade of central cyclooxygenase (COX) pathways enhances the cannabinoid-induced antinociceptive effects on inflammatory temporomandibular joint (TMJ) nociception. Pain. 2007;132:23–32. doi: 10.1016/j.pain.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Albayram O, Alferink J, Pitsch J, Piyanova A, Neitzert K, et al. Role of CB1 cannabinoid receptors on GABAergic neurons in brain aging. Proc Natl Acad Sci U S A. 2011;108:11256–61. doi: 10.1073/pnas.1016442108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Kennerly DA, Stanford N, Majerus PW. Diglyceride lipase: a pathway for arachidonate release from human platelets. Proc Natl Acad Sci U S A. 1979;76:3238–41. doi: 10.1073/pnas.76.7.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellocchio L, Lafenetre P, Cannich A, Cota D, Puente N, et al. Bimodal control of stimulated food intake by the endocannabinoid system. Nat Neurosci. 2010;13:281–3. doi: 10.1038/nn.2494. [DOI] [PubMed] [Google Scholar]
- Berdyshev EV, Schmid PC, Krebsbach RJ, Schmid HH. Activation of PAF receptors results in enhanced synthesis of 2-arachidonoylglycerol (2-AG) in immune cells. FASEB J. 2001;15:2171–8. doi: 10.1096/fj.01-0181com. [DOI] [PubMed] [Google Scholar]
- Best AR, Regehr WG. Serotonin evokes endocannabinoid release and retrogradely suppresses excitatory synapses. J Neurosci. 2008;28:6508–15. doi: 10.1523/JNEUROSCI.0678-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidaut-Russell M, Devane WA, Howlett AC. Cannabinoid receptors and modulation of cyclic AMP accumulation in the rat brain. J Neurochem. 1990;55:21–6. doi: 10.1111/j.1471-4159.1990.tb08815.x. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, et al. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–8. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankman JL, Simon GM, Cravatt BF. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem Biol. 2007;14:1347–56. doi: 10.1016/j.chembiol.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns HD, Van Laere K, Sanabria-Bohorquez S, Hamill TG, Bormans G, et al. [18F]MK-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proc Natl Acad Sci U S A. 2007;104:9800–5. doi: 10.1073/pnas.0703472104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanda PK, Gao Y, Mark L, Btesh J, Strassle BW, et al. Monoacylglycerol lipase activity is a critical modulator of the tone and integrity of the endocannabinoid system. Mol Pharmacol. 2010;78:996–1003. doi: 10.1124/mol.110.068304. [DOI] [PubMed] [Google Scholar]
- Chavez AE, Chiu CQ, Castillo PE. TRPV1 activation by endogenous anandamide triggers postsynaptic long-term depression in dentate gyrus. Nat Neurosci. 2010;13:1511–8. doi: 10.1038/nn.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JK, Chen J, Imig JD, Wei S, Hachey DL, et al. Identification of novel endogenous cytochrome p450 arachidonate metabolites with high affinity for cannabinoid receptors. J Biol Chem. 2008;283:24514–24. doi: 10.1074/jbc.M709873200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–72. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Clapper JR, Moreno-Sanz G, Russo R, Guijarro A, Vacondio F, et al. Anandamide suppresses pain initiation through a peripheral endocannabinoid mechanism. Nat Neurosci. 2010;13:1265–70. doi: 10.1038/nn.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutts AA, Anavi-Goffer S, Ross RA, MacEwan DJ, Mackie K, et al. Agonist-induced internalization and trafficking of cannabinoid CB1 receptors in hippocampal neurons. J Neurosci. 2001;21:2425–33. doi: 10.1523/JNEUROSCI.21-07-02425.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–7. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- Devane WA, Axelrod J. Enzymatic synthesis of anandamide, an endogenous ligand for the cannabinoid receptor, by brain membranes. Proc Natl Acad Sci U S A. 1994;91:6698–701. doi: 10.1073/pnas.91.14.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA, 3rd, Johnson MR, Melvin LS, Howlett AC. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol. 1988;34:605–13. [PubMed] [Google Scholar]
- Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–9. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- Dinh TP, Carpenter D, Leslie FM, Freund TF, Katona I, et al. Brain monoglyceride lipase participating in endocannabinoid inactivation. Proc Natl Acad Sci U S A. 2002;99:10819–24. doi: 10.1073/pnas.152334899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egertova M, Giang DK, Cravatt BF, Elphick MR. A new perspective on cannabinoid signalling: complementary localization of fatty acid amide hydrolase and the CB1 receptor in rat brain. Proc Biol Sci. 1998;265:2081–5. doi: 10.1098/rspb.1998.0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis J, Pediani JD, Canals M, Milasta S, Milligan G. Orexin-1 receptor-cannabinoid CB1 receptor heterodimerization results in both ligand-dependent and -independent coordinated alterations of receptor localization and function. J Biol Chem. 2006;281:38812–24. doi: 10.1074/jbc.M602494200. [DOI] [PubMed] [Google Scholar]
- Elsohly MA, Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005;78:539–48. doi: 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Fan W, Ster J, Gerber U. Activation conditions for the induction of metabotropic glutamate receptor-dependent long-term depression in hippocampal CA1 pyramidal cells. J Neurosci. 2010;30:1471–5. doi: 10.1523/JNEUROSCI.5619-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldy C, Neu A, Jones MV, Soltesz I. Presynaptic, activity-dependent modulation of cannabinoid type 1 receptor-mediated inhibition of GABA release. J Neurosci. 2006;26:1465–9. doi: 10.1523/JNEUROSCI.4587-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaya M, Uchigashima M, Nomura S, Hasegawa Y, Kikuchi H, Watanabe M. Predominant expression of phospholipase Cbeta1 in telencephalic principal neurons and cerebellar interneurons, and its close association with related signaling molecules in somatodendritic neuronal elements. Eur J Neurosci. 2008;28:1744–59. doi: 10.1111/j.1460-9568.2008.06495.x. [DOI] [PubMed] [Google Scholar]
- Gao Y, Vasilyev DV, Goncalves MB, Howell FV, Hobbs C, et al. Loss of retrograde endocannabinoid signaling and reduced adult neurogenesis in diacylglycerol lipase knock-out mice. J Neurosci. 2010;30:2017–24. doi: 10.1523/JNEUROSCI.5693-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of hashish. J. Amer. Chem. Soc. 1964;86:1646–7. [Google Scholar]
- Gaoni Y, Mechoulam R. The absolute configuration of delta-1-tetrahydrocannabinol, the major active constituent of hashish. Tetrahedron Letters. 1967;12:1109–11. doi: 10.1016/s0040-4039(00)90646-4. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Ronesi J, Lovinger DM. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat Neurosci. 2002;5:446–51. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- Gibson HE, Edwards JG, Page RS, Van Hook MJ, Kauer JA. TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron. 2008;57:746–59. doi: 10.1016/j.neuron.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glykys J, Mody I. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron. 2007;56:763–70. doi: 10.1016/j.neuron.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Gobbi G, Bambico FR, Mangieri R, Bortolato M, Campolongo P, et al. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci U S A. 2005;102:18620–5. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffin K, Van Paesschen W, Van Laere K. In vivo activation of endocannabinoid system in temporal lobe epilepsy with hippocampal sclerosis. Brain. 2011;134:1033–40. doi: 10.1093/brain/awq385. [DOI] [PubMed] [Google Scholar]
- Gomes I, Grushko JS, Golebiewska U, Hoogendoorn S, Gupta A, et al. Novel endogenous peptide agonists of cannabinoid receptors. FASEB J. 2009;23:3020–9. doi: 10.1096/fj.09-132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, Brasnjo G, Malenka RC. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat Neurosci. 2010;13:1519–25. doi: 10.1038/nn.2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggenhuber S, Monory K, Lutz B, Klugmann M. AAV vector-mediated overexpression of CB1 cannabinoid receptor in pyramidal neurons of the hippocampus protects against seizure-induced excitoxicity. PLoS One. 2010;5:e15707. doi: 10.1371/journal.pone.0015707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyas AI, Cravatt BF, Bracey MH, Dinh TP, Piomelli D, et al. Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci. 2004;20:441–58. doi: 10.1111/j.1460-9568.2004.03428.x. [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane S, Shen RY. The wake-promoting peptide orexin-B inhibits glutamatergic transmission to dorsal raphe nucleus serotonin neurons through retrograde endocannabinoid signaling. J Neurosci. 2005;25:896–905. doi: 10.1523/JNEUROSCI.3258-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann J, Dragicevic E, Adelsberger H, Henning HA, Sumser M, et al. TRPC3 channels are required for synaptic transmission and motor coordination. Neuron. 2008;59:392–8. doi: 10.1016/j.neuron.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Kano M. Presynaptic monoacylglycerol lipase activity determines basal endocannabinoid tone and terminates retrograde endocannabinoid signaling in the hippocampus. J Neurosci. 2007;27:1211–9. doi: 10.1523/JNEUROSCI.4159-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Tsubokawa H, Ogata H, Emoto K, et al. Phospholipase Cbeta serves as a coincidence detector through its Ca2+ dependency for triggering retrograde endocannabinoid signal. Neuron. 2005;45:257–68. doi: 10.1016/j.neuron.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Hashimotodani Y, Ohno-Shosaku T, Yamazaki M, Sakimura K, Kano M. Neuronal protease-activated receptor 1 drives synaptic retrograde signaling mediated by the endocannabinoid 2-arachidonoylglycerol. J Neurosci. 2011;31:3104–9. doi: 10.1523/JNEUROSCI.6000-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifets BD, Castillo PE. Endocannabinoid signaling and long-term synaptic plasticity. Annu Rev Physiol. 2009;71:283–306. doi: 10.1146/annurev.physiol.010908.163149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimann AS, Gomes I, Dale CS, Pagano RL, Gupta A, et al. Hemopressin is an inverse agonist of CB1 cannabinoid receptors. Proc Natl Acad Sci U S A. 2007;104:20588–93. doi: 10.1073/pnas.0706980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentges ST, Low MJ, Williams JT. Differential regulation of synaptic inputs by constitutively released endocannabinoids and exogenous cannabinoids. J Neurosci. 2005;25:9746–51. doi: 10.1523/JNEUROSCI.2769-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, de Costa BR, Richfield EK. Neuronal localization of cannabinoid receptors in the basal ganglia of rat. Brain Res. 1991;547:267–74. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci U S A. 1990;87:1932–6. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, Miller GE, Carrier EJ, Gorzalka BB, Hillard CJ. Circulating endocannabinoids and N-acyl ethanolamines are differentially regulated in major depression and following exposure to social stress. Psychoneuroendocrinology. 2009;34:1257–62. doi: 10.1016/j.psyneuen.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B, Cheremina O, Brune K. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J. 2008;22:383–90. doi: 10.1096/fj.07-8506com. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin RS, Li CT, Terry GE, Zoghbi SS, et al. Reversible and regionally selective downregulation of brain cannabinoid CB(1) receptors in chronic daily cannabis smokers. Mol Psychiatry. 2011 doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann AG, Suplita RL, Bolton NM, Neely MH, Fegley D, et al. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–12. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- Hu SS, Bradshaw HB, Chen JS, Tan B, Walker JM. Prostaglandin E2 glycerol ester, an endogenous COX-2 metabolite of 2-arachidonoylglycerol, induces hyperalgesia and modulates NFkappaB activity. Br J Pharmacol. 2008;153:1538–49. doi: 10.1038/bjp.2008.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, et al. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry. 2001;58:322–8. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- Jin W, Brown S, Roche JP, Hsieh C, Celver JP, et al. Distinct domains of the CB1 cannabinoid receptor mediate desensitization and internalization. J Neurosci. 1999;19:3773–80. doi: 10.1523/JNEUROSCI.19-10-03773.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–31. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Jung KM, Astarita G, Thongkham D, Piomelli D. Diacylglycerol lipase-alpha and -beta control neurite outgrowth in neuro-2a cells through distinct molecular mechanisms. Mol Pharmacol. 2011;80:60–7. doi: 10.1124/mol.110.070458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KM, Astarita G, Zhu C, Wallace M, Mackie K, Piomelli D. A key role for diacylglycerol lipase-alpha in metabotropic glutamate receptor-dependent endocannabinoid mobilization. Mol Pharmacol. 2007;72:612–21. doi: 10.1124/mol.107.037796. [DOI] [PubMed] [Google Scholar]
- Jung KM, Mangieri R, Stapleton C, Kim J, Fegley D, et al. Stimulation of endocannabinoid formation in brain slice cultures through activation of group I metabotropic glutamate receptors. Mol Pharmacol. 2005;68:1196–202. doi: 10.1124/mol.105.013961. [DOI] [PubMed] [Google Scholar]
- Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. Physiol Rev. 2009;89:309–80. doi: 10.1152/physrev.00019.2008. [DOI] [PubMed] [Google Scholar]
- Katona I, Freund TF. Endocannabinoid signaling as a synaptic circuit breaker in neurological disease. Nat Med. 2008;14:923–30. doi: 10.1038/nm.f.1869. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Magloczky Z, Santha E, Kofalvi A, et al. GABAergic interneurons are the targets of cannabinoid actions in the human hippocampus. Neuroscience. 2000;100:797–804. doi: 10.1016/s0306-4522(00)00286-4. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–58. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona I, Urban GM, Wallace M, Ledent C, Jung KM, et al. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–37. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, et al. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearn CS, Blake-Palmer K, Daniel E, Mackie K, Glass M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: a mechanism for receptor cross-talk? Mol Pharmacol. 2005;67:1697–704. doi: 10.1124/mol.104.006882. [DOI] [PubMed] [Google Scholar]
- Kim D, Jun KS, Lee SB, Kang NG, Min DS, et al. Phospholipase C isozymes selectively couple to specific neurotransmitter receptors. Nature. 1997;389:290–3. doi: 10.1038/38508. [DOI] [PubMed] [Google Scholar]
- Kim J, Alger BE. Inhibition of cyclooxygenase-2 potentiates retrograde endocannabinoid effects in hippocampus. Nat Neurosci. 2004;7:697–8. doi: 10.1038/nn1262. [DOI] [PubMed] [Google Scholar]
- Kim J, Alger BE. Reduction in endocannabinoid tone is a homeostatic mechanism for specific inhibitory synapses. Nat Neurosci. 2010;13:592–600. doi: 10.1038/nn.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci. 2002;22:10182–91. doi: 10.1523/JNEUROSCI.22-23-10182.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Kim YS, Yuan JP, Petralia RS, Worley PF, Linden DJ. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature. 2003;426:285–91. doi: 10.1038/nature02162. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Long JZ, O’Neal ST, Abdullah RA, Poklis JL, et al. Blockade of endocannabinoid-degrading enzymes attenuates neuropathic pain. J Pharmacol Exp Ther. 2009;330:902–10. doi: 10.1124/jpet.109.155465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleckner NW, Dingledine R. Requirement for glycine in activation of NMDA-receptors expressed in Xenopus oocytes. Science. 1988;241:835–7. doi: 10.1126/science.2841759. [DOI] [PubMed] [Google Scholar]
- Kola B, Farkas I, Christ-Crain M, Wittmann G, Lolli F, et al. The orexigenic effect of ghrelin is mediated through central activation of the endogenous cannabinoid system. PLoS One. 2008;3:e1797. doi: 10.1371/journal.pone.0001797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak KR, Rowlinson SW, Marnett LJ. Oxygenation of the endocannabinoid, 2-arachidonylglycerol, to glyceryl prostaglandins by cyclooxygenase-2. J Biol Chem. 2000;275:33744–9. doi: 10.1074/jbc.M007088200. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–27. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- Kunos G, Osei-Hyiaman D, Batkai S, Sharkey KA, Makriyannis A. Should peripheral CB(1) cannabinoid receptors be selectively targeted for therapeutic gain? Trends Pharmacol Sci. 2009;30:1–7. doi: 10.1016/j.tips.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafourcade M, Elezgarai I, Mato S, Bakiri Y, Grandes P, Manzoni OJ. Molecular components and functions of the endocannabinoid system in mouse prefrontal cortex. PLoS One. 2007;2:e709. doi: 10.1371/journal.pone.0000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafourcade M, Larrieu T, Mato S, Duffaud A, Sepers M, et al. Nutritional omega-3 deficiency abolishes endocannabinoid-mediated neuronal functions. Nat Neurosci. 2011;14:345–50. doi: 10.1038/nn.2736. [DOI] [PubMed] [Google Scholar]
- Lee SH, Foldy C, Soltesz I. Distinct endocannabinoid control of GABA release at perisomatic and dendritic synapses in the hippocampus. J Neurosci. 2010;30:7993–8000. doi: 10.1523/JNEUROSCI.6238-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Soltesz I. Requirement for CB1 but not GABAB receptors in the cholecystokinin mediated inhibition of GABA release from cholecystokinin expressing basket cells. J Physiol. 2011;589:891–902. doi: 10.1113/jphysiol.2010.198499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Foldy C, Szabadics J, Soltesz I. Cell-Type-Specific CCK2 Receptor Signaling Underlies the Cholecystokinin-Mediated Selective Excitation of Hippocampal Parvalbumin-Positive Fast-Spiking Basket Cells. J Neurosci. 2011;31:10993–1002. doi: 10.1523/JNEUROSCI.1970-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung HT, Tseng-Crank J, Kim E, Mahapatra C, Shino S, et al. DAG lipase activity is necessary for TRP channel regulation in Drosophila photoreceptors. Neuron. 2008;58:884–96. doi: 10.1016/j.neuron.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JW, Vederas JC. Drug discovery and natural products: end of an era or an endless frontier? Science. 2009;325:161–5. doi: 10.1126/science.1168243. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang L, Harvey-White J, Huang BX, Kim HY, et al. Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology. 2008;54:1–7. doi: 10.1016/j.neuropharm.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, et al. A biosynthetic pathway for anandamide. Proc Natl Acad Sci U S A. 2006;103:13345–50. doi: 10.1073/pnas.0601832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I, Leresche N, Marty A. Calcium entry increases the sensitivity of cerebellar Purkinje cells to applied GABA and decreases inhibitory synaptic currents. Neuron. 1991;6:565–74. doi: 10.1016/0896-6273(91)90059-9. [DOI] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, et al. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat Chem Biol. 2009a;5:37–44. doi: 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long JZ, Nomura DK, Vann RE, Walentiny DM, Booker L, et al. Dual blockade of FAAH and MAGL identifies behavioral processes regulated by endocannabinoid crosstalk in vivo. Proc Natl Acad Sci U S A. 2009b;106:20270–5. doi: 10.1073/pnas.0909411106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonczy A, Biro AA, Nusser Z. Persistently active cannabinoid receptors mute a subpopulation of hippocampal interneurons. Proc Natl Acad Sci U S A. 2004;101:1362–7. doi: 10.1073/pnas.0304752101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lourenco J, Cannich A, Carta M, Coussen F, Mulle C, Marsicano G. Synaptic activation of kainate receptors gates presynaptic CB(1) signaling at GABAergic synapses. Nat Neurosci. 2010;13:197–204. doi: 10.1038/nn.2481. [DOI] [PubMed] [Google Scholar]
- Ludanyi A, Eross L, Czirjak S, Vajda J, Halasz P, et al. Downregulation of the CB1 cannabinoid receptor and related molecular elements of the endocannabinoid system in epileptic human hippocampus. J Neurosci. 2008;28:2976–90. doi: 10.1523/JNEUROSCI.4465-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludanyi A, Hu SS, Yamazaki M, Tanimura A, Piomelli D, et al. Complementary synaptic distribution of enzymes responsible for synthesis and inactivation of the endocannabinoid 2-arachidonoylglycerol in the human hippocampus. Neuroscience. 2011;174:50–63. doi: 10.1016/j.neuroscience.2010.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci. 1996;8:1488–500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Maccarrone M. Good news for CB1 receptors: endogenous agonists are in the right place. Br J Pharmacol. 2008;153:179–81. doi: 10.1038/sj.bjp.0707566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31:463–75. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- Maresz K, Pryce G, Ponomarev ED, Marsicano G, Croxford JL, et al. Direct suppression of CNS autoimmune inflammation via the cannabinoid receptor CB1 on neurons and CB2 on autoreactive T cells. Nat Med. 2007;13:492–7. doi: 10.1038/nm1561. [DOI] [PubMed] [Google Scholar]
- Marinelli S, Pacioni S, Bisogno T, Di Marzo V, Prince DA, et al. The endocannabinoid 2-arachidonoylglycerol is responsible for the slow self-inhibition in neocortical interneurons. J Neurosci. 2008;28:13532–41. doi: 10.1523/JNEUROSCI.0847-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs WR, Blankman JL, Horne EA, Thomazeau A, Lin YH, et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat Neurosci. 2010;13:951–7. doi: 10.1038/nn.2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, et al. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science. 2003;302:84–8. doi: 10.1126/science.1088208. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–4. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–4. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Matyas F, Urban GM, Watanabe M, Mackie K, Zimmer A, et al. Identification of the sites of 2-arachidonoylglycerol synthesis and action imply retrograde endocannabinoid signaling at both GABAergic and glutamatergic synapses in the ventral tegmental area. Neuropharmacology. 2008;54:95–107. doi: 10.1016/j.neuropharm.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Shani A, Edery H, Grunfeld Y. Chemical basis of hashish activity. Science. 1970;169:611–2. doi: 10.1126/science.169.3945.611. [DOI] [PubMed] [Google Scholar]
- Mikasova L, Groc L, Choquet D, Manzoni OJ. Altered surface trafficking of presynaptic cannabinoid type 1 receptor in and out synaptic terminals parallels receptor desensitization. Proc Natl Acad Sci U S A. 2008;105:18596–601. doi: 10.1073/pnas.0805959105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monory K, Blaudzun H, Massa F, Kaiser N, Lemberger T, et al. Genetic dissection of behavioural and autonomic effects of Delta(9)-tetrahydrocannabinol in mice. PLoS Biol. 2007;5:e269. doi: 10.1371/journal.pbio.0050269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monory K, Massa F, Egertova M, Eder M, Blaudzun H, et al. The endocannabinoid system controls key epileptogenic circuits in the hippocampus. Neuron. 2006;51:455–66. doi: 10.1016/j.neuron.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay B, Cinar R, Yin S, Liu J, Tam J, et al. Hyperactivation of anandamide synthesis and regulation of cell-cycle progression via cannabinoid type 1 (CB1) receptors in the regenerating liver. Proc Natl Acad Sci U S A. 2011;108:6323–8. doi: 10.1073/pnas.1017689108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–5. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Nakane S, Oka S, Arai S, Waku K, Ishima Y, et al. 2-Arachidonoyl-sn-glycero-3-phosphate, an arachidonic acid-containing lysophosphatidic acid: occurrence and rapid enzymatic conversion to 2-arachidonoyl-sn-glycerol, a cannabinoid receptor ligand, in rat brain. Arch Biochem Biophys. 2002;402:51–8. doi: 10.1016/S0003-9861(02)00038-3. [DOI] [PubMed] [Google Scholar]
- Navarrete M, Araque A. Endocannabinoids potentiate synaptic transmission through stimulation of astrocytes. Neuron. 2010;68:113–26. doi: 10.1016/j.neuron.2010.08.043. [DOI] [PubMed] [Google Scholar]
- Neu A, Foldy C, Soltesz I. Postsynaptic origin of CB1-dependent tonic inhibition of GABA release at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. J Physiol. 2007;578:233–47. doi: 10.1113/jphysiol.2006.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevian T, Sakmann B. Spine Ca2+ signaling in spike-timing-dependent plasticity. J Neurosci. 2006;26:11001–13. doi: 10.1523/JNEUROSCI.1749-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Spine microdomains for postsynaptic signaling and plasticity. Trends Cell Biol. 2009;19:218–27. doi: 10.1016/j.tcb.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Nyilas R, Dudok B, Urban GM, Mackie K, Watanabe M, et al. Enzymatic machinery for endocannabinoid biosynthesis associated with calcium stores in glutamatergic axon terminals. J Neurosci. 2008;28:1058–63. doi: 10.1523/JNEUROSCI.5102-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyilas R, Gregg LC, Mackie K, Watanabe M, Zimmer A, et al. Molecular architecture of endocannabinoid signaling at nociceptive synapses mediating analgesia. Eur J Neurosci. 2009;29:1964–78. doi: 10.1111/j.1460-9568.2009.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–38. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279:5298–305. doi: 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Baimoukhametova DV, Piet R, Bains JS. Retrograde regulation of GABA transmission by the tonic release of oxytocin and endocannabinoids governs postsynaptic firing. J Neurosci. 2007;27:1325–33. doi: 10.1523/JNEUROSCI.2676-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Wang W, Zhong P, Blankman JL, Cravatt BF, et al. Alterations of endocannabinoid signaling, synaptic plasticity, learning, and memory in monoacylglycerol lipase knock-out mice. J Neurosci. 2011;31:13420–30. doi: 10.1523/JNEUROSCI.2075-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panikashvili D, Simeonidou C, Ben-Shabat S, Hanus L, Breuer A, et al. An endogenous cannabinoid (2-AG) is neuroprotective after brain injury. Nature. 2001;413:527–31. doi: 10.1038/35097089. [DOI] [PubMed] [Google Scholar]
- Parrish JC, Nichols DE. Serotonin 5-HT(2A) receptor activation induces 2-arachidonoylglycerol release through a phospholipase c-dependent mechanism. J Neurochem. 2006;99:1164–75. doi: 10.1111/j.1471-4159.2006.04173.x. [DOI] [PubMed] [Google Scholar]
- Pernia-Andrade AJ, Kato A, Witschi R, Nyilas R, Katona I, et al. Spinal endocannabinoids and CB1 receptors mediate C-fiber-induced heterosynaptic pain sensitization. Science. 2009;325:760–4. doi: 10.1126/science.1171870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB and CB. Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitler TA, Alger BE. Postsynaptic spike firing reduces synaptic GABAA responses in hippocampal pyramidal cells. J Neurosci. 1992;12:4122–32. doi: 10.1523/JNEUROSCI.12-10-04122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter AC, Sauer JM, Knierman MD, Becker GW, Berna MJ, et al. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J Pharmacol Exp Ther. 2002;301:1020–4. doi: 10.1124/jpet.301.3.1020. [DOI] [PubMed] [Google Scholar]
- Prisinzano TE. Natural products as tools for neuroscience: discovery and development of novel agents to treat drug abuse. J Nat Prod. 2009;72:581–7. doi: 10.1021/np8005748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusakiewicz JJ, Duggan KC, Rouzer CA, Marnett LJ. Differential sensitivity and mechanism of inhibition of COX-2 oxygenation of arachidonic acid and 2-arachidonoylglycerol by ibuprofen and mefenamic acid. Biochemistry. 2009;48:7353–5. doi: 10.1021/bi900999z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puighermanal E, Marsicano G, Busquets-Garcia A, Lutz B, Maldonado R, Ozaita A. Cannabinoid modulation of hippocampal long-term memory is mediated by mTOR signaling. Nat Neurosci. 2009;12:1152–8. doi: 10.1038/nn.2369. [DOI] [PubMed] [Google Scholar]
- Rademacher DJ, Patel S, Ho WS, Savoie AM, Rusch NJ, et al. U-46619 but not serotonin increases endocannabinoid content in middle cerebral artery: evidence for functional relevance. Am J Physiol Heart Circ Physiol. 2005;288:H2694–701. doi: 10.1152/ajpheart.00978.2004. [DOI] [PubMed] [Google Scholar]
- Rapoport SI. Arachidonic acid and the brain. J Nutr. 2008;138:2515–20. doi: 10.1093/jn/138.12.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr WG, Carey MR, Best AR. Activity-dependent regulation of synapses by retrograde messengers. Neuron. 2009;63:154–70. doi: 10.1016/j.neuron.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios C, Gomes I, Devi LA. mu opioid and CB1 cannabinoid receptor interactions: reciprocal inhibition of receptor signaling and neuritogenesis. Br J Pharmacol. 2006;148:387–95. doi: 10.1038/sj.bjp.0706757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci U S A. 2002;99:8384–8. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roloff AM, Anderson GR, Martemyanov KA, Thayer SA. Homer 1a gates the induction mechanism for endocannabinoid-mediated synaptic plasticity. J Neurosci. 2010;30:3072–81. doi: 10.1523/JNEUROSCI.4603-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosburg JE, Blankman JL, Long JZ, Nomura DK, Pan B, et al. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci. 2010;13:1113–9. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim JY. Transmembrane helical domain of the cannabinoid CB1 receptor. Biophys Journal. 2009;96:3251–62. doi: 10.1016/j.bpj.2008.12.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Lu L, Yokotani K. Endogenously generated 2-arachidonoylglycerol plays an inhibitory role in bombesin-induced activation of central adrenomedullary outflow in rats. Eur J Pharmacol. 2011;658:123–31. doi: 10.1016/j.ejphar.2011.02.023. [DOI] [PubMed] [Google Scholar]
- Simon GM, Cravatt BF. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for alpha/beta-hydrolase 4 in this pathway. J Biol Chem. 2006;281:26465–72. doi: 10.1074/jbc.M604660200. [DOI] [PubMed] [Google Scholar]
- Simon GM, Cravatt BF. Anandamide biosynthesis catalyzed by the phosphodiesterase GDE1 and detection of glycerophospho-N-acyl ethanolamine precursors in mouse brain. J Biol Chem. 2008;283:9341–9. doi: 10.1074/jbc.M707807200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjostrom PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–54. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- Smith PB, Compton DR, Welch SP, Razdan RK, Mechoulam R, Martin BR. The pharmacological activity of anandamide, a putative endogenous cannabinoid, in mice. J Pharmacol Exp Ther. 1994;270:219–27. [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABAA receptors. Proc Natl Acad Sci U S A. 2003;100:14439–44. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–8. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Straiker A, Wager-Miller J, Hu SS, Blankman JL, Cravatt BF, Mackie K. COX-2 and FAAH can regulate the time course of depolarization induced suppression of excitation. Br J Pharmacol. 2011;164:1672–83. doi: 10.1111/j.1476-5381.2011.01486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC, Malenka RC. Understanding synapses: past, present, and future. Neuron. 2008;60:469–76. doi: 10.1016/j.neuron.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura T, Kondo S, Sukagawa A, Nakane S, Shinoda A, et al. 2-Arachidonoylglycerol: a possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun. 1995;215:89–97. doi: 10.1006/bbrc.1995.2437. [DOI] [PubMed] [Google Scholar]
- Sun YX, Tsuboi K, Okamoto Y, Tonai T, Murakami M, et al. Biosynthesis of anandamide and N-palmitoylethanolamine by sequential actions of phospholipase A2 and lysophospholipase D. Biochem J. 2004;380:749–56. doi: 10.1042/BJ20040031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanimura A, Yamazaki M, Hashimotodani Y, Uchigashima M, Kawata S, et al. The endocannabinoid 2-arachidonoylglycerol produced by diacylglycerol lipase alpha mediates retrograde suppression of synaptic transmission. Neuron. 2010;65:320–7. doi: 10.1016/j.neuron.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Tappe-Theodor A, Agarwal N, Katona I, Rubino T, Martini L, et al. A molecular basis of analgesic tolerance to cannabinoids. J Neurosci. 2007;27:4165–77. doi: 10.1523/JNEUROSCI.5648-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telleria-Diaz A, Schmidt M, Kreusch S, Neubert AK, Schache F, et al. Spinal antinociceptive effects of cyclooxygenase inhibition during inflammation: Involvement of prostaglandins and endocannabinoids. Pain. 2010;148:26–35. doi: 10.1016/j.pain.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Tempia F, Miniaci MC, Anchisi D, Strata P. Postsynaptic current mediated by metabotropic glutamate receptors in cerebellar Purkinje cells. J Neurophysiol. 1998;80:520–8. doi: 10.1152/jn.1998.80.2.520. [DOI] [PubMed] [Google Scholar]
- Toth K, McBain CJ. Afferent-specific innervation of two distinct AMPA receptor subtypes on single hippocampal interneurons. Nat Neurosci. 1998;1:572–8. doi: 10.1038/2807. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Tsutsumi T, Kobayashi T, Ueda H, Yamauchi E, Watanabe S, Okuyama H. Lysophosphoinositide-specific phospholipase C in rat brain synaptic plasma membranes. Neurochem Res. 1994;19:399–406. doi: 10.1007/BF00967316. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Homeostatic signaling: the positive side of negative feedback. Curr Opin Neurobiol. 2007;17:318–24. doi: 10.1016/j.conb.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Turu G, Varnai P, Gyombolai P, Szidonya L, Offertaler L, et al. Paracrine transactivation of the CB1 cannabinoid receptor by AT1 angiotensin and other Gq/11 protein-coupled receptors. J Biol Chem. 2009;284:16914–21. doi: 10.1074/jbc.M109.003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular arrangement of molecules for 2-arachidonoyl-glycerol-mediated retrograde signaling and its physiological contribution to synaptic modulation in the striatum. J Neurosci. 2007;27:3663–76. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchigashima M, Yamazaki M, Yamasaki M, Tanimura A, Sakimura K, et al. Molecular and morphological configuration for 2-arachidonoylglycerol-mediated retrograde signaling at mossy cell-granule cell synapses in the dentate gyrus. J Neurosci. 2011;31:7700–14. doi: 10.1523/JNEUROSCI.5665-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Laere K, Casteels C, Dhollander I, Goffin K, Grachev I, et al. Widespread decrease of type 1 cannabinoid receptor availability in Huntington disease in vivo. J Nucl Med. 2010;51:1413–7. doi: 10.2967/jnumed.110.077156. [DOI] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–32. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci. 2001;21:RC188. doi: 10.1523/JNEUROSCI.21-24-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizi ES, Katona I, Freund TF. Evidence for presynaptic cannabinoid CB(1) receptor-mediated inhibition of noradrenaline release in the guinea pig lung. Eur J Pharmacol. 2001;431:237–44. doi: 10.1016/s0014-2999(01)01413-3. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003a;424:434–8. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Doshi M, Hamazaki T. n-3 Polyunsaturated fatty acid (PUFA) deficiency elevates and n-3 PUFA enrichment reduces brain 2-arachidonoylglycerol level in mice. Prostaglandins Leukot Essent Fatty Acids. 2003b;69:51–9. doi: 10.1016/s0952-3278(03)00056-5. [DOI] [PubMed] [Google Scholar]
- Wettschureck N, van der Stelt M, Tsubokawa H, Krestel H, Moers A, et al. Forebrain-specific inactivation of Gq/G11 family G proteins results in age-dependent epilepsy and impaired endocannabinoid formation. Mol Cell Biol. 2006;26:5888–94. doi: 10.1128/MCB.00397-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JH, Errington ML, Lynch MA, Bliss TV. Arachidonic acid induces a long-term activity-dependent enhancement of synaptic transmission in the hippocampus. Nature. 1989;341:739–42. doi: 10.1038/341739a0. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–92. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Won YJ, Puhl HL, 3rd, Ikeda SR. Molecular reconstruction of mGluR5a-mediated endocannabinoid signaling cascade in single rat sympathetic neurons. J Neurosci. 2009;29:13603–12. doi: 10.1523/JNEUROSCI.2244-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Peng XQ, Li X, Song R, Zhang HY, et al. Brain cannabinoid CB(2) receptors modulate cocaine’s actions in mice. Nat Neurosci. 2011 doi: 10.1038/nn.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki M, Matsui M, Watanabe M. Preferential localization of muscarinic M1 receptor on dendritic shaft and spine of cortical pyramidal cells and its anatomical evidence for volume transmission. J Neurosci. 2010;30:4408–18. doi: 10.1523/JNEUROSCI.5719-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Zhang J, Andreasson K, Chen C. COX-2 oxidative metabolism of endocannabinoids augments hippocampal synaptic plasticity. Mol Cell Neurosci. 2008;37:682–95. doi: 10.1016/j.mcn.2007.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, et al. Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci. 2006;26:4740–51. doi: 10.1523/JNEUROSCI.0054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Uchigashima M, Yamasaki M, Katona I, Yamazaki M, et al. Unique inhibitory synapse with particularly rich endocannabinoid signaling machinery on pyramidal neurons in basal amygdaloid nucleus. Proc Natl Acad Sci U S A. 2011;108:3059–64. doi: 10.1073/pnas.1012875108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino H, Miyamae T, Hansen G, Zambrowicz B, Flynn M, et al. Postsynaptic diacylglycerol lipase alpha mediates retrograde endocannabinoid suppression of inhibition in mouse prefrontal cortex. J Physiol. 2011;589:4857–84. doi: 10.1113/jphysiol.2011.212225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Ives D, Ramesha CS. Synthesis of prostaglandin E2 ethanolamide from anandamide by cyclooxygenase-2. J Biol Chem. 1997;272:21181–6. doi: 10.1074/jbc.272.34.21181. [DOI] [PubMed] [Google Scholar]
- Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, et al. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114:777–89. doi: 10.1016/s0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- Zampronio AR, Kuzmiski JB, Florence CM, Mulligan SJ, Pittman QJ. Opposing actions of endothelin-1 on glutamatergic transmission onto vasopressin and oxytocin neurons in the supraoptic nucleus. J Neurosci. 2010;30:16855–63. doi: 10.1523/JNEUROSCI.5079-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SY, Xu M, Miao QL, Poo MM, Zhang XH. Endocannabinoid-dependent homeostatic regulation of inhibitory synapses by miniature excitatory synaptic activities. J Neurosci. 2009;29:13222–31. doi: 10.1523/JNEUROSCI.1710-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]