Abstract
Exogenous cortisol administration has been used to test the influence of glucocorticoids on a variety of outcomes, including memory and affect. Careful control of factors known to influence cortisol and other endogenous hormone levels is central to the success of this research. While use of hormonal birth control (HBC) is known to exert many physiological effects, including decreasing the salivary cortisol response to stress, it is unknown how HBC influences circulating cortisol levels after exogenous cortisol administration. To determine those effects, we examined the role of HBC on participants’ cortisol levels after receiving synthetic cortisol (hydrocortisone) in two separate studies. In Study 1, 24 healthy women taking HBC and 26 healthy men were administered a 0.1 mg/kg body weight intravenous dose of hydrocortisone, and plasma cortisol levels were measured over 3 hours. In Study 2, 61 participants (34 women; 16 were on HBC) received a 15 mg hydrocortisone pill, and salivary cortisol levels were measured over 6 hours. Taken together, results from these studies suggest that HBC use is associated with a greater cortisol increase following cortisol administration. These data have important methodological implications: (1) when given a controlled dose of hydrocortisone, cortisol levels may increase more dramatically in women taking HBC vs. women not on HBC or men; and (2) in studies manipulating cortisol levels, women on hormonal contraceptives should be investigated as a separate group.
Keywords: hydrocortisone, intravenous, oral, birth control, sex differences, glucocorticoids, HPA axis
Introduction
Glucocorticoid (GC) effects on physiological and psychological outcomes are important from basic research and clinical perspectives. For example, a strong body of research elucidates the complex effects of GCs on learning and memory (de Kloet, Oitzl & Joels, 1999; Wolf, 2008), findings that have important implications for the origins and treatment of psychopathology (e.g., de Quervain & Margraf, 2008).
As part of this research, it is necessary to identify moderating factors, such as sex and medications, which alter cortisol’s bioavailability and psychological effects. Hormonal contraceptives/hormonal birth control (hereafter abbreviated HBC) is known to affect responses to laboratory stress (Kirschbaum et al., 1999; Nielsen et al., 2013). Women taking HBC have distinct HPA axis signatures compared to those not taking HBC, including lower cortisol awakening responses (Pruessner, Hellhammer, & Kirschbaum, 1999); a lower salivary cortisol response to psychosocial stress (Kirschbaum, Pirke, & Hellhammer, 1995); a blunted cortisol response to physical exercise (Bonen, Haynes, & Graham, 1991; Kirschbaum, Pirke, & Hellhammer, 1996); greater glucocorticoid sensitivity to pro-inflammatory cytokines after acute stress (Rohleder, Wolf, Piel, & Kirschbaum 2003); and altered circadian cortisol (Bouma et al., 2009; Pruessner et al., 1997; Reinberg et al., 1996, but refer to Wust et al., 2000). For example, women taking HBC who were administered prednisone or dexamethasone had higher serum cortisol levels (Nickelsen, Lissner, & Schöffling, 1989) and greater cortisol suppression (Seidegård, Simonsson, & Edsbäcker, 2000). Cognitive differences have also been revealed. For example, Kuhlmann & Wolf (2005) found that the effects of hydrocortisone administration on memory retrieval differed for women on vs. off HBC (also see Nielsen et al., 2011, 2013). Taken together, these findings suggest that HBC affects the bioavailability and effects of cortisol.
One approach that researchers have employed to study the effects of GCs in humans is to use environmental manipulations, such as the Trier Social Stress Task (Kirschbaum, Pirke & Hellhammer, 1993) or the Cold Pressor task (e.g., Bentz et al., 2013; Nielsen et al., 2013). These studies are essential for discovering the effects of endogenously generated cortisol in response to psychological stress. However, it is sometimes advantageous to study GCs effects in the absence of emotional arousal due to stress, and to standardize the cortisol dose. For these reasons, researchers administer synthetic cortisol (hydrocortisone) or other GCs in order to evaluate cortisol’s influence on a host of phenomena including memory formation (see, e.g., Lupien & McEwen, 1997; Wolf, 2008; Abercrombie et al., 2011).
No study, to the best of our knowledge, has investigated whether HBC use alters cortisol levels achieved after exogenous cortisol administration. We hypothesized that cortisol levels after cortisol administration would differ for women on vs. off HBC, as well as for women on HBC vs. men. Specifically, we hypothesized that HBC would result in greater cortisol increases, based on past research demonstrating that HBC use is associated with elevated levels of other exogenously administered GCs (e.g., Seidegård et al., 2000). To test our hypotheses, we reanalyzed plasma and salivary cortisol data from two studies involving acute intravenous (IV) and oral cortisol administration, respectively (Wirth, Scherer, Hoks & Abercrombie, 2011; Abercrombie et al., 2011). Study 1 included women on HBC and men, although Study 2 included women on HBC, women not taking HBC, and men.
Study 1 Methods
Participants
Male and female participants aged 18–35, in good health, and fluent in English, were recruited from the University of Wisconsin campus and the local community. Participants were screened for inclusion in the study by phone. For logistical reasons unrelated to the present hypotheses, men and women using HBC were included (Kirschbaum et al., 1999; Wirth et al., 2011). The majority (60%) of women were using a monophasic progestin/estrogen (e.g., Yasmin) while the remaining women were taking a triphasic progestin/estrogen (e.g., Ortho Tri-Cyclen). All study sessions were scheduled so that neither drug administration session fell within the HBC “placebo” week. Exclusion and inclusion criteria are described in more detail in Wirth et al. (2011). Fifty-four participants were enrolled in the study. Two participants served as pilots for a higher dose of hydrocortisone and are not included in the present analyses. Four individuals did not complete all parts of the study. Data from two additional participants was dropped due to failure to comply with instructions. Therefore, 46 participants were included in analyses: 22 men (Mean [SEM] Age: 21.8 [0.8]; BMI: 25.3 [0.6]) and 24 women (Age: 22.4 [0.7]; BMI: 22.9 [0.6]).
Study participants refrained from food, caffeine, and vigorous exercise for 2 h prior to each study session, as these factors can affect cortisol levels (Hansen, Garde, & Perrson 2008; Kirschbaum, Wust, & Faig, 1992; Nicholson, 1989). Participants also refrained from alcohol intake for 24 h prior to Session 1 until completion of Session 2.
Procedures
Each participant received both hydrocortisone (i.e., synthetic cortisol; identical to the endogenous hormone) and placebo, in separate sessions 48 hours apart. Participants completed hydrocortisone and placebo sessions in randomized order, and drug order was double-blinded. Blood samples were collected to assess plasma cortisol at 11 time points; 3 samples were collected before drug administration and 7 were collected after administration.
Study sessions took place at the Clinical and Translational Research Core (CTRC) at the University of Wisconsin Hospital. All procedures received prior approval from the University of Wisconsin Health Sciences Institutional Review Board; participants provided informed consent, and were paid for their participation. Each of the two study sessions began at 1600 h to minimize circadian variation in cortisol. In each session, participants received 0.1 mg/kg body weight IV hydrocortisone or physiological (0.9%) saline placebo, administered over 30 minutes using a programmed pump. Notably, this dose of hydrocortisone produced plasma cortisol levels that were somewhat higher than those caused by a moderate stressor, such as public speaking (Kirschbaum et al., 1993), but still within the physiological range (Fry, Morton, Garcia-Webb, & Keast, 1991; Cydulka & Emerman, 1998). Since the dose was calibrated to body weight, women received a significantly smaller dose than men, receiving on average (SEM) 6.29 (0.16) mg vs. men’s 8.15 (0.24) mg (see Wirth et al., 2011). Blood samples continued to be collected at regular intervals throughout the study sessions, which lasted 5 hours total, ending at 2100 h. Other study procedures, not directly relevant to this investigation, are detailed in Wirth et al. (2011).
Sample Processing and Cortisol Analysis
Blood samples were centrifuged to extract plasma, which was aliquoted and frozen at −80°C until further analysis. All cortisol assays were performed using Coat-A-Count radioimmunoassay (RIA) kits purchased from Siemens Healthcare Diagnostics (Duluth, GA). Average inter-assay coefficient of variation (CV) across all assays was 5.9% and average intra-assay CV was 4.0%. Siemens Healthcare Diagnostics reports a lower limit of detection of 0.2 mg/dl for their Coat-A-Count cortisol RIA kits.
Study 1 Data Analysis
We calculated area under the curve with respect to increase (AUCi; Pruessner et al., 2003) to characterize cortisol elevations after drug infusion. AUCi only reflects the increase in plasma cortisol in response to the infusion, i.e. the amount of cortisol increase above each person’s baseline levels. Therefore, by using AUCi rather than AUCg (or a simple peak or average of the cortisol values), we control for baseline (pre-infusion) differences in cortisol levels that we found between men and women: women had higher baseline cortisol, p’s < 0.03. We calculated AUCi from the pre-infusion sample to the second-to-last sample of the study sessions, Sample 10. We did not include the final sample, Sample 11, as five participants had missing data for their final placebo session sample (also, men’s cortisol levels had returned to baseline by Sample 10). A t-test was used to evaluate whether a sex difference existed in cortisol AUCi.
Study 1 Results
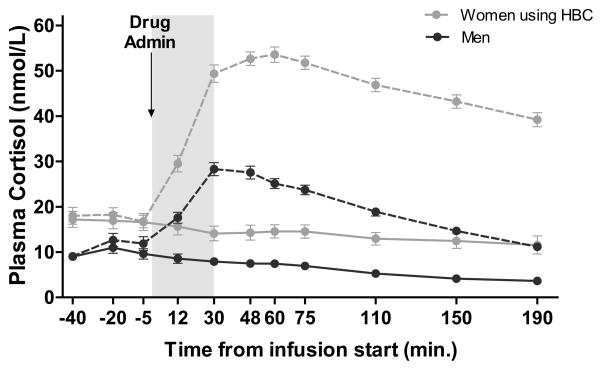
A pairwise t-test was used to examine whether there was a difference by sex in cortisol increase, after hydrocortisone infusion, using AUCi as the dependent variable.1 Results indicated a significant sex difference in AUCi, t(42) = 10.65, p < 0.001, 95% CI [2269.890 to 3331.874], with higher AUCi in women (M: 4016.58, SD: 1034.49) compared to men (M: 1215.70, SD: 672.99). Notably, this difference was not found after placebo infusion, t(42) = −0.973, p = 0.336, 95% CI [−410.938 to 143.602]. Thus, women’s total plasma cortisol rose significantly higher than men’s following cortisol infusion, even though the sexes did not differ in cortisol AUCi on the day that they received placebo (see Figure 1).
Figure 1. Plasma cortisol after hydrocortisone (IV, 0.1 mg/kg) or placebo administration.
Plasma cortisol levels are measured in nmol/L. Dashed lines represent plasma cortisol levels during the session in which hydrocortisone (0.1 mg/kg infused over 30 min) was administered. Solid lines represent plasma cortisol levels during the session in which placebo (0.9% saline) was administered. The gray bar indicates the time of drug infusion (0 to 30 min.). Area under the curve increase (AUCi) in plasma cortisol levels was significantly greater in women, all of whom were taking HBC, vs. men following hydrocortisone administration (t[42] = 10.13, p < 0.001). This difference was not found following placebo infusion.
Results from Study 1 indicate a sex difference in plasma cortisol following an IV infusion of cortisol (hydrocortisone), even though the dose was tailored to each participant’s body weight. Although we suspect that HBC use was at least part of the cause of this difference, since all the women in Study 1 were taking HBC, we cannot distinguish from this study alone whether the increased cortisol in women was due to sex or due to HBC use. Study 2 remedies this issue, as both women using HBC and women not using HBC were included, as well as men.
Study 2 Methods
Participants
Twenty (11 women) un-medicated participants with depression symptoms and 45 (24 women) never-depressed healthy participants were enrolled in the study. Participants were recruited from the community, and screened with the Hamilton Rating Scale for Depression (HRSD; Hamilton, 1960) and the SCID (First, Spitzer, Gibbon, & Williams, 2002). One participant was excluded due to structural brain abnormalities; one was excluded due to abnormally high salivary cortisol levels following hydrocortisone administration (possibly due to chewing the orally administered capsule); and two were excluded due to experimenter error. Altogether, then, our final sample for the present analyses includes 61 participants: 27 men and 34 women (16 taking HBC). All potential participants received a drug screen, and women were required to test negative on a pregnancy test before participation. Other inclusion and exclusion criteria are described in Abercrombie et al. (2011). Mean [SEM] age and BMI, respectively, for the three groups were as follows: women not taking HBC, 30.00 [2.57] and 25.86 [1.42]; women taking HBC, 23.06 [1.17] and 24.66 [1.12]; and men, 27.17 [1.54] and 26.48 [0.80]. Participants in the three groups did not significantly differ in age or BMI (p > 0.05 in all ANOVAs). The University of Wisconsin-Madison Health Sciences Institutional Review Board approved all study procedures. Participants provided written informed consent and were paid for their participation.
Of the 16 women using HBC, 63% (10 women) were prescribed a monophasic progestin/estrogen and the remaining women were taking a triphasic progestin/estrogen. We were unable to control for menstrual phase (in women not taking HBC) or pill cycle phase (for women on HBC) because of a National Institutes of Health stipulation that individuals with depressive symptoms participate in the study within 2 weeks of screening, so as not to require more than 2 weeks without mental health treatment while in the study. The same criteria were applied to individuals who did not endorse depressive symptoms, for consistency. Therefore, those not taking HBC are mixed with regard to menstrual phase; similarly, women on HBC could have been in any stage in their monthly pill cycle.
Procedure
Eligible participants completed an fMRI simulation session, two fMRI scanning sessions, and a memory testing session, in order to test separate hypotheses (see Abercrombie et al., 2011). The fMRI scanning sessions involved cortisol or placebo administration and saliva sampling for cortisol measurement, so only those sessions are detailed here. A repeated-measures design was used in which subjects received cortisol in one scanning session and placebo in the other, with randomized order. Both sessions began between 1630 h and 1730 h, and the two sessions were spaced 48 hours apart. Notably, participants were already acclimated to the scanning environment, using a mock scanning session (fMRI simulation), prior to the sessions when cortisol or placebo administration took place; hence, the fMRI environment was unlikely to cause stress due to novelty, etc. In fact, evidence suggests that if participants have even one single previous exposure to fMRI, they show no cortisol response to a subsequent fMRI experience (Tessner et al., 2006). Further details regarding the memory and fMRI procedures may be found in a previously published manuscript (Abercrombie et al., 2011). Each session lasted approximately 2.75 hours. Participants refrained from eating and exercise within 90 min of all sessions. After providing an initial saliva sample, participants were administered an oral dose of 15 mg hydrocortisone (i.e., cortisol) or placebo.
Salivary Cortisol Measurement
Participants provided a total of 6 saliva samples to examine salivary cortisol levels throughout the study sessions and in their homes after the sessions, using the Salivette saliva collection device (Sarstedt, Newton, NC). One saliva sample was provided 5 minutes before hydrocortisone or placebo administration and five additional samples were provided 20, 95, 130, 200, and 375 minutes after administration. Saliva samples were stored frozen until they were assayed using a chemi-luminescence assay, which has a high sensitivity of 0.16 ng/mL (IBL-International, Hamburg, Germany). Both intra- and inter-assay CVs were below 6%.
Study 2 Data Analysis
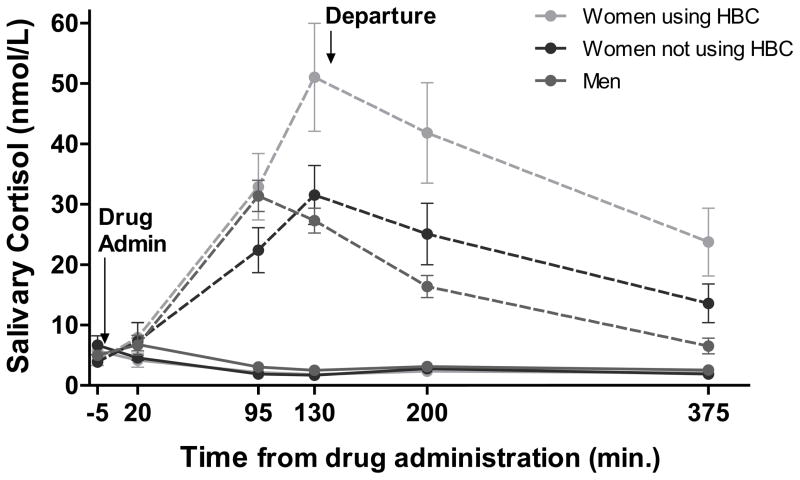
Similarly to data analysis in Study 1, we first tested whether there were differences between groups in baseline salivary cortisol levels – in other words, before the hydrocortisone or placebo treatment. An initial mixed ANOVA comparing the three groups’ (women using HBC; women not on HBC/free-cycling women; men) baseline (Sample 1) cortisol revealed neither any significant between-group differences (F(1,119) = 0.000, p = 0.994), nor were there any differences when comparing pre-hydrocortisone Sample 1 cortisol with the pre-placebo cortisol Sample 1 within each group (Men: F(1,52) = 0.000, p = 0.984; HBC Women: F(1,30) = 0.472, p = 0.497, Control Women: F(1,33) = 1.759, p = 0.194). Also, importantly, there were no significant differences in baseline cortisol between participants with depression symptoms and those without, in any of the three groups (Men: F(1,52) = 0.364, p = 0.549; HBC Women: F(1,30) = 3.001, p = 0.093; free-cycling women: F(1,34) = 0.390, p = 0.537). Because there were no baseline differences in cortisol levels, the primary analyses used area under the curve with respect to ground (AUCg; Pruessner et al., 2003). Cortisol AUCg was calculated from Sample 2 to Sample 6 (~ 375 min. after administration; see Figure 2). Despite no significant difference in baseline cortisol between participants with and without depression symptoms, we controlled for depression symptoms in the ANCOVAs.
Figure 2. Salivary cortisol after hydrocortisone (oral, 15 mg) or placebo administration.
Salivary cortisol levels are measured in nmol/L. Dashed lines represent salivary cortisol levels during the session in which hydrocortisone (15 mg capsule) was administered. Solid lines represent salivary cortisol levels during the session in which placebo capsule was administered. The final two saliva samples were collected in the evening at home after departure from the lab. On the hydrocortisone day, salivary cortisol levels at baseline did not differ between groups. However, area under the curve with respect to ground (AUCg) in salivary cortisol levels was greater in women taking HBC compared to men (M = 5107.77, 95% CI [2405.01, 7810.52], p < 0.001) or compared to women not on HBC (M = 3629.73, 95% CI [686.27, 6573.18], p = 0.012); the latter two groups did not differ. There were no group differences following placebo administration.
Study 2 Results
Two global ANCOVAs, one for each session, were conducted with Group as the independent variable, cortisol AUCg as the dependent variable, and controlling for depression status. As expected, the overall ANCOVA on AUCg after placebo was not significant, F(2,60) = 2.172, p = 0.123, but the overall ANCOVA on cortisol AUCg after receiving hydrocortisone was significant, F(2,60) = 9.872, p < 0.001. Notably, there was no main effect of depression status on AUCg in either ANCOVA. To follow up on this result, we used three post-hoc pairwise ANCOVAs comparing AUCg by group, again controlling for depression. After receiving hydrocortisone, women taking HBC had significantly higher AUCg compared to free-cycling women (i.e., women not on HBC), F(1,33) = 4.767, p = 0.037, and compared to men, F(1,42) = 21.013, p < .001. In the pairwise ANCOVA, free-cycling women did not have a significantly different AUCg compared to men, F(1,44) = 1.954, p = .072. Tukey post-hoc comparisons were also conducted, which cannot control for the depression covariate, but are a more widely-used form of post-hoc test. In these tests, the women taking HBC had a significantly higher AUCg compared to both free-cycling women (M = 3629.73, 95% CI [686.27, 6573.18], p = 0.012) and men (M = 5107.77, 95% CI [2405.01, 7810.52], p < 0.001). The difference between free-cycling women and men was not statistically significant, p = 0.366.
Discussion
In two studies, we generated preliminary evidence that HBC use is associated with greater cortisol levels after an exogenous dose of cortisol. In Study 1, women (all of whom were taking HBC) had greater cortisol increases than men after IV cortisol, despite the fact that the dose of cortisol was calibrated by weight. Based on Study 2 findings, we believe this result was at least partly driven by the women’s HBC use, but we cannot rule out the possible role of sex differences, e.g. in cortisol metabolism. In Study 2, women on HBC had a greater increase in cortisol after a moderate oral dose of cortisol compared with women not using HBC or men. No such differences between these groups were found in cortisol output after placebo administration; in other words, unstimulated cortisol over time did not differ as a function of sex or HBC status. Importantly, salivary cortisol was measured in Study 2, rather than plasma cortisol as in Study 1. Thus, these findings support the idea that women using HBC have greater elevations in free cortisol and potentially also total/plasma cortisol following exogenous cortisol administration.
These findings have important methodological implications. Our findings suggest the importance of testing and/or controlling for the effects of HBC use in studies employing cortisol administration to manipulate cortisol levels. Differences in cortisol levels after exogenous cortisol manipulation in women on vs. off HBC may partially (or wholly) explain any variations in behavioral or physiological effects of exogenous cortisol observed in women on HBC. In addition, variability in outcome measures after exogenous cortisol in women on versus off HBC could create noise in data sets in which HBC use is not controlled or taken into account.
Our study alone cannot speak to potential mechanisms for our findings. However, we speculate that the effect of HBC to increase cortisol’s binding proteins may be one possible mechanism (van der Vange, Blankenstein, Kloosterboer, Haspels, & Thijssen, 1990). HBC-induced elevations of the blood proteins that bind cortisol could delay cortisol’s breakdown and prolong the elevations of the hormone in the blood. Meulenberg et al. (1987) found that HBC use was associated with higher levels of corticosteroid-binding globulins (CBGs). Another study showed that administration of low doses of oral contraceptives increased both corticosteroid- and sex hormone-binding globulins (van der Vange et al., 1990). Presuming that hormone-binding globulins are chronically higher due to HBC use, it is possible that a greater proportion of a dose of exogenous hydrocortisone is bound in participants taking HBC vs. participants not on HBC. Consequently, cortisol may not be subject to the normal breakdown and clearance processes as quickly, as higher levels of binding globulins may protect the exogenous cortisol molecules from metabolism, receptor binding, etc., leading to higher, more prolonged, elevation of cortisol.
On the other hand, our findings could be explained by other factors in addition to, or instead of, greater levels of binding globulins slowing breakdown of cortisol molecules. If increased binding globulins were the only mechanism involved, we might expect to see higher total cortisol but lower salivary/unbound cortisol increases in women on HBC. However, this was not the case in Study 2, in which salivary cortisol was measured. In past work, women taking HBC were found to have a blunted salivary (i.e., free) cortisol response to acute stress (Kirschbaum et al. 1999); Kumsta et al. (2007) found that CBG levels were negatively associated with the salivary cortisol response to the TSST, while CBGs were positively associated with total cortisol levels. Our finding in Study 2 that free/salivary cortisol is also elevated in women on HBC after cortisol administration appears to conflict with these past findings, though it is important to note that endogenous increases in cortisol due to stress are not directly comparable to increases due to exogenous administration. It is also important to note that the relationship between CBG levels and cortisol metabolism is complex. High levels of endogenous versus exogenous cortisol may yield different effects on CBG affinity for cortisol (Schlechte and Hamilton, 2008). Also, there is evidence that binding globulins become saturated less quickly in women on HBC, such that salivary cortisol measurements in women on HBC are indicative of higher total cortisol than equivalent measures in women not taking HBC (Hellhammer et al., 2009). Thus, if anything, our Study 2 salivary cortisol data might underestimate the difference in plasma cortisol between the two groups of women. Finally, HBC affects other metabolic processes as well as binding globulin levels (Cassazza et al., 2004), so there are many possibilities for mechanism(s) behind our findings. As we did not measure binding globulins in these studies, we cannot come to any conclusions yet as to mechanism(s) by which HBC use led to higher cortisol levels.
Cortisol levels after cortisol administration may also depend on the specific type of oral contraceptives that women are taking. Brien (1975) measured human blood plasma concentrations of cortisol, cortisol-resin uptake ratio, and free cortisol in women taking either estrogen/progestogen or progestogen-only HBC. Women taking progestogen-only HBC had levels of cortisol, cortisol reuptake and free cortisol that were similar to controls. However, women taking the combined pill had elevated cortisol and cortisol reuptake ratios in both the morning and afternoon (although no change in free cortisol). All women in our studies were using combined HBC. Nonetheless, examining the effects of different types of HBC on the HPA axis will be an important area of future study.
Two findings unrelated to the main hypothesis deserve mention. First, in Study 1, women on HBC had higher baseline cortisol compared to men. Interpretation of this baseline difference is complicated somewhat by the fact that women in Study 1 were tested during their active pill weeks, whereas pill cycle was uncontrolled in Study 2, wherein there were no group differences in baseline levels. Nonetheless, some past research has also found baseline differences in cortisol between women on and off HBC (e.g., Nielsen et al., 2013). In fact, in Nielsen et al. (2013), elevated baseline cortisol in women on HBC was associated with a lack of cortisol response to a laboratory stressor (cold pressor), whereas there was no such association in women not taking HBC. These findings along with ours point to the importance of controlling for HBC use in studies involving laboratory stressors, not only in studies involving exogenous cortisol administration.
A second interesting pattern in our data is that, in Study 2, women regardless of HBC use seemed to have a later peak in cortisol after cortisol administration compared to men. Although the relatively long time intervals between the saliva samples means we cannot pin-point the exact time of peak cortisol in any group, men’s cortisol was declining by the 130 minute time-point, whereas for women in both groups, 130 minutes was their highest cortisol measurement (Figure 2). We hesitate to draw any conclusions from this pattern, as our saliva sampling was not dense enough to examine peak timing. However, future work should examine potential sex differences in time of cortisol peak, which may or may not depend on hormonal status.
We must acknowledge several methodological limitations with the present research. First, these are post-hoc analyses in studies that were not specifically designed to test associations between HBC and cortisol, although we believe these remain valid and important preliminary data to be expanded on in future work. Second, menstrual phase was not controlled in cycling women in Study 2. We believe this is also a concern to be remedied in future research. Third, Study 2 contained participants with depression symptoms, as part of the original study design; however, depression status did not moderate baseline cortisol or levels of the hormone after cortisol administration. Fourth, Studies 1 and 2 used different designs, and notably, different methods of drug administration and measurement of cortisol levels. Although these methodological differences could be thought to limit the comparability of the studies, the findings from Study 2 expand upon those from Study 1, suggesting that higher cortisol in women taking HBCs is likely due to HBCs, rather than exclusively due to a sex effect. Study 1 also addresses some of the limitations in Study 2, e.g. a less controlled dose of cortisol, and the fact that pill cycle phase was not controlled in Study 2, such that some women may have been tested during their “placebo” week. Obtaining similar results in two studies with different methodologies also indicates that this is a robust, replicable finding, worthy of future investigation in studies designed explicitly to test these hypotheses.
Conclusions
Data from two studies suggest that HBC use results in greater increases in cortisol levels after exogenous cortisol administration. In other words, our findings suggest that, when given a controlled dose of hydrocortisone, cortisol levels may increase more dramatically in women taking HBC vs. women not on HBC or men. Though further research is needed to confirm our preliminary findings, all researchers administering cortisol to humans should be aware of this issue. If women taking HBC are included in cortisol administration studies, the potential effects of HBC use should be examined in data analyses. Our results also suggest future inquiry into the long-term effects of HBC use on cortisol and other aspects of the HPA system. Research is also needed to examine interactions among steroid binding mechanisms, sex steroids (endogenous as well as exogenous), and cortisol. Such investigations could help shed light on potential individual differences underlying HPA dysregulation in a variety of disorders as well as reactivity to acute and chronic stress.
Acknowledgments
Study 1: The authors are grateful to the UW CTRC nursing staff and to research assistants C. Burzinski, C. Cenek, B. Nanzig, S. Scherer, S. Sharma, and K. Swinsky for help with data collection, as well as N. Kalin, G. Nash, and P. Roseboom for assistance with hormone assays.
Study 2: The authors are grateful to E. McAuliff, P. Singh, C. Burzinski, B. Gauthier, K. Krol, A. Benson, L. Burk, J. Gogan, E. Blum Ng, J. Pikorz, N. Shallow, N. Speck, H. Schaefer, J. Halverson, J. Alt, M. Peterson, and the Waisman Laboratory for Brain Imaging and Behavior staff for their assistance with data collection. The authors are also grateful to C. Kirschbaum, who conducted salivary cortisol assays for Study 2, and to N. Kalin, R. Davidson, and E. Young for advice and support.
Footnotes
Using AUCg as the dependent variable, there was also a significant sex difference after hydrocortisone infusion, t(42) = 14.24, p < 0.001, CI: 3052.95 to 4061.05, with higher AUCg in women (M: 6613.74, SD: 951.24) compared to men (M: 3056.74, SD: 683.80).
Declaration of Interest
The authors have no conflicts of interest to report. This research was funded in part by an NIMH K-Award 1K08MH07415-012 and a NARSAD Young Investigator Award to H. Abercrombie. Study 2 was also supported in part by NIMH grants R01-MH43454 and P50-MH084051 to R. Davidson. During Study 1 data collection, M. Wirth was supported by NIH institutional training grant T32MH18931. NSF Graduate Student Fellowships to A. Gaffey and A. Jahn helped support data analysis and writing of the manuscript (A.G.) and Study 2 data collection and analysis (A.J.). Data from Study 1 were collected at the University of Wisconsin Clinical and Translational Research Core (CTRC), which is supported by grant 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the NIH National Center for Research Resources. NARSAD, NIMH, and NSF had no further role in study design; in the collection, analysis, and interpretation of the data; in the writing of the report; or in the decision to submit the paper for publication.
References
- Abercrombie HC, Jahn AL, Davidson RJ, Kern S, Kirschbaum C, Halverson J. Cortisol’s effects on hippocampal activation in depressed patients are related to alterations in memory formation. J Psychiatric Research. 2011;45:15–23. doi: 10.1016/j.jpsychires.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentz D, Michael T, Wilhelm FH, Hartmann FR, Kunz S, von Rohr IR, de Quervain DJ. Influence of stress on fear memory processes in an aversive differential conditioning paradigm in humans. Psychoneuroendocrinol. 2013;38:1186–1197. doi: 10.1016/j.psyneuen.2012.12.018. [DOI] [PubMed] [Google Scholar]
- Bonen A, Haynes FW, Graham TE. Substrate and hormonal responses to exercise in women using oral contraceptives. J Applied Physiology. 1991;70:1917–1927. doi: 10.1152/jappl.1991.70.5.1917. [DOI] [PubMed] [Google Scholar]
- Bouma EM, Riese H, Ormel J, Verhulst FC, Oldehinkel AJ. Adolescents’ cortisol responses to awakening and social stress; effects of gender, menstrual phase and oral contraceptives. The TRAILS study. Psychoneuroendocrinology. 2009;34:884–893. doi: 10.1016/j.psyneuen.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Brien TG. Cortisol metabolism after oral contraceptives: total plasma cortisol and the free cortisol index. Br J Obstet Gynaecol. 1975;82:987–991. doi: 10.1111/j.1471-0528.1975.tb00609.x. [DOI] [PubMed] [Google Scholar]
- Casazza GA, Jacobs KA, Suh SH, Miller BF, Horning MA, Brooks GA. Menstrual cycle phase and oral contraceptive effects on triglyceride mobilization during exercise. Journal of Applied Physiology. 2004;97(1):302–309. doi: 10.1152/japplphysiol.00050.2004. [DOI] [PubMed] [Google Scholar]
- Cydulka RK, Emerman CL. Adrenal function and physiologic stress during acute asthma exacerbation. Annals of Emerg Med. 1998;31:558–561. doi: 10.1016/s0196-0644(98)70201-x. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Oitzl MS, Joels M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- de Quervain DJ, Margraf J. Glucocorticoids for the treatment of post-traumatic stress disorder and phobias: a novel therapeutic approach. Eur J Pharmacol. 2008;583:365–371. doi: 10.1016/j.ejphar.2007.11.068. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; Nov, 2002. [Google Scholar]
- Fry RW, Morton AR, Garcia-Webb P, Keast D. Monitoring exercise stress by changes in metabolic and hormonal responses over a 24-h period. Eur J Appl Physiol Occup Physiol. 1991;63:228–234. doi: 10.1007/BF00233853. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen AM, Garde AH, Persson R. Sources of biological and methodological variation in salivary cortisol and their impact on measurement among healthy adults: a review. Scand J Clin Lab Invest. 2008;68:448–458. doi: 10.1080/00365510701819127. [DOI] [PubMed] [Google Scholar]
- Hellhammer DH, Wust S, Kudielka BM. Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology. 2009;34:163–171. doi: 10.1016/j.psyneuen.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellhammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamus–pituitary–adrenal axis. Psychosom Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Platte CP, Pirke KM, Hellhammer DH. Adrenocortical activation following stressful exercise: Further evidence for attenuated free cortisol responses in women using oral contraceptives. Stress Med. 1996;12:137–143. [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. Preliminary evidence for reduced cortisol responsivity to psychological stress in women using oral contraceptive medication. Psychoneuroendocrinology. 1995;20:509–514. doi: 10.1016/0306-4530(94)00078-o. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The Trier Social Stress Test—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Wust S, Faig HG, Hellhammer DH. Heritability of cortisol responses to human corticotropin-releasing hormone, ergometry, and psychological stress in humans. J Clin Endocrinol and Metab. 1992;75:1526–1530. doi: 10.1210/jcem.75.6.1464659. [DOI] [PubMed] [Google Scholar]
- Kuhlmann S, Wolf OT. Cortisol and memory retrieval in women: Influence of menstrual cycle and oral contraceptives. Psychopharmacology (Berl) 2005;183:65–71. doi: 10.1007/s00213-005-0143-z. [DOI] [PubMed] [Google Scholar]
- Kumsta R, Entringer S, Hellhammer DH, Wüst S. Cortisol and ACTH responses to psychosocial stress are modulated by corticosteroid binding globulin levels. Psychoneuroendocrinology. 2007;32:1153–1157. doi: 10.1016/j.psyneuen.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: integration of animal and human model studies. Brain Res Brain Res Rev. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Meulenberg PM, Ross HA, Swinkels LM, Benraad TJ. The effect of oral contraceptives on plasma-free and salivary cortisol and corticostersone. Clin Chimica Acta. 1987;165:379–385. doi: 10.1016/0009-8981(87)90183-5. [DOI] [PubMed] [Google Scholar]
- Nicholson SA. Stimulatory effect of caffeine on the hypothalamo-pituitary-adrenocortical axis in the rat. Journal of Endocrinol. 1989;122:535–543. doi: 10.1677/joe.0.1220535. [DOI] [PubMed] [Google Scholar]
- Nickelsen T, Lissner W, Schöffling K. The dexamethasone suppression test and long-term contraceptive treatment: Measurement of ACTH or salivary cortisol does not improve the reliability of the test. Exp and Clin Endocrinol. 1989;94:275–280. doi: 10.1055/s-0029-1210910. [DOI] [PubMed] [Google Scholar]
- Nielsen SE, Ertman N, Lakhani YS, Cahill L. Hormonal contraception usage is associated with altered memory for an emotional story. Neurobiol Learn Mem. 2011;96:378–384. doi: 10.1016/j.nlm.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen SE, Segal SK, Worden IV, Yim IS, Cahill L. Hormonal contraception use alters stress responses and emotional memory. Biol Psychol. 2013;92:257–266. doi: 10.1016/j.biopsycho.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosom Med. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: A reliable biological marker for the assessment of adrenocortical activity. Life Sciences. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Reinberg AE, Touitou Y, Soudant E, Bernard D, Bazin R, Mechkouri M. Oral contraceptives alter circadian rhythm parameters of cortisol, melatonin, blood pressure, heart rate, skin blood flow, transepidermal water loss, and skin amino acids of healthy young women. Chronobiol Int. 1996;13:199–211. doi: 10.3109/07420529609012653. [DOI] [PubMed] [Google Scholar]
- Rohleder N, Wolf JM, Piel M, Kirschbaum C. Impact of oral contraceptive use on glucocorticoid sensitivity of pro-inflammatory cytokine production after psychosocial stress. Psychoneuroendocrinology. 2003;28:261–273. doi: 10.1016/s0306-4530(02)00019-7. [DOI] [PubMed] [Google Scholar]
- Schlechte JA, Hamilton D. The effect of glucocorticoids on corticosteroid binding globulin. Clin Endo. 2008;27:197–203. doi: 10.1111/j.1365-2265.1987.tb01145.x. [DOI] [PubMed] [Google Scholar]
- Seidegård J, Simonsson M, Edsbäcker S. Effect of an oral contraceptive on the plasma levels of budesonide and prednisolone and the influence on plasma cortisol. Clin Pharmacol Ther. 2000;67:373–381. doi: 10.1067/mcp.2000.105762. [DOI] [PubMed] [Google Scholar]
- Tessner KD, Walker EF, Hochman K, Hamann S. Cortisol responses of healthy volunteers undergoing magnetic resonance imaging. Hum Brain Mapp. 2006;27:889–895. doi: 10.1002/hbm.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vange N, Blankenstein MA, Kloosterboer HJ, Haspels AA, Thijssen JH. Effects of seven low-dose combined oral contraceptives on sex hormone binding globulin, corticosteroid binding globulin, total and free testosterone. Contraception. 1990;41:345–352. doi: 10.1016/0010-7824(90)90034-s. [DOI] [PubMed] [Google Scholar]
- Wolf OT. The influence of stress hormones on emotional memory: Relevance for psychopathology. Acta Psychol (Amst) 2008;127:513–531. doi: 10.1016/j.actpsy.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Wirth MM, Scherer SM, Hoks RM, Abercrombie HC. The effect of cortisol on emotional responses depends on order of cortisol and placebo administration in a within-subjects design. Psychoneuroendocrinology. 2011;36:945–954. doi: 10.1016/j.psyneuen.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wust S, Wolf J, Hellhammer DH, Federenko I, Schommer N, Kirschbaum C. The cortisol awakening response - normal values and confounds. Noise Health. 2000;2:79–88. [PubMed] [Google Scholar]