Abstract
Temporal lobe epilepsy (TLE) has been modeled in mice using pilocarpine induction, with variable results depending on specific strains. To allow efficient xenotransplantation for the purpose of optimizing potential cell-based therapy of human TLE, we have determined the optimal dosing strategy to produce spontaneous recurring seizures in immunodeficient NodScid mice. Multiple 100 mg/kg injections of pilocarpine have been shown to be more effective than single 300–400 mg/kg injections for inducing spontaneous seizures in NodScid mice. Under our optimal conditions, 88.1+/−2.9% of the mice experienced status epilepticus (SE) with a survival rate of 61.8+/−5.9%. Surviving SE mice displayed spontaneous recurrent seizures at a frequency of 2.8+/−0.9 seizures/day for a duration of 41.1+/−3.5 sec. The widely used method of a single injection of pilocarpine was significantly less efficient in inducing seizures in NodScid mice. Therefore, we have determined that a multiple injection “ramping up” of 100 mg/kg of pilocarpine is optimal for inducing TLE-like spontaneous seizures in NodScid mice. Using this method, mice with SE efficiently developed SRS and expressed mossy fiber sprouting, a signature histopathological feature of TLE.
Keywords: NodScid mice, temporal lobe epilepsy, pilocarpine, seizure, mossy fiber sprouting
1. Introduction
Temporal lobe epilepsy (TLE) is one of the most common forms of human epilepsy, characterized by epileptogenic abnormalities in and around the hippocampal area, resulting in recurrent seizure activity (Engel, 2001). About 30% of individuals with TLE are resistant to anti-epileptic drugs (AEDs) (Engel, 2002), making them potential candidates for surgical resection of affected brain areas. However, such neurosurgical procedures are associated with a high risk of morbidity (Christoph, 2008). Moreover, after surgical resection, most patients continue to rely on AEDs (Shamim et al., 2009). Furthermore, these medicines frequently cause side-effects, such as somnolence, headache, GI disturbance, weight gain, and dizziness (Cramer et al., 2010). Therefore, there remains an urgent unmet medical need for the development of novel therapeutics that can modulate specific brain circuits locally, rather than through systemic pharmacological intervention.
Previous studies provided proof-of-principle that targeted transplantation of GABAergic neurons efficiently controls seizure activity (Baraban et al., 2009; Fine et al., 1990; Hattiangady et al., 2008; Hunt et al., 2013; Lindvall and Bjorklund, 1992; Loscher et al., 1998). In order to characterize and test the effects of human-derived GABAergic neuron transplants, an immunodeficient mouse model would be useful, as it would allow xenografts to be placed without the need for immunosuppression. The pilocarpine model of TLE has been well-characterized in multiple species (Curia et al., 2008), including different strains of mice, such as CF1, C57BL/6, DBA/2J, A/J, NMRI, and CD1 (Borges et al., 2003; Groticke et al., 2007; Mazzuferi et al., 2012; Shibley and Smith, 2002; Winawer et al., 2007). Because the response to pilocarpine differs substantially depending on the mouse strain, one must characterize the dose-response relationship for the particular strain of mouse to be used in a particular investigation. Here, we tested a range of pilocarpine induction conditions to produce spontaneous seizures in NodScid mice. Under our optimal conditions, mice that experienced status epilepticus (SE) at the time of induction, thereafter displayed ongoing spontaneous recurrent seizures (SRS). Histologically, under these conditions, animals demonstrated mossy fiber sprouting into the molecular layer, a well-characterized TLE-like histopathology.
2. Materials and Methods
2.1. Induction of temporal lobe epilepsy (TLE) in Nod-Scid mice
The Animal Care and Use Committee at McLean Hospital approved all animal procedures. Male and female Nod-Scid mice (Charles River Laboratory) at an age of about 7 weeks were housed under a 12-h light-dark cycle with food and water available ad libitum. No significant gender differences in terms of SE induction and survival were observed, therefore the data for both males and females was combined. Mice were administered an intraperitoneal injection (ip) of 1 mg/kg of N-methylscopolamine bromide 15 min prior to injection of pilocarpine to reduce peripheral cholinergic effects (Mazzuferi et al.). Three different dosing protocols of pilocarpine in the mice were compared: (1) a single injection of 300 mg/kg of pilocarpine; (2) a single injection of 400 mg/kg of pilocarpine; (3) ramping up injections of 100 mg/kg of pilocarpine every 10 min until onset of SE, defined as continuous seizures with at least one stage 3–5 seizure (forelimb clonus, rearing, falling), according to the Racine scale (Racine, 1972). The injections were terminated for a mouse if it did not exhibit a response by the sixth injection. Mice that developed SE were administered diazepam (10 mg/kg) ip 90 min after seizure induction to limit the duration of SE and the extent of hippocampal damage.
2.2.Implantation of surface electrodes for EEG recording
Mice that developed SE after pilocarpine injection and naïve mice without pilocarpine injection were surgically implanted with EEG electrodes for further EEG recording using a Leica Angle Two digital stereotaxic instrument (Leica Biosystems) fitted with a Cunningham Mouse Adaptor (Stoelting, Inc, Downers Grove, IL) one week after pilocarpine injection. Mice were anesthetized in an induction chamber supplied with 4–5% isoflurane (Sigma) mixed with 0.8–1.0 L/min oxygen using a calibrated vaporizer. Animals were then administered continuous isoflurane (1–2%) mixed with oxygen (0.8–1.0 L/min) via snout mask for the duration of the anesthesia. Body temperature was maintained using air-activated iron oxide heat packets. Sterile stainless steel bone screw recording electrodes (diameter 0.5 mm, length 1.1 mm; Plastics One) soldered with a lead wire were placed epidurally through rostral burr holes in the skull (AP 1.75 mm, L +2.3 mm) and reference electrodes were implanted caudal to lambda. Electrodes were cemented in place with a rapid-curing dental cement (DenMat Holdings, Lompoc, CA).
2.3. Continuous video-EEG recording of transplanted mice
One week after electrode implantation, EEG recordings of seizure activity in mice were obtained using a MP150 Biopac data acquisition System, EEG100C EEG amplifier module and AcqKnowledge 4.0 EEG Acquisition and Reader Software (BIOPAC Systems Inc.), and Eco Black Box security camera system (Lorex Technology). EEG seizures with high-frequency, high-voltage synchronized polyspike profiles with amplitudes >2-fold background and a duration ≥15 sec (Hunt et al., 2013) were analyzed using AcqKnowledge 4.0 EEG Acquisition and Reader Software (BIOPAC Systems Inc.) by investigators who were blind to treatment conditions. Once EEG seizure activity was observed, it was confirmed by offline review of video recordings. All EEG seizures were accompanied by >stage 3 seizures on the Racine scale (forelimb clonus, rearing, and/or falling). Each animal was recorded for 4 days.
2.4. Immunohistochemistry
One month after seizure induction, mice were terminally anesthetized with an ip overdose of pentobarbital (150 mg/kg, Sigma) and perfused transcardially with heparin saline (0.1% heparin in saline), followed by formaldehyde (4%). The brains were immediately removed, postfixed in 4% formaldehyde for 12 h, cryoprotected in 20% sucrose/PBS solution until equilibrated, and then cryosectioned coronally at 40µm using a freezing microtome. Tissue sections were incubated with blocking buffer (PBS, 10% normal donkey serum (NDS)) containing 0.1% Triton for 10 min. Samples were then incubated with anti-ZnT3 antibody (a kind gift from Dr. Palmiter) or anti-GFAP antibody (DAKO), diluted in PBS containing 2% NDS at 4 °C overnight. After washing with PBS, sections were incubated with fluorochrome-labeled secondary antibodies (Alexa 568-labeled IgG; Invitrogen, Carlsbad, CA) in PBS containing 2% NDS at room temperature for 30 min. After staining, sections were washed with PBS, counterstained with Hoechst 33342 (4 mg/ml), and mounted onto slides in Mowiol 4–88 (Calbiochem, Gibbstown, NJ). Confocal analysis was performed using an Olympus DSU Spinning Disc Confocal on an IX81 inverted microscope (Center Valley, PA), installed with MetaMorph software.
2.5. Statistical analysis
A t-test (alpha=0.05) was performed using Prism6 software (Graph Pad) to compare two groups. For multiple sample comparisons, analysis of variance (ANOVA) with an alpha level of 0.05 was used to determine statistical differences between group means. When significant differences were found, post-hoc analysis was conducted using Fisher’s LSD (a = 0.05) with Prism6 software.
3. Results and discussion
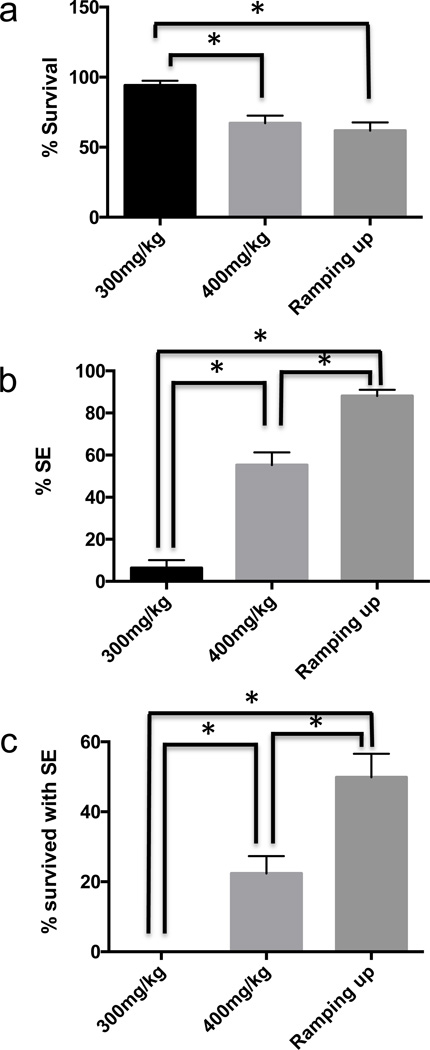
To optimize seizure induction in NodScid mice, we tested three different dosing protocols for delivering pilocarpine in mice: a single injection of 300 mg/kg of pilocarpine, a single injection of 400 mg/kg of pilocarpine, and multiple injections of 100 mg/kg of pilocarpine. For the multiple injection (Groticke et al., 2007), blood levels of pilocarpine were progressively ramped up by administering 100 mg/kg every 10 minutes until the mice exhibited a stage 3–5 seizure, according to the Racine scale (Racine, 1972). The mice started to display > stage 3 seizure activity such as clonus, rearing, or falling by the fourth injection (17.2+/−1.6% of mice) and the majority of the mice displayed >stage 3 seizure activity by the sixth injection (88.1+/− 2.9% of mice). In previous studies, TLE was induced in many other strains of mice with a single injection of 200–300 mg/kg of pilocarpine (Borges et al., 2003; Mazzuferi et al., 2012; Shibley and Smith, 2002; Winawer et al., 2007), while a single dose of 400 mg/kg was frequently lethal (Mazzuferi et al., 2012). However, for NodScid mice in our laboratory, we found that a single injection of 300 mg/kg could not reliably induce SE in NodScid mice, albeit the associated mortality was almost 0% (Fig. 1). The exact cause for such a difference is unclear but it is likely that this is in line with previous observations where immunomodulation/ immunosuppression significantly affected seizure development (Costa-Ferro et al., 2012; Fabene et al., 2013; Louboutin et al., 2011; Marchi et al., 2014). In comparison, a 400 mg/kg single injection provided significantly higher incidence of SE, but with higher mortality (Fig. 1). However, we found that repeated pilocarpine doses until seizure criterion was met (ramping up) were the most efficient protocol in terms of significantly increased rate of generating SE and significantly decreased mortality (Fig 1).
Figure 1. Optimization of pilocarpine-mediated seizure induction in NodScid mice.
Seizures were induced under a single injection of 300mg/kg pilocarpine (n=3), 400mg/kg pilocarpine (n=10), or multiple injections of 100mg/kg pilocarpine (n=9). Each injection session was performed using12–16 mice. (a) Mean percent survival for animals in each session. (b) Mean percent SE for animals in each session. (c) Mean percent survival for animals expressing SE. (Mean ±S.E.M.; P<0.05, ANOVA followed by post hoc analysis using Fisher’s LSD). * denotes significant difference.
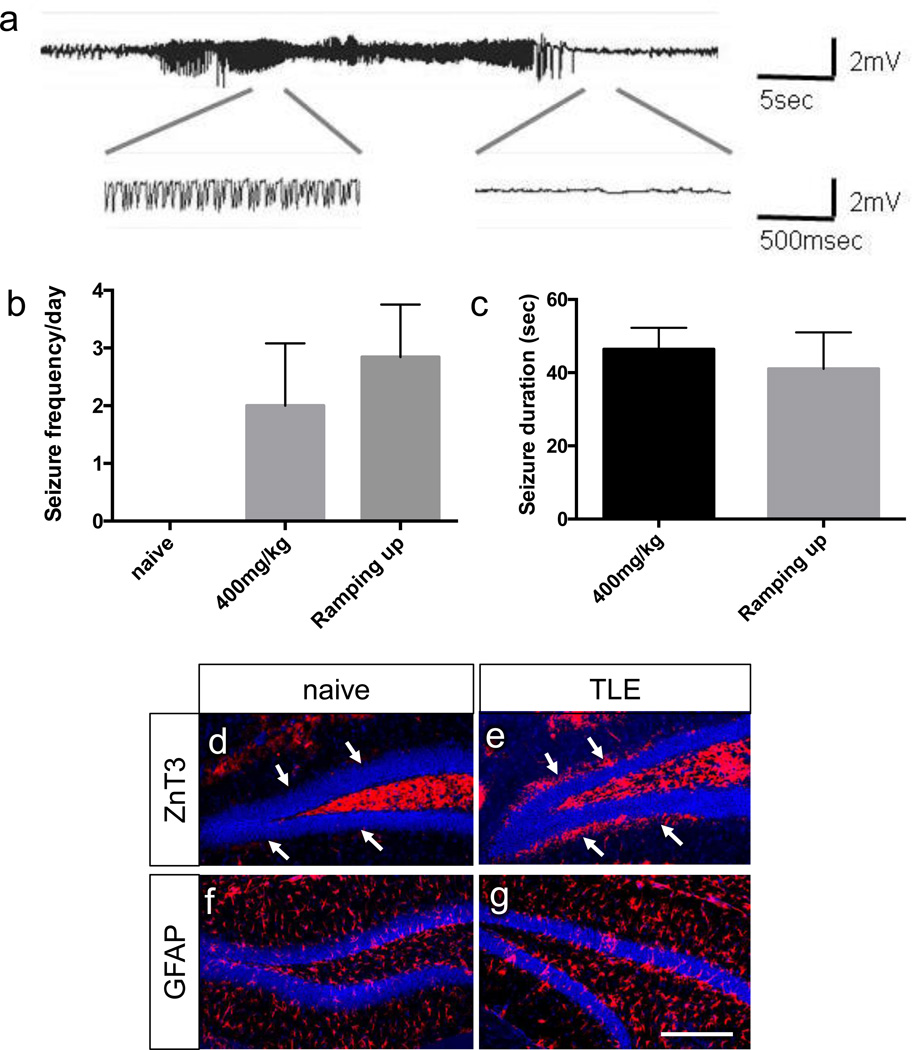
Mice that developed SE were further observed throughout a 4-day period of continuous video-EEG monitoring. This study focused on comparing 400 mg/kg single injection SE mice with 100 mg/kg multiple injection SE mice, since animals given 300 mg pilocarpine rarely achieved SE. Most of the SE mice displayed spontaneous recurrent seizures (SRS) during the 4 day period; 83.3% of the SE mice (10 out of 12) from the 400 mg/kg single injection group and 88.9% of the SE mice (8 out of 9) from the 100 mg/kg multiple injection group experienced spontaneous seizures. The EEG seizure activity, shown by high voltage high frequency polyspikes (Fig. 2a), was unevenly distributed during the 4 days recording period and was accompanied by stage >3 seizures as observed through video monitoring (clonus, rearing, and falling). There was no significant difference in seizure frequency or seizure duration between these two groups (Fig. 2b–c). Histological analysis showed that all three mice with SRS displayed mossy fiber sprouting (Fig. 2d–e) and a slight increase in GFAP staining (Fig. 2f–g), which are hallmark features of TLE development.
Figure 2. Mice with SE induction show spontaneous recurrent seizures and TLE-like histopathology.
Mice with SE were further analyzed for 4 days through continuous video-EEG recording. (a) Representative seizure EEG activity with high-frequency, high-voltage synchronized polyspikes. (b) Seizure frequency per day (Mean ± S.E.M.; P=0.5720, two tailed t-test) for naïve (n=3), 400mg/kg single injection mice (n=10) and ramping up mice (n=8). (c) Seizure duration (Mean ± S.E.M.; P=0.4700, two tailed t-test) for 400mg/kg single injection mice (n=10) and ramping up mice (n=8). (d–g) Immunohistochemical analysis of untreated mice vs. TLE mice. White arrows indicate molecular layer of dentate gyrus, where mossy fiber sprouting is observed in TLE mice but not in untreated mice. scale bars: 200 µm.
In conclusion, repeated administration of lower doses of pilocarpine generated the most efficient TLE induction in NodScid mice, judged by a higher number of SE induction with less mortality, as seen in Fig 1c (the proportion of survived SE mice). This is an important criteria to obtain the maximum number of SRS mice that can be used for subsequent transplantation experiments, considering that majority of mice with SE develop SRS later. However, multiple injections are far more labor-intensive than a single injection, which is a potential drawback of this approach, especially when administered on a large number of mice. Nonetheless, reliably inducing SE and subsequent SRS, while minimizing mortality could outweigh increased labor. We have therefore identified the optimal conditions for generating a TLE model in immunodeficient mice, which should prove valuable in developing novel cell-based therapies for TLE by facilitating efficient xeno-transplantation.
4. Conclusion
In these studies we have demonstrated that a multiple injection ramping of 100 mg/kg of pilocarpine is optimal for inducing TLE-like spontaneous seizures in immunodeficient NodScid mice. Using this method, our results show that mice with SE efficiently develop SRS and express mossy fiber sprouting, a signature histopathological feature of TLE. These findings are significant because the ability to reliably and easily generate a TLE model in immunodeficient mice will facilitate the optimization of human cell transplantation, a powerful approach to developing novel cell-based therapies for treating TLE.
Highlights.
-
-
We tested different conditions for generating a TLE model in NodScid mice.
-
-
Multiple 100 mg/kg injections of pilocarpine have been shown to be optimal in inducing TLE in NodScid mice.
-
-
NodScid mice with SE efficiently developed SRS and expressed mossy fiber sprouting.
Acknowledgement
This study was supported by NIH grants (NS079977), NIH grant (NS070577) and Harvard Stem Cell Institute Seed Grant.
Abbreviations
- TLE
Temporal lobe epilepsy
- SE
Status Epilepticus
- SRS
Spontaneous Recurrent Seizures
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- Baraban SC, Southwell DG, Estrada RC, Jones DL, Sebe JY, Alfaro-Cervello C, Garcia-Verdugo JM, Rubenstein JL, Alvarez-Buylla A. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:15472–15477. doi: 10.1073/pnas.0900141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges K, Gearing M, McDermott DL, Smith AB, Almonte AG, Wainer BH, Dingledine R. Neuronal and glial pathological changes during epileptogenesis in the mouse pilocarpine model. Experimental neurology. 2003;182:21–34. doi: 10.1016/s0014-4886(03)00086-4. [DOI] [PubMed] [Google Scholar]
- Christoph CH. Temporal lobe resection--does the prospect of seizure freedom outweigh the cognitive risks? Nat Clin Pract Neurol. 2008;4:66–67. doi: 10.1038/ncpneuro0657. [DOI] [PubMed] [Google Scholar]
- Costa-Ferro ZS, Souza BS, Leal MM, Kaneto CM, Azevedo CM, da Silva IC, Soares MB, Ribeiro-dos-Santos R, Dacosta JC. Transplantation of bone marrow mononuclear cells decreases seizure incidence, mitigates neuronal loss and modulates pro-inflammatory cytokine production in epileptic rats. Neurobiology of disease. 2012;46:302–313. doi: 10.1016/j.nbd.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Cramer JA, Mintzer S, Wheless J, Mattson RH. Adverse effects of antiepileptic drugs: a brief overview of important issues. Expert Rev Neurother. 2010;10:885–891. doi: 10.1586/ern.10.71. [DOI] [PubMed] [Google Scholar]
- Curia G, Longo D, Biagini G, Jones RS, Avoli M. The pilocarpine model of temporal lobe epilepsy. J Neurosci Methods. 2008;172:143–157. doi: 10.1016/j.jneumeth.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. A Proposed Diagnostic Scheme for People with Epileptic Seizures and with Epilepsy: Report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;42:796–803. doi: 10.1046/j.1528-1157.2001.10401.x. [DOI] [PubMed] [Google Scholar]
- Engel J. Epilepsy in the world today: medical point of view. Epilepsia. 2002;43(Suppl 6):12–13. doi: 10.1046/j.1528-1157.43.s.6.6.x. [DOI] [PubMed] [Google Scholar]
- Fabene PF, Laudanna C, Constantin G. Leukocyte trafficking mechanisms in epilepsy. Molecular immunology. 2013;55:100–104. doi: 10.1016/j.molimm.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Fine A, Meldrum BS, Patel S. Modulation of experimentally induced epilepsy by intracerebral grafts of fetal GABAergic neurons. Neuropsychologia. 1990;28:627–634. doi: 10.1016/0028-3932(90)90038-p. [DOI] [PubMed] [Google Scholar]
- Groticke I, Hoffmann K, Loscher W. Behavioral alterations in the pilocarpine model of temporal lobe epilepsy in mice. Experimental neurology. 2007;207:329–349. doi: 10.1016/j.expneurol.2007.06.021. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty AK. Grafting of striatal precursor cells into hippocampus shortly after status epilepticus restrains chronic temporal lobe epilepsy. Exp Neurol. 2008;212:468–481. doi: 10.1016/j.expneurol.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt RF, Girskis KM, Rubenstein JL, Alvarez-Buylla A, Baraban SC. GABA progenitors grafted into the adult epileptic brain control seizures and abnormal behavior. Nature neuroscience. 2013;16:692–697. doi: 10.1038/nn.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindvall O, Bjorklund A. Intracerebral grafting of inhibitory neurons. A new strategy for seizure suppression in the central nervous system. Advances in neurology. 1992;57:561–569. [PubMed] [Google Scholar]
- Loscher W, Ebert U, Lehmann H, Rosenthal C, Nikkhah G. Seizure suppression in kindling epilepsy by grafts of fetal GABAergic neurons in rat substantia nigra. J Neurosci Res. 1998;51:196–209. doi: 10.1002/(SICI)1097-4547(19980115)51:2<196::AID-JNR8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Louboutin JP, Chekmasova A, Marusich E, Agrawal L, Strayer DS. Role of CCR5 and its ligands in the control of vascular inflammation and leukocyte recruitment required for acute excitotoxic seizure induction and neural damage. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;25:737–753. doi: 10.1096/fj.10-161851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchi N, Granata T, Janigro D. Inflammatory pathways of seizure disorders. Trends in neurosciences. 2014;37:55–65. doi: 10.1016/j.tins.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzuferi M, Kumar G, Rospo C, Kaminski RM. Rapid epileptogenesis in the mouse pilocarpine model: video-EEG, pharmacokinetic and histopathological characterization. Experimental neurology. 2012;238:156–167. doi: 10.1016/j.expneurol.2012.08.022. [DOI] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalography and clinical neurophysiology. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Shamim S, Wiggs E, Heiss J, Sato S, Liew C, Solomon J, Theodore WH. Temporal lobectomy: Resection volume, neuropsychological effects, and seizure outcome. Epilepsy Behav. 2009;16:4–4. doi: 10.1016/j.yebeh.2009.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibley H, Smith BN. Pilocarpine-induced status epilepticus results in mossy fiber sprouting and spontaneous seizures in C57BL/6 and CD-1 mice. Epilepsy research. 2002;49:109–120. doi: 10.1016/s0920-1211(02)00012-8. [DOI] [PubMed] [Google Scholar]
- Winawer MR, Makarenko N, McCloskey DP, Hintz TM, Nair N, Palmer AA, Scharfman HE. Acute and chronic responses to the convulsant pilocarpine in DBA/2J and A/J mice. Neuroscience. 2007;149:465–475. doi: 10.1016/j.neuroscience.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]