Abstract
To analyze the function of cardiac autonomic regulation in patients with obstructive sleep apnea syndrome (OSAS), we enrolled 36 patients with OSAS and divided them according to the apnea hypopnea index (AHI) into 2 groups: Group I (n=19) had mild OSAS (AHI <20) and Group II (n=17) had severe OSAS (AHI ≥20). The findings were compared with those of 24 healthy control subjects who were matched for age, sex, blood pressure, and body mass index. All participants underwent 24-hour Holter monitoring, with continuous time-dependent and spectral analysis of heart rate variability. In addition, we performed arrhythmia analysis. Frequent or repetitive ventricular arrhythmias (≥30 premature ventricular beats/hour) were detected in 15 (42%) patients with OSAS and in 6 (25%) members of the control group. In both mild and severe OSAS, SDNN was significantly lower than in controls, and SDANN findings were similar. In mild OSAS, RMSSD values were not significantly lower than in controls, but in severe OSAS they were. The ULF, VLF, LF and LF/HF values of both groups of OSAS patients were significantly higher than those of controls, but their HF values were lower. The mean LF/HF ratio during the same period was significantly higher in Group II than in Group I and the control group.
Our results suggest that cardiac autonomic activity may be altered in patients with OSAS throughout a 24-hour period, that this alteration occurs even in the absence of hypertension, heart failure, or other disease states, and that it is linked to the severity of OSAS.
Key words: Autonomic nervous system, electrocardiography, heart rate, polysomnography, sleep apnea syndromes/complications
Sleep apnea is a condition in which people stop breathing during sleep. 1 The most common type, obstructive sleep apnea (OSAS) or hypopnea, is the result of a complete or partial pharyngeal occlusion during sleep 2 and is found in 1% to 4% of the adult population worldwide. 3,4 Obstructive sleep apnea occurs more frequently in middle-aged obese men, some of whom have arterial hypertension, 5 heart failure, 6 ischemic heart disease, 7 or stroke. 8 There is also an association of OSAS with cardiac arrhythmia and sudden cardiac death. 9,10
The cardiac autonomic system controls heart rate by means of parasympathetic and sympathetic activity, and the rate reflects the balance or imbalance of these 2 influences. The measurement and analysis of heart rate variability (HRV) is a valuable noninvasive method of evaluating cardiac autonomic functions in an ambulatory setting. Impaired cardiac reflexes have been demonstrated in sleep apnea patients by means of spectral HRV analysis. 11 The analysis of time-dependent HRV measurements has also been considered useful in the screening of sleep apnea patients. 12 However, the relationship between HRV and the cardiac autonomic dysfunction of OSAS is not completely understood. Therefore, we prospectively investigated, by time-dependent and spectral analysis, HRV in patients with OSAS and in a control group.
Patients and Methods
Study Population
In our study, the existence of OSAS was determined by clinical findings and by the apnea hypopnea index (AHI): ≥5 episodes of apnea or hypopnea per hour of sleep. We enrolled 36 patients with OSAS and divided them according to the apnea hypopnea index (AHI) into 2 groups: Group I (n=19) had mild OSAS (AHI <20) and Group II (n=17) had severe OSAS (AHI ≥20). This classification followed earlier studies 13,14 that showed morbidity and mortality rates to be markedly increased in patients who had OSAS and an AHI of ≥20, in comparison with patients who had OSAS and an AHI of <20. We also enrolled 24 healthy control subjects, for a total of 60 participants in the study. Because of the high prevalence of occult OSAS in apparently normal, asymptomatic obese subjects, sleep-disordered breathing was excluded in all control subjects by complete overnight sleep studies.
The 3 groups were closely matched in age, body mass index, and blood pressure. All subjects were male. The patients with sleep apnea were newly diagnosed, normotensive, free of any other known diseases, and receiving no medications; they had never been treated for sleep apnea and were otherwise healthy. All patients with sleep apnea also were free of any cardiac history, limitation or changes in exercise tolerance, and symptoms or signs suggestive of congestive heart failure.
Informed written consent was obtained from all subjects, in accordance with the requirements of our institutional review board.
Sleep Study
For each subject, the overnight sleep study was recorded between 10:30 PM and 6:00 AM in a sleep laboratory, under the observation of a technician. Recordings were done with a POLY-MESAM (PM) unit (MAP; Martinsried, Germany) consisting of a flow sensor for nasal and oral breath flow, a laryngeal microphone, a 3-channel electrocardiograph, 1 stress-sensitive belt each for the thorax and the abdomen, a positional sensor for determination of body movement, and a PLMS (periodic leg movement syndrome) sensor. The data were stored on a computer, and the software (POLY-MESAM, version 1.42) automatically calculated AHI, the apnea index (AI), and the oxygen desaturation index (ODI).
Both automated and hand-scored data analyses were provided and evaluated. The ODI was defined as the number of events per hour of sleep in which oxygen saturation decreased by 4% or more. Obstructive hypopnea was defined as a reduction in airflow or in the sum channel from the inductive plethysmograph of between 20% and 50% for at least 10 seconds, in the presence of chest or abdominal wall motion, and associated with oxyhemoglobin desaturation of 4% or less, possibly followed by arousal from sleep. Apnea was diagnosed in patients in whom respiratory flow was reduced to <20% of normal for at least 10 seconds. The AI was defined as the number of episodes of obstructive apnea per hour of sleep. The AHI was defined as the number of episodes of obstructive apnea and obstructive hypopnea per hour of sleep.
Holter Monitoring
All patients and control subjects underwent 24-hour Holter recording with 3-channel real-time tape recorders (Brentwood by Midmark 8800 Holter; Torrance, Calif), which monitored the bipolar leads I and IV and a modified aVF. Holter flash cards were analyzed with the Brentwood HRV analysis software package, version 5.2. For each patient, the total number of premature ventricular beats, ventricular couplets, and episodes of nonsustained ventricular tachycardia (defined as ≥3 consecutive premature ventricular beats with a rate ≥100 beats/min) were recorded.
Heart Rate Variability Analysis
Heart rate variability was assessed, both in time and frequency domains, on 24-hour Holter recordings after full revision of the electrocardiogram and editing of beats when indicated. During the analysis, only normal beats were measured, and all artifacts were eliminated. Time-domain HRV variables included the mean of all R-R intervals for the entire 24-hour recording; the standard deviation of all R-R intervals (SDNN); the mean of the standard deviations of all R-R intervals for all 5-minute segments (SDANN); the square root of the mean squared differences of successive R-R intervals (RMSSD); and the total number of all normal-normal intervals divided by the height of the histogram of all normal-normal intervals measured on a discrete scale with bins of 7.81 ms. In the frequency domain, HRV was assessed in the range of frequencies of 0 to 0.4 Hz by a fast-Fourier transform spectral analysis algorithm, with a spectral resolution of 0.0005 Hz, with the use of the aforementioned Brentwood software. The amplitude of the following frequency-domain HRV variables was obtained: total power (0–0.4 Hz), ultra-low frequency (ULF, 0–0.0033 Hz), very low frequency (VLF, 0.0033–0.04 Hz), low frequency (LF, 0.04–0.15 Hz), high frequency (HF, 0.15–0.40 Hz), and the low-frequency/high-frequency (LF/HF) ratio. The amplitude values of ULF, LF, HF, and LF/HF ratio were also obtained for each hour of the day.
Statistics
Results are expressed as mean ± SD. Analysis of subgroups according to the severity of the disease were performed with the Mann-Whitney test or the Kruskal-Wallis test. Two-tailed P values <0.05 were considered significant. Data analysis was carried out using the SPSS 9.0 statistical program (SPSS Inc.; Chicago, Ill).
Results
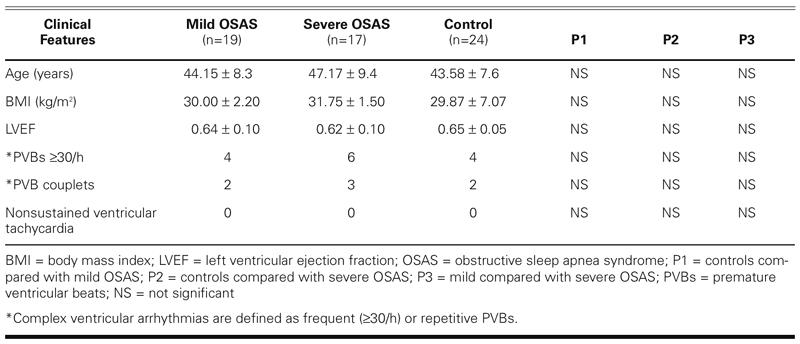
The principal findings obtained from patients and control subjects are summarized in Table I. Age and body mass index values of patients and control subjects were similar. Left ventricular ejection fractions in all OSAS patients were also similar to those in control subjects. Finally, frequent or repetitive ventricular arrhythmias (≥30 premature ventricular beats/hour or PVB couplets) were detected in 15 (42%) patients with OSAS and in 6 (25%) members of the control group.
TABLE I. Main Clinical Features of the 3 Groups
Heart rate variability results are summarized in Table II. In both mild and severe OSAS groups, SDNN was significantly lower than in the control group (P <0.05 and P <0.01, respectively). The comparable SDANN findings for mild and severe OSAS (P <0.05 and P <0.05, respectively) were similar to the SDNN results. In mild OSAS, the RMSSD values were lower than in controls, but not significantly so. However, RMSSD values in severe OSAS were significantly lower than in controls (P < 0.05). Triangular index values were similar in the OSAS and control groups.
TABLE II. Time-Dependent and Spectral Analysis of HRV Results in Patients with OSAS and in Control Subjects
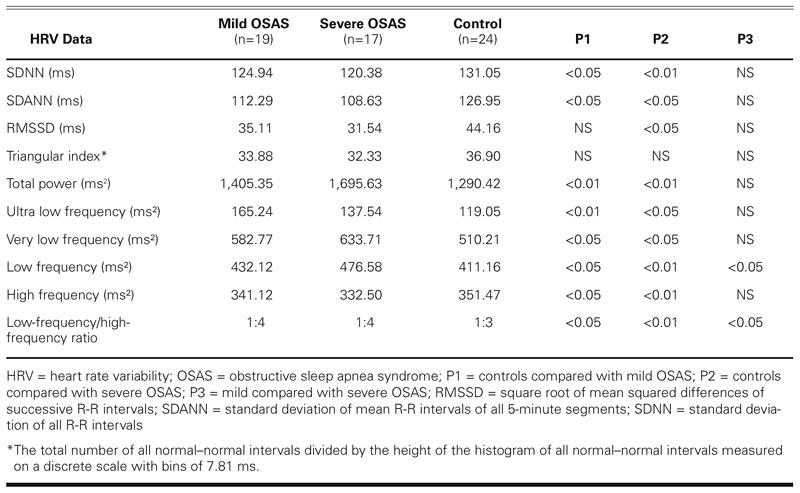
When the total power values of both OSAS groups were compared with those of the control group, there were statistically higher differences (P <0.01 and P <0.01, respectively). The ULF, VLF, LF and LF/HF values of both groups of OSAS patients were found to be significantly higher than those of controls, but their HF values were lower (Table II). In an overview of all values in Table II, there were no statistically significant differences between mild and severe OSAS except in the LF values and the LF/HF ratio.
Discussion
The pathophysiology of OSAS is complex and not completely understood. The individual apneic events in OSAS have a considerable hemodynamic impact that is mediated by a complicated sequence of physiologic events. Several important regulatory mechanisms in cardiovascular homeostasis seem to be affected in OSAS. An altered autonomic balance has been suggested as 1 possible pathogenetic factor, 15–18 and autonomic dysfunction has also been thought to be implicated in the subsequent development of cardiovascular diseases in patients with OSAS. 11 Repetitive episodes of apnea trigger marked fluctuations in both blood pressure and heart rate, with consequent effects on the estimates of cardiovascular variability. 19
Previous studies of cardiac autonomic activity in sleep apnea have focused on measurements obtained during sleep. 20,21 In contrast to previous studies, our study was performed to show the effect of OSAS on cardiac autonomic activity during a 24-hour period.
Time-domain and spectral HRV analysis appear to be powerful tools in investigating OSAS. In the past, time-domain HRV was used frequently, but now spectral analysis of the HRV is a more commonly used method for evaluation of the autonomic modulation of the heart rate. Spectral analysis gives selective information on parasympathetic and sympathetic function. Moreover, because the method is noninvasive, it is appropriate for clinical studies. Spectral analysis is reproducible and has a better sensitivity and specificity than do the previously used time-domain methods in short-term studies of cardiovascular reflexes. 22 In another variation from previous studies, our 24-hour Holter recordings provided information for both spectral and time-domain analyses. In addition, we evaluated Holter arrhythmia findings.
Previous studies on the function of the autonomic nervous system in OSAS have often focused on sympathetic function. Studies of muscle sympathetic nerve activity (MSNA) have shown increased sympathetic activity during sleep apneic events, as well as a sustained increase in MSNA during daytime wakefulness at rest. 11,18,19 A suggested mechanism is that repetitive obstructions to normal breathing during sleep induce hypoxemia and hypercapnia, which (acting through the chemoreflexes) elicit increased sympathetic activity that is especially evident at the end of the apnea episode. 11 The increased sympathetic drive during wakefulness and its repetitive surges during sleep may also be the cause of the decreased baroreceptor reflex sensitivity that has been reported in OSAS. 11,18,23 An increase in serum noradrenalin concentration has also been reported in OSAS patients. 18 Finally, study of MSNA has shown that the increased sympathetic activity seems to be attenuated after treatment with continuous positive airway pressure. 19
Our data indicate that patients with OSAS have a noteworthy impairment of cardiac autonomic function, as assessed by both time-domain and spectral HRV analysis. Our OSAS patients demonstrated that most of their time-dependent parameters were significantly lower than those of the controls. This result may suggest a lack of parasympathetic activity. The rise in sympathetic tone and the withdrawal of parasympathetic activity, which is responsible for increasing the heart rate, could be the physiologic trigger for a complex cascade of events. This sequence of events could lead to an acute cardiovascular incident under suitable pathophysiological conditions. 24 Shiomi and colleagues 21 demonstrated an increase in the VLF component of HRV in OSAS patients. The VLF peak appeared during episodes of apnea in patients with the most severe OSAS and usually disappeared after treatment. The VLF oscillations of heart rate that were described previously in OSAS were synchronized with the absence of air exchange, or hypoxemia. 25 The physiologic interpretation of these VLF oscillations is still a subject of debate. Guilleminault and coworkers 25 found that VLF, HF, and LF activity increased during the night. Those authors claimed that increases in HF during OSAS have been linked to repeated exaggerated bursts of parasympathetic activity during the night, and that the increase in LF comes from an increased sympathetic tone during OSAS, followed by negative feedback on the baroreflex. In contrast to this, we found increases in VLF, LF, and the LF/HF ratio, but a decrease in HF activity. This may mean that parasympathetic activity increases during the night, but sympathetic activity is dominant both day and night.
Our data are in agreement with those obtained in previous studies, 11,12 which also reported an impairment of HRV parameters in patients with OSAS. In our study, we confirmed that the HRV reduction can be linked to the severity of disease. When viewed as a whole, our data suggest that impairment of cardiac autonomic function in patients with OSAS cannot be explained by single abnormalities. It is probably the result of multiple components, which may include respiratory factors, mechanical cardiac dysfunction, and such factors as structural and functional abnormalities of the sinoatrial node, neurohumoral changes associated with physical inactivity, and abnormal mechanoreceptor- and metaboreceptor-mediated reflexes originating from diseased skeletal muscles.
It has been shown that the frequency and severity of arrhythmia can increase in OSAS patients. 11 In our study, the number of premature ventricular beats and PVB-couplet arrhythmias increased in mild and severe cases of OSAS, compared with the control group, but there was no statistical difference (P > 0.05). This may be due to the small number of patients.
This study has several strengths. First, all participants were free of medications. Second, normal control subjects were matched for age and body mass index, so we ruled out any potential confounding influence of age or obesity on our data. Third, all patients with sleep apnea were normotensive, free of other known diseases, newly diagnosed, and never treated for sleep apnea. Fourth, control subjects were selected from normal healthy people who had no sleep problems and negative sleep studies.
In conclusion, we confirmed that cardiac autonomic activity might be altered in patients with OSAS during a 24-hour period. This alteration was evident even in the absence of hypertension, heart failure, or other disease states and was found to be linked to the severity of the OSAS.
Footnotes
Address for reprints: Mustafa Aydin, MD, Zonguldak Karaelmas Universitesi Tip Fakultesi, Kardiyoloji Anabilimdali, 67600 Kozlu, Zonguldak, Turkey
E-mail: drmustafaaydin@hotmail.com
References
- 1.Guilleminault C, Tilkian A, Dement WC. The sleep apnea syndromes. Annu Rev Med 1976;27:465–84. [DOI] [PubMed]
- 2.Remmers JE, deGroot WJ, Sauerland EK. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol 1978; 44:931–8. [DOI] [PubMed]
- 3.Gislason T, Almqvist M, Eriksson G, Taube A, Boman G. Prevalence of sleep apnea syndrome among Swedish men—an epidemiological study. J Clin Epidemiol 1988;41:571–6. [DOI] [PubMed]
- 4.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993;328:1230–5. [DOI] [PubMed]
- 5.Hung J, Whitford EG, Parsons RW, Hillman DR. Association of sleep apnea with myocardial infarction in men. Lancet 1990;336:261–4. [DOI] [PubMed]
- 6.Tkacova R, Rankin F, Fitzgerald FS, Floras JS, Bradley TD. Effects of continuous positive airway pressure on obstructive sleep apnea and left ventricular afterload in patients with heart failure. Circulation 1998;98:2269–75. [DOI] [PubMed]
- 7.Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in men with coronary artery disease. Chest 1996;109:659–63. [DOI] [PubMed]
- 8.Dyken ME, Somers VK, Yamada T, Ren ZY, Zimmerman MB. Investigating the relationship between stroke and obstructive sleep apnea. Stroke 1996;27:401–7. [DOI] [PubMed]
- 9.Guilleminault C, Connolly SJ, Winkle RA. Cardiac arrhythmia and conduction disturbances during sleep in 400 patients with sleep apnea syndrome. Am J Cardiol 1983;52:490–4. [DOI] [PubMed]
- 10.Bliwise DL, Bliwise NG, Partinen M, Pursley AM, Dement W. Sleep apnea and mortality in an aged cohort. Am J Public Health 1988;78:544–7. [DOI] [PMC free article] [PubMed]
- 11.Narkiewicz K, Montano N, Cogliati C, van de Borne PJ, Dyken ME, Somers VK. Altered cardiovascular variability in obstructive sleep apnea. Circulation 1998;98:1071–7. [DOI] [PubMed]
- 12.Roche F, Gaspoz JM, Court-Fortune I, Minini P, Pichot V, Duverney D, et al. Screening of obstructive sleep apnea syndrome by heart rate variability analysis. Circulation 1999; 100:1411–5. [DOI] [PubMed]
- 13.Bradley TD, Phillipson EA. Sleep disorders. In: Murray JF, Nadel JA, Mason RJ, Boushey HA Jr, editors. Textbook of respiratory medicine. 3rd ed. Philadelphia: WB Saunders; 2000. p. 2153–70.
- 14.Guilleminault C, Stoohs R, Partinen M, Kryger M. Mortality and morbidity of obstructive sleep apnea syndrome. In: Saunders NA, Sullivan CE, editors. Sleep and breathing. Lung biology in health and disease. 2nd ed. New York: Marcel Dekker; 1994. p. 557–73.
- 15.Hanly PJ, George CF, Millar TW, Kryger MH. Heart rate response to breath-hold, valsalva and Mueller maneuvers in obstructive sleep apnea. Chest 1989;95:735–9. [DOI] [PubMed]
- 16.Smirne S, Ferini Strambi L, Zucconi M, Pinto P, Franceschi M. Cardiac autonomic dysfunction during sleep in some neurological diseases. Neurophysiol Clin 1990;20:131–6. [DOI] [PubMed]
- 17.Svanborg E, Carlsson-Nordlander B, Larsson H, Sachs C, Kaijser L. Autonomic nervous system function in patients with primary obstructive sleep apnoea syndrome. Clin Auton Res 1991;1:125–30. [DOI] [PubMed]
- 18.Carlson JT, Hedner JA, Sellgren J, Elam M, Wallin BG. Depressed baroreflex sensitivity in patients with obstructive sleep apnea. Am J Respir Crit Care Med 1996;154:1490–6. [DOI] [PubMed]
- 19.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest 1995;96:1897–904. [DOI] [PMC free article] [PubMed]
- 20.Leroy M, Van Surell C, Pilliere R, Hagenmuller MP, Aegerter P, Raffestin B, Foucher A. Short-term variability of blood pressure during sleep in snorers with or without apnea. Hypertension 1996;28:937–43. [DOI] [PubMed]
- 21.Shiomi T, Guilleminault C, Sasanabe R, Hirota I, Maekawa M, Kobayashi T. Augmented very low frequency component of heart rate variability during obstructive sleep apnea. Sleep 1996;19:370–7. [DOI] [PubMed]
- 22.Heart rate variability: standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996;93:1043–65. [PubMed]
- 23.Parati G, Di Rienzo M, Bonsignore MR, Insalaco G, Marrone O, Castiglioni P, et al. Autonomic cardiac regulation in obstructive sleep apnea syndrome: evidence from spontaneous baroreflex analysis during sleep. J Hypertens 1997; 15(12 Pt 2):1621–6. [DOI] [PubMed]
- 24.Molnar J, Zhang F, Weiss J, Ehlert F, Rosenthal JE. Diurnal pattern of QTc interval: how long is prolonged? Possible relation to circadian triggers of cardiovascular events. J Am Coll Cardiol 1996;27:76–83. [DOI] [PubMed]
- 25.Guilleminault C, Connolly S, Winkle R, Melvin K, Tilkian A. Cyclical variation of the heart rate in sleep apnoea syndrome. Mechanisms, and usefulness of 24 h electrocardiography as a screening technique. Lancet 1984;1(8369):126–31. [DOI] [PubMed]


