Rho-kinases are a class of enzyme that have been implicated in the response to painful stimuli including heat and cold. The authors of this study performed experiments to assess the effects of inhibition of rho-kinases (using two small-molecule inhibitors) on nociceptive responses and edema formation in a rat model of peripheral nociception.
Keywords: Fasudil, NO/cGMP/PKG pathway, Nociception, Paw edema, Rho-kinases, Y27632
Abstract
BACKGROUND:
Rho-kinases (ROCKs), a family of small GTP-dependent enzymes, are involved in a range of pain models, and their inhibition typically leads to antinociceptive effects.
OBJECTIVES:
To study the effects of inhibiting ROCKs using two known inhibitors, Y27632 and HA1077 (fasudil), administered locally, on nociception and paw edema in rats.
METHODS:
A range of doses of Y27632 or HA1077 (2.5 μg to 1000 μg) were injected locally into rat paws alone or in combination with carrageenan, a known proinflammatory stimulus. Nociceptive responses to mechanical stimuli and increased paw volume, reflecting edema formation, were measured at 2 h and 3 h, using a Randall-Selitto apparatus and a hydroplethysmometer, respectively.
RESULTS:
Animals treated with either ROCK inhibitor showed biphasic nociceptive effects, with lower doses being associated with pronociceptive, and higher doses with antinociceptive responses. In contrast, a monophasic dose-dependent increase in edema was observed in the same animals. Local injection of 8-bromo-cyclic (c)GMP, an activator of the nitric oxide/cGMP/protein kinase G pathway, also produced biphasic effects on nociceptive responses in rat paws; however, low doses were antinociceptive and high doses were pronociceptive. Local administration of cytochalasin B, an inhibitor of actin polymerization and a downstream mediator of ROCK activity, reversed the antinociceptive effect of Y27632.
CONCLUSIONS:
The results of the present study suggest that ROCKs participate in the local mechanisms associated with nociception/antinociception and inflammation, with a possible involvement of the nitric oxide/cGMP/protein kinase G pathway. Also, drug effects following local administration may differ markedly from the effects following systemic administration. Finally, separate treatment of pain and edema may be needed to maximize clinical benefit in inflammatory pain.
Abstract
HISTORIQUE :
Les rho-kinases (ROCK), une famille de petits enzymes GTPases, participent à une série de modèles de douleur, et leur inhibition a généralement des effets antinociceptifs.
OBJECTIFS :
Étudier les effets de l’inhibition des ROCK au moyen de deux inhibiteurs connus administrés localement, le Y27632 et le HA1077 (fasudil), sur la nociception et l’œdème des pattes des rats.
MÉTHODOLOGIE :
Les chercheurs ont injecté une série de doses de Y27632 et de HA1077 (de 2,5 μg à 1 000 μg) dans les pattes de rats, seules ou en combinaison avec de la carraghénane, un stimulant proinflammatoire connu. Ils ont mesuré les réponses nociceptives aux stimuli mécaniques et à l’augmentation du volume des pattes, reflétant la formation d’œdème au bout de deux et trois heures, au moyen d’un test de Randall-Selitto et d’un hydro-pléthysmomètre, respectivement.
RÉSULTATS :
Les animaux traités avec l’un des inhibiteurs de la ROCK ont présenté des effets nociceptifs biphasiques, les doses moins élevées s’associant à une réponse pronociceptive et les doses plus élevées, à une réponse antinociceptive. Par contre, chez les mêmes animaux, on a observé une augmentation monophasique de l’œdème associée à la dose. L’injection locale de cGMP 8-bromo-cyclique, un activateur du monoxyde d’azote, du cGMP et de la voie de protéine kinase G, produisait également des effets biphasiques sur les réponses nociceptives des pattes de rats. Cependant, de faibles doses étaient antinociceptives et de fortes doses, pronociceptives. L’administration locale de cytochalasine B, un inhibiteur de la polymérisation de l’actine et un médiateur en aval de l’activité des ROCK, renversait l’effet antinociceptif du Y27632.
CONCLUSIONS :
D’après les résultats de la présente étude, les ROCK participent aux mécanismes locaux associés à la nociception ou à l’antinociception et à l’inflammation, peut-être en association avec le monozyde d’azote, le cGMP et la voie de la protéine kinase G. De plus, les effets des médicaments après leur administration locale peuvent différer considérablement de ceux consécutifs à leur administration systémique. Enfin, un traitement distinct de la douleur et de l’œdème peut s’imposer pour en maximiser les avantages cliniques en cas de douleur inflammatoire.
Rho-kinase (ROCK) is a serine/threonine kinase identified as a GTP-Rho-binding protein belonging to the family of GTPases (1). Although many studies have focused on ROCKs in smooth muscle cells (2,3), many other activities have been associated with ROCKs in other cellular systems (3–5). Particularly, the participation of ROCKs in platelet aggregation, formation of stress fibres, focal adhesion, cellular motility (6–9) and monocyte transendothelial migration (10) has been described, which, collectively, suggest the involvement of ROCKs in inflammatory processes (11–13). Closer to the present context, ROCK activity has also been implicated in pain sensitivity responses such as those stimulated by cold (14), heat (15,16), formalin (17) and other stimuli (18–20). In addition, ROCKs have been implicated in the inhibition of acetylcholine release in peripheral cholinergic nerves (21).
In terms of underlying mechanisms, ROCK activity modifies cyto-skeletal structure and function by metabolism of intermediary filaments (vimentin [8]); phosphorylation of myristoylated alanine-rich C-kinase substrate (22); or, especially, via binding proteins that regulate microfilament polymerization (5,23,24). The imbalance in cyto-skeleton assembly caused by ROCK activation may interfere in crucial functions of the central nervous system (19) including inflammatory and neuropathic pain (22). Accordingly, ROCK inhibition increased neurite outgrowth (17,24,25), an important aspect of the cytoskeleton associated with augmented pain sensitivity (26); conversely, its disturbance was reflected in a decrease in the pain sensitivity response (15). Another mechanistic link between ROCKs and nociception (17) is their with the phosphorylation cascade of cofilin, an actindestabilizing protein (27), which implies an important role in nociception mediated via the nitric oxide (NO)/cyclic (c)GMP/protein kinase G (PKG) pathway. Other NO-ROCK interactions lead to a stimulatory activity of ROCKs on NO synthesis (28) and the inverse, activation of ROCK production by NO (29). Thus, although ROCK-NO interactions have been demonstrated, the mechanisms underlying these interactions are not completely understood. The NO/cGMP/PKG pathway is an important component of the peripheral analgesic effects associated with a range of known analgesic compounds (30–39). Despite the well-documented analgesic or, more properly, antinociceptive effects related to this cGMP pathway, nociceptive as well as antinociceptive responses have been observed following injections of NO donors, cGMP analogues or L-arginine, depending on the dose and agent used (40–45). Because biphasic effects are brought about by activation of the cGMP pathway (41,43) and by ROCK inhibition (46), and because both pathways could be involved in nociceptive responses in the periphery, we have analyzed the role(s) played by ROCK inhibitors in a model of peripheral nociception.
We have tested the effects of two ROCK inhibitors, Y27632 and HA1077 (fasudil), alone or in combination with carrageenan, a standard proinflammatory stimulus (47), in a well-established model of inflammatory pain (48), assessing both nociception and edema. Because most of the work using ROCK inhibitors in vivo cited above has used systemic or central administration of these compounds, and because we were particularly interested in their peripheral effects, we administered the ROCK inhibitors locally, by intraplantar (IPL) injection. We also examined the inflammatory effects of 8-bromo-cGMP, a cGMP analogue, to assess the contribution of the NO/cGMP/PKG pain pathway to this model and investigated the participation of the cytoskeleton (actin filaments) in the nociceptive responses to the ROCK inhibitor Y27632.
METHODS
Animals and preparation
All animal care and experimental procedures conformed to the regulations of the International Association for the Study of Pain on Ethical Issues and were approved by the Federal University of Minas Gerais Ethics Committee for Animal Use (Belo Horizonte, Brazil). Male Holtzman rats (150 g to 180 g) were supplied by the Bioterism Center of the Federal University of Minas Gerais. The animals were housed in groups of five animals with a 12 h light/12 h dark cycle and temperature of 24±2°C. They were allowed access to commercial food and water ad libitum. In the first experiment, groups of rats (n=3 to n=5) were injected with Y27632 (2.5 μg to 1000 μg), HA1077 (25 μg to 500 μg), 8-bromo-cGMP (25 μg to 1000 μg) or vehicle (2% dimethyl sulfoxide in saline) IPL at time zero. In a second experiment, groups of animals (n=5) were injected with Y27632 (25 μg or 500 μg) or vehicle 5 min before the proinflammatory stimulus (250 μg carrageenan, injected IPL), considered to represent time 0. In a third set of experiments, cytochalasin B (1 μg) or vehicle was injected IPL 15 min before Y27632 (25 μg or 500 μg; n=3 to n=5).The total volume of all IPL injections was never >100 μL and they were administered with sterile 1 mL syringes and 0.38×13 mm needles. The doses and dilutions of drugs and the times of administration used in the experiments were based on published data for the ROCK inhibitors (15,18) and for 8-bromo-cGMP (41,43).
Measurement of the nociceptive threshold
All assays were performed without knowledge of the treatments administered to the animals. The nociceptive response was evaluated using a pressure algesimeter (Ugo Basile, Italy), in which a progressive compression is applied to a rat’s hindpaw, essentially as proposed by Randall and Sellitto (48). This method consists of the measurement (in g) of the force applied to the pads necessary to trigger a defensive behaviour, such as hindpaw withdrawal, which is a sign of nociception. To avoid tissue damage, the maximum weight applied was fixed at 300 g. The intensity of the nociceptive response (Δ nociceptive threshold) was expressed as the mean (± SEM) difference between nociceptive thresholds (in g) obtained in the right (injected with the test substance) and left (injected or not injected with vehicle) paws at each timepoint. The difference (Δ) of nociceptive thresholds among saline, vehicle and naive-contralateral paws did not change significantly over the experimental period from the values at time 0 (Table 1).
TABLE 1.
Effects of the Rho-kinase inhibitor Y27632 (Y) on nociceptive thresholds in rat paws
| Treatment/dose, μg | Nociceptive threshold g applied/time, min | ||||
|---|---|---|---|---|---|
|
| |||||
| 0 | 30 | 60 | 120 | 180 | |
| Vehicle (n=5) | |||||
| RP | 134±9 | 126±15 | 126±5 | 128±8 | 136±9 |
| LP | 138±8 | 130±12 | 134±9 | 132±11 | 132±8 |
| 2.5 μg Y (n=4) | |||||
| RP | 127±9 | 117±20 | 125±13 | 125±6 | 130±12 |
| LP | 128±10 | 127±10 | 130±12 | 128±10 | 128±10 |
| 25 μg Y (n=7) | |||||
| RP | 133±8 | 83±17 | 81±13 | 84±14 | 101±12 |
| LP | 131±7 | 129±9 | 130±8 | 124±8 | 130±8 |
| 50 μg Y (n=6) | |||||
| RP | 130±9 | 88±8 | 86±20 | 100±31 | 125±14 |
| LP | 130±9 | 133±12 | 135±12 | 128±15 | 130±13 |
| 150 μg Y (n=5) | |||||
| RP | 130±10 | 124±11 | 130±7 | 124±11 | 128±11 |
| LP | 132±8 | 126±11 | 126±9 | 128±8 | 130±10 |
| 500 μg Y (n=7) | |||||
| RP | 126±5 | 197±17 | 191±31 | 151±19 | 131±12 |
| LP | 127±8 | 128±11 | 130±10 | 126±8 | 133±8 |
| 1000 μg Y (n=6) | |||||
| RP | 127±5 | 220±32 | 215±26 | 178±21 | 138±22 |
| LP | 125±5 | 117±5 | 118±4 | 123±10 | 120±6 |
Data presented as mean ± SD. Animals received vehicle or Y in a volume of 100 μL injected intraplantarly into animal's right hind paw(RP). The left hind paw (LP) was injected only with vehicle and served as a control. Measurements of nociceptive thresholds were performed without knowledge of the treatments
The nociceptive responses were measured immediately before (time 0), and 15 min, 30 min, 60 min and 120 min after administration of ROCK inhibitors, and also 180 min after carrageenan administration. To facilitate data comparison, the results were transformed into area under the curve over 120 min or 180 min of observation for each group, using the trapezoidal rule from GraphPad Prism version 6.01 (GraphPad Software, USA).
Assessment of paw edema formation
Paw volumes of the same animals (see above) were measured using a hydroplethysmometer (Ugo Basile, Italy), and data were expressed as the difference (Δ) between right and left hindpaws in millilitres (mean ± SEM) for a group of animals, at the same time points as used in the nociceptive threshold experiments. To facilitate data comparison, these results were also transformed into the area under the curve using the trapezoidal rule, as described above.
Chemicals
The compounds Y27632 ([R]-[+]-trans-4-[1-aminoethyl]-N-[4-pyridyl] cyclohexanecarboxamide dihydrochloride) and HA1077 (fasudil) were purchased from Cayman Chemicals (USA) and LClabs (USA), respectively. Cytochalasin B, an inhibitor of actin polymerization (49), and 8-bromo-cGMP were purchased from Sigma (USA) and λ-carrageenan from Science Lab (USA). All compounds, except carrageenan, were dissolved in 1% to 20% dimethyl sulfoxide in sterile isotonic saline. Carrageenan was dissolved in sterile isotonic saline.
Statistical analysis
Data for each group of four to seven animals (mean ± SEM) were analyzed using one-way ANOVA. Differences between means were further examined using Bonferroni’s test. Differences between means were considered to be statistically significant at P<0.05.
RESULTS
Effects of single IPL injections of ROCK inhibitors or a cGMP analogue on nociceptive threshold and paw edema
To study the role of the ROCK inhibitors on nociception or paw edema, single injections of Y27632 or HA1077 were administered IPL to rat paws. As shown in Figure 1A and 1B, the lower doses of both ROCK inhibitors (25 μg/paw to 50 μg/paw) induced a decrease in the nociceptive threshold, equivalent to the development of hyper-algesia, ie, a pronociceptive response. Intermediate doses were associated with no significant changes in nociceptive thresholds, whereas higher doses (500 μg/paw to 1000 μg/paw) were associated with an elevation of nociceptive threshold above basal levels, equivalent to hypoalgesia or an analgesic response. However, IPL administration of the same doses of Y27632 and HA1077 evoked a continuous dose-response curve of paw edema formation, as shown in Figure 2A and 2B, respectively. It is worth emphasizing that the edema measurements were conducted in the same rats and at the same time as the nociceptive assays. The effects of a cGMP analogue given IPL on nociceptive threshold and paw edema were also studied. As shown in Figure 3, 8-bromo-cGMP also elicited a biphasic effect on nociceptive responses over the dose range used (25 μg to 1000 μg). However, the low dose of 8-bromo-cGMP (25 μg/paw) was associated with an elevation of nociceptive threshold above basal levels (hypoalgesia) whereas the highest dose (1000 μg/paw) was associated with a lowering of nociceptive thresholds (Figure 3). Moreover, the animals injected IPL with 8-bromo-cGMP showed no increases in paw volume, ie, no signs of paw edema (data not shown).
Figure 1).
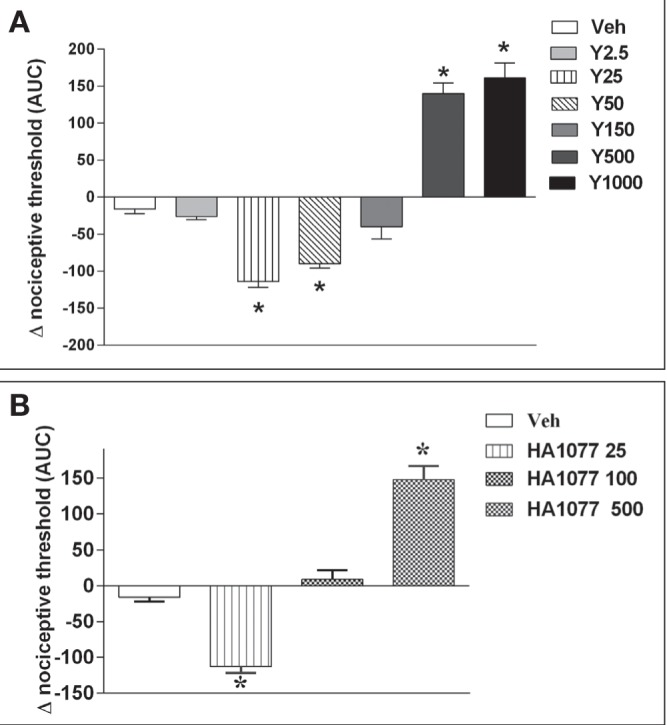
Dual effects of two Rho-kinase (ROCK) inhibitors, Y27632 (A) and HA1077 (B), on nociceptive threshold in rats. Nociceptive thresholds were measured using a pressure algesimeter at 15 min, 30 min, 60 min and 120 min following single injections in rat hind paws. Data were transformed into area under the curve (AUC) over 120 min. Inhibitors were prepared in a solution of dimethyl sulfoxide diluted in iso-tonic saline: vehicle (Veh); Y27632 (Y), 2.5 μg to 1000 μg; HA1077 (HA), 25 μg to 500 μg. *Significantly different from vehicle (P<0.01). n=4 to n=5 animals per group
Figure 2).
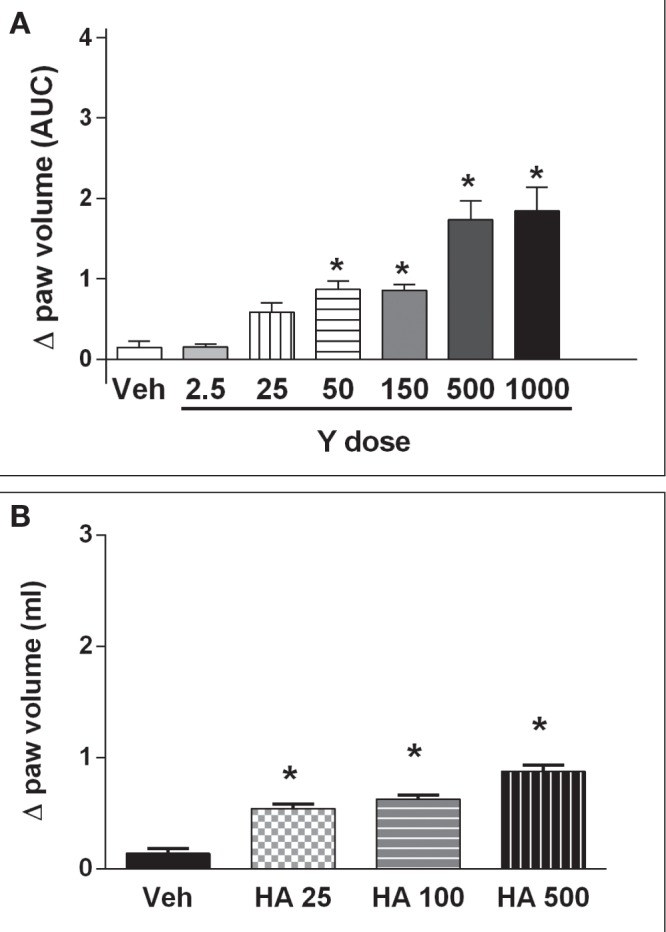
Dose-response curve of Y27632 (Y) (A) and HA1077 (HA) (B) on paw edema formation. The paw volumes (in mL) were measured using a plethysmometer (Ugo Basile, Italy) at 15 min, 30 min, 60 min and 120 min following single injections in rat hindpaws. Inhibitors were prepared in a solution of dimethyl sulfoxide diluted in isotonic saline: vehicle (Veh); Y27632 (Y), 2.5 μg to 1000 μg; HA1077 (HA), 25 μg to 500 μg. *Significantly different from vehicle (P<0.01). n=4 to n=5 animals per group
Figure 3).
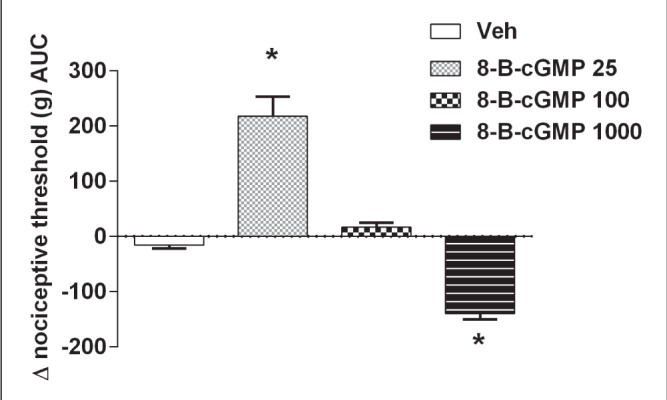
Dual effect of a cyclic (c)GMP analogue, 8-bromo-cGMP (8-B-cGMP) (25 μg to 1000 μg), on nociceptive thresholds in rat paws. Low doses of the cGMP analogue (25 μg) induced analgesia and higher doses induced hyperalgesia. *Significantly different from vehicle (P<0.01); n=4 animals per group. AUC Area under the curve; Veh Vehicle
Treatments administered to the right ipsilateral hindpaw that altered its nociceptive threshold or volume had no effects on the responses in the left contralateral paw that received only vehicle (Table 1).
Effects of Y27632 on responses to carrageenan in rat hindpaws
Because Y27632 administered alone altered the nociceptive threshold of rat paws, the effects of combining Y27632 with carrageenan, a standard proinflammatory stimulus (47), were subsequently examined. In these assays, Y27632 was injected IPL 5 min before carrageenan and nociceptive thresholds and paw volume measured over the subsequent 180 min. At the lower dose, Y27632 (25 μg per paw) which, when given alone, induced significant hyperalgesia (Figure 1A), did not add to the hyperalgesia induced by carrageenan (Figure 4A) or modify its time course (data not shown). At the higher dose (500 μg), however, Y27632 did modify carrageenan-induced hyperalgesia, decreasing the fall of nociceptive threshold (Figure 4A), ie, the ROCK inhibitor behaved as an antinociceptive agent. Hindpaw volumes from these animals injected with carrageenan and Y27632 were also measured (Figure 4B). Again, the low dose of Y27632 did not affect carrageenan-induced paw edema, whereas the higher dose of Y27632 clearly increased the carrageenan-induced edema. The cGMP analogue 8-bromo-cGMP combined with carrageenan showed effects similar to those of Y27632 on carrageenan-induced hyperalgesia, with the lower dose of 8-bromo-cGMP (25 μg) producing an antinociceptive effect (data not shown).
Figure 4).
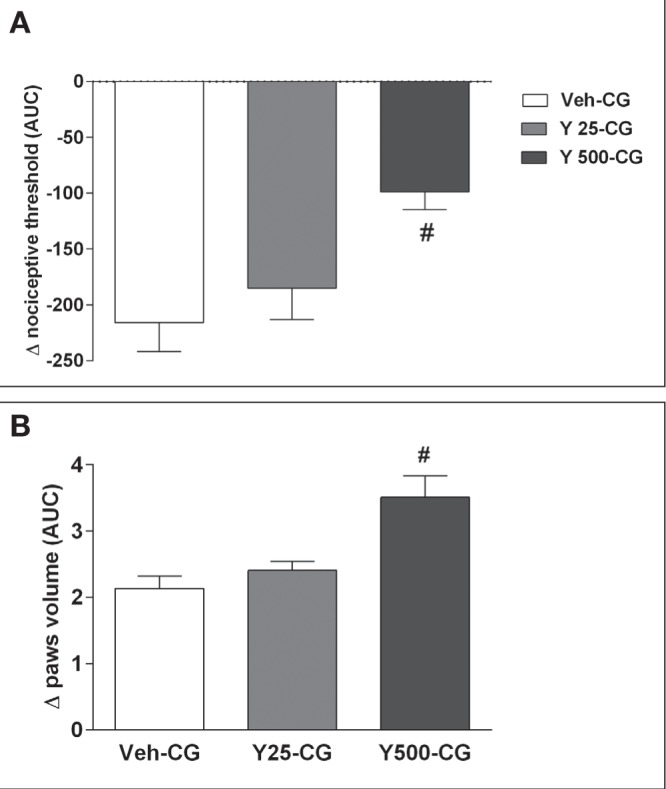
Effects of the combination of Y27632 (Y) (25 μg or 500 μg) with carrageenan (CG) (250 μg) on rat hyperalgesia (A) and paw edema (B). The effects were assessed using a pressure algesimeter and a plethysmometer, respectively. Data were transformed to area under the curve (AUC) over 180 min. #Significantly different from CG (vehicle [Veh]-CG); P<0.05. n=5 animals per group
Effect of cytochalasin B on the pro- and antinociceptive effects of Y27632
Cytochalasin B is known as an inhibitor of actin filament polymerization (49) and has recently been shown to be involved in analgesia induced by morphine in the pain model used in the present study (50). Local pretreatment of rat paws (15 min before) with cytochalasin B (1 μg IPL) did not affect hyperalgesia following the low dose of Y27632 (25 μg) but completely prevented the hypoalgesia induced by the high dose (500 μg) (Figure 5). Pretreatment with cytochalasin B did not affect the paw edema induced by the ROCK inhibitor in these animals (data not shown).
Figure 5).
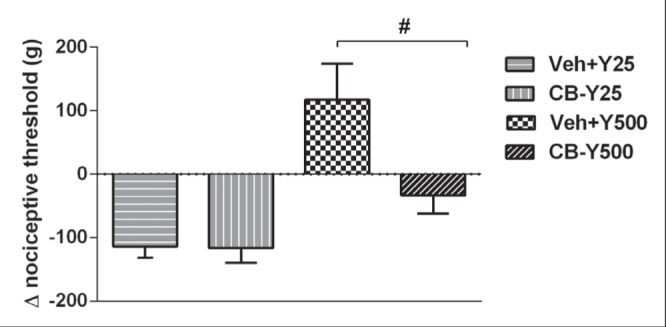
Reversal of the analgesic effect induced by Y27632 (Y) (25 μg or 500 μg) by cytochalasin B (CB) (1 μg) in rat paws. CB was injected 5 min before administration of a low (25 μg) or a high (500 μg) dose of Y locally. CB and Y were diluted in a solution of dimethyl sulfoxide and isotonic saline (vehicle [Veh]). #Significant difference between Veh-Y500 and CB-Y500 (P<0.05). n=3 to n=5 animals per group
DISCUSSION
Our experiments showed that Y27632, a ROCK inhibitor, induced a dose-dependent biphasic effect on nociceptive response, ie, hyper- and hypoalgesia, when administered locally to rat hindpaws. Because both the biphasic nature of the response and the clear pronociceptive effect were unexpected, we used another ROCK inhibitor, HA1077, in our system. Over a similar dose range (25 μg to 500 μg per paw), HA1077 also induced a decrease and an increase in nociceptive threshold, respectively, ie, hyperalgesia at the lowest dose and hypoalgesia at the highest dose used. Interestingly, we had previously shown that analgesic drugs, such as celecoxib and morphine, also induced hypoalgesic or antinociceptive responses under similar experimental conditions (50–52). Because the molecular weight of the two ROCK inhibitors are very close (approximately 300), the doses we used were comparable in molar terms, and the similarity in responses to Y27632 or HA1077 suggests that these biphasic nociceptive responses were more likely to be related to their common activity as ROCK inhibitors.
Our data are comparable with those reported by Chan et al (46), who used a model of cervical column transection, in that beneficial and detrimental effects, respectively, were also observed when a higher or a lower dose of Y27632 was used. However, the predominant effect associated with ROCK inhibitors was antinociception (14–17,53). In particular, HA1077 was associated with antinociceptive properties in a wide variety of pain models (18). In addition, another ROCK inhibitor (AS1892802) tested in a rat model of arthritis was similarly antinociceptive after oral (10 mg/kg) or intra-articular injection (3 μg) in inflamed knees (19,53). Yoshimi et al (20) observed no effect of this ROCK inhibitor, ie, neither nociception nor antinociception, after intra-articular injection in the noninflamed knee. One possible resolution to this paradox is to suggest that other kinases were inhibited by the doses of ROCK inhibitors that we were using (see below), that these other kinases were mediating these opposing effects and overcoming the pronociceptive effects of ROCK inhibition. Another possible solution, also suggested by Zulauf et al (17), is that ROCK, via microfilament modulation, has a role in the cGMP pathway, which has been described as inducing a biphasic effect on nociception (reviewed in Cury et al [45]).
Indeed, the cGMP analogue (8-bromo-cGMP) used in the present study under the same experimental conditions as that of the ROCK inhibitors showed a biphasic nociceptive threshold response, clearly demonstrating a role for this pathway under our conditions. However, this cGMP analogue, which is an agonist of the NO/cGMP/PKG pain pathway, showed antinociceptive effects at low doses, compatible with earlier reports of the involvement of the cGMP pathway in analgesia (33,34,44). The pronociceptive effects of high doses of 8-bromocGMP could be related to the pronociceptive effects reported for NO (43,44) or to effects of other unrelated systems. These data suggest that the NO/cGMP/PKG pathway may be involved in the antinociceptive effect of the ROCK inhibitors we studied.
Our results involving cytochalasin B demonstrated the dependence of the antinociceptive effect of ROCK inhibitors on cytoskeleton integrity, indicating the involvement of microfilaments in nociceptive pathways in the periphery. The present data are compatible with earlier findings using cytochalasin B and the cyclooxygenase-2 inhibitor celecoxib and morphine (50). Because ROCK is a possible target for PKG (54) and ROCK activation can lead to modulation of actin filaments and neurite outgrowth (17,55), it appears that ROCK and microfilaments in the cytoskeleton are important components of the modulation of peripheral pain.
One clear limitation to the present study is the specificity of the ROCK inhibitors. Signalling systems other than the ROCKs may be particularly relevant to the effects of high doses of ROCK inhibitors because both the inhibitors we have used, similar to most kinase inhibitors, are also inhibitors of kinases other than ROCK, usually with lower potencies. Thus, relative to ROCK, Y27632 has a half-maximal inhibitory concentration (IC50) 10-fold greater and HA1077 is approximately threefold less potent toward mitogen and stress activated kinase 1 (56). Potencies against protein kinase A or protein kinase C are also approximately 10- to 20-fold less than against ROCK, for both compounds (56). However, the IC50 values for ROCK inhibition range between 800 nM and 20 μM, concentrations well below those we have applied (78 nmol to 1560 nmol [25 μg to 500 μg] in 100 μL), but there are factors that would further reduce this margin. First, the reported IC50 values have been determined in vitro with cell-free systems, and we applied the compounds to the foot pad, so there will be both diffusion and binding in the extracellular space and passage through the cell membrane to dilute and delay the amounts actually reaching an intracellular kinase. Also, the IC50 measured using a whole-cell assay may be very different from that with cell-free enzymes. For instance, the IC50 for fasudil as an inhibitor of 95D lung carcinoma cells in culture was 0.79 mg/mL (equivalent to 2.1 mM [57]). Chan et al (46) used intrathecal infusions of 2 mM or 20 mM repeated over 14 days to show the effects of Y27632 on rat spinal cord injury. Nevertheless, and in spite of the similarity of the hyperalgesic responses to the two different ROCK inhibitors, we cannot exclude the possibility that the effects we observed were not causally related to inhibition of ROCK.
The present study used one model of inflammatory pain and one mode of stimulation; therefore, it is possible that in other peripheral pain models, such as IPL formalin (43) or rat arthritic knees (19,53), and using other stimuli, the effects of ROCK inhibitors could be different. However, we used a thermal stimulus (Hargreaves method) in our model with carrageenan inflammation (58) and obtained results in good agreement with those obtained with the mechanical stimulus we used here. It is also possible that the outcome of our experiments would be different in female rats because we used only male rats in the present study.
It is important to emphasize that even at the highest doses of the ROCK inhibitors, the contralateral paw that was not injected with Y27632 or HA1077 showed no changes in its nociceptive or edema status. This demonstrated very clearly that the effects we observed in the treated ipsilateral paw were, indeed, local effects and not due to leakage of the locally injected inhibitor into the systemic circulation. These data from the contralateral paw also serve to exclude the possibility of a central or spinal (28,46) action of the ROCK inhibitors, leading to sedation and consequent motor dysfunction. In another model, alterations in the activation of the RhoA-ROCK system were associated with changes in conduction velocity only in sensory, not motor, neurons (59).
An unexpected outcome of our experiments was that the ROCK inhibitors were inflammatory at the lower doses, when the compounds were most likely to be acting as selective inhibitors of ROCK. This action was expressed in our model as hyperalgesia (a pronociceptive effect), and the antinociceptive effects were only observed at the higher doses when selectivity would be less, even when the paw was inflamed by carrageenan. The proinflammatory effect of ROCK inhibition by Y27632 or HA1077 was also expressed as increased microvascular permeability leading to the formation of edema. This component of inflammation was directly dose-dependent, with no indication of a biphasic profile. Thus, at low doses, the ROCK inhibitors were primarily proinflammatory and only partially anti-inflammatory (in terms of pain) at the higher doses.
Our results have also contributed to the evidence for a mechanistic divergence between two classical components of inflammation – pain and edema. Both Y27632 and HA1077 clearly, and without any sign of a biphasic profile, induced paw edema dose-dependently, contrasting sharply with the biphasic effects on nociception. This division between edema and nociception in our model was emphasized by the effect of 8-bromo-cGMP, which elicited no signs of edema over a dose range that induced a biphasic effect on nociception. This discrepancy between the two inflammatory responses did, however, show very clearly that, in our model, changes in nociception and edema were coincident in time but were not mediated by the same pathways. Although these distinct pathways have not yet been fully elucidated, a similar separation has been observed earlier in our model (51) and under other experimental conditions (60).
CONCLUSION
We have demonstrated that the peripheral injection of two ROCK inhibitors exerted a dual effect on nociceptive responses. Biphasic nociceptive responses were also induced by 8-bromo-cGMP, an activator of the NO/cGMP/PKG pathway. Our results are in agreement with most previous reports of antinociceptive effects of ROCK inhibitors but in contrast to their more frequently described anti-inflammatory activity. This difference is most likely to reflect the local and peripheral, as distinct from systemic, application of the ROCK inhibitors. Our data also suggested the possible involvement of the actin filaments of the cytoskeleton in nociceptive responses, which was modulated by ROCK inhibition. Further studies, at the molecular level are warranted to confirm the involvement of ROCKs and the NO/cGMP/PKG pathway in the processes of peripheral nociception, in both inflamed and normal tissues. Such data, however, open new approaches to our understanding of the mechanisms involved in the genesis of pain at the structural level of the cells.
Acknowledgments
This work was supported by Conselho Nacional de Pesquisa, Fundação de Amparo à Pesquisa de Minas Gerais and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior. The technical assistance of Webster Glayser Pimenta dos Reis is greatly appreciated.
Footnotes
DISCLOSURES: The authors have no conflicts of interest to declare.
REFERENCES
- 1.Riento K, Ridley AJ. ROCKs: Multifunctional kinases in cell behaviour. Nature. 2003;4:446–54. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 2.Kawano Y, Fukata Y, Oshiro N, et al. Phosphorylation of myosin-binding subunit (MBS) of myosin phosphatase by Rho-kinase in vivo. J Cell Biol. 1999;147:1023–38. doi: 10.1083/jcb.147.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hilgers RHP, Webb RC. Molecular aspects of arterial smooth muscle contraction: Focus on Rho. Exp Biol Med. 2005;230:829–35. doi: 10.1177/153537020523001107. [DOI] [PubMed] [Google Scholar]
- 4.Nobes C, Hall A. Regulation and function of the Rho family of small GTPases. Curr Opin Genet Dev. 1994;4:77–81. doi: 10.1016/0959-437x(94)90094-9. [DOI] [PubMed] [Google Scholar]
- 5.Fukata Y, Amano M, Kaibuchi K. Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganization of non-muscle cells. Trends Pharm Sci. 2001;22:32–9. doi: 10.1016/s0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- 6.Chihara K, Nakamura N, Yano T, et al. Cytoskeletal rearrangements and transcriptional activation by c-fos serum response element by Rho-kinase. J Biol Chem. 1992;272:25121–7. doi: 10.1074/jbc.272.40.25121. [DOI] [PubMed] [Google Scholar]
- 7.Essler M, Weber PC, Aepfelbacher M. Thrombin inactivates myosin light chain phosphatase via Rho and its target Rho-kinase in human endothelial cells. J Biol Chem. 1998;272:21867–74. doi: 10.1074/jbc.273.34.21867. [DOI] [PubMed] [Google Scholar]
- 8.Kaibuchi K, Kuroda S, Amano M. Regulation of the cytoskeleton and cell adhesion by the Rho family of GTPases in mammalian cells. Annu Rev Biochem. 1999;68:459–86. doi: 10.1146/annurev.biochem.68.1.459. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki Y, Yamamoto M, Wada H, et al. Agonist-induced regulation of myosin phosphatase active in human platelets through activation of Rho-kinase. Blood. 1999;93:3408–17. [PubMed] [Google Scholar]
- 10.Honning H, Van Der Berg TK, Van Der Pol SM, et al. RhoA activation promotes transendothelial migration of monocytes via ROCK. J Leukoc Biol. 2004;75:523–8. doi: 10.1189/jlb.0203054. [DOI] [PubMed] [Google Scholar]
- 11.Hiroki J, Shimokawa H, Higashi M, et al. Inflammatory stimuli upregulate Rho-kinase in human coronary vascular smooth muscle cells. J Mol Chem Cardiol. 2004;37:527–46. doi: 10.1016/j.yjmcc.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Koksel O, Yildirim Ç, Kubat H, et al. Rho-kinase (ROCK-1 and ROCK-2) upregulation in oleic acid-induced lung injury and its restoration by Y27632. Eur J Neurosci. 2005;22:825–34. doi: 10.1016/j.ejphar.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 13.McGown CC, Brown NJ, Hellewell PG, Brookes ZL. ROCK induced inflammation of the microcirculation during endotoxemia mediated by nitric oxide synthase. Microvasc Res. 2011;81:281–8. doi: 10.1016/j.mvr.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Ramer LM, Borisoff JF, Ramer MS. Rho-kinase inhibition enhances axonal plasticity and attenuates cold hyperalgesia after dorsal rhizotomy. J Neurosc. 2004;24:10796–805. doi: 10.1523/JNEUROSCI.3337-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buyukafsar K, Yalçin I, Kurt H, Tiftik N, Sahan-Firat S, Asku F. Rho-kinase inhibitor, Y-27632, has an anti-nociceptive effect in mice. Eur J Pharmac. 2006;541:49–52. doi: 10.1016/j.ejphar.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 16.Ohsawa M, Aasato M, Hayashi SS, Kamei J. RhoA/Rho kinase pathway contributes to the pathogenesis of thermal hyperalgesia in diabetic mice. Pain. 2011;152:114–22. doi: 10.1016/j.pain.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Zulauf L, Coste O, Marian C, Möser C, Brenneis C, Niederberger E. Cofilin phosphorylation is involved in nitric oxide/cGMP-mediated nociception. Biochem Biophys Res Commun. 2009;390:1408–13. doi: 10.1016/j.bbrc.2009.10.166. [DOI] [PubMed] [Google Scholar]
- 18.Boyce-Rustay JM, Simler GH, McGaraughty S, et al. Characterization of Fasudil in preclinical models of pain. J Pain. 2010;11:941–9. doi: 10.1016/j.jpain.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Yoshimi E, Kumakura F, Hatori C, et al. Anti-nociceptive effects of AS1892802, a novel Rho kinase inhibitor, in rat models of inflammatory and non-inflammatory arthritis. J Pharmacol Exp Ther. 2010;334:955–63. doi: 10.1124/jpet.110.167924. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimi E, Yamamoto H, Furuichi Y, Shimizu Y, Takeshita N. Sustained analgesic effect of the Rho kinase inhibitor AS1892802 in rat models of chronic pain. J Pharmacol Sci. 2010;114:119–22. doi: 10.1254/jphs.10158sc. [DOI] [PubMed] [Google Scholar]
- 21.Buyukafsar K, Levent A. Involvement of Rho/Rho-kinase signaling in contractile activity and acetylcholine release in the mouse gastric fundus. Biochem Biophys Res Commun. 2003;303:777–81. doi: 10.1016/s0006-291x(03)00422-4. [DOI] [PubMed] [Google Scholar]
- 22.Tatsumi S, Mabuchi T, Katano T, et al. Involvement of Rho-kinase in inflammatory and neuropathic pain through phosphorylation of myristoylated alanine-rich C-kinase substrate (MARCKs) Neuroscience. 2005;131:491–8. doi: 10.1016/j.neuroscience.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 23.Leung T, Chen Xq, Manser E, Lim L. The p160 RhoA-binding kinase ROCK alpha is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–27. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Z, Ottens AK, Larner SF, et al. Direct Rho-associated kinase inhibition [correction of inhibition] induces cofilin dephosphorylation and neurite outgrowth in PC-12 cells. Cell Mol Biol Lett. 2006;11:12–29. doi: 10.2478/s11658-006-0002-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Govek EE, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19:1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- 26.Takagi K, Okuda-Ashitaka E, Mabuchi T, et al. Involvement of stem cell factor and its receptor tyrosine kinase c-kit in pain regulation. Neuroscience. 2008;153:1278–88. doi: 10.1016/j.neuroscience.2008.02.073. [DOI] [PubMed] [Google Scholar]
- 27.Niwa R, Nagata-Ohashi K, Takeichi M, Mizuno K, Uemura T. Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylate ADF/cofilin. Cell. 2002;108:233–46. doi: 10.1016/s0092-8674(01)00638-9. [DOI] [PubMed] [Google Scholar]
- 28.Matsumura S, Abe T, Mabuchi T, et al. Rho-kinase mediates spinal nitric oxide formation by prostaglandin E2 via EP3 subtype. Biochem Biophys Res Commun. 2005;338:550–7. doi: 10.1016/j.bbrc.2005.09.058. [DOI] [PubMed] [Google Scholar]
- 29.Sunico CR, González-Forero D, Domínguez G, García-Verdugo JM, Moreno-López B. Nitric oxide induces pathological synapse loss by a protein kinase G-, Rho kinase-dependent mechanism preceded by myosin light chain phosphorylation. J Neurosci. 2010;30:973–84. doi: 10.1523/JNEUROSCI.3911-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duarte ID, dos Santos IR, Lorenzetti BB, Ferreira SH. Analgesia by direct antagonism of nociceptor sensitization involves the arginine-nitric oxide-cGMP pathway. Eur J Pharmacol. 1992;217:225–7. doi: 10.1016/0014-2999(92)90881-4. [DOI] [PubMed] [Google Scholar]
- 31.Duarte ID, Lorenzetti BB, Ferreira SH. Peripheral analgesia and activation of the nitric oxide-cyclic GMP pathway. Eur J Pharmacol. 1990;186:289–93. doi: 10.1016/0014-2999(90)90446-d. [DOI] [PubMed] [Google Scholar]
- 32.Ferreira SH, Duarte ID, Lorenzetti BB. The molecular mechanism of action of peripheral morphine analgesia: Stimulation of the cGMP system via nitric oxide release. Eur J Pharmacol. 1991;201:121–2. doi: 10.1016/0014-2999(91)90333-l. [DOI] [PubMed] [Google Scholar]
- 33.Duarte ID, Ferreira SH. The molecular mechanism of central analgesia induced by morphine or carbachol and the L-arginine-nitric oxide-cGMP pathway. Eur J Pharmacol. 1992;221:171–4. doi: 10.1016/0014-2999(92)90789-7. [DOI] [PubMed] [Google Scholar]
- 34.Granados-Soto V, Flores-Murrieta FJ, Castañeda-Hernández G, López-Muñoz FJ. Evidence for the involvement of nitric oxide in the anti-nociceptive effect of ketorolac. Eur J Pharmacol. 1995;277:281–4. doi: 10.1016/0014-2999(95)00123-3. [DOI] [PubMed] [Google Scholar]
- 35.Granados-Soto V, Rufino MO, Gomes Lopes LD, Ferreira SH. Evidence for the involvement of the nitric oxide-cGMP pathway in the anti-nociception of morphine in the formalin test. Eur J Pharmacol. 1997;340:177–80. doi: 10.1016/s0014-2999(97)01399-x. [DOI] [PubMed] [Google Scholar]
- 36.Lorenzetti BB, Ferreira SH. Activation of the arginine-nitric oxide pathway in primary sensory neurons contributes to dipyrone-induced spinal and peripheral analgesia. Inflamm Res. 1996;45:308–11. doi: 10.1007/BF02280997. [DOI] [PubMed] [Google Scholar]
- 37.Soares AC, Leite R, Tatsuo MA, Duarte ID. Activation of ATP-sensitive K(+) channels: Mechanism of peripheral anti-nociceptive action of the nitric oxide donor, sodium nitroprusside. Eur J Pharmacol. 2000;400:67–71. doi: 10.1016/s0014-2999(00)00355-1. [DOI] [PubMed] [Google Scholar]
- 38.Amarante LH, Duarte ID. The kappa-opioid agonist (+/−)-bremazocine elicits peripheral anti-nociception by activation of the L-arginine/nitric oxide/cyclic GMP pathway. Eur J Pharmacol. 2002;454:19–23. doi: 10.1016/s0014-2999(02)02275-6. [DOI] [PubMed] [Google Scholar]
- 39.Romero TR, Resende LC, Duarte ID. The neuronal NO synthase participation in the peripheral anti-nociception mechanism induced by several analgesic drugs. Nitric Oxide. 2011;25:431–5. doi: 10.1016/j.niox.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Kawabata A, Manabe S, Manabe Y, Takagi H. Effect of topical administration of L-arginine on formalin-induced nociception in the mouse: a dual role of peripherally formed NO in pain modulation. Br J Pharmacol. 1994;112:547–50. doi: 10.1111/j.1476-5381.1994.tb13108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sousa AM, Prado WA. The dual effect of a nitric oxide donor in nociception. Brain Res. 2001;897:9–19. doi: 10.1016/s0006-8993(01)01995-3. [DOI] [PubMed] [Google Scholar]
- 42.Prado WA, Schiavon VF, Cunha FQ. Dual effect of local application of nitric oxide donors in a model of incision pain in rats. Eur J Pharmacol. 2002;441:57–65. doi: 10.1016/s0014-2999(02)01413-9. [DOI] [PubMed] [Google Scholar]
- 43.Tegeder I, Schmidtko A, Niederberger E, Ruth P, Geisslinger G. Dual effects of spinally delivered 8-bromo-cyclic guanosine mono-phosphate (8-bromo-cGMP) in formalin-induced nociception in rats. Neurosci Lett. 2002;332:146–50. doi: 10.1016/s0304-3940(02)00938-2. [DOI] [PubMed] [Google Scholar]
- 44.Vivancos GG, Parada CA, Ferreira SH. Opposite nociceptive effects of the arginine/NO/cGMP pathway stimulation in dermal and subcutaneous tissues. Br J Pharmacol. 2003;138:1351–7. doi: 10.1038/sj.bjp.0705181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cury Y, Picolo G, Gutierrez VP, Ferreira SH. Pain and analgesia: The dual effect of nitric oxide in the nociceptive system. Nitric Oxide. 2012;25:243–54. doi: 10.1016/j.niox.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 46.Chan CC, Khodarahmi K, Liu J, et al. Dose-dependent beneficial and detrimental effects of ROCK inhibitor Y27632 on axonal sprouting and functional recovery after rat spinal cord injury. Exp Neurol. 2005;196:352–64. doi: 10.1016/j.expneurol.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 47.Di Rosa M. Biological properties of carrageenan. J Pharm Pharmac. 1972;24:89–102. doi: 10.1111/j.2042-7158.1972.tb08940.x. [DOI] [PubMed] [Google Scholar]
- 48.Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissues. Arch Int Pharmacodyn. 1957;111:409–19. [PubMed] [Google Scholar]
- 49.Brown SS, Spudich JA. Mechanism of action of cytochalasin: Evidence that it binds to actin filament ends. J Cell Biol. 88:487–91. doi: 10.1083/jcb.88.3.487. 1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paiva-Lima P, Rezende RM, Leite R, Duarte ID, Bakhle Y, Francischi JN. Crucial involvement of actin filaments in celecoxib and morphine analgesia in a model of inflammatory pain. J Pain Res. 2012;5:535–45. doi: 10.2147/JPR.S36870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Francischi JN, Chaves CT, Moura AC, et al. Selective inhibitors of cyclo-oxygenase-2 (COX-2) induce hypoalgesia in a rat paw model of inflammation. Br J Pharmacol. 2002;137:837–44. doi: 10.1038/sj.bjp.0704937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rezende RM, Reis WG, Duarte IG, Paiva-Lima P, Bakhle YS, Francischi JN. The analgesic actions of centrally administered celecoxib are mediated by endogenous opioids. Pain. 2009:94–100. doi: 10.1016/j.pain.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 53.Takeshita N, Yoshimi E, Hatori C, Kumakura F, Seki N, Shimizu Y. Alleviating effects of AS1892802, a Rho kinase inhibitor, on osteoarthritic disorders in rodents. J Pharmacol Sci. 2011;115:481–9. doi: 10.1254/jphs.10319fp. [DOI] [PubMed] [Google Scholar]
- 54.Hou Y, Ye RD, Browning DD. Activation of the small GTPase Rac1 by cGMP-dependent protein kinase. Cell Signal. 2004;16:1061–9. doi: 10.1016/j.cellsig.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 55.Tojima T, Ito E. Signal transduction cascades underlying de novo protein synthesis required for neuronal morphogenesis in differentiating neurons. Prog Neurobiol. 2004;72:183–93. doi: 10.1016/j.pneurobio.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 56.Davies SP, Reddy H, Calvano M, Cohen P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem J. 2000;351:5–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang X, Zhang Y, Wang S, Shi W. Effect of fasudil on growth, adhesion, invasion, and migration of 95D lung carcinoma cells in vitro. Can J Physiol Pharmacol. 2010;88:874–9. doi: 10.1139/y10-047. [DOI] [PubMed] [Google Scholar]
- 58.Correa JD, Paiva-Lima P, Rezende RM, et al. Peripheral mu-, kappa-and delta-opioid receptors mediate the hypoalgesic effect of celecoxib in a rat model of thermal hyperalgesia. Life Sci. 2010;86:951–6. doi: 10.1016/j.lfs.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 59.Chan FK, Chung SS, Ng IO, Chung SK. The RhoA GTPase-activating protein DLC2 modulates RhoA activity and hyperalgesia to noxious thermal and inflammatory stimuli. Neurosignals. 2012;20:112–26. doi: 10.1159/000331240. [DOI] [PubMed] [Google Scholar]
- 60.Lorenzetti BB, Ferreira SH. Mode of analgesic action of dipyrone: antagonism of inflammatory hyperalgesia. Eur J Pharmacol. 1985;114:375–81. doi: 10.1016/0014-2999(85)90383-8. [DOI] [PubMed] [Google Scholar]


