Abstract
Treatment interruptions (TIs) limit the therapeutic success of combination antiretroviral therapy and are associated with higher morbidity and mortality. HIV-positive individuals dealing with concurrent health issues, access challenges and competing life demands are hypothesized to be more likely to interrupt treatment. Individuals were included if they initiated cART ≥1 year prior to interview date and had a CD4 cell count or initial regimen recorded at initiation. Using pharmacy recording, TIs were defined as a patient-initiated interruption in treatment ≥90 consecutive days during the 12 months preceding or following the study interview. 117 (15%) of 768 participants included in this study had a TI during the study window. 76.0% of participants were male, 27.5% were of Aboriginal ethnicity and the median age was 46 (interquartile range (IQR): 40–52). In multivariable logistic regression, TIs were significantly associated with current illicit drug use (adjusted odds ratio [aOR]: 1.68, 95% confidence interval [CI]: 1.05–2.68); <95% adherence in the first year of treatment (aOR: 2.68, 95% CI: 1.67–4.12); living with more than one person (aOR: 1.95; 95% CI: 1.22–3.14) or living on the street (aOR: 5.08, 95% CI: 1.72–14.99) compared to living alone; poor perception of overall health (aOR: 1.64 95% CI: 1.05–2.55); being unemployed (aOR: 2.22, 95% CI: 1.16–4.23); and younger age at interview (aOR: 0.57, 95% CI: 0.44–0.75, per 10 year increment). Addressing socioeconomic barriers to treatment retention is vital for supporting the continuous engagement of patients in care.
Keywords: Treatment interruption, HIV, ART, barriers
Introduction
Once engaged in HIV care, it is imperative for HIV-positive individuals to strictly adhere to their prescribed medication protocol in order to maximize the life-extending benefits of combination antiretroviral therapy (cART). One stage of the “cascade of care,” as expounded by Gardner and colleagues and which describes the pathway from initial diagnosis of HIV to viral suppression (1), continuity of treatment is a vital component of care and the best predictor of an HIV-positive individual’s successful management of HIV. Treatment continuity can be examined on a continuum from measures of daily adherence to measures of long-term medication persistence. This distinction represents the difference between asking “how often” and “for how long,” respectively, with respect to a patient’s medication-taking practices (2). As cART is propagated at increasing levels globally, and the impetus to provide treatment earlier in the course of HIV infection for individual and public health benefits gains momentum (3–5), ensuring continuity of treatment becomes even more of a pressing issue.
Until 2006, structured treatment interruptions (TIs) or “drug holidays” were prescribed by physicians in order to minimize treatment-related side effects, improve patient quality of life and decrease the costs of HIV treatment and care (6). These interruption strategies were characterized as either time-defined gaps in treatment, as in the STACCATO trial, or gaps based on CD4 cell count, as demonstrated in the largest trial examining TIs, the SMART trial (7,8). As evidence accumulated that these drug holidays led to a statistically significantly increased risk of HIV disease progression, severe complications and death, the use of structured TIs in the management of HIV-positive individuals were no longer recommended (9,10).
Whether planned or otherwise, TIs result in a heightened risk of opportunistic infection (9,11,12), plasma viral load rebound (13,14), increased risk of person-to-person transmission (15,16), risk of acute viral infection (14), found in 5.9% of participants with TIs in the Staccato trial (7), and the development of new resistance to antiretroviral agents (17–19). Results of the SMART trial showed that there was an increased risk of cardiovascular, hepatic and renal disease in the intermittent treatment group compared to the group receiving continuous treatment (8). Furthermore, a similar large-scale study showed that the increased risk of cardiovascular disease did not abate once treatment was re-initiated (20).
Despite recognition of the detrimental effects of TIs, many studies continue to report on the high prevalence of TIs in their patient populations, which can range anywhere from 6% to 51% (21–26). Research from British Columbia (BC) has identified that almost 40% of patients, followed for a median of 3.3 years, had experienced a TI (21). Despite the frequency of TIs, determinants of unstructured or self-elected TIs are still not well-characterized (27). This study purported to examine gaps in care of 90 consecutive days or longer in antiretroviral treatment and factors associated with these gaps.
Methods
Study design and participant recruitment
The Drug Treatment Program (DTP) at the BC Centre for Excellence in HIV/AIDS is mandated by the government of BC to distribute cART free of charge to eligible HIV-positive individuals. The DTP distributes cART according to standards developed by the BC Therapeutic Guideline Committee, which are consistent with guidelines proposed by the International AIDS Society (28), described previously at length (29).
Briefly, HIV-positive individuals are enrolled into the DTP when they are first prescribed cART by their physicians, and are followed prospectively for clinical and laboratory measurements thereafter. HIV-positive individuals enrolled in the DTP between July 2007 and January 2010 were eligible to participate in the Longitudinal Investigations into Supportive and Ancillary health services (LISA) study. The LISA study was funded to enroll 1,000 HIV-positive individuals over the age of 19 residing in British Columbia who had ever accessed cART. Study participants were actively recruited non-randomly through letters distributed via HIV physicians and pharmacists, by word-of-mouth (snowball sampling) and via advertisements at HIV/AIDS service organizations located throughout the province. Informed consent was obtained from patients prior to conducting the survey. The LISA study oversampled particular sub-populations in order to sufficiently power sub-analyses on women, people who inject drugs and people identifying as Aboriginal.
Study instrument and ethical approval
Cross-sectional socio-demographic data on LISA study participants were collected through a comprehensive interviewer-administered survey which captured a range of variables including: basic demographic data, information about housing, income, social support networks, mental health disorders, drug and alcohol use and quality of life measures. Clinical variables were obtained through longitudinal linkages with the DTP administrative database and integrated with interview data. Ethical approval for the LISA study was obtained from the University of British Columbia/ Providence Health Care, Simon Fraser University, the University of Victoria, and Vancouver Coastal Health Research Ethics Boards.
Inclusion criteria
In order to be included in this analysis, participants were required to have initiated cART at least one year prior to their interview date, which was necessary in order to obtain a complete measure of treatment adherence in the year prior to enrollment. Beginning with the 1,000 LISA sample, patients were excluded if they moved out of the province during the study period, entered a randomized trial, or if they had a prevalent TI at the start of the study window, as determined by clinical linkages to the DTP, which excluded 149 individuals. 83 individuals who initiated treatment outside of BC and did not have a CD4 cell count or initial regimen recorded at initiation were also excluded, leaving a sample size for this analysis of 768 individuals.
Outcome variable
The outcome variable, treatment interruption, was defined as a non-medically supervised interruption in antiretroviral treatment of at least 90 consecutive days during the 12 months preceding or following the study interview. Instances of medically supervised TIs were recorded in the provincial database and could therefore be excluded. Pharmacy prescription refill of cART was used to identify TIs of 90 days; that is, when individuals did not retrieve their prescription, they were recorded in the database as interrupted until they picked up their medication. While there is considerable variability among studies in defining the length of a TI, a period of 90 days or longer was chosen to help decrease misclassification of TIs due to reporting delays or stockpiled medication, and to be consistent with prior literature (21,30,31).
Explanatory variables
A number of covariates were identified as possible factors that might influence TI occurrence. Socio-demographic variables included: age, gender, Aboriginal ancestry, education (<high school vs. ≥high school), current employment status, and current income (dichotomized at $15,000). The survey also asked about incarceration and drug use, both lifetime and current. Lifetime drug use was defined as having ever used cocaine, crack cocaine, heroin, speedball (cocaine and heroin) and methamphetamine; current was defined as drug use in the three months preceding the interview date. Housing was assessed by asking how many other people participants resided with (alone, with more than one person or live on the street) and the type of residence (hotel, treatment centre, shelter or hostel, no fixed address, prison vs. house or apartment). Depression was assessed through affirmative response to the question “have you ever been diagnosed by your doctor with depression” and food insecurity was measured using an eight question modified version of the Radimer and Cornell questionnaire (32,33). Individuals were considered food insecure if they gave at least one positive answer to any of the eight items in the scale, as recommended by Kendall et al. (33). Participants were also asked to report on their perception of their overall health (dichotomized as excellent and very good and good vs., poor and fair) and were asked about a series of non-mutually exclusive possible options for not taking cART medications, “are any of these explanations a reason you have EVER missed taking your HIV meds.”
Clinical variables included in the analysis were CD4 cell count (cells/mm3), HIV viral load at time of treatment interruption (log10 copies/mL), AIDS at treatment initiation (yes or no), adherence in the first year of treatment (calculated as the number of days of antiretroviral medications dispensed, divided by the number of days of follow-up during the 12 months prior to interview date, and expressed as a percentage) and cART regimen.
Statistical Analysis
Bivariate analyses to compare differences between treatment interrupters and non-interrupters were undertaken using the chi-square test for categorical variables and the Wilcoxon rank-sum test for continuous variables. Correlates that were statistically significantly associated at the univariate level (p<0.05) were candidates for inclusion in the multivariable logistic regression model to evaluate the independent association of variables with treatment interruptions. Variables that had been shown to be related to TIs, such as age and gender, were included in this analysis regardless of statistical significance, which was defined as p<0.05. A selection procedure based on the Akaike Information Criterion (AIC) was used to select the variables in the final model. All analyses were conducted using the STATA statistical package version 12.1 (34). A sensitivity analysis was performed to determine whether results changed if the TI observation period was limited to the period 12 months following the study interview. A multivariable model was created using AIC, as above, and compared to the full model to undertake this sensitivity analysis.
Results
Of 768 participants included in the study, 117 (15%) had a recorded TI 90 days or longer within the 24 month window surrounding their interview date (37 prior to the interview and 80 subsequently), as determined by clinical linkage to the DTP. Demographic and clinical differences between those with and without experiences of TIs are presented in Table 1. Individuals with TIs were more likely to be female (42% vs. 21%), younger (median age, interquartile range (IQR): 42 (37–41) vs. 46 (41–52), of Aboriginal ancestry (61% vs.75%), unemployed (87% vs. 73%) and report an income of less than $15,000 CDN annually (74% vs. 55%). They were also significantly more likely to have ever been incarcerated (65% vs. 49%), to have ever injected drugs (78% vs. 55%) and to be using illicit drugs at the time of interview (66% vs. 50%), to have completed less than a high school education (50% vs. 36%) and to rate their overall health poorer than their counterparts who did not interrupt (48% vs. 30%). Importantly, those who interrupted were much more likely to report unstable housing (46% vs. 28%) and to cohabitate with other people (44% vs. 39%) or live on the street (7% vs. 1%) versus living alone. Moreover, individuals who interrupted were more likely to report adherence ≤95% in the first year of treatment (68% vs. 40%) or no cART at study interview (34% vs. 2%) (though for an interruption period less than three months) (all p<0.05).
Table 1.
Characteristics of HIV-positive individuals who did and did not interrupt treatment in the 12 month period before or after LISA interview date (N=768)
| Characteristic | N | No treatment Interruption n (%) n = 651 |
Treatment Interruption n (%) n = 117 |
p – value |
|---|---|---|---|---|
| Gender | 768 | |||
| Male | 516 (79.2) | 68 (58.1) | <0.001 | |
| Female | 135 (20.7) | 49 (41.9) | ||
| Median age (IQR)* | 768 | 46 (41–52) | 42 (37–41) | 0.001 |
| History of IDU† | 766 | |||
| yes | 358 (55.1) | 90 (77.6) | <0.001 | |
| no | 292 (44.9) | 26 (22.4) | ||
| Current illicit drug use | 766 | |||
| yes | 325 (50.0) | 76 (65.5) | 0.002 | |
| no | 325 (50.0) | 40 (34.5) | ||
| Aboriginal | 768 | |||
| yes | 165 (25.4) | 46 (39.3) | 0.002 | |
| no | 486 (74.7) | 71 (60.7) | ||
| Completed High School | 767 | |||
| yes | 418 (64.3) | 58 (49.6) | 0.002 | |
| no | 232 (35.7) | 59 (50.4) | ||
| Earn ≥$15,000 | 762 | |||
| yes | 288 (44.6) | 30 (25.9) | <0.001 | |
| no | 358 (55.4) | 86 (74.1) | ||
| Ever Incarcerated | 767 | |||
| yes | 315 (48.5) | 76 (65.0) | 0.001 | |
| no | 335 (51.4) | 41 (35.0) | ||
| Currently Employed | 768 | |||
| yes | 177 (27.2) | 15 (12.8) | 0.001 | |
| no | 474 (72.8) | 102 (87.2) | ||
| Unstable Housing** | 767 | |||
| yes | 182 (28.0) | 54 (46.1) | <0.001 | |
| no | 468 (72.0) | 63 (53.9) | ||
| Who do you live with? | 766 | |||
| Live alone | 391 (60.1) | 57 (49.1) | <0.001 | |
| With ≥ 1 person | 250 (38.5) | 51 (44.0) | ||
| Homeless | 9 (1.4) | 8 (6.9) | ||
| Food insecure | 755 | 0.012 | ||
| Yes | 404 (62.2) | 87 (74.4) | ||
| No | 245 (37.8) | 30 (25.6) | ||
| Adherence ≥95%‡ | 761 | |||
| yes | 384 (59.6) | 38 (32.5) | <0.001 | |
| no | 260 (40.4) | 79 (67.5) | ||
| Ever depressed | 767 | 0.198 | ||
| Yes | 297 (45.7) | 61 (52.1) | ||
| No | 353 (54.3) | 56 (47.9) | ||
| Median CD4 cell count at treatment initiation* | 763 | 210 (120–330) | 215 (120–330) | 0.294 |
| AIDS at Baseline | 768 | |||
| yes | 94 (14.4) | 17 (14.5) | 0.980 | |
| no | 557 (85.6) | 100 (85.5) | ||
| Overall Health | 768 | |||
| Exc., very good, good | 453 (69.6) | 61 (52.1) | <0.001 | |
| vs. poor, fair | 198 (30.4) | 56 (47.9) | ||
| NRTI combo in cART regimen at interview | 768 | |||
| Tenofovir/Emtricitabine | 303 (46.5) | 46 (39.3) | <0.001 | |
| Abacavir/Lamivudine | 151 (23.2) | 11 (9.4) | ||
| Tenofovir/Lamivudine | 59 (9.1) | 6 (5.1) | ||
| Zidovudine/Lamivudine | 20 (3.1) | 3 (2.6) | ||
| Other | 104 (16.0) | 11 (9.4) | ||
| Not on cART | 14 (2.2) | 40 (34.2) | ||
| Third drug in cART regimen at interview | 768 | |||
| Nevirapine | 69 (10.6) | 2 (1.7) | <0.001 | |
| Efavirenz | 152 (23.4) | 16 (13.7) | ||
| Lopinavir | 103 (15.8) | 12 (10.3) | ||
| Atazanavir | 249 (38.3) | 42 (35.9) | ||
| Other | 64 (9.8) | 5 (4.3) | ||
| Not on cART | 14 (2.2) | 40 (34.2) |
IQR= interquartile range
IDU= injecting drug user
Not living in a house or apartment
In first year of treatment
Several factors were shown to be associated with TI in multivariable analysis, as shown in Table 2. These included younger age at interview (per 10 year increment) (aOR: 0.57, 95% CI: 0.44–0.75); imperfect adherence in the first year of treatment (aOR: 2.68, 95% CI: 1.67–4.12); unemployment (aOR: 2.22, 95% CI: 1.16–4.23); illicit drug use (aOR:1.68, 95% CI: 1.05–2.68); living with many people (aOR: 1.95; 95% CI: 1.22–3.14) or on the street (aOR: 5.08, 95% CI: 1.72–14.99) versus alone; and having a poor impression of one’s overall health (aOR: 1.64 95% CI: 1.05–2.55). Female gender was included in the final model as a variable deemed clinically important but did not achieve statistical significance in the multivariable analysis. Results of the sensitivity analysis showed that there was no difference in the model limiting inclusion of TI events to solely after the interview date.
Table 2.
Factors associated with treatment interruption of ≥90 days among 757 LISA participants in British Columbia, Canada
| Unadjusted odds ratio (95% confidence interval) | p-value | Adjusted odds ratio (95% confidence interval) | p-value | |
|---|---|---|---|---|
| Female vs. Male | 2.75 (1.82–4.16) | <0.001 | 1.58 (0.99–2.52) | 0.054 |
| Age (per 10 year increment) | 0.54 (0.43–0.68) | <0.001 | 0.57 (0.44–0.75) | <0.001 |
| Current illicit drug use | 1.90 (1.26–2.87) | 0.002 | 1.68 (1.05–2.68) | 0.030 |
| Aboriginal ancestry | 1.91 (1.27–2.88) | 0.002 | ||
| Unemployed | 2.54 (1.44–4.49) | 0.001 | 2.22 (1.16–4.23) | 0.016 |
| Completed high school | 1.83 (1.23–2.72) | 0.003 | ||
| Unstable housing* | 2.20 (1.47–3.29) | <0.001 | ||
| Living situation | ||||
| Alone | 1.00 | 1.00 | ||
| With many others | 1.40 (0.93–2.11) | 0.108 | 1.95 (1.22–3.14) | 0.005 |
| On the street | 6.10 (2.26–16.44) | <0.001 | 5.08 (1.72–14.99) | 0.003 |
| Overall health self-rated (poor, fair and neutral) vs. excellent and good) | 2.10 (1.41–3.13) | <0.001 | 1.64 (1.05–2.55) | 0.030 |
| Ever incarcerated | 1.97 (1.31–2.97) | 0.001 | ||
| Currently earn <$15,000 | 2.31 (1.48–3.59) | <0.001 | ||
| Food insecurity | 1.76 (1.34–2.30) | 0.013 | ||
| Ever diagnosed with Hepatitis C** | 2.58 (1.66–4.01) | <0.001 | ||
| < 95% adherence in first year of treatment | 3.07 (2.02–4.66) | <0.001 | 2.68 (1.67–4.12) | <0.001 |
Not living in a house or apartment
The hepatitis C variable was not considered for the final model due to collinearity with current illicit drug use
When asked about possible reasons for missing doses of cART, participants reported a number of barriers and obstacles to consistent medication persistence, which are summarized in Figure 1. Of 768 participants, 168 (22%) responded that the question was not applicable to them because they always take their pills, a group that did however include 20 treatment interrupters. Of the remaining 600 individuals, 89% of those who did interrupt cited “to avoid side effects” as a reason for missing doses versus 68% of those who did not interrupt (p<0.001). Equal proportions of each group (66%) reported that the second most common reason for missing doses was that they “simply forgot.” Traveling or being away from home was reported by 34% of individuals who did not interrupt and 44% of those who did. Significantly more individuals who interrupted missed doses due to nausea and diarrhea (39% vs. 24%, p=0.002), running out of pills (32% vs. 22%, p=0.035), losing or misplacing pills (29% vs. 15%, p=0.001), and not having the right foods or liquids to take with the pills (28% vs. 11%, p<0.001). More than a quarter of those who interrupted stated that they missed doses because they “didn’t feel like the meds really work sometimes” (28% vs. 7%, p<0.001) and because they were “feeling well so they didn’t bother” (26% vs. 10%, p<0.001). Those experiencing TIs were more likely to miss doses because they didn’t want anyone to see or notice them taking HIV meds (18% vs. 7%, p=0.001).
Figure 1.
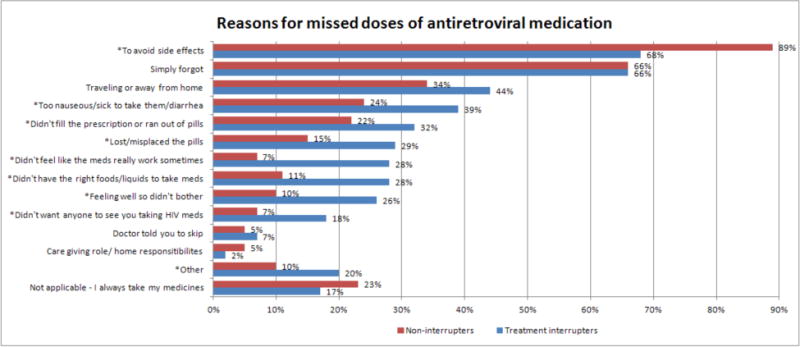
Reasons for missed doses of antiretroviral medication in LISA treatment interrupters (N=651) and non-interrupters (N=117)
*Significant at the p<0.05 level
**Denominator was 600 individuals for all responses except not applicable category, which included the whole sample (N=768)
Discussion
In a population of HIV-positive individuals on treatment in British Columbia, patient-initiated TIs continue to occur; the prevalence of TI was 15% (117/768) in the sample. The TI prevalence in this study is comparable to other studies examining unstructured TIs of similar lengths of three months or longer. For instance, a Swiss cohort found that 27.5% of individuals interrupted therapy between one and three months (35), while a large European study determined that after three years, 16% of patients had interrupted treatment for at least three months (30). Results of this study are a conservative estimate of TIs since those who had not accessed cART at all in the 24-month study window (possibly due to prior interruption) were excluded.
A number of demographic and socio-behavioral factors were independently associated with interrupting treatment such as younger age, illicit drug use, overcrowding or living on the street, unemployment and a poor perception of overall health. Individuals dealing with concurrent issues such as a lack of stable housing or employment, as well as challenges related to addiction may be unable to prioritize adherence to an HIV treatment regimen, resulting in periods of interruptions of cART that may compromise long term prognosis. Individuals with a poor perception of their overall health were more likely to interrupt, indicating that they may be pessimistic about the efficacy of cART in improving their health, leading them to discount the importance of sustained treatment. Similarly, a study of injecting drug users in BC found that lack of faith in the benefits of cART, and poor efficacy expectations (ability to manage treatment schedules and side effects) were independently associated with cART discontinuation (22). Corroborating previous literature, this study also found that suboptimal adherence in the first year of ART was strongly associated with future interruptions in treatment (36). This may signify that individuals who are more likely to embark on lengthy interruptions may effectively be identified and targeted for assistance early on in their course of therapy.
With respect to factors associated with TIs, a number of findings were largely consistent with studies in other settings examining TIs; in particular, younger age was correlated with TIs in this and many other studies (21,30,35), which suggests that younger individuals may be more prone to more chaotic and dynamic lifestyles. Similarly, illicit drug use and hepatitis C co-infection were also associated with TIs in this study and in the literature (21,30,35). However, this study did not find that higher baseline CD4 cell counts were correlated with TIs during the study period as did a number of other studies (21,30,35); in fact, CD4 cell count at treatment initiation was exceedingly low for both groups. It is likely, due to our sampling strategy, that the population under analysis here represents a more marginalized population with lower variability in CD4 count than that examined in other studies. Policy-makers and clinicians should be especially vigilant in preventing TIs in groups of late initiators to prevent significantly compromised patient outcomes.
While female gender was significantly associated with TIs in a number of studies (21,26,30), gender was not significantly associated with TI in this study’s final model. In BC, a higher proportion of women acquire HIV through injecting drug use than men (23% vs. 10% of new diagnoses in 2011 were attributable to IDU in women vs. men (37)) and have demonstrated poorer adherence (38), which may result in less stability in their lives overall and a higher preponderance of TIs. In this study, it is likely that a higher proportion of men were injecting drug users than found in the general HIV-population in the province, leading to higher vulnerability to TIs than found for men in other studies.
Housing status and unemployment have not been described in many studies examining TIs though numerous studies have found associations between unstable housing and suboptimal adherence (39–43) as well as treatment discontinuation (36). It is conceivable that a substandard living situation (including overcrowding) or lack of income from not having a job could exercise a degree of stress on an individual that would preclude attention to a regimen of daily pills. Establishing safe and stable housing for HIV-positive individuals and access to employment is paramount for maintaining engagement in treatment.
In reporting reasons for missing doses, participants cited various physical, social and interpersonal concerns that may act as barriers to treatment continuity, some of which have been described in a meta-analysis of studies in developing and developed countries examining poor adherence (44). Of note, this study found that a number of individuals reported missing doses because they felt well. A study investigating reasons for loss to follow-up in a population of HIV-positive individuals in New York City similarly concluded that 41% left care because they “felt well” (45). Likewise, literature on antibiotic compliance shows similar findings ((46). Clinicians should include information about the importance of continuity of treatment and provide strategies and support for patients in order to prevent resistance and to ensure that patients experience the longterm benefits of cART.
Limitations of the study
Appreciating that many studies solely collect clinical data, LISA provides critical insight into complex socio-economic and demographic characteristics of HIV-positive individuals who have accessed treatment in BC. However, readers should be cautious when interpreting our results. Firstly, the study is a non-probability sample, which limits the generalizability of our results. Specifically, this study is subject to selection bias, as the modest honorarium offered to participants might have led to over-sampling of individuals in need of financial gain. A range of recruitment strategies were employed in an effort to attenuate the effect of this bias.
Additionally, as in many studies that ask for self-reported information collected by interviewers, this study is vulnerable to social desirability and recall biases. As the study design is cross-sectional, temporal and causal relationships cannot be inferred. Further, by design the LISA study only includes individuals who have accessed cART, and thus is not representative of HIV-positive individuals who have yet to access therapy, who may be the most marginalized. Similar studies including all HIV-positive individuals in the province would be beneficial.
This study included TIs both preceding and following the study interview in order to maximize the study sample. Since it is association and not causation (which presupposes a temporal relationship in which the exposure precedes the outcome) that is being investigated in this cross-sectional analysis, including TIs that occur prior to the interview is consistent with our goal of determining correlates of interruption. Results from a sensitivity analysis in which only TIs recorded in the period following the interview date were included in the analysis were consistent with the original analysis.
Conclusions and future directions
Despite universal access to treatment across the province of British Columbia, interruptions in treatment among HIV-positive individuals on cART continue to be pervasive. Future research that includes a qualitative perspective would lead to a deeper understanding of the reasons that people interrupt treatment; for instance, answering a question such as “why is living with many people associated with TI,” would be well-suited to qualitative analysis. In addition, longitudinal research would be able to illustrate how changes in circumstances affect treatment interruptions – for instance, how the loss of housing or a job might create an environment where the risk of an interruption is higher. Following individuals who interrupt treatment over time may also elucidate the longterm effects of interrupting treatment.
In order to ensure the continuity of treatment and the best possible health outcomes for HIV-positive individuals, the barriers to secure, enduring, and accessible treatment must be addressed. Programs to assist individuals adhere to treatment should be developed; simultaneously, researchers, policy-makers, and clinicians alike must work to improve overall quality of life for individuals living with HIV so that lack of adequate housing, employment and addictions do not impede access to life-saving and life-extending treatment for HIV. Only by ensuring that basic life needs are met for individuals living with HIV can there be an expectation of improved retention and the continuous engagement of patients in care.
Contributor Information
Hasina Samji, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA; Division of Epidemiology and Population Health, British Columbia Centre for Excellence in HIV/AIDS, Vancouver, BC, Canada.
Yalin Chen, Division of Epidemiology and Population Health, British Columbia Centre for Excellence in HIV/AIDS, Vancouver, BC, Canada.
Kate Salters, Division of Epidemiology and Population Health, British Columbia Centre for Excellence in HIV/AIDS, Vancouver, BC, Canada; Faculty of Health Sciences, Simon Fraser University, Burnaby, BC, Canada.
Julio S. G. Montaner, Division of Epidemiology and Population Health, British Columbia Centre for Excellence in HIV/AIDS, Vancouver, BC, Canada Department of Medicine, University of British Columbia, Vancouver, BC, Canada.
Robert S. Hogg, Division of Epidemiology and Population Health, British Columbia Centre for Excellence in HIV/AIDS, Vancouver, BC, Canada Faculty of Health Sciences, Simon Fraser University, Burnaby, BC, Canada.
References
- 1.Gardner EM, McLees MP, Steiner JF, et al. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011 Mar 15;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bae JW, Guyer W, Grimm K, Altice FL. Medication persistence in the treatment of HIV infection: a review of the literature and implications for future clinical care and research. AIDS. 2011 Jan 28;25(3):279–290. doi: 10.1097/QAD.0b013e328340feb0. [DOI] [PubMed] [Google Scholar]
- 3.Montaner JS, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010 Aug 14;376(9740):532–539. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lima VD, Johnston K, Hogg RS, et al. Expanded access to highly active antiretroviral therapy: a potentially powerful strategy to curb the growth of the HIV epidemic. J Infect Dis. 2008 Jul 1;198(1):59–67. doi: 10.1086/588673. [DOI] [PubMed] [Google Scholar]
- 5.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walmsley S, Loutfy M. Can structured treatment interruptions (STIs) be used as a strategy to decrease total drug requirements and toxicity in HIV infection? J Int Assoc Physicians AIDS Care (Chic) 2002 Summer;1(3):95–103. doi: 10.1177/154510970200100304. [DOI] [PubMed] [Google Scholar]
- 7.Ananworanich J, Gayet-Ageron A, Le Braz M, et al. CD4-guided scheduled treatment interruptions compared with continuous therapy for patients infected with HIV-1: results of the Staccato randomised trial. Lancet. 2006 Aug 5;368(9534):459–465. doi: 10.1016/S0140-6736(06)69153-8. [DOI] [PubMed] [Google Scholar]
- 8.Strategies for Management of Antiretroviral Therapy (SMART) Study Group. El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006 Nov 30;355(22):2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 9.SMART Study Group. El-Sadr WM, Grund B, Neuhaus J, et al. Risk for opportunistic disease and death after reinitiating continuous antiretroviral therapy in patients with HIV previously receiving episodic therapy: a randomized trial. Ann Intern Med. 2008 Sep 2;149(5):289–299. doi: 10.7326/0003-4819-149-5-200809020-00003. [DOI] [PubMed] [Google Scholar]
- 10.Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008 Aug 6;300(5):555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 11.DART Trial Team. Fixed duration interruptions are inferior to continuous treatment in African adults starting therapy with CD4 cell counts < 200 cells/microl. AIDS. 2008 Jan 11;22(2):237–247. doi: 10.1097/QAD.0b013e3282f2d760. [DOI] [PubMed] [Google Scholar]
- 12.Seminari E, De Silvestri A, Boschi A, Tinelli C. CD4+ guided antiretroviral treatment interruption in HIV infection: a meta-analysis. AIDS Rev. 2008 Oct-Dec;10(4):236–244. [PubMed] [Google Scholar]
- 13.Harrigan PR, Whaley M, Montaner JS. Rate of HIV-1 RNA rebound upon stopping antiretroviral therapy. AIDS. 1999 May 28;13(8):F59–62. doi: 10.1097/00002030-199905280-00001. [DOI] [PubMed] [Google Scholar]
- 14.Kilby JM, Goepfert PA, Miller AP, et al. Recurrence of the acute HIV syndrome after interruption of antiretroviral therapy in a patient with chronic HIV infection: A case report. Ann Intern Med. 2000 Sep 19;133(6):435–438. doi: 10.7326/0003-4819-133-6-200009190-00011. [DOI] [PubMed] [Google Scholar]
- 15.Deeks SG. International perspectives on antiretroviral resistance. Nonnucleoside reverse transcriptase inhibitor resistance. J Acquir Immune Defic Syndr. 2001 Mar 1;26(Suppl 1):S25–33. doi: 10.1097/00042560-200103011-00004. [DOI] [PubMed] [Google Scholar]
- 16.Teicher E, Casagrande T, Vittecoq D. Enhanced risk of HIV sexual transmission during structured treatment interruption. Sex Transm Infect. 2003 Feb;79(1):74. doi: 10.1136/sti.79.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bangsberg DR, Hecht FM, Charlebois ED, et al. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000 Mar 10;14(4):357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- 18.Dybul M. Structured Treatment Interruption: Approaches and Risks. Curr Infect Dis Rep. 2002 Apr;4(2):175–180. doi: 10.1007/s11908-002-0059-2. [DOI] [PubMed] [Google Scholar]
- 19.Pai NP, Tulsky JP, Lawrence J, Colford JM, Jr, Reingold AL. Structured treatment interruptions (STI) in chronic suppressed HIV infection in adults. Cochrane Database Syst Rev. 2005 Oct 19;(4):CD005482. doi: 10.1002/14651858.CD005482. [DOI] [PubMed] [Google Scholar]
- 20.Calmy A, Nguyen A, Montecucco F, for the STACCATO Study Team HIV activates markers of cardiovascular risk in a randomized treatment interruption trial: STACCATO. Program and abstracts of the 15th Conference on Retroviruses and Opportunistic Infections. 2008 [Google Scholar]
- 21.Moore DM, Zhang W, Yip B, et al. Non-medically supervised treatment interruptions among participants in a universally accessible antiretroviral therapy programme. HIV Med. 2010 May;11(5):299–307. doi: 10.1111/j.1468-1293.2009.00779.x. [DOI] [PubMed] [Google Scholar]
- 22.Kerr T, Marshall A, Walsh J, et al. Determinants of HAART discontinuation among injection drug users. AIDS Care. 2005 Jul;17(5):539–549. doi: 10.1080/09540120412331319778. [DOI] [PubMed] [Google Scholar]
- 23.Yuan Y, L’italien G, Mukherjee J, Iloeje UH. Determinants of discontinuation of initial highly active antiretroviral therapy regimens in a US HIV-infected patient cohort. HIV Med. 2006 Apr;7(3):156–162. doi: 10.1111/j.1468-1293.2006.00355.x. [DOI] [PubMed] [Google Scholar]
- 24.Ahdieh Grant L, Silverberg MJ, Palacio H, et al. Discontinuation of potent antiretroviral therapy: predictive value of and impact on CD4 cell counts and HIV RNA levels. AIDS. 2001 Nov 9;15(16):2101–2108. doi: 10.1097/00002030-200111090-00005. [DOI] [PubMed] [Google Scholar]
- 25.O’Brien ME, Clark RA, Besch CL, Myers L, Kissinger P. Patterns and correlates of discontinuation of the initial HAART regimen in an urban outpatient cohort. J Acquir Immune Defic Syndr. 2003 Dec 1;34(4):407–414. doi: 10.1097/00126334-200312010-00008. [DOI] [PubMed] [Google Scholar]
- 26.Touloumi G, Pantazis N, Antoniou A, et al. Highly active antiretroviral therapy interruption: predictors and virological and immunologic consequences. J Acquir Immune Defic Syndr. 2006 Aug 15;42(5):554–561. doi: 10.1097/01.qai.0000230321.85911.db. [DOI] [PubMed] [Google Scholar]
- 27.Murri R, Guaraldi G, Lupoli P, et al. Rate and predictors of self-chosen drug discontinuations in highly active antiretroviral therapy-treated HIV-positive individuals. AIDS Patient Care STDS. 2009 Jan;23(1):35–39. doi: 10.1089/apc.2007.0248. [DOI] [PubMed] [Google Scholar]
- 28.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012 Jul 25;308(4):387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 29.Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001 Nov 28;286(20):2568–2577. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 30.Holkmann Olsen C, Mocroft A, Kirk O, et al. Interruption of combination antiretroviral therapy and risk of clinical disease progression to AIDS or death. HIV Med. 2007 Mar;8(2):96–104. doi: 10.1111/j.1468-1293.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- 31.d’arminio Monforte A, Cozzi-Lepri A, Phillips A, et al. Interruption of highly active antiretroviral therapy in HIV clinical practice: results from the Italian Cohort of Antiretroviral-Naive Patients. J Acquir Immune Defic Syndr. 2005 Apr 1;38(4):407–416. doi: 10.1097/01.qai.0000147529.57240.b0. [DOI] [PubMed] [Google Scholar]
- 32.Radimer KL, Olson CM, Campbell CC. Development of indicators to assess hunger. J Nutr. 1990 Nov;120(Suppl 11):1544–1548. doi: 10.1093/jn/120.suppl_11.1544. [DOI] [PubMed] [Google Scholar]
- 33.Kendall A, Olson CM, Frongillo EA., Jr Validation of the Radimer/Cornell measures of hunger and food insecurity. J Nutr. 1995 Nov;125(11):2793–2801. doi: 10.1093/jn/125.11.2793. [DOI] [PubMed] [Google Scholar]
- 34.StataCorp LP. Stata Statistical Software: Release 12. College Station; TX: 2011. [Google Scholar]
- 35.Taffe P, Rickenbach M, Hirschel B, et al. Impact of occasional short interruptions of HAART on the progression of HIV infection: results from a cohort study. AIDS. 2002 Mar 29;16(5):747–755. doi: 10.1097/00002030-200203290-00010. [DOI] [PubMed] [Google Scholar]
- 36.Moss AR, Hahn JA, Perry S, et al. Adherence to highly active antiretroviral therapy in the homeless population in San Francisco: a prospective study. Clin Infect Dis. 2004 Oct 15;39(8):1190–1198. doi: 10.1086/424008. [DOI] [PubMed] [Google Scholar]
- 37.BC Centre for Disease Control. HIV in British Columbia: Annual Surveillance Report 2011. 2012 (Retrieved from http://www.bccdc.ca/util/about/annreport/default.htm).
- 38.Tapp C, Milloy MJ, Kerr T, et al. Female gender predicts lower access and adherence to antiretroviral therapy in a setting of free healthcare. BMC Infect Dis. 2011 Apr 6;11 doi: 10.1186/1471-2334-11-86. 86-2334-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spire B, Duran S, Souville M, et al. Adherence to highly active antiretroviral therapies (HAART) in HIV-infected patients: from a predictive to a dynamic approach. Soc Sci Med. 2002 May;54(10):1481–1496. doi: 10.1016/s0277-9536(01)00125-3. [DOI] [PubMed] [Google Scholar]
- 40.Lieb S, Brooks RG, Hopkins RS, et al. Predicting death from HIV/AIDS: a case-control study from Florida public HIV/AIDS clinics. J Acquir Immune Defic Syndr. 2002 Jul 1;30(3):351–358. doi: 10.1097/00126334-200207010-00012. [DOI] [PubMed] [Google Scholar]
- 41.Johnson MO, Catz SL, Remien RH, et al. Theory-guided, empirically supported avenues for intervention on HIV medication nonadherence: findings from the Healthy Living Project. AIDS Patient Care STDS. 2003 Dec;17(12):645–656. doi: 10.1089/108729103771928708. [DOI] [PubMed] [Google Scholar]
- 42.Berg KM, Demas PA, Howard AA, Schoenbaum EE, Gourevitch MN, Arnsten JH. Gender differences in factors associated with adherence to antiretroviral therapy. J Gen Intern Med. 2004 Nov;19(11):1111–1117. doi: 10.1111/j.1525-1497.2004.30445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leaver CA, Bargh G, Dunn JR, Hwang SW. The effects of housing status on health-related outcomes in people living with HIV: a systematic review of the literature. AIDS Behav. 2007 Nov;11(6 Suppl):85–100. doi: 10.1007/s10461-007-9246-3. [DOI] [PubMed] [Google Scholar]
- 44.Mills EJ, Nachega JB, Bangsberg DR, et al. Adherence to HAART: a systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006 Nov;3(11):e438. doi: 10.1371/journal.pmed.0030438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Udeagu CC, Webster TR, Bocour A, Michel P, Shepard CW. Lost – or just not following up?: Public health effort to re-engage HIV-infected persons lost to follow-up into HIV medical care: 108 (120) AIDS. 2013 May 10; doi: 10.1097/QAD.0b013e328362fdde. [DOI] [PubMed] [Google Scholar]
- 46.Pechere JC. Parameters important in short antibiotic courses. J Int Med Res. 2000;28(Suppl 1):3A–12A. [PubMed] [Google Scholar]


