Abstract
Toll-like receptor 7 (TLR7) signaling predominantly regulates production of type I Interferons (IFNs), which has been suggested in clinical studies to be anti-fibrotic. However, the mechanistic role of the TLR7-type I IFN axis in liver fibrosis has not been elucidated. In the present study, liver fibrosis was induced in wild-type (WT), TLR7-deficient, and IFN-α/β receptor-1 (IFNAR1)-deficient mice and TLR7-mediated signaling was assessed in liver cells isolated from these mice. TLR7-deficient and IFNAR1-deficient mice were more susceptible to liver fibrosis than WT mice, indicating that TLR7-type I IFN signaling exerts a protective effect against liver fibrosis. Notably, the hepatic expression of IL-1ra was suppressed in TLR7- or IFNAR1-deficient mice compared with respective WT mice, and treatment with recombinant IL-1ra reduced liver fibrosis. In vivo activation of TLR7 significantly increased IFNa4 and IL-1ra expression in the liver. Interestingly, each cytokine had different cellular source showing that dendritic cells (DCs) are responsible cell type for production of type I IFN, while Kupffer cells (KCs) mainly produce IL-1ra in response to type I IFN. Furthermore, TLR7 activation by R848 injection suppressed liver fibrosis and production of pro-inflammatory cytokines, and these effects were dependent on type I IFN signaling. Consistent with in vivo data, IFNα significantly induced IL-1ra production in primary KCs.
Conclusions
TLR7 signaling activates DCs to produce type I IFN, which in turn induces anti-fibrogenic IL-1ra production in KCs. Thus, manipulation of the TLR7-type I IFN-IL-1ra axis may be a new therapeutic strategy for the treatment of liver fibrosis.
Keywords: TLR7, liver fibrosis, Type I IFN, IL-1ra
Introduction
Liver fibrosis is a sequel of a chronic wound healing response to a continuous hepatocellular insult. Such injuries induce an inflammatory response and activation of hepatic stellate cells (HSCs) in the liver, leading to excessive production of extracellular matrix proteins (1). Liver cirrhosis, the end stage of liver fibrosis is associated with development of complications such as ascites, renal failure, hepatic encephalopathy, variceal bleeding, and portal hypertension, and may progress to hepatocellular carcinoma (2). Thus, investigation of liver fibrosis is the first step in understanding a number of life-threatening complications in chronic liver disease.
Toll-like receptors (TLRs) are pattern recognition receptors (PRRs) involved in sensing and eliminating foreign antigens. Interaction of TLRs with their specific ligands leads to the production of various cytokines necessary for innate or adaptive immune responses (3). TLR7 is generally considered to recognize ssRNA derived from viruses and bacteria (4, 5). However, host-derived denatured RNA from apoptotic cells is also reported to be an endogenous ligand of TLR7 (6, 7). Several lines of evidence suggest that intestinal bacterial overgrowth and increased bacterial translocation are associated with liver fibrosis. Upon liver injury, levels of bacterial products increase in the portal or systemic circulation (8, 9). Thus, liver cells might be exposed to effective levels of TLR7 ligands during the pathogenesis of liver fibrosis. Although critical roles of TLR4 and TLR9 signaling in liver fibrosis have been demonstrated (10, 11), the role of TLR7 in the development of liver fibrosis has not been studied.
TLR7 signaling predominantly induces type I Interferons (IFN-α and IFN-β) in an interferon regulatory factor 7 (IRF7)-dependent manner (12). Interestingly, it has been suggested that type I IFNs participate in suppression of liver diseases (13–16). It has been noted that type I IFNs directly suppress progressive fibrosis and prevent the subsequent occurrence of hepatocellular carcinoma in patients with hepatitis C who do not respond to treatment with type I IFN (17, 18). Therapy with IFN-α has been reported to ameliorate fibrosis in histopathological analysis of biopsy specimens (19) and reduce the serum levels of fibrotic markers, such as the N-terminal propeptide of procollagen type III (20). Furthermore, type I IFN directly inhibits several pro-fibrogenic factors (Col1α1, Timp1, and Tgfb1) that promote liver fibrosis in concanavalin-induced liver fibrosis (16). Some reports have suggested that IFN-α also possesses anti-fibrogenic properties in cultured fibroblasts or human chondrocytes (21, 22). Recently, it has been demonstrated that treatment of activated rat HSCs and rat T6 cells with CL075, a TLR7 and TLR8 ligand, significantly inhibits cell proliferation and migration (23). Although multiple studies evaluating the role of type I IFN or TLR7 have been shown in vitro, it is unclear what mechanism exerts this function by TLR7 signaling in vivo.
The present study examined the in vivo role of TLR7 by assessing liver fibrosis induced by bile duct ligation (BDL) or carbon tetrachloride (CCl4) injection in wild-type (WT) mice and mice deficient in TLR7 or type I IFN receptor (IFNAR1). Our results demonstrated that TLR7 signaling protects liver fibrosis by up-regulating interleukin-1 receptor antagonist (IL-1ra) through type I IFNs.
Materials and Methods
Mice
TLR7-deficient and IFNAR1-deficient mice (8–10 weeks of age, 25–30 g body weight) were used in these studies. Dr. Akira (Osaka University, Suita, Japan) kindly provided the TLR7-deficient mice (on a BALB/c and C57BL/6 background). IFNAR1-deficient mice (on C57BL/6 background) were purchased from B&K Universal Limited (Hull, UK) and backcrossed on C57BL/6 mice for at least ten generations. CD11c-DTR transgenic mice (on C57BL/6 background) were purchased from the Jackson Laboratory. The mice received humane care according to US National Institutes of Health recommendations outlined in the “Guide for the Care and Use of Laboratory Animals”. Experimental procedures and animal management procedures were undertaken in accordance with the requirements of the Animal Care and Ethics Committees of Chonbuk National University. The animal facility of the Chonbuk National University is fully accredited by the National Association of Laboratory Animal Care.
Liver fibrosis model
This study was performed using two different murine fibrosis models. In the first model, BDL was performed in genetic modified mice and corresponding control WT mice (n = 12 mice per group) as previously described (24). In the second model, CCl4 (Sigma-Aldrich; 10% in corn oil) or vehicle (corn oil) was administered i.p. at a dose of 2 ml/kg body weight three times per week for up to 12 weeks.
Other materials and methods
Other materials used in this study and methods for isolation of liver cell fractions, in vivo depletion of dendritic cells and Kupffer cells, hepatic cytokine measurement, immunohistochemistry, immunofluorescence, quantitative real-time PCR (qRT-PCR), and assessment of hydroxyproline contents are described in the Supplementary Materials and Methods section.
Statistical Analysis
All data were expressed as the mean ± standard error. Differences between multiple groups were compared using one-way analysis of variance (ANOVA) using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA). Duncan’s Multiple Range Test (DMRT) was used for individual comparisons. Differences between two groups were compared using a two-tailed Student’s t-test. A p-value < 0.05 was considered statistically significant.
Results
TLR7-deficient mice are susceptible to chronic liver fibrosis
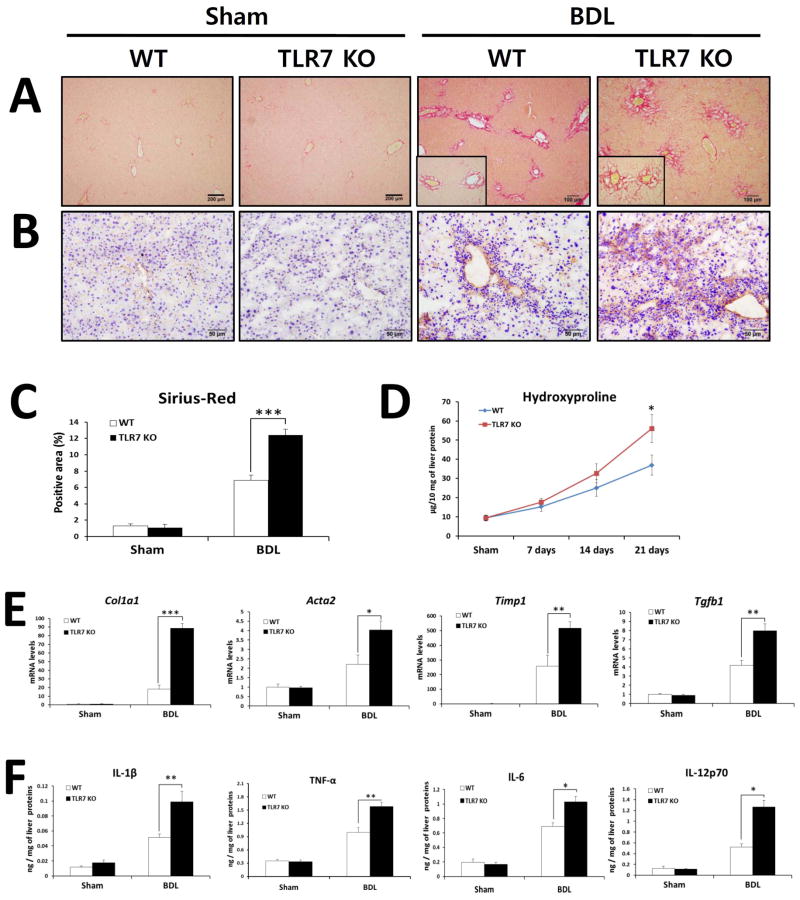
To investigate the importance of TLR7 in liver fibrosis, WT and TLR7-deficient mice were subjected to BDL or CCl4 injection. At 21 days after BDL, TLR7-deficient mice displayed significantly increased liver fibrosis compared with WT mice as determined by quantification of Sirius red- and α-SMA-positive areas and measurement of hydroxyproline content (Fig. 1A–D). The exacerbation of liver fibrosis was also confirmed by qRT-PCR analysis of hepatic expression of fibrogenic genes (Fig. 1E). The mRNA levels of Col1a1, Acta2, Timp1, and Tgfb1 were significantly elevated in TLR7-deficient mice compared with WT mice. Furthermore, inflammatory responses of TLR7-deficient mice were significantly higher than those of WT mice as demonstrated by hepatic protein expression of pro-inflammatory cytokines including IL-1β, TNF-α, IL-6, and IL-12p70 (Fig. 1F).
Fig. 1. TLR7-deficient mice are prone to BDL-induced liver fibrosis and inflammation.
(A–F) TLR7-deficient and respective WT (Balb/c) mice underwent sham operation (n = 3 per group) or BDL (n = 8 per group). After 21 days, mice were evaluated as follows: Fibrillar collagen deposition was determined by Sirius red staining (A) and quantification of the Sirius red–positive area (C). (B) The expression of α-SMA was determined by immunohistochemistry. (D) Hydroxyproline levels were determined. (E) Expression of pro-fibrogenic markers was determined by qRT-PCR and shown as fold change compared with WT sham-operated mice. (F) Hepatic inflammatory cytokines were quantified by ELISA. The results are expressed as ng of each cytokine per mg of liver protein. Data are presented as means ± SEM per group. Two-tailed Student’s t-test, *P < 0.05, **P < 0.01. Original magnification, ×100 (Sirius red), ×200 (α-SMA).
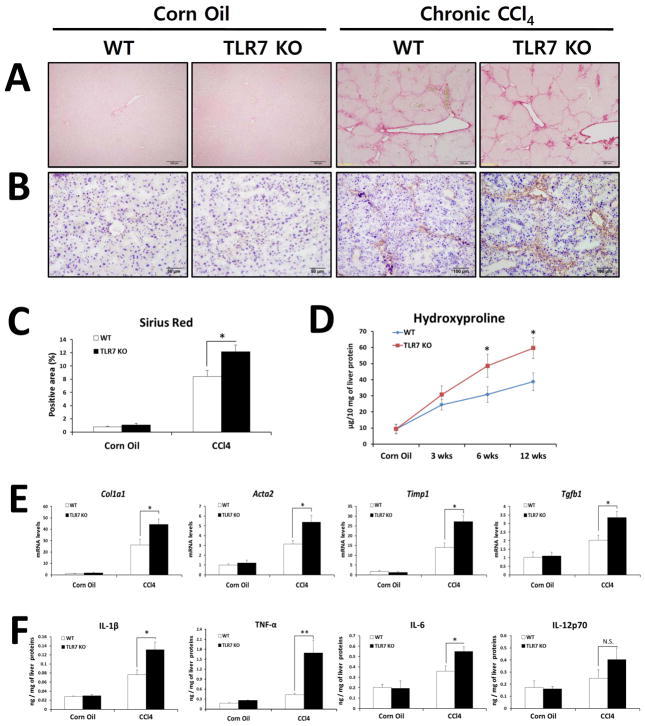
Similar to the BDL model, CCl4 treatment for 12 weeks resulted in aggravated fibrosis in TLR7-deficient mice compared with WT mice as assessed by quantification of Sirius red- and α-SMA-positive areas and hydroxyproline content (Figures 2A–D). In accordance with the extent of fibrosis, mRNA expression of Col1a1, Acta2, Timp1, and Tgfb1 mRNA was significantly higher in TLR7-deficient mice than in WT mice (Fig. 2E) and hepatic inflammation was significantly increased in TLR7-deficient mice compared with WT mice (Fig. 2F). These data indicate that loss of TLR7 exacerbates liver fibrosis in two different models of chronic liver injury and suggest that TLR7 signaling plays a protective role during the course of chronic liver fibrosis.
Fig. 2. TLR7-deficient mice are susceptible to CCl4-induced chronic liver fibrosis and inflammation.
(A–F) TLR7-deficient and respective WT (Balb/c) mice were treated with corn oil (n = 3 per group) or with 36 injections of CCl4 (2 μl/g body weight, 10% of CCl4, three times per week; n = 10 per group). Fibrillar collagen deposition was determined by Sirius red staining (A) and quantification of the Sirius red-positive area (C). (B) The expression of α-SMA was determined by immunohistochemistry. (D) Determination of hydroxyproline levels. (E) Expression of pro-fibrogenic markers determined by qRT-PCR shown as fold change compared with WT corn oil-treated mice. (F) Quantification of hepatic inflammatory cytokines by ELISA. The results are expressed as ng of each cytokine per mg of liver protein. Data are presented as means ± SEM per group. Two-tailed Student’s t-test, *P < 0.05, **P < 0.01. N.S., not significant. Original magnification, ×100 (Sirius red, BDL groups of α-SMA), ×200 (α-SMA).
Given that there are strain-specific differences of T helper cytokine responses in liver fibrosis (25), we sought to examine whether TLR7-mediated signaling is affected by mouse strain. To rule out the confounding effect of genetic background in our study, we performed same set of BDL experiments on C57BL/6 background TLR7-KO versus respective WT mice. As shown in Supplementary Figure 1, fibrosis results from C57BL/6 background showed similar pattern with those from Balb/C background, suggesting that protective role of TLR7 in liver fibrosis is highly preserved, at least between these two different strains of mice.
TLR7 deficiency reduces induction of type I IFN signaling in DCs
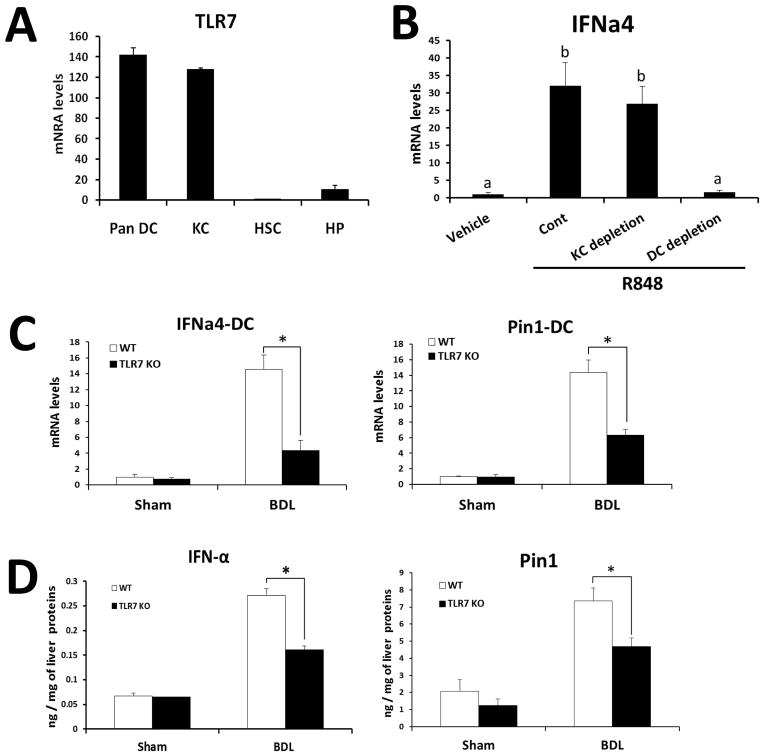
To investigate the cellular source of TLR7 in the liver, we isolated various cell populations [dendritic cells (DCs), Kupffer cells (KCs), hepatic stellate cells (HSCs), and hepatocytes (HPs)] from WT normal livers. Quantification of Tlr7 mRNA expression in each cell population indicated that DCs and KCs are the primary source of TLR7 in the liver (Fig. 3A). Since the TLR7-mediated signaling pathway is predominantly responsible for type I IFN production (26, 27) and these cytokines are mainly produced by macrophages and DCs (5, 28), the cell types required for induction of TLR7-type I IFN axis, we focused on macrophages and DCs in the liver. To elucidate the responsible cell type for production of type I IFN upon TLR7 activation, we administered R848 to CD11c-DTR transgenic mice after depletion of DCs by diphtheria toxin injection or to WT mice after depletion of KCs by clodronate liposome injection. As shown in Fig 3B, R848 injection significantly increased IFNa4 expression in the liver. Notably, this induction was completely abrogated in DC-depleted mice, but not in KC-depleted mice. Because the BDL model induces more prominent phenotypes than the CCl4 model, we predominantly focused on the BDL model in subsequent analyses of liver injury. To further investigate the role of TLR7 and type I IFN in DCs of BDL liver, DCs were isolated from BDL liver of WT or TLR7 KO mice and the levels of type I IFN related genes were measured. Consistent with ex vivo results, mRNA levels of IFN-a4 and Pin1, a signal molecule that activates IRF7 and type I IFN signaling (29), were significantly decreased in DCs of TLR7-deficient mice compared with those of WT mice (Fig. 3C). Furthermore, hepatic protein levels of IFN-α and Pin1 were significantly lower in TLR7-deficient mice than in WT mice (Fig. 3D). These results indicate that TLR7 deficiency decreases type I IFN signaling in fibrotic DCs.
Fig. 3. TLR7 deficiency decreases the induction of type I IFN signaling in DCs.
(A) mRNA levels of TLR7 was determined by qRT-PCR in isolated liver cell fractions representing HPs, KCs, DCs, and HSCs in WT. (B) mRNA of IFNa4 was determined by qRT-PCR in the WT liver at 30 min after injection of TLR7 ligand (R848). Depletion of DCs was achieved by diphtheria toxin injections (4 ng/g of BW) to CD11c-DTR mice for two consecutive days, and depletion of KCs was done by clodronate liposome injections (200 μl/mouse) for two consecutive days. (C) mRNA levels of IFNa4 and Pin1 were determined by qRT-PCR in isolated DC fraction. The results are shown as fold change compared with normal cells from WT mice. Values presented are from three independent experiments and each sample was assayed in duplicate. (D) Hepatic IFN-α and Pin1 were quantified by Luminex and ELISA, respectively. The results were expressed as ng of IFN-α per mg of liver protein. Data are presented as means ± SEM per group. Different letters indicate significant differences (p < 0.01) according to ANOVA and DMRT (B). Two-tailed Student’s t-test, *P < 0.05, **P < 0.01 (C–D).
Decreased type I IFNs contributes to the exacerbation of BDL-induced fibrosis in TLR7-deficient mice
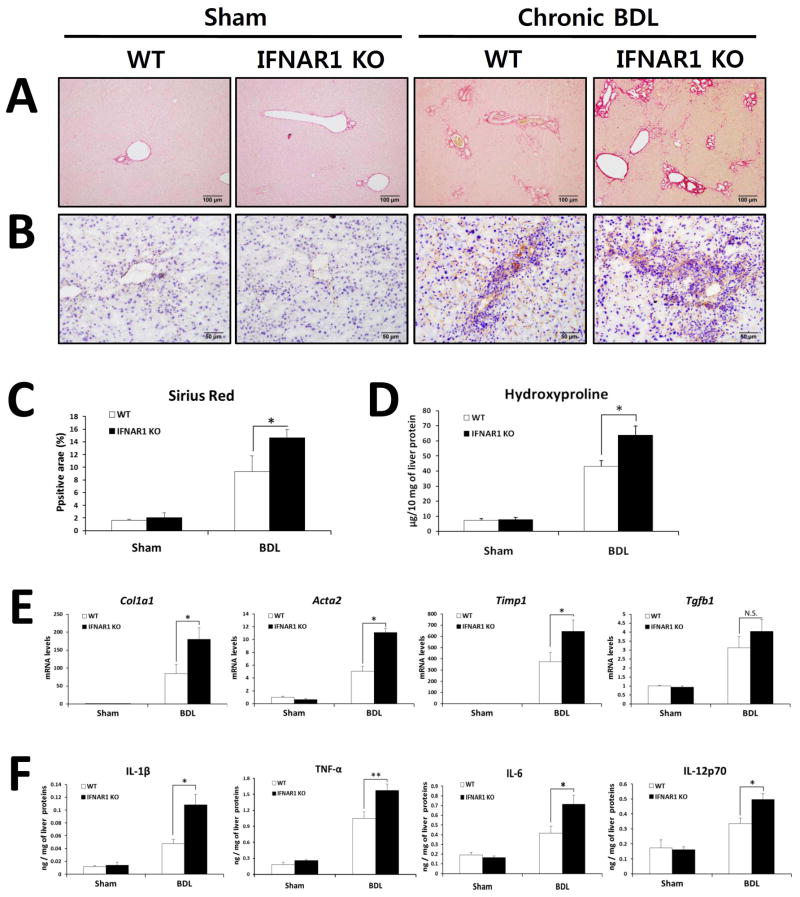
The effects of TLR7 are transduced by two cascades, NF-κB and IRF7 pathways, that induce production of pro-inflammatory cytokines and type I IFNs respectively (3, 30). To differentiate between these two arms of TLR7 signaling in liver fibrosis, we examined liver fibrosis in WT and IFNAR1-deficient mice after 21 days of BDL. We hypothesized that decreased type I IFN signaling is responsible for the exacerbation of liver fibrosis in TLR7-deficient mice. Similar to our findings in TLR7-deficient mice, IFNAR1-deficient mice were more susceptible to BDL-induced fibrosis than WT mice, as demonstrated by an increase in Sirius red- and α-SMA-positive area and hydroxyproline content in IFNAR1-deficient mice (Fig. 4A–D). These results were further supported by qRT-PCR data showing a significantly higher induction of mRNA expression of Col1a1, Acta2, and Timp1 (but not Tgfb1) in IFNAR1-deficient mice compared with WT mice (Fig. 4E). Additionally, liver inflammatory responses were significantly increased in IFNAR1-deficient mice compared with WT mice, as demonstrated by hepatic protein expression of pro-inflammatory cytokines including IL-1β, TNF-α, IL-6, and IL-12p70 (Fig. 4F). These data indicate that type I IFN signaling prevents the liver fibrosis that is triggered by BDL. These findings, together with our earlier results (Figs. 1, 2 and 3), suggest that the exacerbated liver fibrosis in TLR7-deficient mice is mediated by the loss of protective type I IFN signaling.
Fig. 4. BDL-induced fibrosis is exacerbated in IFNAR1-deficient mice compared with WT mice.
(A–F) IFNAR1-deficient and respective WT (C57BL/6) mice underwent a sham operation (n = 3 per group) or BDL (n = 6 per group). After 21 days, mice were evaluated as follows: (A) Fibrillar collagen deposition was determined by Sirius red staining and (C) quantification of the Sirius red–positive area. (B) The expression of α-SMA was determined by immunohistochemistry. (D) Hydroxyproline levels were determined. (E) Expression of pro-fibrogenic markers was determined by qRT-PCR and shown as fold change compared with WT sham-operated mice. (F) Hepatic inflammatory cytokines were quantified by ELISA. The results are expressed as ng of each cytokine per mg of liver protein. Data are presented as means ± SEM per group. Two-tailed Student’s t-test, *P < 0.05, **P < 0.01. Original magnification, ×100 (Sirius red), ×200 (α-SMA).
TLR7 activates DCs to produce type I IFN, which in turn induces IL-1ra production in KCs
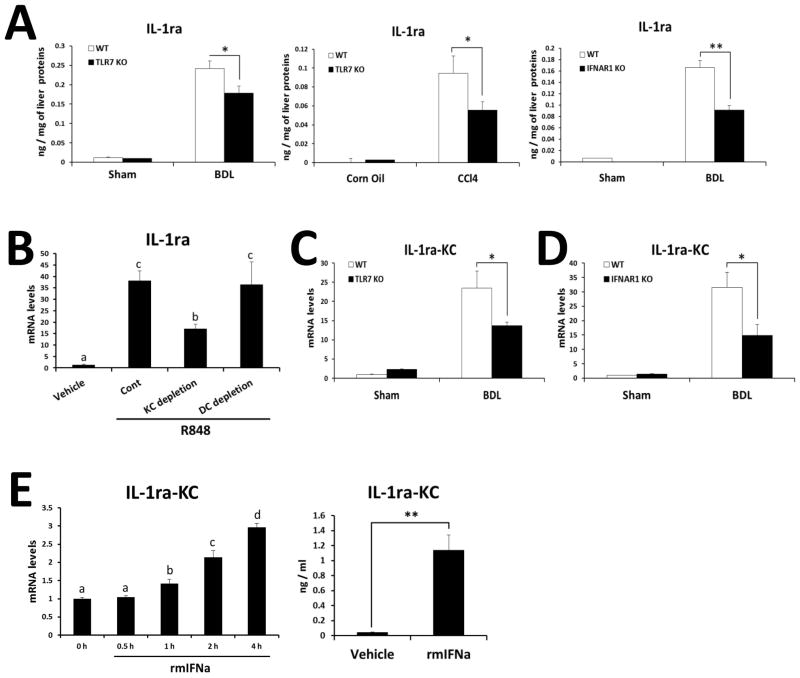
The protective effect of TLR7-mediated type I IFN signaling against liver fibrosis implied the involvement of type I IFN-dependent anti-inflammatory factors. We therefore explored the expression of another IFN-inducible molecule, IL-1ra, an endogenous antagonist for IL-1β (31). Hepatic IL-1ra protein levels in the fibrotic liver were significantly decreased in both TLR7- and IFNAR1-deficient mice compared with WT (Fig. 5A). To clarify the responsible cell type for production of IL-1ra upon TLR7 activation, R848 was administered to CD11c-DTR transgenic mice after either depletion of DCs or KC. As shown in Fig 5B, TLR7 activation by R848 significantly increased IL-1ra expression in the liver. Of note, such induction was 50% decreased in KC-depleted mice, but not changed in DC-depleted mice. Furthermore, IL-1ra expression was significantly reduced in KCs of bile duct ligated TLR7 or IFNAR1-deficient mice compared with those of WT mice (Fig. 5C and 5D). These results prompted us to test whether KCs can be stimulated by Type I IFN, thereby producing IL-1ra by the paracrine manner. Indeed, the treatment of mouse recombinant IFNα significantly induced IL-1ra production in primary KCs (Fig. 5E). Collectively, our results suggest that TLR7 signaling activates DCs to produce type I IFN, which in turn induces IL-1ra production in KCs.
Fig. 5. TLR7- and IFNAR1-deficiency reduce IL-1ra production in KCs.
(A) Hepatic IL-1ra in BDL and CCl4 murine models were quantified by ELISA. The results are expressed as ng of cytokine per mg of liver protein. (B) mRNA of IL-1ra was determined by qRT-PCR in the WT liver at 2 h after injection of TLR7 ligand (R848). Depletion of DCs was achieved by diphtheria toxin injection (4 ng/g) to CD11c-DTR mice for two consecutive days, and depletion of KCs was done by clodronate liposome injection (200μl/mouse) for two consecutive days. (C) mRNA levels of IL-1ra were determined by qRT-PCR in normal KCs, and in vivo activated (21 days post-BDL) KCs from WT and TLR7-deficient mice. (D) mRNA levels of IL-1ra were determined by qRT-PCR in normal KCs, and in vivo activated (21 days post-BDL) KCs from WT and IFNAR1-deficient mice. Values presented are from three independent experiments and each sample was assayed in duplicate. The results are shown as fold change compared with normal cells from WT mice. (E) KCs isolated from WT mice were stimulated with recombinant murine IFNa (500IU/ml), and mRNA and protein levels of IL-1ra were measured with qRT-PCR and ELISA, respectively. Data are presented as means ± SEM per group. Two-tailed Student’s t-test, *P < 0.05, **P < 0.01 (A, C, D). Different letters indicate significant differences (B, p < 0.01; E, p < 0.05) according to ANOVA and DMRT.
Restoration of IL-1ra ameliorated BDL-induced liver fibrosis in TLR7 or IFNAR1-deficient mice
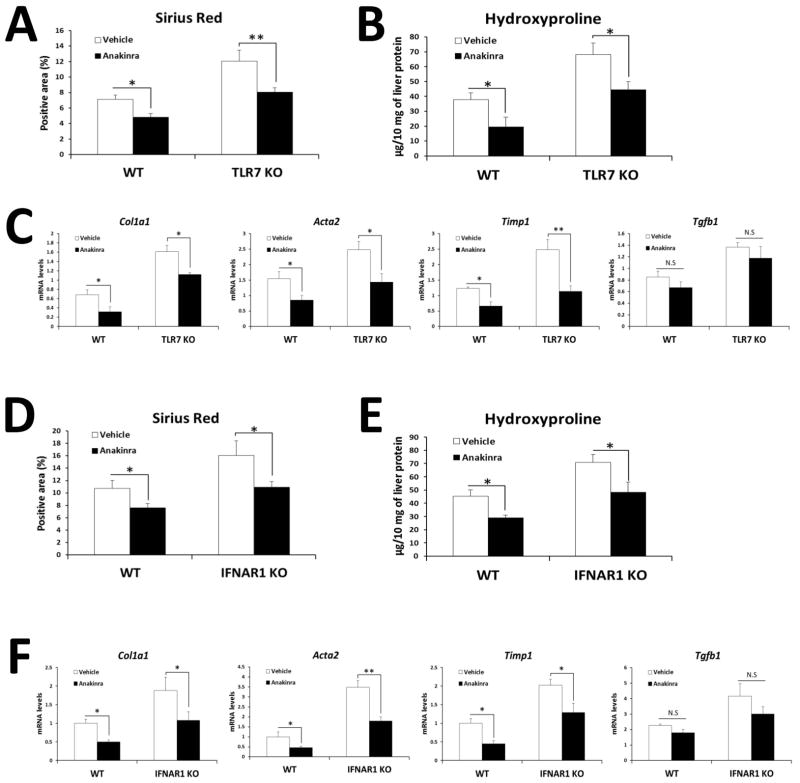
To test the therapeutic potential of IL-1ra in the pathogenesis of liver fibrosis, we treated bile duct ligated TLR7 or IFNAR1-deficient mice with recombinant IL-1ra (Anakinra®). Consistent with the results in Fig. 1 and 4, BDL-induced fibrosis was exacerbated in TLR7 or IFNAR1-deficient mice compared with each WT mice. Notably, treatment with Anakinra restored the fibrosis in TLR7 or IFNAR1-deficient mice to levels comparable to those of WT mice (Fig. 6A–E, Supplementary Fig. 4). Amelioration of liver fibrosis in TLR7 or IFNAR1-deficient mice was verified by quantification of fibrogenic genes expression with the exception of Tgfb1 (Fig. 6C and F). These results suggest that TLR7-dependent IL-1ra is responsible for the protection against BDL-induced liver fibrosis.
Fig. 6. Administration of recombinant IL-1ra ameliorates the liver fibrosis induced by TLR7 or IFNAR1 deficiency.
(A–F) Recombinant IL-1ra (Anakinra®) or saline-injected TLR7 and IFNAR1-deficient mice versus respective WT mice, underwent BDL (n = 8 per group). After 21 days, mice were evaluated as follows: (A and D) Fibrillar collagen deposition was determined by quantification of the Sirius red-positive area. (B and E) Hydroxyproline levels were determined. (C and F) Expression of pro-fibrogenic markers was determined by qRT-PCR and shown as fold change compared with WT sham-operated mice. Data are presented as means ± SEM per group. Two-tailed Student’s t-test, *P < 0.05, **P < 0.01. N.S., not significant.
TLR7-induced protection of liver fibrosis through IL-1ra is dependent on type I IFN signaling
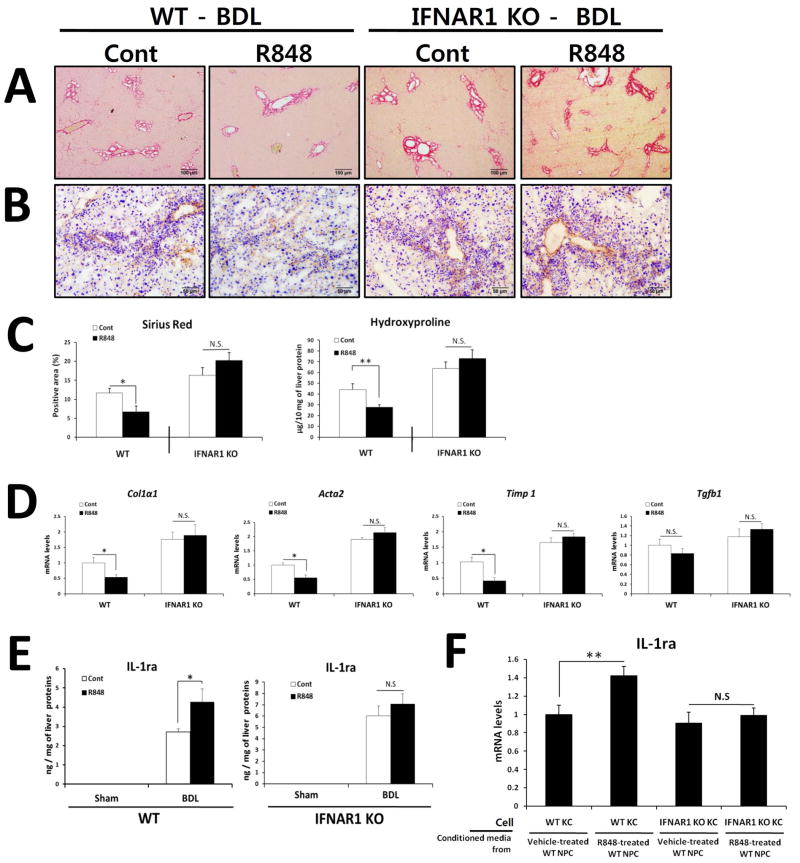
We next treated bile duct ligated WT mice with R848, a selective ligand for TLR7, to investigate whether TLR7 activation prevents liver fibrosis. As shown in Fig. 7, treatment with R848 ameliorated BDL-induced liver fibrosis compared with vehicle-treated animals, as demonstrated by decreased histopathology (Sirius-red and αSMA) and hydroxyproline content (Fig. 7A–C). In accordance with the histological findings, treatment with R848 significantly decreased the expression of fibrogenic genes (Fig. 7D) and resulted in induction of IL-1ra protein in the liver of WT mice (Fig. 7E).
Fig. 7. R848-induced protection of liver fibrosis is dependent on type I IFN signaling.
(A–E) R848 or Saline-injected WT and IFNAR1-deficient mice underwent BDL (n = 12–14 per group). After 21 days, mice were evaluated as follows: (A) Fibrillar collagen deposition was determined by Sirius red staining and (C) quantification of the Sirius red–positive area. (B) The expression of α-SMA was determined by immunohistochemistry. (C) Hydroxyproline levels were determined. (D) Expression of pro-fibrogenic markers was determined by qRT-PCR and shown as fold change compared with saline-treated mice. (E) Hepatic IL-1ra was quantified by ELISA and expressed as ng of IL-1ra protein per mg of liver protein. (F) Gene expression was determined by qRT-PCR analysis of KCs isolated from WT or IFNAR1 KO mice and stimulated for 6 h by conditioned media from vehicle- or R848-treated WT NPCs. Values are presented from three independent experiments and each sample was assayed in duplicate. Data are presented as means ± SEM per group. Two-tailed Student’s t-test, *P < 0.05, **P < 0.01. Original magnification, ×100 (Sirius red), ×200 (α-SMA).
Finally, we investigated whether TLR7-mediated amelioration of liver fibrosis is dependent on type I IFN signaling using mice deficient in IFNAR1. In contrast to WT mice, treatment with TLR7 ligand did not inhibit the progression of BDL-induced liver fibrosis in IFNR-deficient mice (Fig. 7A–D). In addition, hepatic production of IL-1ra was not increased by R848 treatment in IFNAR1-deficient mice (Fig. 7E). To further explore the functional importance of type I IFN signaling in IL-1ra production, we treated isolated KCs from WT and IFNAR1-deficient mice with conditioned media from R848- or vehicle-treated WT hepatic non-parenchymal cells (WT-NPCs). Conditioned media from R848-treated WT-NPCs increased expression of the IL-1ra gene in WT KCs and this effect was dampened in IFNAR1-deficient KCs (Fig. 7F), which is consistent with data showing type I IFN signaling-dependent anti-inflammatory effect of R848 in vivo (Supplementary Fig. 5). These findings support the notion that the TLR7-mediated anti-fibrotic activity via production of IL-1ra is dependent on type I IFN signaling.
Discussion
TLR7 signaling induces production of type I IFNs through IRF7 and activation of Pin1 (12, 29). Accumulating evidence suggests that type I IFNs have a protective function against liver fibrosis (13–16, 20) and that TLR7 agonist-induced activation inhibits HSC activation (32). However, the mechanistic role of TLR7 signaling in liver fibrosis has not been fully studied. The present study demonstrated that TLR7-deficient mice have greater liver fibrosis than WT mice after BDL or CCl4 treatment. Furthermore, our data demonstrated that type I IFN-dependent IL-1ra is pivotal in the protection against liver fibrosis, based on experiments using IFNAR1- or TLR7-deficient mice. Consequently, we concluded that TLR7-type I IFN signaling inhibits liver fibrosis through induction of IL-1ra.
Several lines of evidence support a mechanism in which inhibition of type I IFN-dependent IL-1ra enhances liver fibrosis in bile duct ligated and CCl4-treated TLR7-deficient mice. First, the hepatoprotective role of the type I IFN-dependent IL-1ra axis and the therapeutic potential of IL-1ra have been well documented in various hepatic injury models (15, 33, 34). Second, hepatic production of IFN-α and IL-1ra and expression of hepatic Pin1, a key player in the activation of the IRF7-type I IFN axis (29), are significantly lower in TLR7-deficient mice than in WT mice. Third, similar to TLR7-deficiency, inactivation of type I IFN signaling exacerbated liver fibrosis and blocked IL-1ra production, and decreased IL-1ra expression aggravated liver fibrosis in TLR7 or IFNAR1-deficient mice. Fourth, DCs are responsible cell type for production of type I IFN upon TLR7 activation while KCs mainly produce IL-1ra in response to type I IFN. Finally, TLR7 activation with R848 ameliorated liver fibrosis in vivo and activated DCs to produce type I IFN, which subsequently induced IL-1ra production in KCs in vitro. Taken together, our results suggest that TLR7 activation-mediated type I IFNs and IL-1ra production is responsible for the anti-fibrotic effect.
For clarification of events downstream of TLR7, we additionally studied IFNAR1-deficient mice. Similar to TLR7-deficient mice, IFNAR1-deficient mice showed aggravated liver fibrosis compared with WT mice, suggesting that type I IFN signaling, a common denominator in signaling between TLR7 and IFNAR1 deficiency, has a protective role against liver fibrosis. In response to liver injury caused by BDL or CCl4 treatment, increased production of IL-1ra was evident in the fibrotic liver of WT mice (Supplementary Fig. 3), whereas TLR7-deficient and IFNAR1-deficient mice showed lower induction of IL-1ra. Therefore, we speculate that BDL- or CCl4-induced IL-1ra production is partially mediated by Type I IFNs and TLR7. This notion is consistent with a previous report (15) and our data showing significantly decreased IL-1ra production in TLR7-deficient mice compared with those of WT mice. Moreover, treatment with IL-1ra ameliorated the exacerbated liver fibrosis in TLR7 or IFNAR1-deficient mice. Accordingly, these findings suggest that dysregulation of IL-1ra is responsible for exacerbated liver fibrogenesis in TLR7 or IFNAR1-deficient mice.
To elucidate the cellular source of type I IFN and IL-1ra upon TLR7 activation, we depleted DCs and KCs by diphtheria toxin injection in CD11c-DTR mice and clodronate injection in WT mice, respectively. In vivo activation of TLR7 by R848 injection significantly increased the expression of type I IFN and IL-1ra in liver. DC depletion completely inhibited type I IFN production and KC depletion significantly decreased 50% of IL-1ra expression in liver. These results suggest that type I IFN-producing cells are predominantly DCs while IL-1ra is mainly produced by KCs in response to type I IFN.
The previous report demonstrated an anti-fibrotic effect of TLR7 ligand in primary activated HSCs (14 days after isolation) and T6 cell line (23). However, it may not be reflected the real fibrogenic responses of TLR7 on HSCs because fully activated HSCs were used in their study. We investigated the effect of TLR7 ligand on primary quiescent (day 1 post isolation), intermediate (day 3) and activated (day 7) HSCs. Consequently, we found that TLR7 ligand has no effects on fibrogenic responses of quiescent, intermediated and activated HSCs (Supplementary Fig. 2). Notably, R848 significantly suppressed expression of anti- apoptotic molecules (Bcl-2, Bcl-xL and Mcl-1) in intermediated HSCs (day 3), suggesting that TLR7 activation in HSC mediates survival mechanism. These results corroborate the previous report showing TLR7 ligand-induced inhibition of proliferation and migration of HSCs (23).
We further showed that administration of the TLR7 ligand R848 significantly ameliorated BDL-induced liver fibrosis. Moreover, R848 treatment induced the expression of hepatic IL-1ra in vivo and in vitro. Interestingly, these effects were dampened in IFNAR1-deficient liver and KCs. This finding was consistent with previous studies demonstrating type I IFN-dependent induction of IL-1ra in liver injury (15). Originally, we questioned whether R848 treatment induces pro-inflammatory cytokines in IFNAR1-deficient mice. But significant changes were not found between groups. Of note, we found R848 treatment suppresses BDL-induced liver inflammation in WT mice, but not in IFNAR1-deficient mice, suggesting that R848-induced type I IFN may exerts anti-inflammatory effect in liver fibrosis (Supplementary Fig. 5). Several reports demonstrated that type I IFN inhibits the production of pro-inflammatory cytokines, such as IL-1β, TNF-α, IL-12, and Th1 and Th17 type cytokines (35–39). Furthermore, type I IFN directly suppresses IL-1β production and inflammasome activation via signal transducer and activator of transcription 3 (STAT3) and STAT1 (35). In addition to previous reports, our data suggest that TLR7-induced IL-1ra for the prevention of liver fibrosis is dependent on type I IFN signaling. Also, we concluded that our treatment condition of R848 does not induce the additional increment of pro- inflammatory responses in exceed of surgery (BDL)-induced inflammation.
TLR7 signaling activates two bifurcating pathways through the adapter protein MyD88 (myeloid differentiation primary response gene 88) (40). The first cascade leads to the activation of pro-inflammatory cytokines and requires NF-κB, which is achieved by early endosomal activation (VAMP3+) (41). The second cascade leads to the activation of type I interferon (IFN) genes through phosphorylation of IRF7, which is achieved by late endosomal activation (LAMP2+) (42). A recent study showed that AP3-deficient DCs fail to produce type I IFNs despite normal to moderately enhanced NF-κB–dependent cytokine production (43). In our study, mice deficient for TLR7 signaling showed significantly decreased AP3 expression and a lower LAMP2/VAMP3 ratio in the liver and specifically in DCs. Furthermore, we demonstrated that late endosomal activation is required for type I IFN production in response to R848 (Supplementary Fig. 6). Taken together, these findings suggest that the enhanced liver fibrosis in TLR7-deficient mice is mediated by suppression of bifurcation toward type I IFN signaling through inhibition of AP3 and LAMP2, and this notion is being further investigated.
In summary, TLR7 signaling activates DCs to induce type I IFN signaling, which triggers the production of IL-1ra in KCs. This pathway plays a pivotal role in endogenous protection against liver fibrosis. Furthermore, we clarified that DCs are major source of TLR7-induced type I IFN production, and KCs mainly produce IL-1ra in response to type I IFN by paracrine manner. Our findings demonstrate for the first time that stimulation of TLR7 protects against chronic liver injury by anti-inflammatory type I IFN-dependent induction of IL-1ra. Understanding of TLR7-type I IFN protective mechanisms will offer valuable insights for the prevention and treatment of fibrosis.
Supplementary Material
Acknowledgments
Financial Support
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0064313), (2010-0003644), by the Brain Korea 21 Plus Program in 2013, Republic of Korea, and NIH/NIDDK R01DK085252 and NIH/NIEHS P42ES010337 (to Ekihiro Seki).
List of Abbreviations
- AP3
adaptor protein 3
- α-SMA
alpha-smooth muscle actin
- BDL
bile duct ligation
- DCs
dendritic cells
- HSC
hepatic stellate cells
- IFNAR1
IFN-α/β receptor-1
- IRF7
interferon regulatory factor 7
- IL-1ra
interleukin-1 receptor antagonist
- KCs
Kupffer cells
- LAMP2
lysosomal-associated membrane protein 2
- NF-κB
nuclear factor κB
- PRRs
pattern recognition receptors
- TGF
transforming growth factor
- TLR7
Toll-like receptor 7
- VAMP3
vesicle-associated membrane protein 3
- WT
wild-type
Footnotes
Conflicts of interest: None of the authors have any conflicts of interest to disclose.
References
- 1.Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 (Suppl 1):S38–53. doi: 10.1016/s0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 2.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 4.Eberle F, Sirin M, Binder M, Dalpke AH. Bacterial RNA is recognized by different sets of immunoreceptors. Eur J Immunol. 2009;39:2537–2547. doi: 10.1002/eji.200838978. [DOI] [PubMed] [Google Scholar]
- 5.Mancuso G, Gambuzza M, Midiri A, Biondo C, Papasergi S, Akira S, Teti G, et al. Bacterial recognition by TLR7 in the lysosomes of conventional dendritic cells. Nat Immunol. 2009;10:587–594. doi: 10.1038/ni.1733. [DOI] [PubMed] [Google Scholar]
- 6.Guiducci C, Tripodo C, Gong M, Sangaletti S, Colombo MP, Coffman RL, Barrat FJ. Autoimmune skin inflammation is dependent on plasmacytoid dendritic cell activation by nucleic acids via TLR7 and TLR9. J Exp Med. 2010;207:2931–2942. doi: 10.1084/jem.20101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrat FJ, Meeker T, Gregorio J, Chan JH, Uematsu S, Akira S, Chang B, et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med. 2005;202:1131–1139. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwabe RF, Seki E, Brenner DA. Toll-like receptor signaling in the liver. Gastroenterology. 2006;130:1886–1900. doi: 10.1053/j.gastro.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 9.Frances R, Benlloch S, Zapater P, Gonzalez JM, Lozano B, Munoz C, Pascual S, et al. A sequential study of serum bacterial DNA in patients with advanced cirrhosis and ascites. Hepatology. 2004;39:484–491. doi: 10.1002/hep.20055. [DOI] [PubMed] [Google Scholar]
- 10.Seki E, De Minicis S, Osterreicher CH, Kluwe J, Osawa Y, Brenner DA, Schwabe RF. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe A, Hashmi A, Gomes DA, Town T, Badou A, Flavell RA, Mehal WZ. Apoptotic hepatocyte DNA inhibits hepatic stellate cell chemotaxis via toll-like receptor 9. Hepatology. 2007;46:1509–1518. doi: 10.1002/hep.21867. [DOI] [PubMed] [Google Scholar]
- 12.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Inagaki Y, Nemoto T, Kushida M, Sheng Y, Higashi K, Ikeda K, Kawada N, et al. Interferon alfa down-regulates collagen gene transcription and suppresses experimental hepatic fibrosis in mice. Hepatology. 2003;38:890–899. doi: 10.1053/jhep.2003.50408. [DOI] [PubMed] [Google Scholar]
- 14.Fort J, Pilette C, Veal N, Oberti F, Gallois Y, Douay O, Rosenbaum J, et al. Effects of long-term administration of interferon alpha in two models of liver fibrosis in rats. Journal of Hepatology. 1998;29:263–270. doi: 10.1016/s0168-8278(98)80012-3. [DOI] [PubMed] [Google Scholar]
- 15.Petrasek J, Dolganiuc A, Csak T, Kurt-Jones EA, Szabo G. Type I interferons protect from Toll-like receptor 9-associated liver injury and regulate IL-1 receptor antagonist in mice. Gastroenterology. 2011;140:697–708. e694. doi: 10.1053/j.gastro.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanabe J, Izawa A, Takemi N, Miyauchi Y, Torii Y, Tsuchiyama H, Suzuki T, et al. Interferon-beta reduces the mouse liver fibrosis induced by repeated administration of concanavalin A via the direct and indirect effects. Immunology. 2007;122:562–570. doi: 10.1111/j.1365-2567.2007.02672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alric L, Duffaut M, Selves J, Sandre K, Mularczyck M, Izopet J, Desmorat H, et al. Maintenance therapy with gradual reduction of the interferon dose over one year improves histological response in patients with chronic hepatitis C with biochemical response: results of a randomized trial. J Hepatol. 2001;35:272–278. doi: 10.1016/s0168-8278(01)00110-6. [DOI] [PubMed] [Google Scholar]
- 18.Omata M, Shiratori Y. Long-term effects of interferon therapy on histology and development of hepatocellular carcinoma in hepatitis C. J Gastroenterol Hepatol. 2000;15 (Suppl):E134–140. doi: 10.1046/j.1440-1746.2000.02115.x. [DOI] [PubMed] [Google Scholar]
- 19.Hiramatsu N, Hayashi N, Kasahara A, Hagiwara H, Takehara T, Haruna Y, Naito M, et al. Improvement of liver fibrosis in chronic hepatitis C patients treated with natural interferon alpha. J Hepatol. 1995;22:135–142. doi: 10.1016/0168-8278(95)80420-x. [DOI] [PubMed] [Google Scholar]
- 20.Fort J, Pilette C, Veal N, Oberti F, Gallois Y, Douay O, Rosenbaum J, et al. Effects of long-term administration of interferon alpha in two models of liver fibrosis in rats. J Hepatol. 1998;29:263–270. doi: 10.1016/s0168-8278(98)80012-3. [DOI] [PubMed] [Google Scholar]
- 21.Duncan MR, Berman B. Persistence of a reduced-collagen-producing phenotype in cultured scleroderma fibroblasts after short-term exposure to interferons. J Clin Invest. 1987;79:1318–1324. doi: 10.1172/JCI112956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldring MB, Sandell LJ, Stephenson ML, Krane SM. Immune interferon suppresses levels of procollagen mRNA and type II collagen synthesis in cultured human articular and costal chondrocytes. J Biol Chem. 1986;261:9049–9055. [PubMed] [Google Scholar]
- 23.Chou MH, Huang YH, Lin TM, Du YY, Tsai PC, Hsieh CS, Chuang JH. Selective activation of Toll-like receptor 7 in activated hepatic stellate cells may modulate their profibrogenic phenotype. Biochemical Journal. 2012;447:25–34. doi: 10.1042/BJ20112058. [DOI] [PubMed] [Google Scholar]
- 24.Georgiev P, Navarini AA, Eloranta JJ, Lang KS, Kullak-Ublick GA, Nocito A, Dahm F, et al. Cholestasis protects the liver from ischaemic injury and post-ischaemic inflammation in the mouse. Gut. 2007;56:121–128. doi: 10.1136/gut.2006.097170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi Z, Wakil AE, Rockey DC. Strain-specific differences in mouse hepatic wound healing are mediated by divergent T helper cytokine responses. Proc Natl Acad Sci U S A. 1997;94:10663–10668. doi: 10.1073/pnas.94.20.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 27.Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stockinger S, Kastner R, Kernbauer E, Pilz A, Westermayer S, Reutterer B, Soulat D, et al. Characterization of the interferon-producing cell in mice infected with Listeria monocytogenes. PLoS Pathog. 2009;5:e1000355. doi: 10.1371/journal.ppat.1000355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tun-Kyi A, Finn G, Greenwood A, Nowak M, Lee TH, Asara JM, Tsokos GC, et al. Essential role for the prolyl isomerase Pin1 in Toll-like receptor signaling and type I interferon-mediated immunity. Nat Immunol. 2011;12:733–741. doi: 10.1038/ni.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hemmi H, Kaisho T, Takeuchi O, Sato S, Sanjo H, Hoshino K, Horiuchi T, et al. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat Immunol. 2002;3:196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- 31.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 32.Chou MH, Huang YH, Lin TM, Du YY, Tsai PC, Hsieh CS, Chuang JH. Selective activation of Toll-like receptor 7 in activated hepatic stellate cells may modulate their profibrogenic phenotype. doi: 10.1042/BJ20112058. [DOI] [PubMed] [Google Scholar]
- 33.Mancini R, Benedetti A, Jezequel AM. An interleukin-1 receptor antagonist decreases fibrosis induced by dimethylnitrosamine in rat liver. Virchows Arch. 1994;424:25–31. doi: 10.1007/BF00197389. [DOI] [PubMed] [Google Scholar]
- 34.Isoda K, Sawada S, Ayaori M, Matsuki T, Horai R, Kagata Y, Miyazaki K, et al. Deficiency of interleukin-1 receptor antagonist deteriorates fatty liver and cholesterol metabolism in hypercholesterolemic mice. J Biol Chem. 2005;280:7002–7009. doi: 10.1074/jbc.M412220200. [DOI] [PubMed] [Google Scholar]
- 35.Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Forster I, Farlik M, et al. Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity. 2011;34:213–223. doi: 10.1016/j.immuni.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Jungo F, Dayer JM, Modoux C, Hyka N, Burger D. IFN-beta inhibits the ability of T lymphocytes to induce TNF-alpha and IL-1beta production in monocytes upon direct cell-cell contact. Cytokine. 2001;14:272–282. doi: 10.1006/cyto.2001.0884. [DOI] [PubMed] [Google Scholar]
- 37.Nagai T, Devergne O, van Seventer GA, van Seventer JM. Interferon-beta mediates opposing effects on interferon-gamma-dependent Interleukin-12 p70 secretion by human monocyte-derived dendritic cells. Scandinavian Journal of Immunology. 2007;65:107–117. doi: 10.1111/j.1365-3083.2006.01880.x. [DOI] [PubMed] [Google Scholar]
- 38.McRae BL, Nagai T, Semnani RT, van Seventer JM, van Seventer GA. Interferon-alpha and -beta inhibit the in vitro differentiation of immunocompetent human dendritic cells from CD14(+) precursors. Blood. 2000;96:210–217. [PubMed] [Google Scholar]
- 39.Guo B, Chang EY, Cheng G. The type I IFN induction pathway constrains Th17-mediated autoimmune inflammation in mice. Journal of Clinical Investigation. 2008;118:1680–1690. doi: 10.1172/JCI33342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uematsu S, Sato S, Yamamoto M, Hirotani T, Kato H, Takeshita F, Matsuda M, et al. Interleukin-1 receptor-associated kinase-1 plays an essential role for Toll-like receptor (TLR)7- and TLR9-mediated interferon-{alpha} induction. J Exp Med. 2005;201:915–923. doi: 10.1084/jem.20042372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takaoka A, Yanai H, Kondo S, Duncan G, Negishi H, Mizutani T, Kano S, et al. Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors. Nature. 2005;434:243–249. doi: 10.1038/nature03308. [DOI] [PubMed] [Google Scholar]
- 42.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 43.Sasai M, Linehan MM, Iwasaki A. Bifurcation of Toll-like receptor 9 signaling by adaptor protein 3. Science. 2010;329:1530–1534. doi: 10.1126/science.1187029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.