Abstract
Axial flow ventricular assist devices show great promise as a potential treatment for patients with end-stage heart failure. The HeartMat® II system, redesigned on the basis of initial clinical experiences in Europe, is now in clinical trials in the United States. We report on the 1st use of the newly redesigned HeartMate II in the United States. The system has unique features, which include an accurate flow estimator and the ability to automatically detect and correct excessive left ventricular unloading. The new design also incorporates changes in the texturing of the blood-contacting surfaces to prevent thrombosis. The implantation technique and configuration are similar to those of the HeartMate XVE LVAS and require a median sternotomy for access. The 1st patient to receive the device in the United States has had an uncomplicated perioperative course and now awaits transplantation.
Key words: Equipment design, heart assist devices, heart failure, human, male
The ever-growing population of patients with end-stage heart failure emphasizes the need for mechanical support devices that can provide long-term durability and reliability and can be implanted with a low operative risk. This improvement should safely extend their use to a broader patient population, rather than restricting implantation to the terminally ill. Axial flow pumps are designed to be smaller and more reliable than pulsatile pumps. The Jarvik 2000 FlowMaker (Jarvik Heart, Inc.; New York, NY) and the MicroMed DeBakey VAD® (MicroMed Technology, Inc.; Houston, Tex) ventricular assist devices are axial flow pumps that have shown promising results in early clinical trials.1,2 The HeartMate® II left ventricular assist system (LVAS) (Thoratec Corporation; Pleasanton, Calif), another promising new device, was first used in a European study that ended early due to poor outcomes related to device design issues. On the basis of the problems encountered, the HeartMate II was redesigned and is now beginning clinical trials in the United States. We report the 1st clinical use of the device in the United States and describe the implantation procedure, the clinical results to date, and the unique features of this circulatory support system.
The Device
The HeartMate II LVAS is an axial flow pump that has a spinning rotor as its only moving part (Fig. 1). The implantation technique is similar to that used to implant the original HeartMate devices (HeartMate Implantable Pneumatic [IP] and HeartMate XVE LVAS). The redesigned HeartMate II has a left ventricular apical inflow cannula with a sintered titanium blood-contacting surface. The bladed impeller spins on a bearing and is powered by an electromagnetic motor. No compliance chamber or valves are necessary, and a single driveline exits the right lower quadrant of the abdomen. The inlet cannula is placed in the ventricular appendage, and the pump is placed either intraperitoneally or extraperitoneally. The outflow cannula is connected to a Dacron graft, which is then anastomosed to the ascending aorta in a similar fashion to that done with the original HeartMate XVE. The external controller and batteries resemble those of the original HeartMate as well. The pump is designed to spin at 6,000 to 15,000 rpm and to deliver as much as 10 L/min of cardiac output. A computerized algorithm is used to continuously estimate flow from the device.
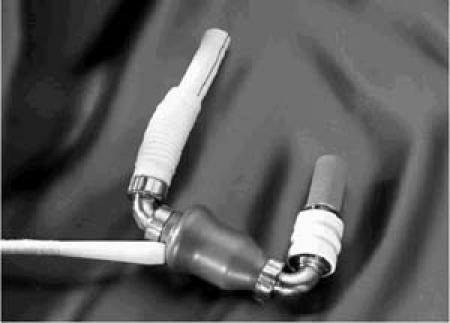
Fig. 1 HeartMate® II left ventricular assist device.
In the 1st clinical trial, thrombus was observed at the inlet and outlet stators (near the fore and aft bearings).3 As a result, engineers reexamined the textured surface and flow paths. Blood flow near the inlet and outlet stators was characterized by relatively narrow channels with high velocities and flow disturbances, similar to the flow around the impeller and its surrounding housing, neither of which is textured. By contrast, the rest of the pump—the inflow and outflow cannulas—is characterized by relatively large, unobstructed flow paths similar to those in the HeartMate XVE, wherein the textured surfaces have proved beneficial. Therefore, by shifting the start of the smooth surface slightly upstream and ending the smooth surface slightly downstream of its original location, the designers created a pump with smooth and textured surfaces more logically matched to their intended functions (Fig. 2).

Fig. 2 Regions of the HeartMate II that were previously textured.
Case Report
This redesigned HeartMate II was first implanted in an 18-year-old man with a history of nonischemic cardiomyopathy of unknown cause, which was diagnosed approximately 6 months before he was admitted to our hospital. At the time of admission (he was transferred from an outside hospital), he had severe, decompensated heart failure and end-organ dysfunction. Despite medical therapy, his symptoms had progressively worsened. He was extremely lethargic due to hepatic encephalopathy, low cardiac output syndrome, and impending organ failure. He was treated with intravenous medications, including milrinone and nesiritide. His condition did not improve, and an intra-aortic balloon pump (IABP) was inserted on the 2nd day of hospitalization. An echocardiogram on admission revealed an ejection fraction <0.15, with a possible apical mural thrombus and global hypokinesis. In addition, he had a dilated right ventricle, dilated atria, and moderate mitral regurgitation. His estimated pulmonary artery pressure was 50 to 55 mmHg, and his left ventricular diastolic dimension was 6.6 cm. Despite the IABP and intravenous medical therapies, he remained severely hemodynamically compromised, although his organ dysfunction improved. The encephalopathy and hepatic dysfunction improved, and he regained normal renal function. He could not, however, be weaned from the IABP. In November 2003, 17 days after admission, he underwent implantation of the redesigned HeartMate II LVAS.
A median sternotomy was performed, and cardiopulmonary bypass (CPB) was instituted. A small patent foramen ovale was closed. The outflow graft was first anastomosed to the ascending aorta in an end-to-side fashion. The inflow cannula was placed in the left ventricular apex after apical coring. Sutures were used to secure the sewing ring to the ventricle under direct visualization. The inlet cannula was inserted into the sewing ring and secured, and the pump was activated. Following de-airing, the patient was weaned from CPB in the usual fashion. The pump was placed intra-abdominally, and the inlet cannula was placed through the diaphragm. Figure 3 shows the device in place. The initial postoperative transthoracic echocardiogram revealed that the aortic valve was opening at 8,000 and 9,000 rpm, but not at 10,000 rpm. Hence, the initial pump rate was kept at 9,000 rpm. Additional studies approximately 3 weeks after implantation revealed that the optimal pump speed was 8,600 rpm (∼4.4 L/min), which has been maintained since that time. During exercise, the patient adjusts the pump speed to 9,000 rpm (∼5.3 L/min) to provide more cardiac output support.
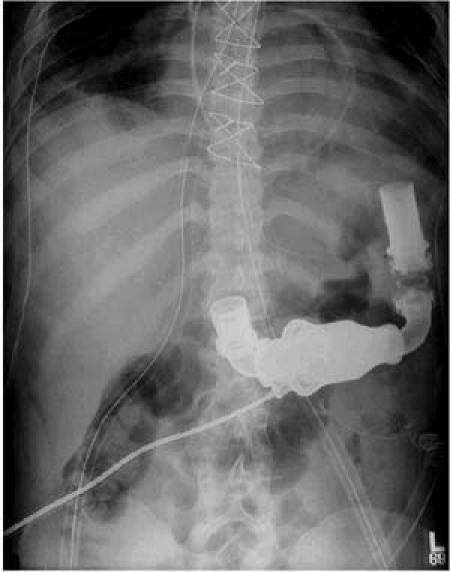
Fig. 3 Radiograph showing the redesigned HeartMate II in place in the 1st patient in the United States to have the device implanted.
Histologic examination of the myocardial plug revealed myocyte hypertrophy and increased interstitial collagen, but no evidence of myocarditis. The patient had a benign postoperative course and was weaned from the ventilator approximately 48 hours later. He began a rehabilitation program and quickly began to walk. At first, anticoagulation was given intravenously. Then oral warfarin was given to achieve an international normalized ratio of 2 to 3. The patient was also given aspirin. No other significant clinical events occurred during the postoperative period, and he began an aerobic exercise program approximately 1 week postoperatively. He remains in New York Heart Association functional class I with no symptoms and is awaiting transplant more than 6 months after implantation. Plasma-free hemoglobin levels have been in the 7-mg/dL range, indicating a low level of hemolysis throughout the period of support. There have been no complications, and the patient has been successfully rehabilitated.
Discussion
Numerous devices are now FDA-approved for therapy in acute heart failure, bridge-to-transplant, and, most recently, destination therapy in patients ineligible for transplantation.4 The new generation of axial flow devices offers significant potential advantages over earlier devices, because they are smaller, simpler, and less obtrusive to the patient, yielding a better quality of life. In addition, because of their smaller size, they can be used in smaller patients, including children.
Challenges still exist, however, in developing devices that are nonthrombogenic and are durable. Also challenging is the management of patients with these devices, which impart an altered physiology with partial pulsatile flow. The inflow and outflow of the HeartMate II have a configuration similar to that of the original HeartMate. The success of the original device in these areas serves as a good basis for the newer generation pump. Again, the challenge is to make the pump mechanism durable and nonthrombogenic.
To bring LVAD technology to the estimated 100,000 patients each year who might benefit, the new devices must be durable and safe, and offer an extended, improved quality of life. Operative risk should be low and reliability high. Late operative complications (infection and thromboembolism) should be minimal. The new HeartMate II has been redesigned on the basis of experience gained by a large group of investigators and is a promising addition to this field. Nimbus Medical, Inc. (Rancho Cordova, Calif), initiated work on the HeartMate II in 1989. Richard Wampler had developed the Hemopump, which was first implanted in April 1988 at the Texas Heart Institute. The senior author (OHF) suggested that blood-immersed bearings were feasible and might be applied to an implantable modification of the Hemopump. A subsequent iteration of this pump was developed by Thermo Cardiosystems (Woburn, Mass)—after it acquired Nimbus—and the University of Pittsburgh. The pump (acquired by Thoratec; Pleasanton, Calif) was first used clinically in June 2001. This initial clinical experience was unfavorable and led to the modifications described above.
Ours is the 1st patient in the United States to be supported with the redesigned HeartMate II as a bridge to transplant. More than 6 months post-implant, there have been no complications, and the patient has been successfully rehabilitated. We await the results in larger numbers of patients with great anticipation.
Footnotes
Address for reprints: O.H. Frazier, MD, Texas Heart Institute, MC 3-114, P.O. Box 20345, Houston, TX 77225-0345
E-mail: knowlin@heart.thi.tmc.edu
References
- 1.Frazier OH, Myers TJ, Westaby S, Gregoric ID. Clinical experience with an implantable, intracardiac, continuous flow circulatory support device: physiologic implications and their relationship to patient selection. Ann Thorac Surg 2004;77:133–42. [DOI] [PubMed]
- 2.Noon GP, Morley DL, Irwin S, Abdelsayed SV, Benkowski RJ, Lynch BE. Clinical experience with the MicroMed DeBakey ventricular assist device. Ann Thorac Surg 2001;71(3 Suppl):S133–8. [DOI] [PubMed]
- 3.Griffith BP, Kormos RL, Borovetz HS, Litwak K, Antaki JF, Poirier VL, Butler KC. HeartMate II left ventricular assist system: from concept to first clinical use. Ann Thorac Surg 2001;71(3 Suppl):S116–20. [DOI] [PubMed]
- 4.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, et al. Long-term mechanical left ventricular assistance for end-stage heart failure. N Engl J Med 2001;345:1435–43. [DOI] [PubMed]


