Abstract
Mycotic aneurysms are rarely seen in patients who have infective endocarditis, and the management of these patients remains controversial. We present the case of a patient who had infective endocarditis complicated by a mycotic aneurysm of the left middle cerebral artery. There was substantial mitral regurgitation, and Streptococcus viridans was isolated from the blood samples. Dysarthria appeared during the 4th week of the antibiotic therapy, but resolved completely 8 hours after onset. The left middle cerebral artery was embolized with platinum detachable coils. On the 7th day after the radiologic intervention, the native mitral valve was replaced with a 33-mm St. Jude Medical® bileaflet mechanical mitral prosthesis. Most mycotic aneurysms show notable regression of symptoms with effective antibiotic treatment, and a very few may diminish in size. However, it is impossible to predict the response of these aneurysms to therapy. To prevent the perioperative rupture of mycotic aneurysms and intracranial hemorrhage, priority should be given to endovascular interventions to treat cerebrovascular aneurysms in patients such as ours.
Key words: Aneurysm, infected; embolization, therapeutic; endocarditis, bacterial; intracranial aneurysm/therapy; mitral valve/surgery
Mycotic aneurysms are rarely seen in patients who have infective endocarditis, and the management of these patients remains controversial.1,2 If surgical intervention is directed first at treatment of the infective endocarditis, there is danger of aneurysmal rupture due to heparinization. On the other hand, if a craniotomy is performed first for the treatment of a mycotic intracerebral aneurysm, there is danger of a cardiac complication such as congestive heart failure. Nowadays, endovascular treatment of mycotic aneurysms before cardiac surgery is a developing solution for these patients.3
In this report, we present the case of a patient who had infective endocarditis complicated by a mycotic aneurysm of the left middle cerebral artery. Before surgery for infective endocarditis, we treated the aneurysm by means of embolization with platinum detachable coils.
Case Report
In April 2003, a 53-year-old man presented at our hospital with serious headache, fatigue, nausea, and vomiting. A subarachnoid hemorrhage originating from the left middle cerebral artery was detected in the computed tomographic (CT) scan. Cerebral angiography was performed, and a mycotic aneurysm with a radius of 3 mm was seen (Fig. 1A). The patient had a mild fever and a pansystolic murmur in the mitral area. Upon transthoracic echocardiography, a vegetation was detected on the mitral valve, as was a ruptured chorda tendinea. Streptococcus viridans was isolated from the blood samples. The diagnosis of infective endocarditis was made, and the patient was placed on a therapy of aqueous penicillin G, 24 million IU per day, and 80 mg gentamicin, 3 times per day. Transesophageal echocardiography revealed a 1- × 2-cm vegetative mass originating from the posterior leaflet of the mitral valve and a ruptured chorda tendinea of the posterior leaflet (Fig. 2). There was significant regurgitation of the mitral valve.
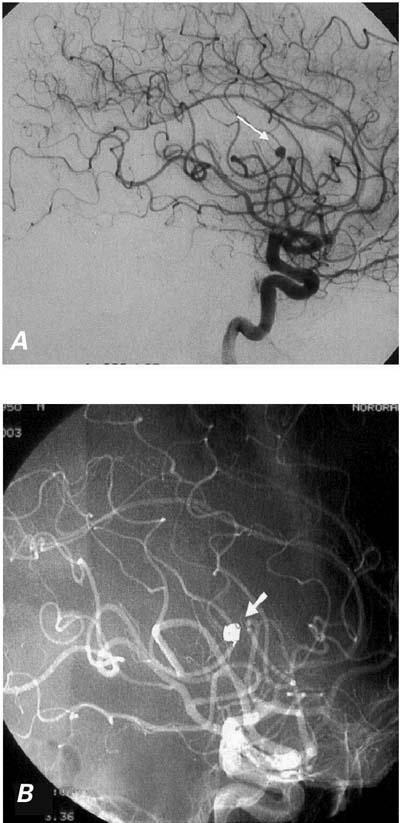
Fig. 1 Cerebral aneurysm (arrow) before (A) and after (B) therapeutic embolization.
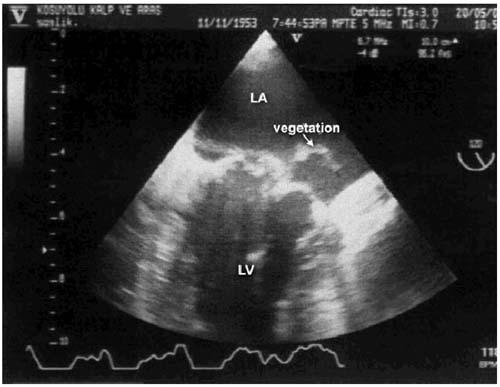
Fig. 2 Vegetation on the posterior leaflet as seen in preoperative echocardiogram.
LA = left atrium; LV = left ventricle
Dysarthria appeared during the 4th week of antibiotic treatment, but it resolved completely 8 hours after its onset. Cerebral angiography was performed once again, and the radius of the mycotic aneurysm had not changed. We then decided to treat the aneurysm via the endovascular route. With the patient under general anesthesia, the left internal carotid artery was entered with a 6F guiding catheter. With a Rapid Transit® microcatheter (Cordis Corporation; Miami, Fla) and Terumo-16 microguidewire (Terumo Corporation; Tokyo, Japan) combination, we entered the aneurysm. The sac was successfully embolized with platinum detachable coils (Boston Scientific Corporation; Boston, Mass). After angiography confirmed the total occlusion, the procedure was terminated (Fig. 1B). Upon awakening, the patient showed no new neurologic deficit.
The cardiac operation was performed 1 week after the therapeutic embolization. There was a 1- × 2-cm vegetative mass on the free margin of the posterior leaflet of the mitral valve, and chordal attachments were ruptured (Fig. 3). Because of this significant damage, we replaced the native valve with a standard 33-mm St. Jude Medical® bileaflet mechanical mitral valve (St. Jude Medical; St. Paul, Minn). The vegetation did not yield any microorganisms. The antibiotic treatment was continued for 6 weeks after the operation. There were no pathologic findings on the follow-up echocardiogram. Prosthetic valve function was considered to be normal. The patient was discharged from the hospital 3 weeks after the operation.
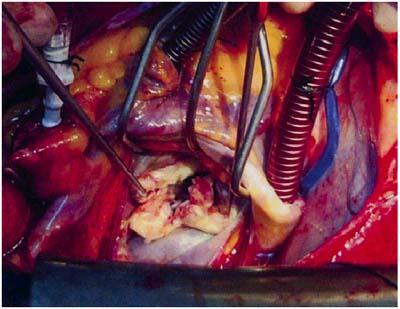
Fig. 3 Vegetative mass and ruptured chordal attachments.
Discussion
Central nervous system complications affect the mortality rate associated with surgical intervention for endocarditis. The results are better in those patients who require and undergo surgery early in the course of their illness.4 About 10% of patients with infective endocarditis have associated cerebral complications.5 The incidence of mycotic aneurysm is 2% to 3% in patients with infective endocarditis.1 Three percent of intracerebral aneurysms have a mycotic origin. The mortality rate of patients with untreated mycotic aneurysms is nearly 30%, and, if the aneurysm ruptures, the mortality rate of course increases.6 Therefore, when a neurologic deficit is detected, cerebral angiography is indicated. Most mycotic aneurysms show notable regression of symptoms and may diminish in size with proper treatment.7 However, it is impossible to predict the responses of these aneurysms to therapy. In our patient, there was no regression of the aneurysm after 4 weeks of antibiotic treatment. Newly arising cardiac failure in union with systemic embolization is a major indication for surgical intervention in patients who have bacterial endocarditis. In the management of these patients, the choice between cardiac surgery and mycotic aneurysmal intervention is controversial.
If cardiac surgery is performed as a first step, intracranial hemorrhage may occur during cardiopulmonary bypass due to systemic heparinization. If an intracerebral aneurysm undergoes clip therapy through a craniotomy before cardiac surgery, the delay in surgical treatment of the endocarditis can result in cardiac failure. Therefore, a craniotomy may have to be performed substantially before cardiac failure has begun. If bacteremia and cardiac failure can be controlled in patients who have bacterial endocarditis, antibiotic regimens and the direct attachment of clips may be effective therapies.
In our patient, a large, hyperkinetic vegetative mass on the free margin of the posterior leaflet, together with dysarthria in the 4th week of antibiotic therapy, necessitated an urgent cardiac operation. We gave priority to treating the aneurysm by endovascular procedures; then on the 5th day after the endovascular procedure, we successfully performed cardiac surgery for valve replacement.
To prevent perioperative rupture of mycotic aneurysms and consequent intracranial hemorrhage, we recommend endovascular intervention to treat cerebrovascular aneurysms in patients such as ours.
Footnotes
Address for reprints: Dr. Hasan Basri Erdogan, Department of Cardiovascular Surgery, Kosuyolu Heart and Research Hospital, 81020 Kadikoy, Istanbul, Turkey
E-mail: gulayhasan@superonline.com
References
- 1.Oohara K, Yamazaki T, Kanou H, Kobayashi A. Infective endocarditis complicated by mycotic cerebral aneurysm: two case reports of women in the peripartum period. Eur J Cardiothorac Surg 1998;14:533–5. [DOI] [PubMed]
- 2.Gillinov AM, Shah RV, Curtis WE, Stuart RS, Cameron DE, Baumgartner WA, Greene PS. Valve replacement in patients with endocarditis and acute neurologic deficit. Ann Thorac Surg 1996;61:1125–30. [DOI] [PubMed]
- 3.Chapot R, Houdart E, Saint-Maurice JP, Aymard A, Mounayer C, Lot G, Merland JJ. Endovascular treatment of cerebral mycotic aneurysms. Radiology 2002;222:389–96. [DOI] [PubMed]
- 4.Parrino PE, Kron IL, Ross SD, Shockey KS, Kron AM, Towler MA, Tribble CG. Does a focal neurologic deficit contraindicate operation in a patient with endocarditis? Ann Thorac Surg 1999;67:59–64. [DOI] [PubMed]
- 5.Eishi K, Kawazoe K, Kuriyama Y, Kitoh Y, Kawashima Y, Omae T. Surgical management of infective endocarditis associated with cerebral complications. Multi-center retrospective study in Japan. J Thorac Cardiovasc Surg 1995; 110:1745–55. [DOI] [PubMed]
- 6.Utoh J, Miyauchi Y, Goto H, Obayashi H, Hirata T. Endovascular approach for an intracranial mycotic aneurysm associated with infective endocarditis. J Thorac Cardiovasc Surg 1995;110:557–9. [DOI] [PubMed]
- 7.Asai T, Usui A, Miyachi S, Ueda Y. Endovascular treatment for intracranial mycotic aneurysms prior to cardiac surgery. Eur J Cardiothorac Surg 2002;21:948–50. [DOI] [PubMed]


