Abstract
Background
Inefficient central processing and integration of visual, vestibular, and somatosensory information may contribute to poor balance and diminished postural control in children with fetal alcohol spectrum disorders (FASD).
Objectives
This pilot study examined sensorimotor performance and the sensory control of balance using a battery of clinical tests in combination with an experimental laboratory assessment that quantifies sensory subsystem use (i.e., sensory weighting) among a systematically diagnosed sample of children with FASD and children with typical development.
Methods
Using a case-control design, 10 children with FASD (8.0-15.9 years; 20% female) were compared to 10 age- and sex-matched controls on standardized clinical measures and on kinematic outcomes from the Multimodal Balance Entrainment Response system (MuMBER), a computerized laboratory assessment whereby visual, vestibular, and somatosensory input is manipulated at different frequencies during standing balance.
Results
Children with FASD showed poorer sensorimotor performance across clinical outcomes with significant group differences (p < .05) on parent-reported movement behaviors (Sensory Processing Measure and Movement Assessment Battery for Children-2 Checklist) and performance on the Dynamic Gait Index. Experimental kinematic outcomes yielded statistically significant group differences (p <.10) on a small proportion of somatosensory and vestibular sensory weighting fractions and postural sway velocity in response to the manipulation of sensory input.
Conclusions
Preliminary findings showed small group differences in sensorimotor and sensory weighting behaviors, specifically those that rely on the integration of vestibular sensation. Differences must be examined and replicated with a larger sample of children with FASD to understand the impact on balance control and functional sensorimotor behaviors.
Keywords: Fetal alcohol spectrum disorder, prenatal alcohol exposure, postural control, balance, sensory weighting
Alcohol is a well-established neurobehavioral teratogen. Prenatal alcohol exposure (PAE) can increase the risk for lifelong neurobehavioral problems, as well as developmental and intellectual disabilities that fall under the umbrella term of fetal alcohol spectrum disorders (FASD). Balance deficits are a more frequent and persistent sensorimotor impairment reported among individuals with FASD.1-5 Studies examining brain structures in association with PAE have identified cerebellar differences and deficits in cerebellar-dependent behaviors (e.g., gait, balance, and coordination) in both animal models6,7 and in children.8,9 Impairments on standardized clinical tests of balance and motor function have also been described among clinically affected children1,2 and adults.5 The nature of these balance deficits are not yet fully understood, and clinical assessment and intervention guidance remain limited.
Efficient balance and postural control provide the stability needed to support participation in many higher-level physical, play, and learning activities that promote function and healthy child development.10,11 Children with impaired or inefficient balance and postural stability may have difficulty with simple tasks that rely on good postural control, such as sitting in a desk, maintaining attention, and learning and controlling complex movements. Poor balance and postural control may also underlie undesirable behaviors that may be misinterpreted as aggression or inattention and contribute to frustration, anxiety, and decreased self-esteem.12,13,14 Accurately identifying and developing interventions for balance and postural control impairments has the potential to improve postural stability and adaptive motor function as a foundation for participation in childhood activities (e.g., playground activities, organized sports) that promote health and well-being (e.g., social interaction, self-esteem) among this high-risk and underserved clinical population.15
Postural control is a complex and dynamic perceptual-motor process that involves the integration of three sensory subsystems (visual, vestibular, and somatosensory), which cue the neuromuscular system to activate postural muscles in response to specific task or environmental conditions.10,16 The inefficient use of, or adaptation to, sensory information has been explored as a potential mechanism for balance impairments in children with PAE; however, there has been limited replication or expansion of previous findings.3,4 Roebuck et al3 used computer posturography to evaluate postural sway while sensory information was manipulated both in accuracy and complexity during standing balance.
The children with PAE had significantly lower composite scores (p < 0.01) on the Sensory Organization Test than controls. Results indicated that the children with PAE had adequate postural stability when environmental conditions were stable (i.e., accurate visual and vestibular information), but had more postural sway when somatosensory information was inaccurate (i.e., they were less able to compensate with visual and vestibular information).
Roebuck et al4 also examined whether the balance deficits among children with PAE were central or peripheral in nature. Twelve children with PAE were compared to 12 controls on postural and neuromuscular responses to movement perturbations using electromyographic (EMG) activity of the lower limbs. No significant group differences were found on short-latency and medium-latency EMG responses, suggesting intact peripheral responsiveness. However, the children with PAE demonstrated more delayed and variable long-latency responses, suggesting poor central processing of sensorimotor information during balance.
Taken together, these study results suggest that inefficient central processing and integration of visual, vestibular, and somatosensory information may play a role in balance and postural control impairments in children affected by PAE. While important, these findings do not precisely specify a child’s ability to continually integrate the three sensory subsystems during balance, which is needed to adapt to changing environmental situations. For example, when standing on a moving surface (e.g., boat, escalator, bus) both the feet and the eyes may not give accurate orientation information, therefore, one needs to weight vestibular input to adequately maintain stability.
Recently, an experimental technique has been developed to examine how sensory subsystems integrate to permit successful stabilization for postural control.16 This technique involves the manipulation of small, barely perceptible oscillating sensory stimuli (visual field movement or tactile movement) provided at low frequencies in a standing position. The frequency and amplitude of an individual’s body sway relative to a particular sensory stimulus frequency and amplitude are examined to determine how a person differentially weights each sensory subsystem for balance control (i.e., sensory weighting).17,18
Developmentally, children as young as 4 years have been shown to weight visual and tactile stimuli and shift their response between the two sensory stimuli when stimulus amplitude becomes too large.19 This capacity to prioritize the reliance on a particular sensory input enhances postural stability in response to changing environmental conditions. In contrast, the inability to weight certain sensory inputs to activate the most efficient and effective postural motor adjustments has been implicated in individuals with postural control impairments and motor deficits.20
Sensory weighting between visual and tactile subsystems as a potential mechanism for balance and postural control impairments has not been examined in children with FASD. In addition, manipulations to discover vestibular weighting capabilities in both children with FASD and children with typical development (TD) have not been studied. A clearer understanding of balance impairments among children with FASD is needed in order to develop and evaluate the effects of sensory directed rehabilitation programs, especially in light of the potential for therapeutic motor training to ameliorate motor deficits described in alcohol-exposed animal models.6,7
This study had two primary objectives. The first objective was to describe clinical sensorimotor profiles, with an emphasis on the sensory control of balance, of a systematically diagnosed clinical sample of children with FASD compared to an age- and sex-matched control group of children with TD. The second objective was to examine visual, vestibular, and somatosensory weighting during standing balance between the two groups with an experimental laboratory test using kinematic outcome measures. We hypothesized that the children with FASD would show poorer performance on clinical sensorimotor outcomes compared to controls. On kinematic outcomes, we expected that compared to controls, children with FASD would demonstrate poorer postural control (higher velocity and greater area of postural body sway) and would show different sensory weighting fractions as compared to controls when the frequency of sensory input increased.
MATERIALS AND METHODS
This study was approved by the University of Washington Human Subjects Division Institutional Review Board. A sample of children with FASD was recruited from the University of Washington Fetal Alcohol Syndrome Diagnostic and Prevention Network (FAS DPN) clinical registry and database of over 2,500 patients. Participants in the database represent a clinical population of individuals with confirmed prenatal alcohol exposure systematically diagnosed by an interdisciplinary team21 using the FASD 4-Digit Diagnostic Code.22,23 The 4-Digit Diagnostic Code is a rigorously defined and extensively validated diagnostic system.22,24,25 The four digits of the code reflect the magnitude of expression of the key diagnostic features of FASD: (a) growth deficiency, (b) facial features, (c) central nervous system (CNS) structural and/or functional abnormalities, and (d) maternal alcohol consumption during pregnancy. The magnitude of expression of each feature is ranked independently on a 4-point Likert scale with 1 reflecting complete absence of the FAS feature and 4 reflecting a strong "classic" presence of the FAS feature. Each 4-Digit Diagnostic Code falls into unique clinical diagnostic categories, including the following that fall broadly under the designation of FASD: (a) fetal alcohol syndrome/alcohol-exposed (FAS/AE), (b) partial FAS/alcohol-exposed (PFAS/AE), (c) static encephalopathy/alcohol-exposed (SE/AE), and (d) neurobehavioral disorder/alcohol-exposed (ND/AE). See Astley23 for a full explanation of the 4-Digit Diagnostic Code, diagnostic methods, and categories, and Astley26 for a profile of the FAS DPN clinical population.
Participants
Ten children with FASD and 10 age- and sex-matched children with typical development (TD) participated in the study. Children in both groups were included in the study if they were ages 8-16 years, male or female, and of any race/ethnicity. Specific inclusion criteria for children with FASD were: confirmed prenatal alcohol exposure at any level, a diagnosis on the fetal alcohol spectrum of FAS, PFAS, SE/AE, or ND/AE, and a previously identified sensorimotor impairment based on clinical diagnostic assessment results. Exclusion criteria for the children with FASD included an IQ < 60 to assure children could adequately follow test directions. In addition, children with severe neuromotor conditions (e.g., cerebral palsy) that impaired ambulation or independent standing for less than 2 minutes, a history of serious head injury, seizures, a visual acuity impairment not corrected by glasses, or a lower limb or back injury within the previous six months were also excluded to control for severe neurological, visual, and orthopedic conditions. Children were recruited and enrolled in accordance with IRB guidelines established for the University of Washington FAS DPN.
Children with TD were recruited from a university research participant pool whose caregivers consented at the time of the child’s birth to be contacted for university research studies, through flyers posted in the university community, and by word-of-mouth. Each child with TD was matched to a child in the FASD group by age (± 6 months) and sex. Children with TD were not eligible if, by parent report, they had a sensory or motor impairment, were enrolled in special education or occupational or physical therapy programs, reported any neurological illness/injury, seizure, genetic, metabolic, behavioral, cognitive disorders, or had a lower limb or back injury within the previous six months. Children in the comparison group were excluded from the study if during the enrollment screen caregivers reported prenatal alcohol exposure of ≥ 3 drinks (defined as ½ oz absolute alcohol = 12 oz beer = 5 oz wine= 1 oz of 100 proof spirit) prior to pregnancy recognition or during the pregnancy.
Procedures
Children were tested during a 2.5-hour laboratory visit. Assessments were administered in the same order by an occupational therapist or physical therapist trained in the assessment battery and who was masked to group status. Each parent completed three questionnaires.
Instrumentation
Measures to describe the participants were:
Demographic Questionnaire: Parent questionnaire to gather demographic, child health, and developmental information.
Anthropometric Measures: Child’s height, weight, and foot size.
Clinical Sensory Screen:27 Child’s responses to light touch and light pressure on each foot and lower leg. A limb position test and limb movement test examined proprioception and kinesthesia. Responses were scored as pass or fail.
Clinical Strength, Range of Motion, and Posture Screen: Child’s lower extremity range of motion and strength were examined, and standing posture for scoliosis, kyphosis, lordosis, leg length difference, and standing foot position were observed.
Kaufman Brief Intelligence Test-2, Matrices Subtest (K-BIT-2):28 A measure of non-verbal intelligence for persons 4-90 years to estimate cognitive level. For children 4-18 years, good internal reliability (α = .86 - .92), test-retest reliability (r = .83 - 91), and construct validity have been reported. The Matrices Subtest standard score was the variable of interest with higher scores indicating better performance.
Standardized clinical measures of sensorimotor behaviors and adaptation during static and dynamic balance activities were:
Movement Assessment Battery for Children- 2nd edition (MABC-2):29 A measure of motor skills in children 3-16 years. Internal reliability (α = 0.90) and test-retest reliability for the total score are high (ICC = 0.97).30 The Balance subtest scaled score is reported with higher scores indicating better performance.
Sensory Processing Measure (SPM):31 A standardized parent questionnaire for children 5-12 years that examines child behavioral responses to touch, movement, visual, and auditory input. Internal reliability (α = .77-.95), inter-rater reliability (r > .94), and construct validity have been reported. Balance subsection and total score T scores are reported, with higher scores indicating more behaviors indicative of poor sensory processing.
Pediatric Clinical Test of Sensory Interaction for Balance-2 (P-CTSIB-2):32 Static balance was assessed under six systematically altered sensory conditions. The conditions were: (1) eyes open standing on floor, (2) eyes closed standing on floor, (3) sway-referenced vision via wearing a dome with eyes open standing on floor, and (4-6) the three visual conditions repeated while standing on medium-density memory foam. Total ordinal score and vestibular (condition 5 + 6) sensory system scores are reported; higher scores indicate better performance. Test-retest reliability (ICC[2,1]=.67-.89), inter-rater reliability (ICC[2,1]=.49-.92), and validity are adequate.33,34
Movement Assessment Battery for Children- 2nd edition Checklist (MABC-2 Checklist):29 Parent questionnaire that examines child movement behaviors in everyday situations, including movement in static or predictable environments and in dynamic or unpredictable environments. Raw scores for each subtest are reported with lower scores indicating better performance.
Dynamic Gait Index (DGI):35 Eight walking tasks test dynamic balance during vestibular challenges (e.g., walking 20’ while turning head right and left, stepping over obstacles). Adequate test-retest reliability (ICC[2,1] = .71) and construct validity have been demonstrated in children.36 The total raw score is reported with higher scores indicating better performance.
Laboratory measure of sensory weighting:
MultiModal Balance Entrainment Response (MuMBER) system: This computerized laboratory assessment system was developed based, in part, on the work of Jeka et al.18 This experimental method measures sensory weighting across sensory subsystems by determining the fraction between body sway frequency and sensory stimulus frequency during standing balance under varying sensory conditions. The construct validity of the MuMBER system as sensitive measure of sensory system weighting has been demonstrated, as children with and without FASD showed changes in sensory weighting behaviors as sensory input was introduced and as sensory stimuli frequency was increased. Within session trial-to-trial reliability of sensory weighting is fair.
During the MuMBER protocol, participants stood on a piece of medium-density memory foam (5 cm thick) overlaid on a platform that tilts (vestibular stimulus), faced a visual screen that displayed a field of horizontally moving dots (visual stimulus), and rested the right index finger on a moveable touch pole (somatosensory stimulus) (Figure 1). The finger pressure on the touch pole was regulated to light touch indicated by a tone. If the child pressed too hard (>5N), the tone would stop and the examiner would assist the child to the correct touch pressure. A helmet (weighing 1.5 kg) restricted peripheral vision to the screen (approximately 110° horizontally and 45°vertically) and white noise was played through earphones during the trials to mask touch pole and platform noise. A safety harness was attached to an overhead trolley to prevent falls. Participants were instructed to stand with feet together, keep their finger resting on the touch pole, look at the screen, and then do whatever felt natural.
FIG. 1.
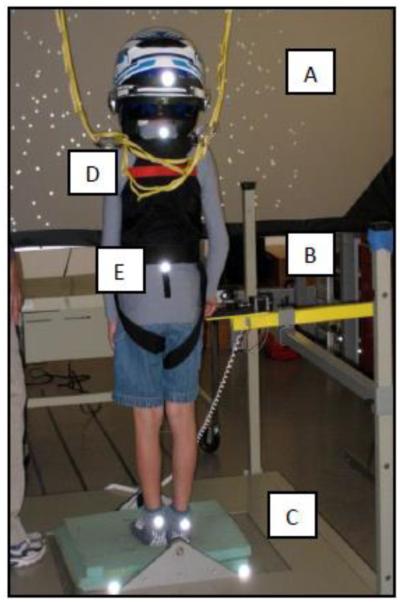
MultiModal Balance Entrainment Response (MuMBER) system components:
A. Moveable visual screen dots (visual stimulus), B. touch pole for right index finger (somatosensory stimulus), C. moveable platform surface with foam overlay (vestibular stimulus). D. Safety harness for fall protection. E. Reflective markers for motion capture on heels, sacrum, spine, and head (helmet).
Sensory stimuli were provided in various combinations of Low (L), Medium (M), and High (H) frequencies for visual (dots on screen), somatosensory (touch pole), and vestibular (platform support surface) inputs, under fixed amplitudes (Table 1). For example, as the frequencies increased the dots, touch pole, and platform oscillated medially-laterally at a faster rate. Stimulus conditions were grouped in a sequence where the frequency of one sensory system increased from L, to M, to H while the other two were held at low frequency stimulation. Each stimulus was triggered to move in a medial-lateral direction at a specific frequency unique to each sensory stimulus and at consistent small amplitudes. Two trials of 60 seconds duration each, over seven test conditions, were applied (Table 1). A Qualysis Oqus 300 motion analysis system37 captured body sway movements at 120 Hz through five cameras located posterior and lateral to the child. The system tracked five reflective markers located on the head (helmet), seventh cervical (C7) vertebra, sacrum between the posterior superior iliac crests, and Achilles tendons, approximately 5 cm above the inferior surface of each heel. Postural sway was tracked in response to the changing sensory stimulation, and kinematic data were used to determine the proportion that the child weighted each stimulus as the frequency of sensory movement was altered. The expectation was that children would decrease sensory weighting to high frequency stimuli due to the potential destabilizing effect on their balance.
TABLE 1.
Multi Modal Balance Entrainment and Response (MuMBER) Protocol: Visual, Somatosensory and Vestibular Conditions
| Condition1, 2 | Acronym3 | Visual Freq. [Hz] (V) |
Somatosensory Freq. [Hz] (T) |
Vestibular [Hz] (P) |
Freq. |
|---|---|---|---|---|---|
| All systems low | LLL | 0.32 | 0.24 | 0.40 | |
| Medium V, low T, low P | MLL | 0.57 | 0.24 | 0.40 | |
| High V, low T, low P | HLL | 1.01 | 0.24 | 0.40 | |
| Low V, medium T, low P | LML | 0.32 | 0.59 | 0.40 | |
| Low V, high T, low P | LHL | 0.32 | 1.11 | 0.40 | |
| Low V, low T, medium P | LLM | 0.32 | 0.24 | 0.52 | |
| Low V, low T, high P | LLH | 0.32 | 0.24 | 0.86 |
Amplitude range was kept constant as follows: visual dots (visual stimulus) = 9 mm medial-lateral; touch-pole (somatosensory stimulus) = 4 mm medial-lateral; platform tilt (vestibular stimulus) = 0.25º tilt;
V = Visual; T = Somatosensory; P = Vestibular;
L = low frequency movement; M = medium frequency movement; H = high frequency movement
Data Reduction
All kinematic data were examined frame by frame for missing data. If markers momentarily disappeared due to the child's body movements (e.g., if the child extended his head, the helmet could obscure the C7 marker; if the child's safety vest shifted it could obscure the C7 or sacral marker), those segments were interpolated. Any trials with a marker gap (>300 ms) were discarded. Each marker component was centered, by subtracting the mean, before further processing. Spectral analysis was conducted using custom LabVIEW software to derive the magnitude of the sacrum marker movement at the three sensory stimuli movement frequencies for the seven conditions tested. To derive the sensory weighting variables, we calculated the fraction, defined as the magnitude of (medial-lateral) body sway at each sensory frequency divided by all other peaks of body sway movement at other frequencies (Figure 2).
FIG. 2.
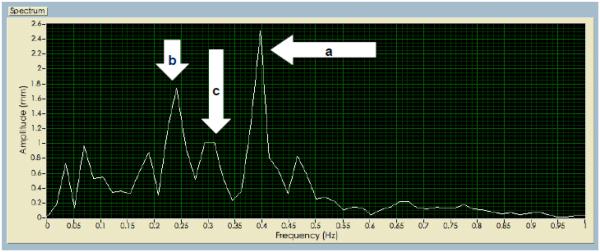
Spectral analysis from the sacrum marker of one participant derived from Labview software for an LLL condition. a) Peak at the vestibular frequency 0.4Hz; b) Peak at the somatosensory frequency 0.24Hz; c) Peak at the visual frequency 0.32Hz.
The vestibular weighting fraction, somatosensory weighting fraction and visual weighting fraction are calculated as a/N, b/N and c/N, respectively, where N is the sum of all peaks observed. The methodology relies on stimulating the various balance subsystems, each with its own unique stimulus frequency, to reveal how much each subsystem contributes to the whole. The total response (i.e., marker motion) is broken down into frequency and amplitude components (i.e., spectral analysis), and the results across stimulus frequencies are compared. For each stimuli response the amplitude vs. frequency data form distinct amplitude peaks at specific frequencies (i.e., response peaks), which often, but not exclusively, correspond to the stimulus frequencies. For example, a response peak at the frequency of the somatosensory stimulus would suggest a large somatosensory subsystem contribution (weighting). The fraction of the somatosensory response peak to the total of all response peaks provides a relative weighting metric for the somatosensory subsystem. Two kinematic postural control variables were derived by sensory condition using MatLab software: (1) velocity of body sway movement (root mean square mm/sec), and (2) area of the ellipse of body sway (cm2). The mean of two trials per sensory condition was used for all analyses. Outcomes from all body markers yielded similar trends, however, because the sacral marker is closer to the body center of mass and had fewer missing data points (as compared to the C7 marker where the helmet obscured more data points) only data from the sacral marker are reported for all analyses.38
Data Analysis
Descriptive statistics were used to summarize demographic, standardized sensorimotor clinical outcomes and sensory weighting/postural sway kinematic outcomes. Due to the small sample size, we used a non-parametric Wilcoxon signed-rank test for paired data to compare the performance of children with FASD to children with TD. Two-tailed significance levels were set at α = 0.05 for clinical outcomes and α = 0.10 for experimental outcomes (MuMBER). Since this is the first study to use MuMBER outcome variables, the study has an exploratory and preliminary nature, and corrections for multiple comparisons were not applied.
RESULTS
Sample Demographics
Personal characteristics for both groups are presented in Table 2. Matching during sampling produced comparable age and sex distributions. Only one child with FASD was in the care of a biological parent, whereas all 10 children with TD had a biological parent listed as primary caregiver. Groups were similar on parent level of education; however, all families of the TD group had income greater than $75K, compared to three families of the group with FASD. The mean intellectual estimate was lower for the children with FASD than children with TD. We refrained from doing hypothesis testing for group differences since the sample size was small, and due to self-selection (especially of the control group), the groups are not likely to be representative of the entire population of caregivers.
TABLE 2.
Personal and Demographic Characteristics by Group
| Characteristic | FASD (n= 10 ) | TD (n = 10) |
|---|---|---|
| Age in months | ||
| Mean (SD) | 142.2 (30.4) | 144.5 (30.2) |
| Median (Min, Max) | 130.5 (111, 186) | 133.0 (113, 187) |
| Sex, % of females | 20.0 | 20.0 |
| Caregiver relationship to child, % | ||
| Biological parent | 10.0 | 100.0 |
| Adoptive/legal guardian/other | 90.0 | 0.0 |
| Caregiver level of Education,% | ||
| High School Diploma | 10.0 | 0.0 |
| Some College | 20.0 | 10.0 |
| College or Professional Degree | 70.0 | 90.0 |
| Annual income | ||
| < $25,000 | 10.0 | 0.0 |
| $25,000 to $50,000 | 20.0 | 0.0 |
| $50,000 to $75,000 | 40.0 | 0.0 |
| > $75,000 | 30.0 | 100.0 |
| K-BIT-2 Matrices1 | ||
| Mean (SD) | 90.8 (19.2) | 112.7 (12.4) |
| Median (min, max) | 97 (56, 100) | 116.5 (93,129) |
| FASD diagnosis, % | ||
| FAS/pFAS2 | 20.0 | 0.0 |
| Static encephalopathy/alcohol exposed | 50.0 | 0.0 |
| Neurobehavioral disorder/alcohol exposed | 30.0 | 0.0 |
Kaufman Brief Intelligence Test-2, Matrices Subtest (K-BIT-2).
Fetal Alcohol Syndrome (FAS)/partial fetal alcohol syndrome
Clinical sensory, strength, range of motion, and posture screen measures were grossly intact and comparable between groups with one exception. A higher proportion of children with FASD (50%) than children with TD (10%) were unable to complete trunk force flexion testing for 30 seconds. A similar proportion of children in each group demonstrated minor postural or musculoskeletal differences that included postural lordosis (FASD 40%; TD 60%), hyper-pronated feet (FASD 50%; TD 40%), and borderline to mild signs of scoliosis (FASD 30%; TD 20%).
Standardized Clinical Measures of Sensorimotor Behaviors and Balance
Mean and median scores on child performance and caregiver-reported sensorimotor outcomes are reported by group in Table 3. The median scores for children with FASD suggested clinically poorer performance than the children with TD across all clinical sensorimotor measures. Statistically significant differences (p < 0.05) were seen on the MABC-2 checklist and SPM, indicating more parent-reported balance problems during functional movement for the children with FASD. The children with FASD also had significantly lower median scores on the DGI; lower scores on the MABC-2 Balance and P-CTSIB-2 total ordinal and vestibular scores approached significance.
TABLE 3.
Standardized clinical measures of sensorimotor behaviors and adaptation during static and dynamic balance by group
| Measures of Sensorimotor Behaviors and Balance |
FASD | TD | p value5 |
|---|---|---|---|
| MABC Balance Total1 | |||
| Mean (SD) | 8.5 (2.7) | 11.3 (3.1) | |
| Median (min, max) | 9.0 (5.0, 14.0) | 12.0 (6.0, 14.0) | .08 |
| MABC Checklist A Static (raw score) | |||
| Mean (SD) | 7.1 (4.8) | .8 (1.9) | |
| Median (min, max) | 7.0 (1.0, 15.0) | 0.0 (0.0, 6.0) | .02 |
| MABC Checklist B Dynamic (raw score) | |||
| Mean (SD) | 10.5 (7.2) | .4 (1.0) | |
| Median (min, max) | 10.5 (0.0, 24.0) | 0.0 (0.0, 3.0) | .01 |
| SPM Total Score2 | |||
| Mean (SD) | 63.7 (6.6) | 46.8 (8.2) | |
| Median (min, max) | 65.0 (47, 69) | 43.0 (40, 60) | .007 |
| SPM Balance 2 | |||
| Mean (SD) | 58.0 (10.7) | 49.4 (9.6) | |
| Median (min, max) | 59.5 (40, 71) | 47.0 (40, 61) | .04 |
| DGI Total Score3 | |||
| Mean (SD) | 21.4 (1.4) | 23.2 (1.1) | |
| Median (min, max) | 21.0 (19, 24) | 24.0 (21, 24) | .02 |
| P-CTSIB-2 Static Ordinal Total4 | |||
| Mean (SD) | 27.6 (2.3) | 29.1 (1.1) | |
| Median (min, max) | 28.5 (22,30) | 29.5 (27, 30) | .07 |
| P-CTSIB-2 Vestibular | |||
| Mean (SD) | 8.2 (1.4) | 9.3 (1.1) | |
| Median (min, max) | 8.5 (5, 10) | 10.0 (7, 10) | .06 |
Movement Assessment Battery for Children 2nd edition (MABC-2; standard score M = 10, SD=3);
Sensory Processing Measure (SPM; T score; M = 50, SD=10).
Dynamic Gait Index (DGI; raw score) – missing 1 pair;
Pediatric Clinic Test of Sensory Interaction for Balance-2 (P-CTSIB-2; raw score).
Wilcoxon signed-rank test for paired data.
Laboratory (kinematic) Measures
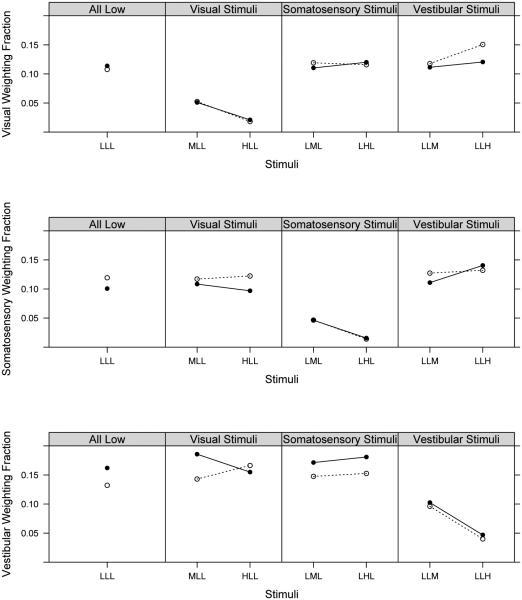
Table 4 and Figure 3 show the visual, somatosensory, and vestibular fractions across sensory conditions manipulated at low, medium, and high frequencies and group comparisons. Overall, both children with and without FASD systematically decreased sensory weighting as the stimulus frequency increased. Visual median weighting fractions were not statistically different between groups across the seven conditions tested. Somatosensory median weighting fractions were significantly different for two of the conditions tested: LLL (p = 0.09), with the children with FASD showing a lower median weighting fraction, and HLL (p = 0.07), with the children with FASD showing a slightly higher median weighting fraction. A statistically significant difference in one Vestibular median weighting fraction for the LLL condition (p = 0.09) was found. Clinical significance cannot yet be determined as this is a new measure utilized only in the sample tested for this study. Postural control outcomes (median body sway area and velocity) were almost uniformly higher for the group of children with FASD; with a statistically significant group difference in sway velocity in the LLH condition (p = .07) (Table 5) suggesting poorer postural control in the children with FASD compared to children with TD.
TABLE 4.
Sensory weighting fractions: Sensory condition by group
|
| ||||||
|---|---|---|---|---|---|---|
| Sensory Condition1,2 |
Visual Fraction | Somatosensory Fraction | Vestibular Fraction | |||
|
| ||||||
| TD | FASD | TD | FASD | TD | FASD | |
|
| ||||||
| LLL | ||||||
| Mean (SD) | .11 (.03) | .11 (.03) | .12 (.03) | .10 (.02) | .13 (.04) | .16 (.06) |
| Median | .11 | .12 | .12 | .11 | .13 | .15 |
| (Min, Max) | (.07, .15) | (.06, .17) | (.07, .18) | (.06, .13) | (.06, .23) | (.10, .29) |
| p value3 | 0.51 | 0.09 | 0.09 | |||
|
| ||||||
| MLL | ||||||
| Mean (SD) | .05 (.01) | .05 (.01) | .12 (.02) | .11 (.03) | .14 (.05) | .19 (.05) |
| Median | .05 | .05 | .12 | .11 | .14 | .19 |
| (Min, Max) | (.04, .06) | (.02, .07) | (.08, .15) | (.06,. 14) | (.05, .22) | (.11, .26) |
| p value3 | 0.57 | 0.45 | 0.20 | |||
|
| ||||||
| HLL | ||||||
| Mean (SD) | .02 (.005) | .02 (.02) | .12 (.03) | .10 (.02) | .17 (.06) | .15 (.02) |
| Median | .02 | .02 | .12 | .09 | .16 | .15 |
| (Min, Max) | (.01, .03) | (.01, .08) | (.07, .16) | (.08, .12) | (.06, .29) | (.12, .20) |
| p value3 | 0.72 | 0.07 | 0.45 | |||
|
| ||||||
| LML | ||||||
| Mean (SD) | .12 (.04) | .11 (.03) | .05 (.02) | .05 (.02) | .15 (.04) | .17 (.06) |
| Median | .11 | .12 | .04 | .04 | .16 | .15 |
| (Min, Max) | (.08, .20) | (.07, .15) | (.03, .08) | (.03, .08) | (.06, .20) | (.12, .27) |
| p value3 | 0.88 | 0.65 | 0.39 | |||
|
| ||||||
| LHL | ||||||
| Mean (SD) | .12 (.02) | .12 (.03) | .01 (.003) | .02 (.006) | .15 (.04) | .18 (.06) |
| Median | .11 | .12 | .01 | .01 | .15 | .17 |
| (Min, Max) | (.08, .15) | (.08, .17) | (.01, .02) | (.01, .03) | (.08, .21) | (.11, .29) |
| p value3 | 0.58 | 0.39 | 0.14 | |||
|
| ||||||
| LLM | ||||||
| Mean (SD) | .12 (.03) | .11 (.04) | .13 (.03) | .11 (.04) | .10 (.04) | .10 (.04) |
| Median | .11 | .11 | .13 | .12 | .09 | .09 |
| (Min, Max) | (.06, .18) | (.06, .17) | (.07,. 16) | (.05, .16) | (.02,. 15) | (.06, .17) |
| p value3 | 0.45 | 0.33 | 0.96 | |||
|
| ||||||
| LLH | ||||||
| Mean (SD) | .15 (.03) | .12 (.05) | .13 (.03) | .14 (.05) | .04 (.01) | .05 (.01) |
| Median | .15 | .11 | .13 | .13 | .04 | .05 |
| (Min, Max) | (.09, .20) | (.09, .23) | (.08, .19) | (.09, .23) | (.02, .05) | (.03, .07) |
| p value3 | 0.20 | 0.65 | 0.20 | |||
|
| ||||||
Sensory conditions are presented in the following order visual, somatosensory, vestibular (e.g., MLL = visual stimulus at Medium frequency; somatosensory stimulus at Low frequency; vestibular stimulus at Low frequency).
L = low frequency movement; M = medium frequency movement; H = high frequency movement.
Wilcoxon signed-rank tests for paired data.
FIG. 3.
Visual, somatosensory, and vestibular weighting fractions across conditions: Children with FASD are depicted in solid circles and children with TD are depicted in open circles.
TABLE 5.
Postural control measures by group
|
| ||||
|---|---|---|---|---|
| Sensory Condition1,2 |
Ellipse of body sway area (mm2) Sacrum |
Velocity (RMS)3
Sacrum |
||
|
|
||||
| TD | FASD | TD | FASD | |
|
| ||||
| LLL | ||||
| Mean (SD) | 15.5 (6.5) | 14.2 (6.7) | 10.4 (1.5) | 10.9 (1.5) |
| Median | 14.1 | 12.9 | 10.3 | 10.3 |
| (min, max) | (5.2, 24.3) | (6.8, 26.2) | (7.9, 12.4) | (9.6, 14.2) |
| p value4 | 0.44 | 0.59 | ||
|
| ||||
| MLL | ||||
| Mean (SD) | 14.7 (6.3) | 19.9 (14.1) | 10.8 (1.5) | 13.0 (4.4) |
| Median | 12.7 | 16.1 | 11.0 | 12.1 |
| (min, max) | (8.7, 28.2) | (8.0, 54.3) | (8.5, 13.4) | (9.8, 24.2) |
| p value4 | 0.52 | 0.26 | ||
|
| ||||
| HLL | ||||
| Mean (SD) | 15.3 (6.2) | 16.6 (7.9) | 10.4 (1.6) | 12.8 (2.3) |
| Median | 13.3 | 15.6 | 10.4 | 12.0 |
| (min, max) | (8.4, 25.5) | (6.7, 32.4) | (8.0, 13.6) | (10.2, 17.6) |
| p value4 | 0.95 | 0.11 | ||
|
| ||||
| LML | ||||
| Mean (SD) | 14.4 (6.8) | 14.0 (6.0) | 10.8 (1.4) | 11.4 (1.5) |
| Median | 13.3 | 14.3 | 11.4 | 11.5 |
| (min, max) | (5.6, 26.4) | (5.2, 25.2) | (8.6, 13.0) | (9.0, 14.0) |
| p value4 | 0.95 | 0.59 | ||
|
| ||||
| LHL | ||||
| Mean (SD) | 17.0 (8.1) | 16.7 (7.5) | 10.5 (2.0) | 11.8 (1.2) |
| Median | 15.5 | 17.2 | 10.2 | 11.7 |
| (min, max) | (7.2, 31.8) | (6.3, 26.2) | (7.4, 14.1) | (9.9, 13.8) |
| p value4 | 0.77 | 0.26 | ||
|
| ||||
| LLM | ||||
| Mean (SD) | 16.5 (11.6) | 15.7 (9.2) | 10.4 (1.8) | 12.0 (2.8) |
| Median | 11.9 | 12.7 | 10.2 | 11.5 |
| (min, max) | (8.0, 45.0) | (9.3, 38.8) | (7.8, 13.1) | (9.2, 19.0) |
| p value4 | 0.95 | 0.37 | ||
|
| ||||
| LLH | ||||
| Mean (SD) | 15.2 (5.2) | 16.8 (12.8) | 10.8 (1.8) | 14.0 (5.8) |
| Median | 16.3 | 14.8 | 10.9 | 12.3 |
| (min, max) | (6.5, 22.0) | (6.0, 48.2) | (7.6, 13.2) | (10.3, 29.0) |
| p value4 | 0.37 | 0.07 | ||
|
| ||||
Sensory conditions are presented in the following order visual, somatosensory, vestibular (e.g., MLL = visual stimulus at Medium frequency; somatosensory stimulus at Low frequency; vestibular stimulus at Low frequency).
L = low frequency movement; M = medium frequency movement; H = high frequency movement;
RMS = root mean square;
Wilcoxon signed-rank tests for paired data.
DISCUSSION
This pilot study is the first to describe the sensory control of balance using an experimental assessment system that quantifies the sensory weighting of visual, somatosensory, and vestibular stimuli in combination with clinical sensorimotor tests among a systematically diagnosed sample of children with FASD and children with TD. Our overall results describe and replicate evidence of diminished functional sensorimotor performance on clinical measures for children with FASD compared to matched controls with TD. Our experimental outcome of sensory weighting yielded a small proportion of statistically significant group differences on somatosensory and vestibular sensory weighting fractions and postural sway velocity. Significant group contrasts were not found on other clinical and kinematic (postural control) measures that focused on the sensory control of balance. We interpret findings from our experimental measure as evidence of possible differences in how efficiently children with FASD adapt to and use sensory subsystems, namely vestibular input, for balance control. These findings are small and preliminary given our limited sample size, and the clinical significance of our experimental sensory weighting outcomes needs further validation.
Clinical Measures
As expected, given the range of alcohol-related diagnoses of the children with FASD in this sample, standardized clinical measures yielded a heterogeneous descriptive profile of sensorimotor performance. As a group, the children with FASD generally had intact strength, musculoskeletal integrity, and peripheral responses to sensation. Mean and median scores on standardized clinical sensorimotor measures suggested poorer balance and functional sensorimotor performance for the children with FASD than their counterparts with TD. The children with FASD scored approximately 1 standard deviation lower than controls on the MABC-2 Balance subtest. Caregivers of the children with FASD almost uniformly reported difficulties with day-to-day movement behaviors on the SPM and MABC-2 checklist. This demonstrates the difficulties children with FASD have using motor and postural skills efficiently in the context of complex, dynamic tasks and environments.2 While the results of the caregiver-reported measures (SPM and MABC-2 checklist) for participants older than age 12 (30%) should be interpreted with caution, as they were beyond the upper age limits of the normative sample, the latter findings are consistent with previous research.2
Mixed results were seen on clinical measures that examined the sensory control of balance under static and dynamic conditions. We found statistically significant group differences on the DGI, which measures gait quality during dynamic vestibular conditions. The lower score for the children with FASD on this measure suggests that the children with FASD demonstrated less efficient vestibular processing in response to dynamic vestibular challenges. However, the clinical significance of these subtle differences requires more investigation as the DGI has not been utilized extensively with children.
Group differences on the P-CTSIB-2, a clinical measure of the sensory control of balance, approached statistical significance for the total score and vestibular score. We consider the lower mean scores for the children with FASD as clinically significant and suggestive of decreased postural stability under complex sensory conditions (i.e., inaccurate or conflicting sensory information). We base this on previous testing of children with balance disorders with the P-CTSIB and P-CTSIB-2 in which most children ages 4-9 years receive the maximum total score.39,40 Therefore, any deviation from a maximum total point score suggests a clinically significant difference, especially since the mean age of the children with FASD in our sample was 11.9 years. Poorer performance on the P-CTSIB-2 is aligned with previous research findings in children with PAE of similar age who showed less efficient vestibular processing during standing balance under altered sensory conditions.3
Sensory Weighting Measures
Using our novel laboratory MuMBER measurements, we have described and quantified how children in both groups weight visual, vestibular, and somatosensory information with the aim of examining how children differentially utilize each subsystem to control overall stability. Children in both groups showed similar decreases in sensory weighting for all subsystems as the frequency of the sensory stimuli increased. This suggests that all of the children could unweight from potentially destabilizing visual, somatosensory, and vestibular sensory stimuli. However, two interesting group differences in the sensory weighting outcomes were seen. First, the children with FASD showed consistently higher vestibular sensory weighting fractions than controls across most conditions. Second, the children with FASD showed consistently higher velocity of body sway (observed as “jerky” movements) to control standing balance as compared to controls across all of the sensory conditions.
These findings suggest that the children with FASD had less efficient postural stability specifically in response to the platform stimuli (±0.25 degree of medial-lateral tilt). We hypothesize that the children with FASD were less able to actively synchronize their body sway to the subtle platform support surface movement. This, in turn, may have caused their body movement to be “dragged” by the platform movement, resulting in higher vestibular sensory weighting fractions in comparison to the children with TD. Children with TD appeared to react to the tilting by coordinating their movements opposite to the platform movement, resulting in smaller sway amplitudes and fractions. This hypothesis will need to be tested further with larger samples of children with FASD.
Taken together, our clinical and experimental findings are consistent with, and expand, previous research that suggests that children affected by PAE demonstrate poorer postural stability and static balance2 and have more difficulty adapting when sensory (i.e., vestibular) information is inaccurate.3 However, this study was preliminary and had a small sample size, and therefore, any statistically significant group differences need to be corroborated in larger studies powered to confirm findings. Results from our laboratory measurement system need replication in larger samples of children with and without FASD. Further, we observed that younger children (8-9 years) with and without FASD demonstrated poorer postural control during the computerized laboratory assessment compared to older controls. This agrees with other findings that suggest that sensory integration for postural control does not fully mature until adolescence.41 We did not test enough children in either group to estimate the effect of developmental differences, but this warrants further study.
The overall severity of postural disability in the children with FASD was minimal to moderate, despite our effort to include children with FASD who had a history of sensorimotor dysfunction. In further exploration of the data utilizing only the seven matched pairs with children with FASD who had more “severe” diagnosis on the fetal alcohol spectrum (i.e., FAS or SE/AE) there were statistically significant differences between the groups on all clinical measures, but differences on the MuMBER sensory weighting fractions were unchanged. Examining larger samples of children with more moderate to severe postural control impairments is needed to better understand the clinical utility of the MuMBER system.
Despite study limitations, our results are congruent with other descriptive studies that report a range of sensorimotor abilities (from subtle to clinically significant impairments) in individuals clinically affected by PAE.2,5 Our focus on the measurement of sensory weighting behaviors provides new and more precise information about how children with and without FASD integrate sensory information to control balance. Our comprehensive sensorimotor profile that included both clinical and kinematic outcomes was an attempt to more precisely test whether the inefficient central processing of sensation, in particular vestibular input, plays a role in diminished postural control and functional sensorimotor performance among children with FASD. Further investigation is warranted to more fully understand how children with FASD use and integrate visual, vestibular, and somatosensory information during balance under both experimental and naturalistic conditions and how this capacity affects functional movement behaviors.
Acknowledgements
This project was supported by Award Number R21AA019579 from the National Institute on Alcohol Abuse and Alcoholism. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Alcohol Abuse and Alcoholism.
REFERENCES
- 1.Kalberg WO, Provost B, Tollison SJ, et al. Comparison of motor delays in young children with fetal alcohol syndrome to those with prenatal alcohol exposure and with no prenatal alcohol exposure. Alcohol Clin Exp Res. 2006;12:2037–2045. doi: 10.1111/j.1530-0277.2006.00250.x. [DOI] [PubMed] [Google Scholar]
- 2.Kooistra L, Ramage B, Crawford S, et al. Can attention deficit hyperactivity disorder and fetal alcohol spectrum disorder be differentiated by motor and balance deficits? Hum Mov Sci. 2009;28:529–542. doi: 10.1016/j.humov.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 3.Roebuck TM, Simmons RW, Mattson SN, Riley EP. Prenatal exposure to alcohol affects the ability to maintain postural balance. Alcohol Clin Exp Res. 1998;22:252–258. [PubMed] [Google Scholar]
- 4.Roebuck TM, Simmons RW, Mattson SN, Riley EP. Neuromuscular responses to balance. Alcohol Clin Exp Res. 1998b;22:1192–1997. [PubMed] [Google Scholar]
- 5.Connor PD, Sampson PD, Streissguth AP, Bookstein FL, Barr HM. Effects of prenatal alcohol exposure on fine motor coordination and balance: A study of two adult samples. Neuropsychologia. 2006;44:744–751. doi: 10.1016/j.neuropsychologia.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Klintsova A, Cowell RM, Swain RA, Napper RMA, Goodlett CR, Greenough WT. Therapeutic effects of complex motor training on motor performance deficits induced by neonatal binge-like alcohol exposure in rats: I. Behavioral results. Brain Res. 1998;8:48–61. doi: 10.1016/s0006-8993(98)00495-8. [DOI] [PubMed] [Google Scholar]
- 7.Klintsova A, Goodlett CR, Greenough WT. Therapeutic motor training ameliorates cerebellar effects of postnatal binge alcohol. Neurotoxicol Teratol. 1999;22:125–132. doi: 10.1016/s0892-0362(99)00052-5. [DOI] [PubMed] [Google Scholar]
- 8.Astley SJ, Aylward E, Brooks A, et al. Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2009;33:1–19. doi: 10.1111/j.1530-0277.2009.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sowell ER, Jernigan TL, Mattson SN, Riley EP, Sobel DF, Jones KL. Abnormal development of the cerebellar vermis in children prenatally exposed to alcohol: Size reduction in lobules I–V. Alcohol Clin Exp Res. 1996;20:31–34. doi: 10.1111/j.1530-0277.1996.tb01039.x. [DOI] [PubMed] [Google Scholar]
- 10.Horak FB, MacPherson JM. Postural orientation and equilibrium. In: Rowell LB, Sheperd JT, editors. Handbook of Physiology. Oxford University Press; New York: 1996. pp. 255–292. [Google Scholar]
- 11.Westcott SL, Burtner PA. Postural control in children: Implications for pediatric practice. Phys Occup Ther Pediatr. 2004;24:5–55. doi: 10.1300/j006v24n01_02. [DOI] [PubMed] [Google Scholar]
- 12.Rivard LM, Missiuna C, Hanna S, Wishart L. Understanding teachers' perceptions of the motor difficulties of children with developmental coordination disorder. Br J Educ Psychol. 2007;77:633–648. doi: 10.1348/000709906X159879. [DOI] [PubMed] [Google Scholar]
- 13.Missiuna C, Moll S, King S, King G, Law MA. Trajectory of troubles: parents' impressions of the impact of developmental coordination disorder. Phys Occup Ther Pediatr. 2007;27:81–101. [PubMed] [Google Scholar]
- 14.Skinner RA, Piek JP. Psychosocial implications of poor motor coordination in children and adolescents. Hum Mov Sci. 2001;20:73–94. doi: 10.1016/s0167-9457(01)00029-x. [DOI] [PubMed] [Google Scholar]
- 15.Johnson CC. The benefits of physical activity for youth with developmental disabilities: a systematic review. Am J Health Promot. 2009:157–67. doi: 10.4278/ajhp.070930103. [DOI] [PubMed] [Google Scholar]
- 16.Peterka RJ. Sensorimotor integration in human postural control. J Neurophysiol. 2002;88:1097–1118. doi: 10.1152/jn.2002.88.3.1097. [DOI] [PubMed] [Google Scholar]
- 17.Oie KS, Kiemel T, Jeka JJ. Multisensory fusion: simultaneous re-weighting of vision and touch for the control of human posture. Brain Res Cogn Brain Res. 2002;14:164–176. doi: 10.1016/s0926-6410(02)00071-x. [DOI] [PubMed] [Google Scholar]
- 18.Jeka JJ, Oie KS, Kiemel T. Multisensory information for human postural control: integrating touch and vision. Exp Brain Res. 2000;134:107–125. doi: 10.1007/s002210000412. [DOI] [PubMed] [Google Scholar]
- 19.Bair WN, Kiemel T, Jeka JJ, Clark JE. Development of multisensory reweighting for posture control in children. Exp Brain Res. 2007;183:435–446. doi: 10.1007/s00221-007-1057-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeka JJ, Allison LK, Saffer M, Zhang Y, Carver S, Kiemel T. Sensory reweighting with translational visual stimuli in young and elderly adults: the role of state-dependent noise. Exp Brain Res. 2006;174:517–527. doi: 10.1007/s00221-006-0502-y. [DOI] [PubMed] [Google Scholar]
- 21.Clarren SK, Carmichael Olson H, Clarren SGB, Astley SJ. A child with fetal alcohol syndrome. In: Guralnick MJ, editor. Interdisciplinary clinical assessment of young children with developmental disabilities. Paul H. Brookes Publishing; Baltimore, MD: 2000. pp. 307–326. [Google Scholar]
- 22.Astley SJ, Clarren SK. Diagnosing the full spectrum of fetal alcohol exposed individuals: Introducing the 4-digit diagnostic code. Alcohol Alcohol. 2000;23:400–410. doi: 10.1093/alcalc/35.4.400. [DOI] [PubMed] [Google Scholar]
- 23.Astley SJ. Diagnostic guide for fetal alcohol spectrum disorders: The 4-digit diagnostic code. 3rd FAS Diagnostic and Prevention Network, University of Washington; Seattle, WA: 2004. [Google Scholar]
- 24.Astley SJ, Aylward E, Brooks A, et al. Neuropsychological and behavioral outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Can J Clin Pharmacol. 2009;16:e178–201. [PMC free article] [PubMed] [Google Scholar]
- 25.Astley SJ, Aylward E, Brooks A, et al. Magnetic resonance imaging outcomes from a comprehensive magnetic resonance study of children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2009;33:1–19. doi: 10.1111/j.1530-0277.2009.01004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Astley SJ. Profile of the first 1,400 patients receiving diagnostic evaluations for fetal alcohol spectrum disorder at the Washington State Fetal Alcohol Syndrome Diagnostic & Prevention Network. Can J Clin Pharmacol. 2010;17:e132–e164. [PubMed] [Google Scholar]
- 27.Schilling DL. Implementation of a strength training program for a 5-year-old child with poor body awareness and developmental coordination disorder. Phys Ther. 2007;87:455–467. doi: 10.2522/ptj.20060170. [DOI] [PubMed] [Google Scholar]
- 28.Kaufman A, Kaufman N. Kaufman Brief Intelligence Test-2nd edition. Western Psychological Services; Los Angeles: 2004. [Google Scholar]
- 29.Henderson SE, Sugden DA. Movement Assessment Battery for Children. 2nd Psychological Corporation; London, UK: 2007. [Google Scholar]
- 30.Wuang YP, Su JH, Su CY. Reliability and responsiveness of the Movement Assessment Battery for Children-Second Edition Test in children with developmental coordination disorder. Dev Med Child Neurol. 2012;54:160–165. doi: 10.1111/j.1469-8749.2011.04177.x. [DOI] [PubMed] [Google Scholar]
- 31.Parham LD, Ecker C, Miller Kuhanek H, Glennon T. Sensory Processing Measure Manual. Western Psychological Services; Los Angeles: 2007. [Google Scholar]
- 32.Crowe TK, Deitz JC, Richardson PK, Atwater SW. Interrater reliability of the Clinical Test for Sensory Interaction for Balance. Phys Occup Ther Ped. 1990;10(4):1–27. [Google Scholar]
- 33.Garner J, Haas A, Antone A, Fenlason C, Vessey S, Westcott SL. Reliability of a new single-rater version of the Pediatric Clinical Test of Sensory Interaction for Balance. Pediatr Phys Ther. 2005;17:68. [Google Scholar]
- 34.Vessey SK, Antone A, Fenlason C, Garner J, Haas A, Westcott SL. Validity of a new single-rater version of the Pediatric Clinical Test of Sensory Interaction for Balance. Ped Phys Ther. 2005;17:88–89. [Google Scholar]
- 35.Shumway-Cook A, Woollacott MH. Motor Control: Translating Research into Clinical Practice. 3rd Lippincott, Williams, & Watkins; Philadelphia: 2007. [Google Scholar]
- 36.Vilnai-Lubetsky A, Jirikowic TL, McCoy SW. Investigation of the Dynamic Gait Index in children: A pilot study. Ped Phys Ther. 2011;23:268–273. doi: 10.1097/PEP.0b013e318227cd82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qualisys Motion Capture System Available at http://www.qualisys.com/. Accessed August 3, 2012.
- 38.Gard SC, Miff A, Kuo D. Comparison of kinematic and kinetic methods for computing the vertical motion of the body center of mass during walking. Hum Mov Sci. 2004;22:597–610. doi: 10.1016/j.humov.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 39.Deitz JC, Richardson PK, Atwater SW, Crowe TK. Performance of normal children on the Clinical Test of Sensory Interaction for Balance. Occup Ther J Res. 1991;11(6):336–356. doi: 10.5014/ajot.46.9.793. [DOI] [PubMed] [Google Scholar]
- 40.Richardson PR, Atwater SW, Crowe TK, Deitz JC. Performance of preschoolers on the Pediatric Clinical Test of Sensory Interaction for Balance. Am J Occup Ther. 1992;46(9):793–800. doi: 10.5014/ajot.46.9.793. PMID:1514565. [DOI] [PubMed] [Google Scholar]
- 41.Rine RM, Rubish K, Feeney C. Measurement of sensory system effectiveness and maturational changes in postural control in young children. Pediatr Phys Ther. 1998;10:16–22. [Google Scholar]