Abstract
Much of the research on the humoral response to allografts has focused on circulating serum antibodies and the long-lived plasma cells that produce these antibodies. In contrast, the interrogation of the quiescent memory B cell compartment is technically more challenging and thus has not been incorporated into the clinical diagnostic or prognostic toolkit. In this review, we discuss new technologies that have allowed this heretofore enigmatic subset of B cells to be identified at quiescence and during a recall response. These technologies in experimental models are providing new insights into memory B cell heterogeneity with respect to their phenotype, cellular function and the antibodies they produce. Similar technologies are also allowing for the identification of comparable memory alloreactive B cells in transplant recipients. While much of the focus in transplant immunology has been on controlling the alloreactive B cell population, long-term transplant patient survival is critically dependent on protection by pathogen-specific memory B cells. Techniques are also available that allow the interrogation of memory B cell response to pathogen re-encounter. Thus we are poised in our ability toinvestigate how immunosuppression affects allo- as well as pathogen-specific memory B cells, and reason that these investigation can yield new insights that will be beneficial for graft as well as patient survival.
Introduction
The advent of sensitive solid-phase assays for quantifying donor-specific antibodies (DSA) has resulted in the delineation of DSA as being one of the most important biomarkers for predicting allograft injury and loss (1, 2). Latest statistics indicate that detection of DSA either pre-transplantation or post-transplantation significantly increases the probability of graft loss (3, 4). Circulating DSA is pathologic to the allograft because it can directly bind to the graft to cause local inflammation and tissue damage through complement activation and FcγR-mediated cytotoxicity, and also function as opsonins to enhance antigen uptake and presentation by antigen-presenting cells to T cells (5–9). Currently, high-titer DSA is reduced by plasmapheresis, or their effects are mitigated by the administration of intravenous immunoglobulin (IVIG) or treatment with eculizumb, an anti-C5 antibody (10).
DSA likely derives from two sources of memory B cells; the quiescent memory B cell and the long-lived plasma cell (LLPC). Data from mouse models suggest that the biology and repertoire of each are distinct, and thus their involvement pre- and post-transplantation could impact graft loss differently. The quiescent memory B cell rapidly and vigorously reactivates upon alloantigen re-exposure, such as in secondary transplantation of sensitized individuals, and accounts for the generation of de novo DSA from their plasma cell progeny. In contrast, the LLPC constitutively secrete antibodies and are critical for the maintenance of long-term circulating DSA but do not mobilize upon alloantigen re-exposure. The DSA repertoire of memory B cells is predicted to be initially of lower affinity, yet still retaining the ability to undergo affinity maturation and to generate new types of high affinity LLPC, while the DSA repertoire of LLPC is predicted to be static and of higher affinity.
Much of the research on the humoral response to allografts has focused on circulating serum antibodies and the LLPC that produce these antibodies. The standardization of high throughput solid phase-based assays has greatly contributed to the relatively ease in quantifying the presence of DSA. While the secretion of antibodies by LLPC is resistant to current immunosuppression, plasma cell depletion has been successfully achieved in experimental models with drugs such as bortezomib and atacicept (TACI-Ig), and clinical trials testing their efficacy in transplantation or autoimmune disease are ongoing (10, 11). In contrast, the interrogation of the quiescent memory B cell compartment is technically more challenging, and has not been incorporated into the clinical diagnostic or prognostic toolkit. In this review, we argue that successful transplantation may benefit from a better understanding of this under appreciated and potentially pathogenic alloreactive memory B cell compartment.
Memory B cells in mice
i. Generation of differentiated B cell subsets
Naïve B cells that bear antigen receptors specific for antigen are induced to activate and, in conjunction with signals from specialized helper CD4+ T cells (T follicular helper cells), to undergo clonal expansion and differentiation into unique B cell types with qualitatively and quantitatively distinct B cell antigen receptors (12) (Figure 1). To secrete antibody into the tissue and blood, activated B cells must differentiate into plasma cells; interestingly two variants of plasma cells have been documented, short- and long-lived (13–15). To generate diversity in the repertoire of antigen specific cells, activated B cells must transiently repress plasma cell differentiation and undergo class switch recombination to IgG isotypes or progress towards the Germinal Center (GC) B cell fate trajectory and generate high affinity clonal variants (16–18). Lastly, to generate memory, activated B cells must repress their activation program and undergo quiescence (19–21). Importantly, memory B cell reactivation following antigen re-exposure results in the same cell fate choices in order to execute effector function. Ultimately the antibodies that are produced serve to protect us from infections, or conversely, to induce tissue pathology, including allograft rejection.
Figure 1.
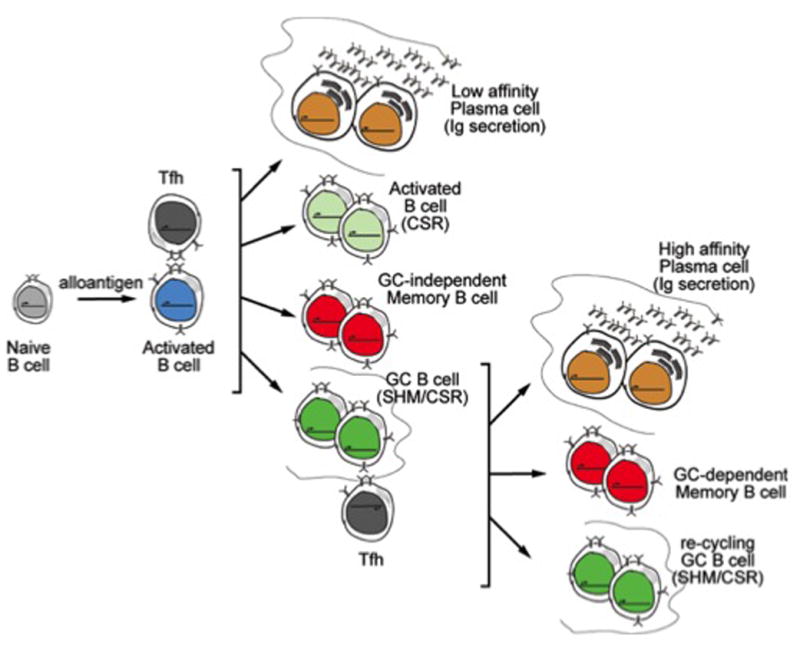
Cell fate map of alloantigen-specific B cells during graft recognition. Early alloantigen-dependent activation of B cells and subsequent cognate interactions with T follicular helper cells at the T-B interface results into the differentiation of B cells into indicated cell fates. Within the GC, representation of alloantigen by B cells results in renewed cognate interactions with Tfh cells that shapes the post-GC B cell repertoire and causes differentiation into the indicated cell fates. Quiescent memory B cells revisit these differentiation trajectories upon alloantigen reencounter.
ii. Long-lived plasma cells
Because the long-lived plasma cell (LLPC) has been shown to function for years, it has been considered part of the memory B cell compartment (22). In contrast, the plasmablast response that produces spikes in antigen specific serum antibody following antigenic exposure is comparatively much more transient lasting only a few days (23, 24). In the mouse, LLPC are thought to derive following selection in GCs and then to migrate to sinusoidal regions in the bone marrow; however, such cells have also been shown to exist in the spleen, lymph node and sites of inflammation (15). Importantly, LLPC constitutively secrete antibody at a high rate and thus is the main source of steady-state serum antibody. Because it is likely that a limited number of bone marrow sinusoid niches exist, a turnover of the LLPC is predicted to occur during every B cell response to infection (25). However, the rate or the signals that regulate LLPC turnover and disappearance over time is incompletely understood. Inflammatory cell types including basophils and eosinophils have been shown to provide cytokines and receptor-ligand interactions that promote LLPC survival (26–30); however, whether changes in these populations during infections affect turnover remains to be determined.
Except for antibody secretion, LLPC are thought to be terminally differentiated and have lost many B cell properties including activation and clonal expansion and have down-regulated expression of many cell surface receptors typical of B cells (14). Despite down-regulated expression of MHC and co-stimulatory receptor genes, one report suggests that the remaining pool of membrane-deposited MHC and co-stimulatory proteins is stable and continues to be functional by modulating T cell responses in distinct ways (31). Notably, along with other B cell-specific markers, expression of the target of Rituximab, CD20, is down regulated; this makes LLPC experimentally challenging to quantify and therapeutically challenging to target with cell-specific antibodies (15). Lastly, it has been shown that the repertoire of LLPC is skewed to relatively higher affinity antibody (32, 33). Overall, in the context of transplantation, the LLPC compartment secretes DSA for many years after sensitization that is of higher affinity for alloantigen and presents as a difficult cell population to target therapeutically without also depleting the protective antibody response to pathogens.
iii. Memory B cells
Recent experimental breakthroughs in tracking memory B cell differentiation and function in vivo have revealed new insights into this heretofore elusive B cell subset, which we summarize below and in Table 1.
a. Increased numbers and greater functionality
Following antigen exposure within a given immune response, select B cells are retained in a quiescent state for long periods of time and are termed memory cells (19, 34, 35). As a result, the immune host exhibits greater numbers of antigen-specific B cells compared to a naïve one. Although memory B cells are quiescent (do not cycle or secrete antibody) they exhibit enhanced functional properties that are evident upon antigen re-exposure. This includes the participation in a secondary immune response by activation, clonal expansion, differentiation into plasma cells and germinal GC B cells, as well as functioning as antigen presenting cells and directly facilitating the T cell response (36, 37). Although these properties are shared by naïve B cells, memory B cells exhibit faster kinetics with enhanced reactivation potential, thus making them the dominant B cell type at the onset of the recall response.
Understanding the molecular determinants that control the enhanced re-activation properties of memory compared to naive B cells is an area of intense study because their identification will improve our ability to therapeutically target the reactivation potential of this subset of cells in pathologic conditions, including transplantation. Three notable determinants have been identified: greater B cell receptor (BCR) affinity (see below) and hence greater BCR signal strength (38–42); a distinct expression pattern of transcriptional determinants of B cell differentiation (43); and the cytoplasmic tail of the IgG BCR that confers greater signaling capacity compared to that of the IgM receptor (44, 45). Because recent findings suggest that the memory B cell pool is heterogeneous, a propos the expression of the IgM or IgG receptor, the latter possibility cannot completely explain the difference between a primary and recall response (see below), and thus the former two or other, yet to be discovered, mechanisms are thought to predominate. One implication of these findings to transplantation is that, in the context of chronic rejection, a continuous low-grade activation and differentiation of memory B cells into plasma cells would result in gradual DSA accumulation, leading ultimately to antibody-mediated pathology and graft dysfunction. In contrast, in the setting of secondary transplantation of sensitized patients, allospecific memory B cells would efficiently and preferentially reactivate upon alloantigen recognition, and if not effectively controlled, result in strong de novo DSA production.
b. Distinct repertoire
The BCR repertoire of memory B cells is distinct from naïve B cells and the antibodies secreted by LLPC. It has long been recognized that the repertoire of memory B cells is distinct from that of naïve B cells based on the extent of class switch recombination that had occurred during the primary response (46). This results in a switch from primarily IgM (naïve) to diverse IgG, IgE, and IgA subclasses; the distribution of which is determined by the nature of T cell help and the cytokine environment. This distribution of switched Ig subclasses in memory B cells may or may not differ from the LLPC compartment.
At the level of antigen specificity, because of the process of affinity maturation, which involves a somatic hypermutation-based mechanism, selects for higher affinity variants during the GC phase of the primary response, memory B cells exhibit mutations in the antigen combining site that confer greater affinity for the antigen (47, 48). Thus, the antigen-specific repertoire of memory B cells differs from that of naïve B cells. For the same reason, the repertoire of LLPC differs greatly from naïve B cells, but interestingly, the repertoire of LLPC is skewed towards even greater affinity than memory B cells (32, 33). The basis of this is not well understood; but it is thought to be mediated by differential requirements for the Bcl2 family of anti-apoptotic factors (49, 50) as well as differential signaling through the antigen receptor and selection by GC-resident T follicular helper cells to favor LLPC differentation (18). Importantly, because of the differences in repertoire, memory B cells may be more cross-reactive than antibodies produced by LLPC (32). In that study it was reported that, during a secondary infection, serum could only protect when challenged with a homologous virus whereas memory B cells could protect when challenged with either a homologous or heterologous variant virus. This finding has dramatic implications for transplantation. First, it suggests that cataloguing the HLA reactivity pattern of sensitized patients using serum alone may be insufficient for appropriate and/or optimal HLA matching. Second, it suggests that, following secondary transplantation in sensitized patients, de novo DSA associated with unpredicted or multiple HLA specificities may be arising from memory B cell precursors.
c. Heterogeneity
Classically, the experimental identification of memory B cells was based on persisting cells that exhibited evidence of participation in the primary response (19). However, because of the paucity of stable markers that persisted long-term after primary activation, investigators have focused on only the memory B cells that had undergone class switch recombination. Because of this technical limitation, the memory B cell field had largely ignored the potential of IgM memory B cells. More recently, innovative cell fate tracking techniques have uncovered that the memory B cell compartment is remarkably heterogenous, with a significant fraction exhibiting a lack of switch recombination (and continues to express IgM) or somatic hypermutation (51, 52). These observations imply that the generation of each type of memory B cells proceeds through fundamentally distinct developmental pathways (20). Importantly, the phenotypic heterogeneity of memory B cells translates into functional variation including their longevity, responsiveness to antigen and propensity to differentiate to certain effector cell fates (Table I) (51–56), thus ultimately, affecting the dynamics of the recall response.
Table I.
Summary of the functional and phenotypic heterogeneity evident of mouse memory B cells. Because memory B cells are quiescent, their characteristics are juxtaposed with those of naïve B cells. The PD-L2 and CD80 nomenclature is a new addition to the memory B cell cannon and not all parameters have been determined yet.
| naïve | Heterogeneity in B cell memory | ||||||
|---|---|---|---|---|---|---|---|
| Memory B cells | Switched or Unswitched Memory B Cells | ||||||
| IgM | IgM+ | IgG+ | IgA+ | LLPC | PD-L2loCD80lo | PD-L2hiCD80hi | |
| developmental route | n.d. | pre- & post-GC | pre- & post-GC | post-GC | post-GC | not known | not known |
| tissue tropism | recirculating | recirculating | recirculating | Mucosal | bone marrow and mucosal | not known | not known |
| S0HM | none | low or high | low or high | high | low or high | low | high |
| life span | brief | indefinite | decays | not known | indefinite | indefinite | indefinite |
| propensity for effector cell fates | germinal center ≫ plasma cell | limited by presence of high affinity antibody | plasma cell ≫ germinal center | plasma cell ≫ germinal center | Inert | germinal center ≫ plasma cell | plasma cell ≫ germinal center |
| repertoire | broad/low affinity | archival? | broad/low affinity | unknown | narrow/high affinity | broad/low affinity | not known |
| CD19 expression | high | high | high | high | low/absent | high | high |
| CD20 expression | high | high | high | high | low/absent | high | high |
| BAFFR expression | high | high | high | high | high | high | high |
| BCMA expression | low/absent | low/absent | low/absent | low/absent | high | low/absent | low/absent |
| Potential therapeutics | Alemtuzumab Rituximab |
Belimumab Atacicept Rituximab |
Belimumab Atacicept Rituximab |
Belimumab Atacicept Rituximab |
Bortezomib Atacicept IVIG Plasmaphoresis |
Belimumab Atacicept Rituximab |
Belimumab Atacicept Rituximab |
Current research in this area is intense and focused on how phenotypic and functional heterogeneity in memory B cells is generated and the extent by which they influence the recall response – particularly in pathologic conditions. Furthermore, determining whether these concepts are manifest in humans is an important goal of current human immunologic research. In the context of transplantation, variation of the types of memory B cells in a sensitized patient could determine the kinetics and/or magnitude of de novo DSA upon successive transplantation. Alternatively, the variation in the type of memory B cells generated may contribute to the dynamics of chronic rejection.
iv. Methods of measuring memory B cells in murine models of allograft rejection
To understand the behavior of memory B cells in allograft rejection, it is critical that such cells be identified both in vitro and in vivo. A number of laboratories, including ours, have used fluorescence-based flow cytometry that employs MHC-tetramer technology to detect allospecific B cells with excellent specificity in mouse models of experimental transplantation (57, 58). This approach enables the fate of rare allospecific B cells to be tracked following MHC-incompatible transplantation. In fact, combining this technology with phenotypic and functional analyses using ELISPOT assays (59) allows the determination of the frequency of MHC class I Kd - specific cells that differentiate into plasma cells, GC B cells, and memory B cells following transplantation. These technologies poise us, at least in this experimental setting, to address the role of memory B cells in graft rejection in sensitized mice as well as the fate of allospecific B cells following tolerance induction. Indeed, in a model of chronic rejection, MHC-tetramer tracking technology was used to reveal that the expansion of allospecific B cells coincided with the detection of DSA and graft pathology (57). Overall, these cutting edge approaches promise to be useful in identifying parameters of allospecific memory B cell generation, as well as a means with which to identify approaches to modulate their activity.
Memory B cells in human transplantation
Understanding the behavior of memory B cells in human transplantation is just beginning, as new assays to identify these cells in the peripheral blood of transplant recipients are being developed (Figure 2). Here we discuss some of these in vitro or ex vivo assays that have been used to quantify memory antigen-specific B cells.
Figure 2.
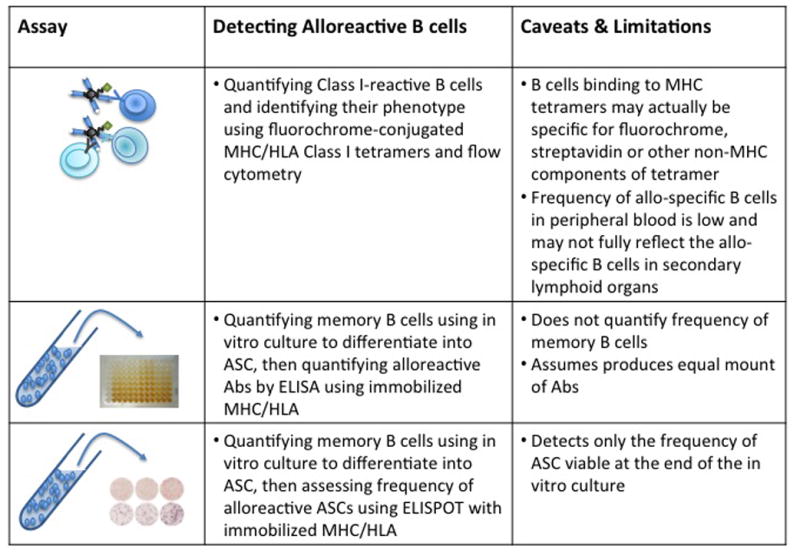
Ex vivo assays for detecting alloreactive B cells or alloantibody secreting cells (ASC).
i. Memory Donor-HLA specific B cells: quantification by flow cytometry
The ability to assess the function of memory alloreactive B cells in transplant patients requires an assay to detect these cells. The identification of alloreactive B cells from peripheral blood was initially reported by Zachary et al. (60, 61) using HLA tetramers (tet), which are streptavidin-biotin complexes of four peptide-loaded HLA molecules conjugated to a fluorescent protein. The tetramer-binding B cells were then enumerated by flow cytometry to allow for a rapid and sensitive quantitation of a patient’s B cell response to the given MHC antigen. Additionally, the CD27 and CD38 markers were used to define the tet+memory B cells and plasma cells, respectively. Contrary to expectation, they did not observe a significant correlation between the frequencies of memory CD27+tet+ (33%–44% vs. 34%–36%) B cells or CD38+tet+ (57%–65% vs. 59%–66%) plasma cells and the corresponding HLA mismatch for a previous transplant. However they observed an increased frequency of CD27+ cells among the tet+ B cells compared to overall CD19+ cells, and an increased frequency of donor-specific HLA-tetramer binding B cells among the patients who were DSA-negative at time of transplantation but who went on to develop de novo antibodies post-transplantation. The latter observations suggested the presence of memory B cells in sensitized patients, and their relative resistance to conventional immunosuppression upon retransplantation.
Despite these notable observations, there are a number of caveats. Firstly, it is now clear that there exists a significant frequency of B cells that recognize the non-HLA portions of the tetramer, such as the streptavidin-biotin and fluorochrome itself (54, 56). Thus additional controls are necessary to isolate the B cells binding to HLA away from those binding to the streptavidin-biotin-fluorochrome complex (57, 58). The elimination of these non-HLA-specific B cells, while reducing the total number of detected tetramer-binding B cells, may result in a more robust correlation between prior sensitization to alloantigen and the number of memory and or CD38+ allo-specific B cells. Secondly, while this approach affords excellent resolution of the memory B cell type(s) associated with pathological anti-graft responses, it suffers from the very low frequency of these cells in the peripheral blood. Thus approaches that incorporate the enrichment of tetramer-binding B cells or more sensitive functional readouts would increase the precision for detecting low frequency alloreactive memory B cells and make their quantification more robust.
ii. Memory Donor-HLA specific B cells: functional quantification
Memory alloreactive B cells can also be indirectly quantified by the in vitro culture of B cells from peripheral blood mononuclear cells (PBMCs) to induce the differentiation into antibody secreting cells, and then measuring the amount of alloreactive IgG in the culture supernatant or quantifying the number of IgG-secreting cells in an ELISPOT assay using immobilized HLA monomers as capture (62) (63, 64). A number of anti-CD40-based culture conditions have been described, which frequently include a cocktail of cytokines such as IL-2, IL-10 and IL-21, and a TLR-9 ligand, CpG, (65) (62, 64) (63). The culture supernatants tend to be more sensitive as it allows for the secreted IgG to accumulate in the supernatant over the entire culture period, and the assessment of IgG specificity can use the same HLA-beads used to assess DSA in the serum of transplant recipients. In contrast, the ELISPOT assay measures only the total number of HLA-specific IgG-secreting cells surviving at the end of the culture period, usually 5–14 days, but it can provide an estimate of the frequency of memory B cells that differentiated into antibody-secreting cells (ASCs) in vitro.
Han et al. (64) investigated the presence of memory B cells in 13 of 16 allograft recipients, 8 of 12 transfusion-sensitized patients, 3 of 3 multiparous women with serum HLA antibodies. Using in vitro culture to differentiate memory B cells into plasma cells and then assessing for the presence of HLA-specific antibody in the culture supernatants with single HLA-antigen beads, they observed that HLA antibody-producing B cells were detected in sensitized individuals, but none in the non-sensitized controls. Furthermore, in 13 of 16 allograft recipients, IgG antibodies against mismatched donor HLA antigens were observed. Interestingly, DSA were sometimes produced in B-cell cultures when serum reactions were negative. Thus, of a total of 50 antibody specificities detected, 35 of them were found in both the serum and the B-cell cultures, 11 only in the serum, and 4 only in the supernatants of B-cell cultures. By separating the B cells into CD27+ and CD27− subsets prior to in vitro culture, they showed that the donor-specific IgG were produced by the CD27+ memory B cell subset. Collectively their study demonstrated that an expanded population of HLA-specific memory B cells can be detected in sensitized recipients, and the discordance between the specificity of circulating antibody and the antibodies generated in vitro suggests a potential divergence in the repertoire of plasma cells and quiescent memory B cells. Nevertheless, it is possible that these differences may reflect limitations in the in vitro memory B cell assay, and thus there is an urgent need for sensitive and specific assays to quantify memory alloreactive B cells and to assess their roles in defining the outcome of allografts in the clinic.
While HLA-specific IgG titers in the culture supernatant can be extrapolated to the total numbers of memory B cells, this quantification is indirect and makes the assumption that all plasma cells secrete a constant about of IgG. To quantify the frequency of memory B cells, Heidt et al. (62) used the same in vitro culture system but used the ELISPOT assay to quantify the frequency of ASC secreting antibodies specific for donor HLA at the end of the 7-day culture. In a study of 11 individuals that had been HLA-immunized by previous pregnancies, 8 kidney transplant patients who were on the waiting list for a retransplant after rejection of a previous transplant, and 14 control blood donors. HLA-immunized individuals sensitized by pregnancies had frequencies of HLA-specific 0–182 ASC per million CD19+ cells recovered from the in vitro culture, and transplant recipients who had rejected their grafts had similar frequencies of 1–143 HLA-specific ASC per CD19+ million. In contrast, non-immunized individuals had none. The authors reasoned that the relatively low frequencies of ASCs detected could be explained by the low Luminex MFI values of the circulating antibodies. It is also possible that the low frequencies of alloreactive memory B cells in the study reflected limitations in the sensitivity of assay or the low frequency of memory B cells in the peripheral blood compartment, which may not necessarily reflect frequencies in secondary lymphoid organs. Also, only IgG-secreting cells were quantified, and it remains unclear whether the majority of alloreactive memory B cells in sensitized individuals persist as IgG+ or IgM+ memory B cells, as has been suggested by recent studies in mice (51, 52). Thus further experimentation is required to improve on the quantification these memory alloreactive B cells so that their sensitivity to current immunosuppression can be assessed, as well as their activation requirements compared to those of pathogen-specific memory B cells.
iii. Pathogen-specific memory B cells
While much of the focus in transplantation has been on the pathogenic effects of memory B cells, it is important to be mindful that pathogen-specific memory B cells are critical for the long-term health of the recipients. Thus, an ideal immunosuppressive regimen would be one that results in memory alloreactive B cells being inhibited, while preserving the function of pathogen-specific memory B cells. The study of Turner et al. (66) support such a possibility, in which they reported that sirolomus enhanced the magnitude and quality of the viral-specific but inhibited graft-reactive CD8+ T cell responses. Whether specific types pharmacological suppression can have differential effects on pathogen-specific versus allo-specific memory B cells is currently not known but is an important area of investigation. Indeed, recent technological breakthroughs are now allowing memory B cells specific for pathogens and HLA alloantigen to be assessed in the clinic; notwithstanding the caveat that arises from peripheral blood sampling that may not be adequately reflective of the tissue-resident anti-graft response.
Influenza infection is major cause of morbidity in transplant recipients, and current guidelines call for annual influenza vaccination of all transplant recipients from 3 months post-transplantation. Notably, Wrammert et al. (67) reported that vaccination of healthy human subjects with an annual trivalent influenza vaccine resulted in a rapid and robust influenza-specific IgG+ ASC response that peaked at approximately day 7 and that the ASCs were characterized by a highly restricted BCR repertoire. Sequencing of the immunoglobulin variable regions isolated from sorted single ASCs producing high affinity influenza-specific antibodies confirmed that the majority of the ASC response arose from memory B cells. Cowan et al. (68) built on these observations to quantify the influenza-specific ASC response in stable renal transplant recipients. They observed that the early influenza-specific ASCs response to influenza vaccination was significantly reduced compared to healthy controls. Similar reductions were observed in the seroresponse and rates of seroconversion. Their study therefore extended previous observations of blunted serological responses in transplant patients by demonstrating that the rapid differentiation from influenza-specific memory B cells into ASC is significantly inhibited by immunosuppression in stable renal transplant recipients. Whether this is the case for live influenza infection, which elicits a distinct immune response compared to the inactivated influenza vaccine (69), is a critical question that requires further assessment.
Summary
New technologies are allowing antigen experienced B cells generated in vivo to be tracked and enumerated in mice, and are providing insights into the heterogeneity of plasma cells as well as memory B cells in mice, and into how each memory B cell subset is generated and functions during a recall response. Understanding the basic biology of these cells will allow for the rationale use of therapeutics (Table 1); for instance, anti-CD20 (Rituximab) should successfully curtail memory and naïve B cell responses, targeting the B cell survival factor with anti-BAFF/BLyS (anti-BAFF; belimumab) should reduce the mature B cell pool as well GC B cell responses while the combined inhibition of BAFF and APRIL with TACI-Ig; (atacicept) should additionally deplete plasma cells (70), and the depletion of short-lived ASC can be achieved with the small molecule inhibitor of proteasome, bortezomib (Velcade), which promotes plasma cell apoptosis and inhibits cell proliferation (71). Finally, comparable methodologies are being used to quantify LLPC/ASCs and memory B cells specific for alloantigens or pathogens in transplant patients. These assays will allow clinicians to quantify the efficacy of immunosuppression targeting each B cell or plasma cell subset, and whether these drugs can be titrated to suppress alloreactive B cells while preserving memory B cells involved in protective immunity.
Acknowledgments
This work was supported in part by a grant (1R01AI110513-01) from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health.
Abbreviations
- ASC
antibody secreting cells
- BCR
B cell receptor
- DSA
donor specific antibody
- GC
germinal center
- LLPC
long-lived plasma cells
- Tet
tetramer
Footnotes
The authors declare no conflicts of interest
References
- 1.Reed EF, Rao P, Zhang Z, Gebel H, Bray RA, Guleria I, et al. Comprehensive assessment and standardization of solid phase multiplex-bead arrays for the detection of antibodies to HLA-drilling down on key sources of variation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(11):3050–1. doi: 10.1111/ajt.12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loupy A, Jordan SC. Transplantation: Donor-specific HLA antibodies and renal allograft failure. Nature reviews Nephrology. 2013;9(3):130–1. doi: 10.1038/nrneph.2013.18. [DOI] [PubMed] [Google Scholar]
- 3.Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. The New England journal of medicine. 2013;369(13):1215–26. doi: 10.1056/NEJMoa1302506. [DOI] [PubMed] [Google Scholar]
- 4.Mohan S, Palanisamy A, Tsapepas D, Tanriover B, Crew RJ, Dube G, et al. Donor-specific antibodies adversely affect kidney allograft outcomes. Journal of the American Society of Nephrology: JASN. 2012;23(12):2061–71. doi: 10.1681/ASN.2012070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns AM, Chong AS. Alloantibodies prevent the induction of transplantation tolerance by enhancing alloreactive T cell priming. Journal of immunology. 2011;186(1):214–21. doi: 10.4049/jimmunol.1001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns AM, Ma L, Li Y, Yin D, Shen J, Xu J, et al. Memory alloreactive B cells and alloantibodies prevent anti-CD154-mediated allograft acceptance. Journal of immunology. 2009;182(3):1314–24. doi: 10.4049/jimmunol.182.3.1314. [DOI] [PubMed] [Google Scholar]
- 7.Carroll MC, Isenman DE. Regulation of humoral immunity by complement. Immunity. 2012;37(2):199–207. doi: 10.1016/j.immuni.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nimmerjahn F, Ravetch JV. Antibody-mediated modulation of immune responses. Immunological reviews. 2010;236:265–75. doi: 10.1111/j.1600-065X.2010.00910.x. [DOI] [PubMed] [Google Scholar]
- 9.Wasowska BA, Lee CY, Halushka MK, Baldwin WM., 3rd New concepts of complement in allorecognition and graft rejection. Cellular immunology. 2007;248(1):18–30. doi: 10.1016/j.cellimm.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stegall MD, Moore N, Taner T, Li H, Dean PG. Down-regulating humoral immune responses: implications for organ transplantation. Transplantation. 2014;97(3):247–57. doi: 10.1097/TP.0b013e3182a72115. [DOI] [PubMed] [Google Scholar]
- 11.Vincent FB, Saulep-Easton D, Figgett WA, Fairfax KA, Mackay F. The BAFF/APRIL system: emerging functions beyond B cell biology and autoimmunity. Cytokine & growth factor reviews. 2013;24(3):203–15. doi: 10.1016/j.cytogfr.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goodnow CC, Vinuesa CG, Randall KL, Mackay F, Brink R. Control systems and decision making for antibody production. Nature immunology. 2010;11(8):681–8. doi: 10.1038/ni.1900. [DOI] [PubMed] [Google Scholar]
- 13.Sciammas R, Davis MM. Blimp-1; immunoglobulin secretion and the switch to plasma cells. Curr Top Microbiol Immunol. 2005;290:201–24. doi: 10.1007/3-540-26363-2_9. [DOI] [PubMed] [Google Scholar]
- 14.Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005;5(3):230–42. doi: 10.1038/nri1572. [DOI] [PubMed] [Google Scholar]
- 15.Tarlinton DM, Hodgkin PD. Targeting plasma cells in autoimmune diseases. The Journal of experimental medicine. 2004;199(11):1451–4. doi: 10.1084/jem.20040719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen CD, Okada T, Cyster JG. Germinal-center organization and cellular dynamics. Immunity. 2007;27(2):190–202. doi: 10.1016/j.immuni.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8(1):22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 18.Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. 2012;30:429–57. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- 19.Good-Jacobson KL, Shlomchik MJ. Plasticity and heterogeneity in the generation of memory B cells and long-lived plasma cells: the influence of germinal center interactions and dynamics. Journal of immunology. 2010;185(6):3117–25. doi: 10.4049/jimmunol.1001155. [DOI] [PubMed] [Google Scholar]
- 20.Good-Jacobson KL, Tarlinton DM. Multiple routes to B-cell memory. Int Immunol. 2012;24(7):403–8. doi: 10.1093/intimm/dxs050. [DOI] [PubMed] [Google Scholar]
- 21.Tarlinton D, Good-Jacobson K. Diversity among memory B cells: origin, consequences, and utility. Science. 2013;341(6151):1205–11. doi: 10.1126/science.1241146. [DOI] [PubMed] [Google Scholar]
- 22.Slifka MK, Ahmed R. Long-lived plasma cells: a mechanism for maintaining persistent antibody production. Curr Opin Immunol. 1998;10(3):252–8. doi: 10.1016/s0952-7915(98)80162-3. [DOI] [PubMed] [Google Scholar]
- 23.Cunningham AF, Gaspal F, Serre K, Mohr E, Henderson IR, Scott-Tucker A, et al. Salmonella induces a switched antibody response without germinal centers that impedes the extracellular spread of infection. Journal of immunology. 2007;178(10):6200–7. doi: 10.4049/jimmunol.178.10.6200. [DOI] [PubMed] [Google Scholar]
- 24.Smith KG, Hewitson TD, Nossal GJ, Tarlinton DM. The phenotype and fate of the antibody-forming cells of the splenic foci. Eur J Immunol. 1996;26(2):444–8. doi: 10.1002/eji.1830260226. [DOI] [PubMed] [Google Scholar]
- 25.Xiang Z, Cutler AJ, Brownlie RJ, Fairfax K, Lawlor KE, Severinson E, et al. FcgammaRIIb controls bone marrow plasma cell persistence and apoptosis. Nature immunology. 2007;8(4):419–29. doi: 10.1038/ni1440. [DOI] [PubMed] [Google Scholar]
- 26.Chu VT, Beller A, Rausch S, Strandmark J, Zanker M, Arbach O, et al. Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis. Immunity. 2014;40(4):582–93. doi: 10.1016/j.immuni.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 27.Chu VT, Frohlich A, Steinhauser G, Scheel T, Roch T, Fillatreau S, et al. Eosinophils are required for the maintenance of plasma cells in the bone marrow. Nature immunology. 2011;12(2):151–9. doi: 10.1038/ni.1981. [DOI] [PubMed] [Google Scholar]
- 28.Denzel A, Maus UA, Rodriguez Gomez M, Moll C, Niedermeier M, Winter C, et al. Basophils enhance immunological memory responses. Nature immunology. 2008;9(7):733–42. doi: 10.1038/ni.1621. [DOI] [PubMed] [Google Scholar]
- 29.Valujskikh A, Bromberg JS. The house that Jack built: expanding the concept of plasma cell niches. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(3):533. doi: 10.1111/ajt.12202. [DOI] [PubMed] [Google Scholar]
- 30.Winter O, Moser K, Mohr E, Zotos D, Kaminski H, Szyska M, et al. Megakaryocytes constitute a functional component of a plasma cell niche in the bone marrow. Blood. 2010;116(11):1867–75. doi: 10.1182/blood-2009-12-259457. [DOI] [PubMed] [Google Scholar]
- 31.Pelletier N, McHeyzer-Williams LJ, Wong KA, Urich E, Fazilleau N, McHeyzer-Williams MG. Plasma cells negatively regulate the follicular helper T cell program. Nature immunology. 2010;11(12):1110–8. doi: 10.1038/ni.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Purtha WE, Tedder TF, Johnson S, Bhattacharya D, Diamond MS. Memory B cells, but not long-lived plasma cells, possess antigen specificities for viral escape mutants. The Journal of experimental medicine. 2011;208(13):2599–606. doi: 10.1084/jem.20110740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith KG, Light A, Nossal GJ, Tarlinton DM. The extent of affinity maturation differs between the memory and antibody-forming cell compartments in the primary immune response. Embo J. 1997;16(11):2996–3006. doi: 10.1093/emboj/16.11.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takemori T, Kaji T, Takahashi Y, Shimoda M, Rajewsky K. Generation of memory B cells inside and outside germinal centers. Eur J Immunol. 2014;44(5):1258–64. doi: 10.1002/eji.201343716. [DOI] [PubMed] [Google Scholar]
- 35.Taylor JJ, Jenkins MK, Pape KA. Heterogeneity in the differentiation and function of memory B cells. Trends Immunol. 2012;33(12):590–7. doi: 10.1016/j.it.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Constant S, Sant’Angelo D, Pasqualini T, Taylor T, Levin D, Flavell R, et al. Peptide and protein antigens require distinct antigen-presenting cell subsets for the priming of CD4+ T cells. Journal of immunology. 1995;154(10):4915–23. [PubMed] [Google Scholar]
- 37.Constant S, Schweitzer N, West J, Ranney P, Bottomly K. B lymphocytes can be competent antigen-presenting cells for priming CD4+ T cells to protein antigens in vivo. Journal of immunology. 1995;155(8):3734–41. [PubMed] [Google Scholar]
- 38.Batista FD, Neuberger MS. Affinity dependence of the B cell response to antigen: a threshold, a ceiling, and the importance of off-rate. Immunity. 1998;8(6):751–9. doi: 10.1016/s1074-7613(00)80580-4. [DOI] [PubMed] [Google Scholar]
- 39.Dintzis HM, Dintzis RZ, Vogelstein B. Molecular determinants of immunogenicity: the immunon model of immune response. Proc Natl Acad Sci U S A. 1976;73(10):3671–5. doi: 10.1073/pnas.73.10.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kouskoff V, Famiglietti S, Lacaud G, Lang P, Rider JE, Kay BK, et al. Antigens varying in affinity for the B cell receptor induce differential B lymphocyte responses. The Journal of experimental medicine. 1998;188(8):1453–64. doi: 10.1084/jem.188.8.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mongini PK, Blessinger CA, Dalton JP. Affinity requirements for induction of sequential phases of human B cell activation by membrane IgM-cross-linking ligands. Journal of immunology. 1991;146(6):1791–800. [PubMed] [Google Scholar]
- 42.Myers CD, Kriz MK, Sullivan TJ, Vitetta ES. Antigen-induced changes in phospholipid metabolism in antigen-binding B lymphocytes. Journal of immunology. 1987;138(6):1705–11. [PubMed] [Google Scholar]
- 43.Kometani K, Nakagawa R, Shinnakasu R, Kaji T, Rybouchkin A, Moriyama S, et al. Repression of the transcription factor Bach2 contributes to predisposition of IgG1 memory B cells toward plasma cell differentiation. Immunity. 2013;39(1):136–47. doi: 10.1016/j.immuni.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 44.Horikawa K, Martin SW, Pogue SL, Silver K, Peng K, Takatsu K, et al. Enhancement and suppression of signaling by the conserved tail of IgG memory-type B cell antigen receptors. The Journal of experimental medicine. 2007;204(4):759–69. doi: 10.1084/jem.20061923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin SW, Goodnow CC. Burst-enhancing role of the IgG membrane tail as a molecular determinant of memory. Nature immunology. 2002;3(2):182–8. doi: 10.1038/ni752. [DOI] [PubMed] [Google Scholar]
- 46.Coffman RL, Lebman DA, Rothman P. Mechanism and regulation of immunoglobulin isotype switching. Adv Immunol. 1993;54:229–70. doi: 10.1016/s0065-2776(08)60536-2. [DOI] [PubMed] [Google Scholar]
- 47.Radic M. Tracking and trapping somatic mutations in Ig genes. Journal of immunology. 2008;180(9):5763–4. doi: 10.4049/jimmunol.180.9.5763. [DOI] [PubMed] [Google Scholar]
- 48.Tarlinton DM. Evolution in miniature: selection, survival and distribution of antigen reactive cells in the germinal centre. Immunol Cell Biol. 2008;86(2):133–8. doi: 10.1038/sj.icb.7100148. [DOI] [PubMed] [Google Scholar]
- 49.Smith KG, Light A, O’Reilly LA, Ang SM, Strasser A, Tarlinton D. bcl-2 transgene expression inhibits apoptosis in the germinal center and reveals differences in the selection of memory B cells and bone marrow antibody-forming cells. The Journal of experimental medicine. 2000;191(3):475–84. doi: 10.1084/jem.191.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vikstrom I, Carotta S, Luthje K, Peperzak V, Jost PJ, Glaser S, et al. Mcl-1 is essential for germinal center formation and B cell memory. Science. 2010;330(6007):1095–9. doi: 10.1126/science.1191793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Anderson SM, Tomayko MM, Ahuja A, Haberman AM, Shlomchik MJ. New markers for murine memory B cells that define mutated and unmutated subsets. The Journal of experimental medicine. 2007;204(9):2103–14. doi: 10.1084/jem.20062571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dogan I, Bertocci B, Vilmont V, Delbos F, Megret J, Storck S, et al. Multiple layers of B cell memory with different effector functions. Nature immunology. 2009;10(12):1292–9. doi: 10.1038/ni.1814. [DOI] [PubMed] [Google Scholar]
- 53.Kaji T, Ishige A, Hikida M, Taka J, Hijikata A, Kubo M, et al. Distinct cellular pathways select germline-encoded and somatically mutated antibodies into immunological memory. The Journal of experimental medicine. 2012;209(11):2079–97. doi: 10.1084/jem.20120127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science. 2011;331(6021):1203–7. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zuccarino-Catania GV, Sadanand S, Weisel FJ, Tomayko MM, Meng H, Kleinstein SH, et al. CD80 and PD-L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nature immunology. 2014;15(7):631–7. doi: 10.1038/ni.2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor JJ, Pape KA, Jenkins MK. A germinal center-independent pathway generates unswitched memory B cells early in the primary response. The Journal of experimental medicine. 2012;209(3):597–606. doi: 10.1084/jem.20111696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kwun J, Oh BC, Gibby AC, Ruhil R, Lu VT, Kim DW, et al. Patterns of de novo allo B cells and antibody formation in chronic cardiac allograft rejection after alemtuzumab treatment. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(10):2641–51. doi: 10.1111/j.1600-6143.2012.04181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen J, Yin H, Xu J, Wang Q, Edelblum KL, Sciammas R, et al. Reversing endogenous alloreactive B cell GC responses with anti-CD154 or CTLA-4Ig. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(9):2280–92. doi: 10.1111/ajt.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sicard A, Phares TW, Yu H, Fan R, Baldwin WM, 3rd, Fairchild RL, et al. The spleen is the major source of antidonor antibody-secreting cells in murine heart allograft recipients. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(7):1708–19. doi: 10.1111/j.1600-6143.2012.04009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zachary AA, Kopchaliiska D, Montgomery RA, Leffell MS. HLA-specific B cells: I. A method for their detection, quantification, and isolation using HLA tetramers. Transplantation. 2007;83(7):982–8. doi: 10.1097/01.tp.0000259017.32857.99. [DOI] [PubMed] [Google Scholar]
- 61.Zachary AA, Kopchaliiska D, Montgomery RA, Melancon JK, Leffell MS. HLA-specific B cells: II. Application to transplantation. Transplantation. 2007;83(7):989–94. doi: 10.1097/01.tp.0000259019.68244.d7. [DOI] [PubMed] [Google Scholar]
- 62.Heidt S, Roelen DL, de Vaal YJ, Kester MG, Eijsink C, Thomas S, et al. A NOVel ELISPOT assay to quantify HLA-specific B cells in HLA-immunized individuals. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2012;12(6):1469–78. doi: 10.1111/j.1600-6143.2011.03982.x. [DOI] [PubMed] [Google Scholar]
- 63.Lynch RJ, Silva IA, Chen BJ, Punch JD, Cascalho M, Platt JL. Cryptic B cell response to renal transplantation. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2013;13(7):1713–23. doi: 10.1111/ajt.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Han M, Rogers JA, Lavingia B, Stastny P. Peripheral blood B cells producing donor-specific HLA antibodies in vitro. Human immunology. 2009;70(1):29–34. doi: 10.1016/j.humimm.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 65.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. Journal of immunological methods. 2004;286(1–2):111–22. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 66.Turner AP, Shaffer VO, Araki K, Martens C, Turner PL, Gangappa S, et al. Sirolimus enhances the magnitude and quality of viral-specific CD8+ T-cell responses to vaccinia virus vaccination in rhesus macaques. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11(3):613–8. doi: 10.1111/j.1600-6143.2010.03407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wrammert J, Smith K, Miller J, Langley WA, Kokko K, Larsen C, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus. Nature. 2008;453(7195):667–71. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cowan M, Chon WJ, Desai A, Andrews S, Bai Y, Veguilla V, et al. Impact of immunosuppression on recall immune responses to influenza vaccination in stable renal transplant recipients. Transplantation. 2014;97(8):846–53. doi: 10.1097/01.TP.0000438024.10375.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakaya HI, Wrammert J, Lee EK, Racioppi L, Marie-Kunze S, Haining WN, et al. Systems biology of vaccination for seasonal influenza in humans. Nature immunology. 2011;12(8):786–95. doi: 10.1038/ni.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stohl W, Hilbert DM. The discovery and development of belimumab: the anti-BLyS-lupus connection. Nature biotechnology. 2012;30(1):69–77. doi: 10.1038/nbt.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ejaz NS, Alloway RR, Halleck F, Durr M, Budde K, Woodle ES. Review of Bortezomib Treatment of Antibody-Mediated Rejection in Renal Transplantation. Antioxidants & redox signaling. 2014 doi: 10.1089/ars.2014.5892. [DOI] [PubMed] [Google Scholar]


