Abstract
Background
Inexpensive, non-invasive tools for assessing Alzheimer-type pathophysiologies are needed. Computerized cognitive assessments are prime candidates.
Methods
Cognitively normal participants, aged 51-71, with MRI, FDG-PET, amyloid PET, CogState computerized cognitive assessment, and standard neuropsychological tests were included. We first examined the association between the CogState battery and neuroimaging measures. We then compared that association to the one between standard neuropsychological z-scores and neuroimaging.
Results
Slower reaction times for CogState Identification and One Back, and lower memory and attention z-scores, were associated (P<.05) with FDG-PET hypometabolism. Slower time on the Groton Maze Learning Task and worse One Card Learning accuracy were associated (P<.05) with smaller hippocampal volumes. There were no associations with amyloid PET. Associations of CogState and neuropsychological z-scores with neuroimaging were small and of a similar magnitude.
Conclusions
CogState subtests were cross-sectionally comparable to standard neuropsychological tests in their relatively weak associations with neurodegeneration imaging markers.
Keywords: Preclinical Alzheimer's disease, Neuropsychology, Computerized cognitive battery, Neuroimaging, Amyloid-beta, Hippocampal volume
1. Introduction
Evidence of amyloid (cerebrospinal fluid (CSF) amyloid-beta or amyloid imaging) and neurodegeneration (CSF tau, hippocampal volume, or FDG-PET hypometabolism) are the defining components of the preclinical stages of Alzheimer's disease (AD) [1]. Psychometrically evident, subtle changes in cognition are proposed to occur later in the pre-clinical phase of AD (i.e., in stage 3) and have a stronger correlation with neurodegeneration compared to amyloid. Inexpensive, non-invasive tools for identifying the early stages of the Alzheimer-type pathophysiologic process, and subtle cognitive changes, are needed. Computerized tests may have logistic and cost advantages over standard pencil-and-paper tests. The aim of the present study was to examine the cross-sectional association between the CogState computerized cognitive battery and neuroimaging measures of amyloid PET and neurodegeneration (hippocampal volume and FDG-PET) in cognitively normal individuals, aged 51-71, enrolled in the population-based Mayo Clinic Study of Aging. We then compared the cross-sectional relationship between CogState and neuroimaging to that between standard neuropsychological global- and domain-specific z-scores and neuroimaging.
2. Methods
2.1. Participants
The Mayo Clinic Study of Aging (MCSA) is a population-based study of cognitive aging among Olmsted County, MN, residents that began in October 2004, and initially enrolled individuals aged 70 to 89 years. The details of the study design and sampling procedures have been previously published [2]. Given the importance of understanding risk factors and the development and progression of AD pathophysiology in middle-age, we expanded the study to also enroll a population-based sample of individuals aged 50-69 using the same stratified random sampling methodology as in the original cohort. The Olmsted County population, aged 50-69 (n = 31,502), was sampled by 5-year age groups and sex on November 1, 2011. Of the 948 participants who enrolled in the study the first year, the present study includes 324 who were cognitively normal, completed a cognitive assessment (CogState computerized battery and standard pencil and paper battery) and also had a MRI within 5 months of the visit. Of the 324 individuals, 261 (81%) consented to additional amyloid imaging (PiB-PET) and 259 (80%) to FDG-PET. There were no demographic or cognitive differences between those who did and did not consent to PET imaging.
2.2. Standard protocol approvals, registrations, and patient consents
The study protocols were approved by the Mayo Clinic and Olmsted Medical Center Institutional Review Boards. All participants provided written informed consent to participate in the study and in the imaging protocols.
2.3. Participant assessment
Study visits included a neurologic evaluation by a physician, an interview by a study coordinator, and neuropsychological testing administered by a psychometrist [2]. The physician examination included a medical history review, a complete neurological examination, and administration of the Short Test of Mental Status [3] and the Unified Parkinson's Disease Rating Scale [4]. The study coordinator interview included questions about memory to both the participant and an informant using the Clinical Dementia Rating scale [5]. A psychometrist administered a neuropsychological battery that included nine tests covering four domains: 1) memory (Auditory Verbal Learning Test Delayed Recall Trial [6], Wechsler Memory Scale-Revised Logical Memory & Visual Reproduction II) [7]; 2) language (Boston Naming Test [8] and Category Fluency) [9]; 3) executive function (Trail Making Test (TMT) B [10] and WAIS-R Digit Symbol subtest) [11]; and 4) visuospatial skills (WAIS-R Picture Completion and Block Design subtests) [11].
2.3. CogState computerized battery
Several computerized batteries are available, with advantages and limitations for each. We chose to include the CogState battery in the MCSA because it is brief (20 minutes); requires minimal administrative oversight and has a web-based platform; is easy to understand, even for non-English speakers and people with little computer experience (e.g., [12-14]); has minimal practice effects after initial familiarization (e.g., [13,15,16]); does not have ceiling or floor effects; and has good test-retest reliability (e.g., [13,15,16]). However, some limitations should be noted. For example, the card tasks have relatively low face validity as they are game-like and remote from traditional neuropsychological tests [17]). Further, the four card tasks primarily load on only two factors – “learning efficiency” and “problem solving” [18,19].
The administration of the CogState computerized cognitive battery was overseen by the study coordinator and included four card tasks and the Groton Maze Learning Test (GMLT), which has previously been described in detail [13,18,20]. The four card tasks consisted of the following tests (in this order):
Detection (DET) task – a simple reaction time paradigm that measures psychomotor speed. Reaction time was the primary outcome measure.
Identification (IDN) task – a choice reaction time paradigm that measures visual attention. Reaction time was the primary outcome measure.
One Card Learning (OCL) task – a continuous visual recognition learning task that assesses memory and attention. Reaction time and accuracy were the primary outcome measures.
One Back (ONB) task– a task that assesses working memory and attention. Reaction time and accuracy were the primary outcome measures.
The GMLT was given after the four card tasks and is a hidden pathway maze learning test that measures problem solving, reasoning, recent memory, and executive function. The primary outcome measures were the number of moves per second.
Criterion and construct validity for these tests have been reported [14]. For example, performance on the Detect task correlated highly with the Grooved Pegboard Dominant Hand (r = .81, P <.001) and TMT Part A (r = .70, P < .001). Performance on the Identification task correlated highly with the Grooved Pegboard Dominant Hang (r = 0.53, P < .01), TMT Parts A (r = .76, P < .001) and B (r = .78, P<.001), and Symbol Digit Modality Test (r = .81, P <.01). The One Back task correlated highly with the TMT Parts A (r = .69, P <.01) and B (r = .71, P <.01), Symbol Digit Modality Test (r = .81, P <.01), Spatial Span Subtest [21] (r = .80, P <.01) and Benton Visual Memory Test (BVMT) (r = .54, P <.01). The One Card Learning test most highly correlated with the Spatial Span Subtest (r = .69, P <.01), BVMT (r = .83, P <.01), and Rey Complex Figure Test – Delayed Recall (r = .79, P <.01).
2.5. Diagnostic Determination
The performance of a person in a particular cognitive domain on standard neuropsychological testing (i.e., not CogState) was measured by comparing the person's domain score with the score from age- and education-adjusted scores of cognitively normal individuals previously obtained using Mayo's Older American Normative Studies (MOANS) [22]. This approach relies on previous normative work and extensive experience with the measurement of cognitive abilities of the population from which the study participants were drawn. Subjects with scores of 1.0 SD or greater below the age-specific mean in the general population were considered for a possible cognitive impairment. However, a final decision to diagnose MCI was based on a consensus agreement between the interviewing nurse, examining physician, and neuropsychologist, taking into account education, prior occupation, visual or hearing deficits, and other information [2,23]. A diagnosis of dementia was made according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria [24]. Individuals not meeting criteria for MCI or dementia were deemed to be cognitively normal.
2.6. Amyloid PET methods
PET images were acquired using a PET/CT scanner (DRX, GE Healthcare). A CT image was obtained for attenuation correction. The 11C PIB PET scan, consisting of four 5-minute dynamic frames, was acquired from 40–60 minutes after injection. Image analysis was done using our in-house fully automated image processing pipeline, which uses MRI to guide PET region of interest (ROI) placement [25]. An amyloid PET standardized uptake value ratio (SUVR) was formed by calculating the median uptake over voxels in the prefrontal, orbitofrontal, parietal, temporal, anterior cingulate, and posterior cingulate/precuneus ROIs for each subject and dividing this meta ROI by the median uptake over voxels in the cerebellar gray matter ROI of the Automated Anatomical Labeling atlas [26].
2.7. FDG-PET methods
FDG-PET images were obtained on the same day one hour after the amyloid PET scan. FDG-PET scans were analyzed using the pipeline described above [25]. The angular gyrus, posterior cingulate, and inferior temporal cortical ROIs defined an “Alzheimer signature” meta-ROI [27] which was normalized to the pons and vermis.
2.8. Structural MRI methods
All subjects underwent MR scanning at 3T with a standardized protocol that included a 3D-MPRAGE sequence. Hippocampal volume was measured with FreeSurfer software [28,29].
2.9. Statistical methods
The present study is a cross-sectional study and can only determine associations, not demonstrate cause and effect, so it is plausible to examine either variable as the outcome. However, based on our knowledge of the underlying pathology and the disease process, we hypothesize that changes in Alzheimer pathology, as quantified by neuroimaging biomarkers (X), cause subsequent cognitive changes (Y), as measured by the computerized cognitive battery or standard neuropsychological tests. Therefore, we evaluated how well the neuroimaging measures predicted the cognitive measures. This analytic method also allowed us to better compare the associations between the CogState and neuroimaging measures and the standard neuropsychological tests and neuroimaging measures.
To characterize the relationship between imaging and CogState measures, we performed a linear regression analysis treating the CogState measure as the dependent variable, the imaging measure as the primary predictor, and including age, sex, and education as covariates. As recommended, these covariates were included in the model because they were considered potential confounders a priori and were not screened for significance. We modeled the imaging variables using a restricted cubic spline with three knots because we could not assume a linear relationship between imaging and CogState, especially for amyloid PET, which had few subjects with high levels. The knots were chosen based on the distribution of the data. The knots for FDG were at 1.6, 1.8, and 2.0; the knots for HV were 7, 8, and 9 cm3; the knots for amyloid PET were at 1.3, 1.4, and 1.5. For models with hippocampal volume, we included total intracranial volume (TIV) as a covariate.
We created scatter plots of the CogState measure vs. the imaging measure and plotted the estimated regression equation (the mean CogStage value as it varied over the imaging values) for a 60 year old male with 16 years of education. Since we did not include interactions, we made the assumption that the regression equation would be parallel, yet shifted up or down depending on the levels of the adjustment variables (e.g., assuming men vs. women or age 65 vs. 60). In each scatter plot we reported a P-value from a 2 d.f. test of a model with vs. without the imaging variable. This can be interpreted as a global test of a linear or non-linear association with CogState. Although amyloid PET was not modeled on the log scale, in the scatter plots we used a log-transformed x-axis to avoid overemphasizing the few high amyloid values. Given that our analyses were parametric in nature, we transformed the CogState measures to achieve approximate conditionally normal distributions. Reaction times tended to be skewed and were log10 transformed to make them more normal. The arcsine of the square root of the accuracy scores was analyzed to make proportions more normally distributed. These two translations are suggested by the test developers and have been used in analyses of CogState data [13,16,18,20]. However, we plotted the regression equation and confidence limits on the back-transformed scale. We did this by simulating from the fitted model [30]. We applied the same regression analysis approach for global and domain-specific z-scores and individual memory tests (based on raw neuropsychological scores rather than MOANS-adjusted scores). For this analysis, we used z-scores calculated from raw cognitive test scores. This is different from the MOANS-adjusted scores used for diagnostic purposes. As CogState scores are not age-adjusted, we chose to also use non-adjusted neuropsychological scores. A two-sided P-value < .05 was considered statistically significant.
3. Results
The characteristics of the (cognitively normal) participants are shown in Table 1. The median age (Interquartile range [IQR]) was 64 (60, 67) years, 54% were men, and 25% were APOE E4 carriers. There were no significant demographic or cognitive differences between the 324 MCSA participants with neuroimaging vs. the 622 without (data not shown). The median number of months between the cognitive tests and MRI was 2.3 months (IQR: 1.5-3.1). Of the 324 subjects with MRI, 261 (81%) had available amyloid imaging and 259 (80%) had FDG-PET.
Table 1.
Characteristics of participants with MRI and CogState measurements (n=324)
| Characteristic | Summary |
|---|---|
| Men, no. (%) | 175 (54) |
| Age, median years (IQR) | 64 (60, 67) |
| Education level, median years (IQR) | 16 (13, 17) |
| Short Test of Mental Status, median (IQR) | 36 (34, 37) |
| Auditory Verbal Learning Task (AVLT) | |
| AVLT DR, median (IQR) | 9 (7, 11) |
| AVLT sum of trials 1-5, median (IQR) | 46 (40, 52) |
| Composite z-scores, median (IQR) | |
| Global | 1.33 (0.77, 1.83) |
| Memory | 1.18 (0.63, 1.76) |
| Attention | 1.14 (0.72, 1.66) |
| Language | 0.86 (0.34, 1.44) |
| Visuospatial | 0.97 (0.35, 1.50) |
| Months from the visit with baseline CogState and standard neuropsychological tests to the MRI | |
| Median (IQR) | 2.3 (1.5, 3.1) |
| Min, Max | 0.6, 4.6 |
| Hippocampus | |
| Volume median (IQR), cm3 | 7.9 (7.3, 8.5) |
| Volume as % of TIV, median (IQR) | 0.55% (0.51%, 0.59)% |
| Amyloid PET | |
| Scan completed, n % | 261 (81) |
| Number with SUVR > 1.5, % | 19 (7) |
| Median (IQR), SUVR | 1.30 (1.25, 1.35) |
| Range, SUVR | 1.12 to 2.44 |
| FDG-PET | |
| Scan completed, n % | 259 (80) |
| Median (IQR), SUVR | 1.76 (1.66, 1.87) |
Abbreviations: MRI, magnetic resonance imaging; IQR, interquartile range; AVLT, Auditory Verbal Learning Task; AVLT DR, Auditory Verbal Learning Task delayed response; TIV, Total intracranial volume; PET, positron emission tomography; SUVR = Standard uptake value ratio; FDG-PET, fluorodeoxyglucose-positron emission tomography
3.1. Cogstate computerized tests and neuroimaging
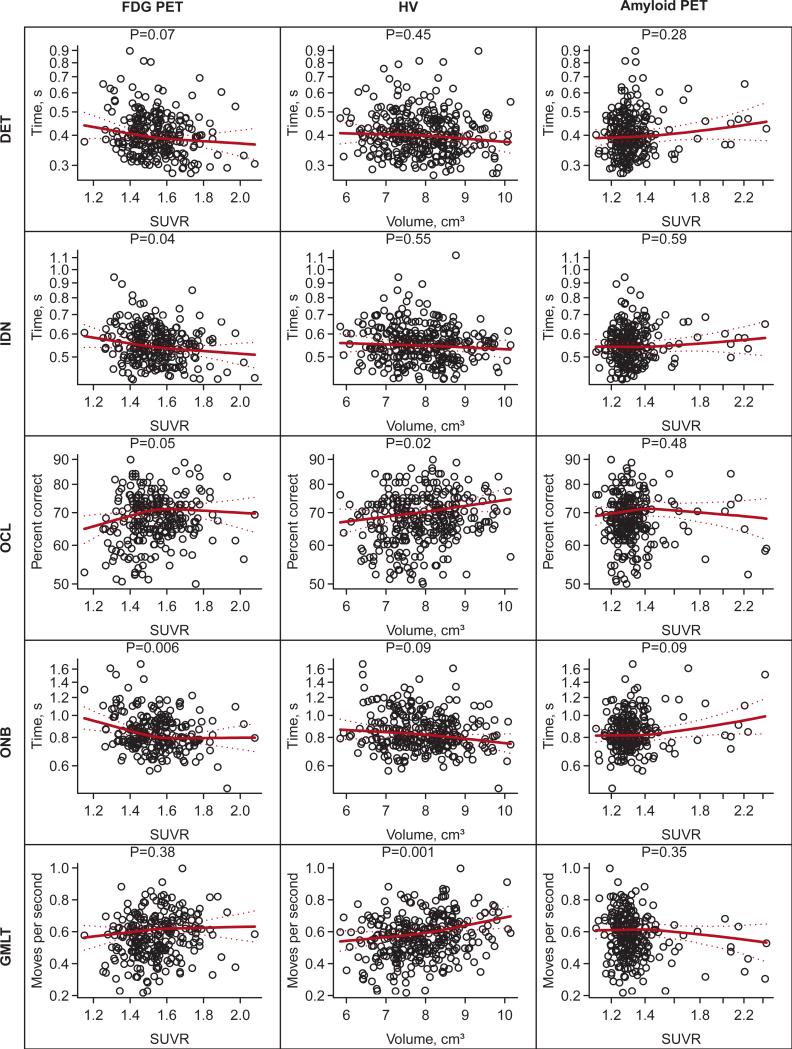
Scatter plots and regression lines summarizing the associations between each of the CogState computerized tasks and FDG-PET, HV, and amyloid PET and are shown in Fig. 1. After controlling for age, sex, and education, FDG-PET hypometabolism in the Alzheimer signature meta-ROI was associated with slower reaction times for the CogState IDN (P = .04) and ONB (P = .006) tasks. Smaller HVs were associated with fewer moves per second (slower time) on the GMLT (P = .001) and poorer accuracy on the OCL (P = .02) after controlling for age, sex, education, and TIV. There were no significant associations between any CogState test and amyloid PET. We did not find evidence of interactions between imaging measures and APOE E4 genotype in predicting CogState performance.
Fig. 1.
Scatter plots and fitted regression lines for CogState measures vs. neuroimaging measures. All regression models are adjusted for age, sex, and education. The HV models are also adjusted for total intracranial volume (TIV). The regression lines summarize the fit for a 60 year old male with 16 years of education and, for hippocampus models only, a TIV of 1.4 L. The P-values shown are based on a 2 d.f. test of the significance of the neuroimaging variable. The associated R2 values can be found in Table 2.
3.2. Global- and domain-specific Z-scores and neuroimaging
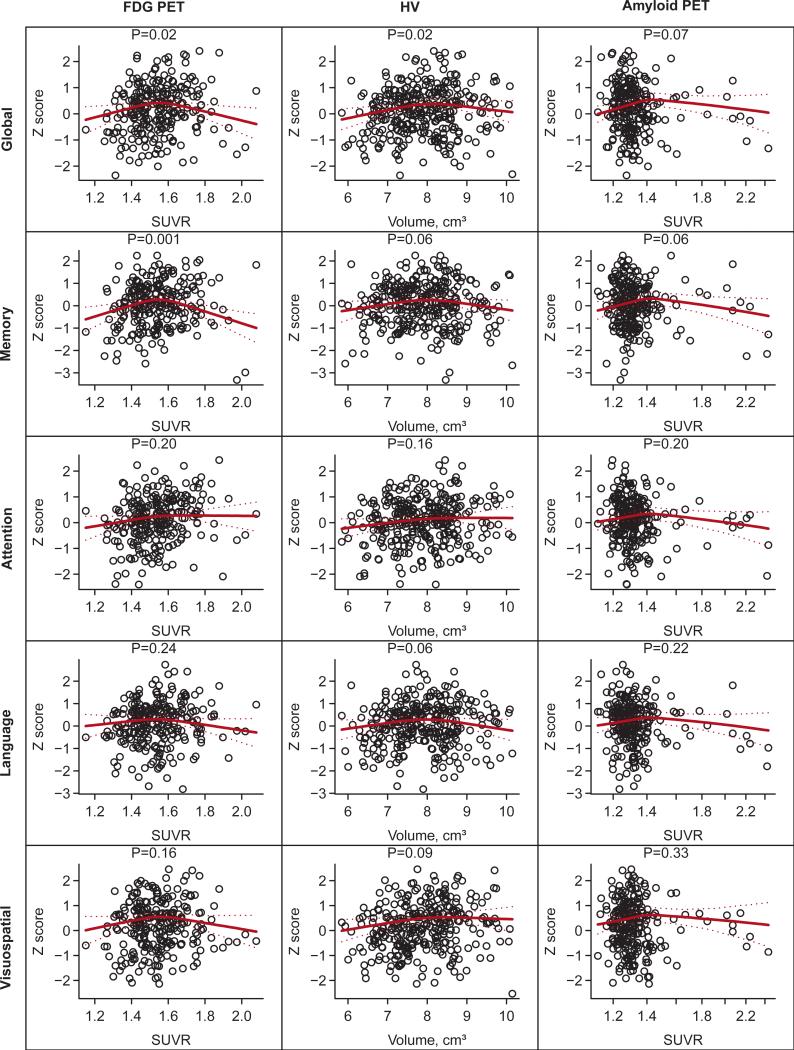
Scatter plots and regression lines summarizing the associations between global and domain-specific z-scores from standard neuropsychological tests and HV, amyloid PET, and FDG-PET are shown in Fig. 2. Both global z-scores (P = .02) and memory z-scores (P = .001) were associated with FDG-PET hypometabolism. However, the relationship was not linear but rather inverse U-shaped. Global z-scores were also associated with HV (P = .02) in the same inverse U-shaped manner. There were no significant associations between any of the z-scores and amyloid PET. We did not observe interactions between APOE E4 genotype and neuroimaging measures in predicting the cognitive z-scores.
Fig. 2.
Scatter plots and fitted regression lines for z-score measures vs. neuroimaging measures. All regression models are adjusted for age, sex, and education. The HV models are also adjusted for total intracranial volume (TIV). The regression lines summarize the fit for a 60 year old male with 16 years of education and, for hippocampus models only, a TIV of 1.4 L. The P-values shown are based on a 2 d.f. test of the significance of the neuroimaging variable. The associated R2 values can be found in Table 2.
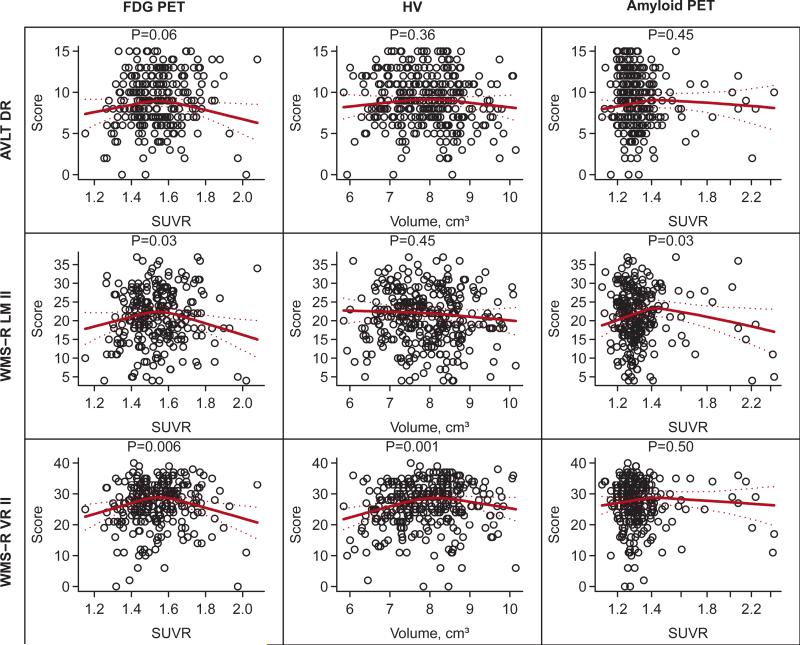
Cross-sectional studies have previously shown associations between amyloid burden and memory performance, and have suggested that some memory tests may be more sensitive than others. Therefore, we further examined the individual tests comprising the memory domain (Fig. 3). The AVLT delayed recall was not associated with any neuroimaging measures. The WMS-R logical memory II had an inverse U- shaped association with FDG-PET (P = .03) and amyloid PET (P = .03). The WMS-R visual reproduction II also had significant inverse U-shaped associations with FDG-PET (P = .006) and HV (P = .001).
Fig. 3.
Scatter plots and fitted regression lines for the three components of the memory z-score. All regression models are adjusted for age, sex, and education. The HV models are also adjusted for total intracranial volume (TIV). The regression lines summarize the fit for a 60 year old male with 16 years of education and, for hippocampus models only, a TIV of 1.4 L. The P-values shown are based on a 2 d.f. test of the significance of the neuroimaging variable. The associated R2 values can be found in Table 2. AVLT DR = Auditory Verbal Learning Task, Delayed Recall; WMS-R LM II = Wechsler Memory Scale-Revised Logical Memory II; WMS-R VR II = Wechsler Memory Scale-Revised Visual Reproduction II.
3.3. Independent Comparison of the CogState Computerized Battery versus Standard Neuropsychological Measures with Neuroimaging
Although associations between neuroimaging and cognitive measures in this cohort were generally weak, we compared the associations between CogState and neuroimaging and between standard neuropsychological measures and neuroimaging. For each regression model fit, we reported the increase in R2 upon adding the neuroimaging predictor to a model with age, sex, and education (Table 2). We also reported the total R2 for the full model for comparison. For reference, the P-values are also shown in parentheses. The improvements in R2 were small with values ranging from nearly 0 to .04, (i.e., neuroimaging predictors accounted for up to an additional 4% of total variability after adjusting for age, sex, and education). The improvements tended to be of a similar magnitude for the CogState tests, neuropsychological test z-scores, and individual memory tests. The total R2 values were higher for the neuropsychological test z-scores and individual memory tests compared to the CogState tests. This illustrates that the neuropsychological measures were influenced to a greater extent by age, sex, and education compared to the CogState measures.
Table 2.
R2 for regression models. The first value shown is the improvement in R2 upon adding the neuroimaging predictor to a model with age, sex, and education. The second value (following ;) is the total R2 for the model (P-value for the imaging predictor based on a 2 d.f. test). R2 can be interpreted as the proportion of variability in the response accounted for by the regression model.
| Response Variable | FDG-PET | HV | Amyloid PET |
|---|---|---|---|
| CogState | |||
| Detection (DET) time | 0.02 ; 0.09 (P = .07) | 0.00 ; 0.07 (P = .45) | 0.01 ; 0.08 (P = .28) |
| Identification (IDN) time | 0.02 ; 0.06 (P = .04) | 0.00 ; 0.04 (P = .55) | 0.00 ; 0.04 (P = .59) |
| One card learning (OCL) accuracy | 0.02 ; 0.10 (P = .05) | 0.01 ; 0.08 (P = .48) | 0.02 ; 0.03 (P = .09) |
| One card back (ONB) time | 0.04 ; 0.09 (P = .006) | 0.02 ; 0.07 (P = .09) | 0.02 ; 0.07 (P = .09) |
| GMLT moves per second | 0.01 ; 0.15 (P = .38) | 0.04 ; 0.15 (P = .001) | 0.01 ; 0.15 (P = .35) |
| Z scores derived from neuropsychological test battery | |||
| Global | 0.02 ; 0.34 (P = .02) | 0.02 ; 0.28 (P = .02) | 0.01 ; 0.32 (P = .07) |
| Memory | 0.04 ; 0.23 (P = .001) | 0.02 ; 0.17 (P = .06) | 0.02 ; 0.20 (P = .06) |
| Attention | 0.01 ; 0.24 (P = .20) | 0.01 ; 0.20 (P = .16) | 0.01 ; 0.23 (P = .20) |
| Language | 0.01 ; 0.25 (P = .24) | 0.01 ; 0.22 (P = .06) | 0.01 ; 0.25 (P = .22) |
| Visuospatial | 0.01 ; 0.16 (P = .16) | 0.01 ; 0.15 (P = .09) | 0.01 ; 0.15 (P = .33) |
| Components of memory Z score | |||
| AVLT DR | 0.02 ; 0.21 (P = .06) | 0.03 ; 0.19 (P = .36) | 0.01 ; 0.20 (P = .45) |
| WMS-R LM II | 0.03 ; 0.15 (P = .03) | 0.01 ; 0.11 (P = .45) | 0.02 ; 0.15 (P = .03) |
| WMS-R VR II | 0.04 ; 0.12 (P = .006) | 0.04 ; 0.11 (P = .001) | 0.01 ; 0.08 (P = .50) |
Abbreviations: FDG-PET, fluorodeoxyglucose-positron emission tomography; HV, hippocampal volume; PET, positron emission tomography; GMLT, Groton Maze Learning Test; AVLT DR, Auditory Verbal Learning Task delayed recall; WMS-R LM II, Wechsler Memory Scale-Revised Logical Memory II; WMS-R VR II, = Wechsler Memory Scale-Revised Visual Reproduction II.
4. Discussion
In the present study, we examined the association between performance on the CogState computerized cognitive battery and neuroimaging measures and compared it to the association between standard neuropsychological tests and neuroimaging measures. We had three main findings: 1) Overall, there was a weak cross-sectional association between either CogState or standard neuropsychological tests and neuroimaging; 2) CogState and standard neuropsychological tests correlated more with neurodegenerative measures than amyloid; and 3) CogState variables explained approximately as much variance in the neuroimaging measures as the standard neuropsychological battery. Thus, while CogState, which possesses logistical advantages over standard neuropsychological testing, was comparable to neuropsychological testing for detecting associations with neurodegeneration, neither test was particularly sensitive and cannot be used as cross-sectional predictors of imaging pathology in cognitively normal middle-aged individuals.
Several studies have examined the cross-sectional relationship between standard neuropsychological tests and neuroimaging or CSF measures of amyloid, the initial pathological hallmark of AD, among cognitive normal individuals, with mixed results. A recent meta-analysis reported statistically significant associations between amyloid burden and episodic memory, but not with other memory (i.e., semantic) or cognitive domains [31]. However, the effect sizes were small and accounted for less than 2% of the total variance in cognitive performance [31]. Notably, these studies primarily included participants aged 70 and older. In the present study of individuals aged 50-69, we also observed weak cross-sectional associations between amyloid imaging and some, but not all, tests of memory (i.e., Wechsler Logical Memory paragraph recall, memory z-score, and CogState One Back).
In contrast to the lack of association with amyloid imaging, we found several more significant associations between both the CogState tests and cognitive z-scores with markers of neurodegeneration (e.g., hippocampal volume and FDG hypometabolism), even after adjusting for age, sex, and education. Based on the current pathophysiological cascade for pre-clinical AD [32-34], neurodegenerative markers are altered after the appearance of brain amyloid and closer in time to the development of cognitive symptoms. Therefore, it is reasonable that we are finding stronger cross-sectional associations with neurodegenerative markers. In support of our findings, a recent study also reported that FDG-PET hypometabolism and brain atrophy, but not amyloid, was cross-sectionally associated with cognition [35]. While a focus of pre-clinical secondary prevention trials has been on amyloid, identifying those who also have neurodegeneration would likely identify a subgroup of individuals who will decline the fastest. This information would also be helpful for determining who best to target for treatment purposes in the population.
Interestingly, we found several inverse U-shaped associations between amyloid or FDG-PET neuroimaging and the standard neuropsychological battery. For example, there were positive associations between most z-scores and memory tests up to PIB SUVR of 1.4, after which there was a negative association. Thus, individuals at the lowest and highest PIB SUVR values had similar cognitive performance. The explanation for this phenomenon is currently unclear but previous studies of cognitively normal individuals counterintuitively have reported better cognitive performance in those with high versus low amyloid [36,37]. Notably, however, associations of amyloid or FDG-PET and CogState tests generally appeared to be more linear than U-shaped. The reasons for these different associations are not well understood.
We and others have previously reported that APOE genotype may modify the association between amyloid imaging and cognition among individuals aged 70 and older [38,39]. In the present study of cognitively normal individuals aged 51 to 71 years, APOE genotype did not modify the association between amyloid imaging and cognition. However, because we had only a small number of individuals with high amyloid levels (i.e., SUVR > 1.5) in this age group, it is possible we may not have had enough power to observe an association.
When we compared the CogState computerized battery and standard neuropsychological tests, using percent of explained variance of the models, the results were similar. As the CogState tests take approximately 20-25 minutes to complete, do not require a trained psychometrician, performance is not as influenced by age, sex, and education compared too many standard neuropsychological tests, and could be taken online at home, CogState might have significant advantages. The CogState tests do not appear to be cross-sectionally associated with amyloid imaging, but this is not different from standard neuropsychological tests. Previous studies have shown that tests from the CogState battery are sensitive to cognitive impairment in mild AD and MCI compared with healthy controls and have little practice effect in healthy adults [20,40]. The latter point is important for longitudinal analyses of cognitive change and warrants future research to assess the longitudinal associations between CogState and neuroimaging measures.
Some limitations of the study warrant consideration. First, the study is cross-sectional. While previous cross-sectional studies suggested little association between amyloid and cognition, longitudinal studies have shown that high brain amyloid can predict memory decline in cognitively normal individuals [35,41,42]. Therefore, amyloid may be a better predictor of memory decline. As we continue to accrue longitudinal follow-up, future research will examine whether amyloid imaging predicts memory decline (using the CogState computerized battery and the standardized neuropsychological battery) in the MCSA. Second, the present study focused on neuroimaging biomarkers of amyloid and neurodegenerative pathology, the major Alzheimer-type pathologies. However, other brain changes, such as vascular pathology, also contribute to cognitive decline. Future research is needed to assess the relationship between the CogState computerized battery and other brain pathologies.
Research in context.
Systematic review: We reviewed the literature in PubMed examining the relationships between either computerized cognitive batteries or standard neuropsychological testing with neuroimaging measures of Alzheimer disease (AD) pathophysiologies. While studies have cross-sectionally examined the relationships between standard neuropsychological tests and neuroimaging measures, there has not been a comprehensive comparison of standard neuropsychological measures to computerized batteries as indicators of AD-related pathologies.
Interpretation: The results of our study suggest that the CogState tests explained about as much variance in the neuroimaging measures as the standard neuropsychological battery in middle-aged cognitively normal individuals. However, while CogState was comparable to neuropsychological testing, neither test was particularly sensitive and cannot be used cross-sectionally as predictors of imaging. The ease of administration and shorter time required for the CogState battery makes it advantageous for examining cognitive change in longitudinal studies.
Future Directions: Continued follow-up of our cohort will help to determine the longitudinal, predictive value of the CogState computerized tests for neuroimaging changes in comparison to standard neuropsychological tests.
Acknowledgments
This study was supported by funding from the National Institutes of Health/National Institute on Aging P50 AG016574, U01 AG006786, R01 AG041851, and R01 AG011378; the Robert H. and Clarice Smith and Abigail van Buren Alzheimer's Disease Research Program, the Driskill Foundation, and was made possible by the Rochester Epidemiology Project (R01 AG034676). The funding organizations did not have a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Abbreviations
- AD
Alzheimer's disease
- APOE
apolipoprotein E
- AVLT
Auditory Verbal Learning Task
- AVLT DR
Auditory Verbal Learning Task, Delayed Recall
- CSF
cerebrospinal fluid
- DET
detection
- FDG-PET
fluorodeoxyglucose-positron emission tomography
- GMLT
Groton Maze Learning Test
- HV
hippocampal volume
- IDN
identification
- IQR
interquartile range
- MCI
mild cognitive impairment
- MCSA
Mayo Clinic Study of Aging
- MOANs
Mayo's Older American Normative Studies
- MRI
magnetic resonance imaging
- OCL
One Card Learning
- ONB
One Back
- PiB-PET
Pittsburgh compound B-positron emission tomography
- ROIs
regions of interest
- SUVR
standardized update value ratio
- TIV
total intracranial value
- WMS-R LM II
Wechsler Memory Scale-Revised Logical Memory II
- WMS-R VR II
Wechsler Memory Scale-Revised Visual Reproduction II
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures
Dr. Mielke served as a consultant to Eli Lilly and receives research support from the NIH/NIA, the Alzheimer Drug Discovery Foundation, and the Michael J. Fox Foundation. Mr. Weigand reports no disclosures. Ms. Wiste reports no disclosures. Dr. Vemuri receives support from the NIH/NIA and Alzheimer's Association. Dr. Machulda reports no disclosures. Dr. Knopman serves as Deputy Editor for Neurology®; served on a Data Safety Monitoring Board for Lilly Pharmaceuticals; served as a consultant to TauRx Pharmaceuticals, was an investigator in clinical trials sponsored by Baxter, Elan Pharmaceuticals, and Forest Pharmaceuticals in the past 2 years; and receives research support from the NIH. Dr. Lowe serves on scientific advisory boards for Bayer Schering Pharma and GE Healthcare and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals, the NIH (NIA, NCI), the MN Partnership for Biotechnology and Medical Genomics, and the Leukemia & Lymphoma Society. Dr. Roberts receives research support from the NIH/NIA and from Abbvie Health Economics and Outcomes Research. Dr. Kantarci serves on the data safety monitoring board for Pfizer Inc. and Jansen Alzheimer's Immunotherapy, Takeda Global Research & Development Center, Inc.; and she is funded by the NIH [R01AG040042, P50 AG16574/P1, P50 AG44170/P 2]. Dr. Rocca receives research support from the NIH and the Michael J. Fox Foundation. Dr. Jack provides consulting services for Janssen Research & Development, LLC. He receives research funding from the National Institutes of Health (R01 AG011378, R01 AG041851, R01 AG037551, U01 HL096917, U01 AG032438, U01 AG024904), and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation.
Dr. Petersen serves on scientific advisory boards for Pfizer, Inc., Janssen Alzheimer Immunotherapy, Roche, Inc., Merck, Inc, and Genentech, Inc.; receives royalties from the publication of Mild Cognitive Impairment (Oxford University Press, 2003); and receives research support from the NIH/NIA.
References
- 1.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–92. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kokmen E, Smith GE, Petersen RC, Tangalos E, Ivnik RC. The short test of mental status. Correlations with standardized psychometric testing. Arch Neurol. 1991;48:725–8. doi: 10.1001/archneur.1991.00530190071018. [DOI] [PubMed] [Google Scholar]
- 4.Fahn S, Elton R. Committee MotUD. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden C, Caine D, Lieberman A, editors. Recent Developments in Parkinson's Disease. Macmillan Health Care Information; Florham Park, NJ: 1987. pp. 153–63. [Google Scholar]
- 5.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412–4. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 6.Rey A. L'examen Clinique en Psychologie. Presses Universitaires de France; Paris: 1964. [Google Scholar]
- 7.Wechsler D. Manual for the Wechsler Memory Scale-Revised. The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- 8.Kaplan E, Goodglass H, Weintraub S. The Boston Naming Test. Lea & Febiger; Philadelphia: 1983. [Google Scholar]
- 9.Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests. Oxford University Press; New York: 2006. [Google Scholar]
- 10.Reitan R. Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–6. [Google Scholar]
- 11.Wechsler D. Wechsler Adult Intelligence Scale-Revised [Manual] Psychological Corporation; San Antonio, TX: 1981. [Google Scholar]
- 12.Dingwall KM, Lewis MS, Maruff P, Cairney S. Reliability of repeated cognitive testing in healthy Indigenous Australian adolescents. Aust Psychol. 2009;44:224–34. [Google Scholar]
- 13.Fredrickson J, Maruff P, Woodward M, Moore L, Fredrickson A, Sach J, et al. Evaluation of the usability of a brief computerized cognitive screening test in older people for epidemiological studies. Neuroepidemiology. 2010;34:65–75. doi: 10.1159/000264823. [DOI] [PubMed] [Google Scholar]
- 14.Maruff P, Thomas E, Cysique L, Brew B, Collie A, Snyder P, et al. Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol. 2009;24:165–78. doi: 10.1093/arclin/acp010. [DOI] [PubMed] [Google Scholar]
- 15.Falleti MG, Maruff P, Burman P, Harris A. The effects of growth hormone (GH) deficiency and GH replacement on cognitive performance in adults: a meta-analysis of the current literature. Psychoneuroendocrinology. 2006;31:681–91. doi: 10.1016/j.psyneuen.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Collie A, Maruff P, Darby DG, McStephen M. The effects of practice on the cognitive test performance of neurologically normal individuals assessed at brief test-retest intervals. J Int Neuropsychol Soc. 2003;9:419–28. doi: 10.1017/S1355617703930074. [DOI] [PubMed] [Google Scholar]
- 17.Crook TH, Kay G, Larrabee G. Computer Based Cognitive Testing. In: Grant I, Adams KM, editors. Neuropsychological Assessment of Neuropsychiatric Disorders. Third Edition. Oxford University Press; New York, NY: 2009. [Google Scholar]
- 18.Pietrzak RH, Maruff P, Mayes LC, Roman SA, Sosa JA, Snyder PJ. An examination of the construct validity and factor structure of the Groton Maze Learning Test, a new measure of spatial working memory, learning efficiency, and error monitoring. Arch Clin Neuropsychol. 2008;23:433–45. doi: 10.1016/j.acn.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Snyder PJ, Jackson CE, Petersen RC, Khachaturian AS, Kaye J, Albert MS, et al. Assessment of cognition in mild cognitive impairment: a comparative study. Alzheimer's Dement. 2011;7:338–55. doi: 10.1016/j.jalz.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lim YY, Ellis KA, Harrington K, Ames D, Martins RN, Masters CL, et al. Use of the CogState Brief Battery in the assessment of Alzheimer's disease related cognitive impairment in the Australian Imaging, Biomarkers and Lifestyle (AIBL) study. J Clin Exp Neuropsychol. 2012;34:345–58. doi: 10.1080/13803395.2011.643227. [DOI] [PubMed] [Google Scholar]
- 21.Wechsler D. Wechsler Memory Scale-III. Third Edition. Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- 22.Ivnik RJ, Malec JF, Smith GE, Tangalos EG, Petersen RC, Kokmen E, et al. Mayo's Older Americans Normative Studies: WAIS-R norms for ages 56 to 97. Clin Neuropsychol. 1992;6:1–30. [Google Scholar]
- 23.Petersen RC, Roberts RO, Knopman DS, Geda YE, Cha RH, Pankratz VS, et al. Prevalence of mild cognitive impairment is higher in men: the Mayo Clinic Study of Aging. Neurology. 2010;75:889–97. doi: 10.1212/WNL.0b013e3181f11d85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 4th ed. American Psychiatric Association; Washington, D.C.: 1994. [Google Scholar]
- 25.Jack CR, Jr., Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain. 2008;131:665–80. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 27.Landau SM, Harvey D, Madison CM, Reiman EM, Foster NL, Aisen PS, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75:230–8. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97:11050–5. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jack CR, Jr., Knopman DS, Weigand SD, Wiste HJ, Vemuri P, Lowe V, et al. An operational approach to National Institute on Aging-Alzheimer's Association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71:765–75. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gelman A, Hill J. Data Analysis Using Regression and Multilevel/Hierarchical Models. Cambridge University Press; New York: 2007. [Google Scholar]
- 31.Hedden T, Oh H, Younger AP, Patel TA. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology. 2013;80:1341–8. doi: 10.1212/WNL.0b013e31828ab35d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 33.Shaw LM, Korecka M, Clark CM, Lee VM, Trojanowski JQ. Biomarkers of neurodegeneration for diagnosis and monitoring therapeutics. Nat Rev Drug Discov. 2007;6:295–303. doi: 10.1038/nrd2176. [DOI] [PubMed] [Google Scholar]
- 34.Jack CR, Jr., Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12:207–16. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ossenkoppele R, van der Flier WM, Verfaillie SC, Vrenken H, Versteeg A, van Schijndel RA, et al. Long-term effects of amyloid, hypometabolism, and atrophy on neuropsychological functions. Neurology. 2014;82:1768–75. doi: 10.1212/WNL.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 36.Chetelat G, Villemagne VL, Pike KE, Baron JC, Bourgeat P, Jones G, et al. Larger temporal volume in elderly with high versus low beta-amyloid deposition. Brain. 2010;133:3349–58. doi: 10.1093/brain/awq187. [DOI] [PubMed] [Google Scholar]
- 37.Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–17. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kantarci K, Lowe V, Przybelski SA, Weigand SD, Senjem ML, Ivnik RJ, et al. APOE modifies the association between Aβ and cognition in cognitively normal older adults. Neurology. 2012;78:232–40. doi: 10.1212/WNL.0b013e31824365ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lim YY, Ellis KA, Ames D, Darby D, Harrington K, Martins RN, et al. Aβ amyloid, cognition, and APOE genotype in healthy older adults. Alzheimer's Dement. 2013;9:538–45. doi: 10.1016/j.jalz.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 40.Lim YY, Jaeger J, Harrington K, Ashwood T, Ellis KA, Stoffler A, et al. Three-month stability of the CogState brief battery in healthy older adults, mild cognitive impairment, and Alzheimer's disease: results from the Australian Imaging, Biomarkers, and Lifestyle-rate of change substudy (AIBL-ROCS). Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists. 2013;28:320–30. doi: 10.1093/arclin/act021. [DOI] [PubMed] [Google Scholar]
- 41.Lim YY, Maruff P, Pietrzak RH, Ellis KA, Darby D, Ames D, et al. Aβ and cognitive change: examining the preclinical and prodromal stages of Alzheimer's disease. Alzheimers Dement. 2014 doi: 10.1016/j.jalz.2013.11.005. in press. [DOI] [PubMed] [Google Scholar]
- 42.Lim YY, Ellis KA, Pietrzak RH, Ames D, Darby D, Harrington K, et al. Stronger effect of amyloid load than APOE genotype on cognitive decline in healthy older adults. Neurology. 2012;79:1645–52. doi: 10.1212/WNL.0b013e31826e9ae6. [DOI] [PubMed] [Google Scholar]