Abstract
Immune control of many intracellular pathogens, including Trypanosoma cruzi, is reported to be dependent on the production of nitric oxide. In this study, we show that mice deficient in inducible nitric oxide synthase (iNOS or NOS2) exhibit resistance to T. cruzi infection that is comparable to that of wild-type mice. This is the case for two iNOS-deficient mouse strains, Nos2tm1Lau and Nos2 N5, infected with the Brazil or Tulahuen strain of T. cruzi. In all cases, blood parasitemia, tissue parasite load, and survival rates are similar between wild-type and iNOS-deficient mice. In contrast, both wild-type and Nos2tm1Lau mice died within 32 days postinfection when treated with the nitric oxide synthase inhibitor aminoguanidine. Increased transcription of NOS1 or NOS3 is not found in iNOS-knockout (KO) mice, indicating that the absence of nitric oxide production through iNOS is not compensated for by increased production of other NOS isoforms. However, Nos2tm1Lau mice exhibit enhanced expression of tumor necrosis factor alpha, interleukin-1, and macrophage inflammatory protein 1α compared to that of wild-type mice, and these alterations may in part compensate for the lack of iNOS. These results clearly show that iNOS is not required for control of T. cruzi infection in mice.
Trypanosoma cruzi is the protozoan parasite that causes Chagas' disease. Approximately 18 million people in Latin America are infected with T. cruzi, and an additional 40 million people are at risk of becoming infected. T. cruzi infection is estimated to result in 21,000 deaths annually (77). The infection is initiated by the entry of metacyclic trypomastigotes into the mammalian host and the subsequent invasion by these parasites of a wide variety of host cell types. Within host cells, T. cruzi converts into amastigote forms that replicate in the host cell cytoplasm. As immune control of the infection is established and the infection progresses into the chronic phase, parasites are restricted predominantly to muscle tissues. Control of T. cruzi infection requires the activation of multiple immune effector mechanisms as demonstrated by the fact that mice lacking CD4+ T cells, CD8+ T cells, or B cells fail to control the infection (31, 58, 60, 68-70). CD4+ T cells and CD8+ T cells are important sources of cytokines, including the type 1 cytokine gamma interferon (IFN-γ), and production of IFN-γ in the acute phase of infection is strongly associated with resistance (46, 57). Infection of IFN-γ-depleted (55) and IFN-γ- or IFN-γ receptor-knockout (KO) mice (21, 72) results in death early in infection, demonstrating that IFN-γ is essential for control of T. cruzi infection.
Among the best-studied functions of IFN-γ is the induction of the microbicidal product nitric oxide (NO). NO is an important cytotoxic and cytostatic factor in cell-mediated immune responses to many intracellular pathogens including Leishmania spp. (18, 33, 45) and Toxoplasma gondii (2, 24, 63). NO production is catalyzed by the enzyme NO synthase (NOS), which exists in three isoforms, NOS1, NOS2, and NOS3. Expression of NOS2, also known as inducible NOS (iNOS), is regulated by a combination of cytokines and microbial products, bacterial lipopolysaccharide (LPS) and the cytokine IFN-γ being among the most potent inducers (19, 37). T. cruzi itself (39) and glycoconjugates isolated from T. cruzi (13) can enhance levels of nitrite, a stable degradation product of NO, in macrophages. While primarily studied as a product of macrophages in the case of T. cruzi infection, iNOS is produced by a wide variety of cell types, including NK cells, dendritic cells, neutrophils, endothelial cells, myocytes, and fibroblasts (15, 20, 25, 26, 73, 74), in response to various pathogens or cytokines. Induction of iNOS expression in T. cruzi-infected macrophages, myocytes, and fibroblasts results in a dramatic reduction in the ability of amastigotes to survive and replicate in these cells in vitro (44). This regulation of parasite growth is reversed by the inclusion of inhibitors of iNOS in the culture, supporting the role of NO, rather than other cytokine-induced activities, in the control of T. cruzi infection in vitro (17, 44). In vivo studies have also suggested a critical role for NO in the control of T. cruzi infection. Mice administered iNOS inhibitors N-monomethyl-l-arginine (l-NMMA), Nω-nitro-l-arginine (NOARG), aminoguanidine (AG), or nitrosoguanidine l-arginine methyl ester (l-NAME) exhibit higher parasite levels and greater mortality than do untreated mice (52, 54, 62, 73). Although these in vivo and in vitro studies are strongly suggestive of a role for NO in immunity to T. cruzi, this conclusion rests primarily on the use of chemical inhibitors of iNOS, the absolute specificity of which for iNOS has not been proven.
In this study, we explored further the role of IFN-γ-inducible NOS in the control of T. cruzi infection. The two strains of iNOS-deficient mice used for this purpose, Nos2tm1Lau mice (32) and Nos2 N5 (36), were found to be as resistant to T. cruzi as were wild-type (WT) mice, and the similarity in responses of WT and iNOS-KO mice was seen during infection with both the Brazil and Tulahuen strains of T. cruzi. This study clearly demonstrates that iNOS is not required for control of T. cruzi infection in mice but suggests that iNOS-KO mice may compensate for the absence of NO production with the upregulation of cytokines important in immune control of T. cruzi infection.
MATERIALS AND METHODS
Mice and parasites.
WT C57BL/6J (WT), C57BL/6-Ifngtm1Ts (GKO), and C57BL/6-Nos2tm1Lau (Nos2tm1Lau) (32) mice were obtained from The Jackson Laboratory (Bar Harbor, Maine) or were bred in our facilities. C57BL/6Ai-Nos2 N5 (Nos2 N5) mice, deficient in iNOS (36), were obtained from Taconic (Germantown, N.Y.). Five female mice per strain were injected intraperitoneally with 103, 104, or 105 blood-form trypomastigotes (BFT) of the Brazil strain of T. cruzi or with 15 fibroblast-derived trypomastigotes of the Tulahuen strain of T. cruzi as indicated. All animal research complied with federal guidelines. Parasitemia was determined weekly, and survival was monitored daily.
DNA preparation.
Heart (50 mg) and skeletal muscle (300 mg) tissues were minced using surgical blades and added to a 5× volume of sodium dodecyl sulfate-proteinase K lysis buffer (27). The lysis buffer consisted of 10 mM Tris-HCl (pH 7.6; Bio-Rad Laboratories, Hercules, Calif.), 0.1 M NaCl (J. T. Baker, Phillipsburg, N.J.), 10 mM EDTA (J. T. Baker), 0.5% sodium dodecyl sulfate (Bio-Rad Laboratories), and 300 μg of proteinase K (Roche, Indianapolis, Ind.)/ml. The samples were then heated for 2 h at 55°C and extracted twice with phenol-chloroform-isoamyl alcohol (25:24:1; Sigma, St. Louis, Mo.). Cold ethanol (AAPER Alcohol and Chemical Co., Shelbyville, Ky.), twice the volume of the extracted sample, was then added, and samples were stored at −80°C for 30 min. Samples were centrifuged for 30 min at 16,000 × g, washed with 70% ethanol, vacuum dried, and resuspended in water. Fifty nanograms of DNA was assayed per real-time PCR.
mRNA preparation.
Skeletal and cardiac muscle and spleen tissues were harvested, and 1 ml of TRI reagent (Sigma) per 100 mg of tissue was added. Tissues were homogenized and stored at −70°C prior to completion of the RNA extraction. Samples were centrifuged at 16,000 × g for 10 min at 4°C, and the supernatants were transferred to fresh tubes with 0.2 ml of chloroform per ml of TRI reagent. Samples were mixed and incubated on ice for 15 min and centrifuged at 16,000 × g for 15 min at 4°C. The aqueous phase was transferred to a fresh tube, and RNA was precipitated by adding 0.5 ml of isopropanol per ml of TRI reagent used for the initial homogenization. Samples were incubated at 4°C for 20 min and centrifuged at 16,000 × g for 15 min at 4°C. The RNA pellet was washed twice with 75% ethanol and centrifuged at 16,000 × g for 5 min at 4°C before being resuspended in 200 μl of RNase-free water.
DNA was removed by adding 30 μg of RNA, 30 μl of RQ1 RNase-free DNase 10× reaction buffer, 30 U of RQ1 RNase-free DNase (Promega, Madison, Wis.), and nuclease-free water to 30 μg of RNA and incubating the mixture at 37°C for 30 min. RQ1 DNase stop solution (30 μl) was than added, and tubes were incubated at 65°C for 10 min to inactivate the DNase. RNA was cleaned using the RNeasy Mini kit (Qiagen, Valencia, Calif.). For first-strand cDNA synthesis, 10 μg of total RNA and 0.5 μg of oligo(dT)12-18 primer (Invitrogen Life Technologies, Carlsbad, Calif.) in a final volume of 20 μl were heated at 65°C for 10 min. A mixture of 5× first-strand buffer (Invitrogen), 0.1 M dithiothreitol (Invitrogen), and 10 mM deoxynucleoside triphosphate mix and RNasin was added to RNA and heated to 42°C. Two hundred units of SuperScript II RNase H− reverse transcriptase (Invitrogen) was added to each reaction mixture and incubated for 90 min at 42°C. An 0.5-μl quantity of RNase H was added and incubated at 37°C for 30 min. The obtained cDNA was diluted 1/10 with water, and 2 μl was used for amplification.
Generation of PCR standards for quantification of T. cruzi.
The standards for the PCRs were generated using 500 mg of minced healthy tissue, either skeletal or cardiac muscle, to which 107 T. cruzi epimastigotes were added. The tissue was then treated, extracted, and precipitated as described above. Once resuspended, the DNA was serially diluted with 25 mg of DNA/ml from healthy tissue. The standard 10-fold dilutions ranged from 0.01 to 1,000 parasite equivalents per 50 ng of total DNA. A standard curve was generated from these dilutions to determine the parasite load of DNA from infected tissue samples as previously described (14).
Oligonucleotides.
The following primer pair amplified the T. cruzi 195-bp repeat DNA: TCZ-F* (5′-GCT CTT GCC CAC AMG GGT GC-3′, where M = A or C) and TCZ-R (5′-CCA AGC AGC GGA TAG TTC AGG-3′) (modified from the work of Moser et al. [41]). For amplification of murine tumor necrosis alpha (TNF-α), the primer pair TNF-5241 (5′-TCC CTC TCA TCA GTT CTA TGG CCC A-3′) and TNF-5411 (5′-CAG CAA GCA TCT ATG CAC TTA GAC CCC-3′) (designed by Vector NTI) was used. Murine cDNA was amplified with the following primers: iNOS F (5′-CAG CTG GGC TGT ACA AAC CTT-3′) and R (5′-CAT TGG AAG TGA AGC GGT TCG-3′) (51), nNOS F (5′-ACT GAC ACC CTG CAC CTG AAG A-3′) and R (5′-GTG CGG ACA TCT TCT GAC TTC C-3′), eNOS F (5′-CCT CGA GTA AAG AAC TGG GAA GTG-3′) and R (5′-AAC TTC CTT GGA AAC ACC AGG G-3′), IFN-γ F (5′-TTC TTC AGC AAC AGC AAG GCG A-3′) and R (5′-TCC TTT TCC GCT TCC TGA GGC T-3′), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) F (5′-TGT CGT GGA GTC TAC TGG TGT CTT C-3′) and R (5′-CGT GGT TCA CAC CCA TCA CAA-3′).
Real-time PCR.
Reaction mixtures contained DNA or cDNA, 0.5 μM primer mix, 10 μl of Qiagen QuantiTect Sybr Green PCR Master Mix, and PCR-grade H2O (Qiagen) to a final total volume of 20 μl. Amplification of T. cruzi DNA and murine TNF-α DNA by the Roche LightCycler (Roche) was as previously described (14). The real-time PCR program for amplification of cDNA was as follows: 95°C at a 20°C/s ramp and holding for 15 min and 70 cycles of 95°C at a 20°C/s ramp for 5 s, then an increase to 55°C at a 20°C/s ramp, for a 5-s hold, and 72°C at a 2°C/s ramp for an 8-s hold, at the end of which fluorescence intensity was acquired. For melting curve generation, samples were heated to 95°C at a 20°C/s ramp, for a 0-s hold, then 60°C at a 20°C/s ramp, for a 30-s hold, and finally 90°C at an 0.2°C/s ramp, for an 0-s hold. Finally, the samples were cooled for 1 min at 40°C at a 20°C/s ramp.
Each DNA sample was quantified in triplicate. Triplicate values for each T. cruzi-specific DNA sample were averaged, and values were corrected by calculating the ratio of T. cruzi-specific product to averaged murine TNF-α product. Corrected values for each experimental group were then averaged, and the standard error of the mean was determined. Statistical significance was determined by t test where P was <0.05. For quantification of mRNA expression levels, copy number was calculated from a standard curve, obtained by plotting known input concentrations of control plasmids at log dilutions to the PCR cycle number at which the fluorescence intensity is above background. PCR products for eNOS, nNOS, iNOS, IFN-γ, and GAPDH, amplified with the above primers and PCR program, were cloned into the pCR2.1-TOPO vector (Invitrogen). cDNA from murine muscle tissue and splenocytes was amplified by real-time PCR with the eNOS, nNOS, iNOS, IFN-γ, and GAPDH primers listed above. Separately, reactions with reaction mixtures containing serially diluted control plasmids (107 to 101 copies), 0.5 μM plasmid-specific primers, 10 μl of Qiagen QuantiTect Sybr Green PCR Master Mix (Qiagen), and PCR-grade H2O (Qiagen) to a final total volume of 20 μl were used to generate standard curves. Individual sample values for NOS isoforms and IFN-γ were normalized by the housekeeping gene GAPDH and presented as number of transcripts per 107 copies of GAPDH. Samples were run in triplicate, and corrected values for each sample were averaged. Individual sample averages were then averaged per mouse strain, standard deviations were determined, and standard errors of the means were calculated. No amplification of nonspecific products was observed. Statistical significance was determined by t test where P was <0.05.
NO assay.
Healthy and infected (103 BFT of the Brazil strain) C57BL/6J and Nos2tm1Lau mice were sacrificed 108 days after infection. Thioglycolate-elicited peritoneal exudate cells and spleen cells were plated in complete RPMI at 5 × 106 cells per well. Complete RPMI consisted of RPMI 1640 (Mediatech, Herndon, Va.) supplemented with 10% fetal bovine serum (HyClone Laboratories, Logan, Utah), 2 mM l-glutamine (Life Technologies, Rockville, Md.), 1 mM sodium pyruvate (Sigma), 50 μg of gentamicin (Sigma)/ml, and 1 M β-mercaptoethanol (Sigma). For splenocytes, 0.1 mM nonessential amino acids (Life Technologies) were added to complete RPMI. Adherent cells were obtained by culture in 24-well plates at 37°C and 5% CO2 for 3 to 4 h followed by three washes with RPMI. Complete medium plus or minus 100 U of IFN-γ (Genzyme, Cambridge, Mass.)/ml and 10 ng of LPS (Calbiochem, San Diego, Calif.)/ml was added, and the cells were incubated at 37°C and 5% CO2 for an additional 48 h. Supernatants were collected and assayed for nitrite levels with the Griess reaction (Promega). Briefly, 50 μl of sample was dispensed in triplicate in 96-well flat-bottomed plates and incubated at room temperature with 50 μl of 1% sulfanilamide (Sigma) for 10 min. Fifty microliters of 0.1% N-1-naphtheylethylenediamine dihydrochloride (Sigma) was added and allowed to incubate for 10 min. Absorbance at 540 nm was measured and compared to a sodium nitrite (Promega) standard.
NOS inhibitor studies.
C57BL/6J, Nos2tm1Lau, and Nos2 N5 mice, five females per strain, were infected with 103 BFT of the Brazil strain of T. cruzi. Two days after infection, five mice per strain received drinking water containing 1% AG (Sigma). Survival was monitored daily, and parasitemia was monitored weekly.
Bio-Plex mouse cytokine assay.
Splenocytes from infected C57BL/6J and Nos2tm1Lau mice were harvested and plated (106 cells/well) in triplicate and stimulated with medium alone, phorbol myristate acetate (PMA; 50 ng/ml) and calcium ionophore (500 ng/ml), a mixture of T. cruzi-specific peptides shown to be targets of cytotoxic T-lymphocyte response (2.5 μM) (35; D. L. Martin, unpublished data), or a T. cruzi lysate (25 μg/ml). T. cruzi lysate generation was as follows: culture-grown parasites, approximately a 1:1 ratio of trypomastigotes to amastigotes, were pelleted, washed, frozen at −70°C, thawed, and sonicated. Freezing, thawing, and sonication were repeated, and particulates were removed by centrifugation at 16,000 × g for 30 min at 4°C. Protein concentration was determined, and the lysate was filter sterilized (0.22-μm pore size) and stored at −20°C until use. Culture supernatants were collected after 24 h of stimulation and stored at −70°C. The Bio-Plex Mouse 18-Plex cytokine assay (Bio-Rad Laboratories) was conducted according to the manufacturer's protocol. Cytokine standards were diluted fourfold with complete RPMI in final concentrations of 32,000 to 1.95 pg/ml. The mouse cytokines granulocyte-macrophage colony-stimulating factor (GM-CSF), IFN-γ, TNF-α, interleukin-1α (IL-1α), IL-β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-10, IL-12p40, IL-12p70, IL-17, granulocyte CSF (G-CSF), KC, macrophage inflammatory protein 1α (MIP-1α), and RANTES were analyzed. Calculated concentrations for each cytokine were averaged, and the standard deviations were determined. Statistical significance was determined by t test where * (P < 0.05) designates increased cytokine production by Nos2tm1Lau cells.
RESULTS
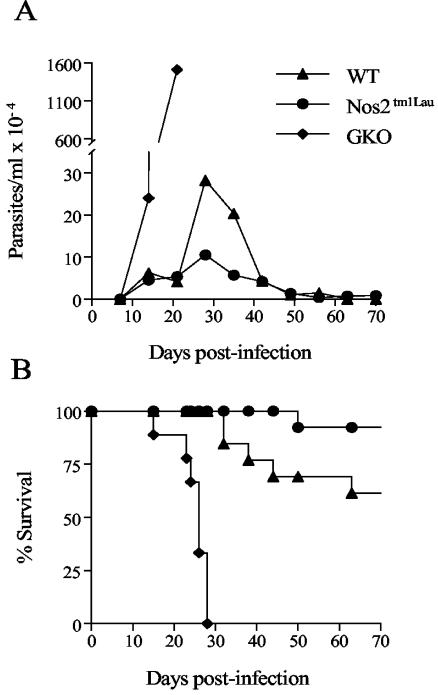
To clarify the contribution of IFN-γ-inducible NOS to control of T. cruzi infection, WT, Nos2tm1Lau, and GKO mice were infected with 103 BFT of the Brazil strain of T. cruzi. With this typically nonlethal infective dose, WT and Nos2tm1Lau mice survived acute T. cruzi infection with similar parasitemia and survival rates whereas the GKO mice exhibited a 50-fold-higher parasitemia (Fig. 1A) and early death (Fig. 1B). Approximately 60% of WT and 90% of Nos2tm1Lau mice survived beyond 120 days postinfection (dpi) (Fig. 1B). Increasing the infective dose to 105 BFT also resulted in similar survival rates in Nos2tm1Lau and WT mice (data not shown). Thus, in contrast to previous reports (21, 40), in our system iNOS-KO mice are not more susceptible than WT mice to T. cruzi-induced death.
FIG. 1.
Parasitemia and survival of Nos2tm1Lau mice infected with T. cruzi. C57BL/6J (WT), Nos2tm1Lau, and GKO mice were infected with 103 BFT of the Brazil strain of T. cruzi, and parasitemia (A) and survival (B) were observed. Results were pooled from three experiments with a total of 15 to 20 mice per group.
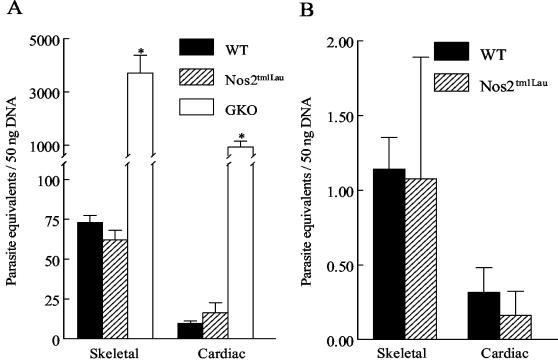
Although WT and iNOS-KO mice had comparable blood parasite levels, we considered it possible that the number of parasites in tissues, the level at which NO would be expected to exert its primary activity, could be significantly increased in iNOS-KO mice. To evaluate this possibility, real-time PCR, which reliably detects 0.01 parasite equivalents/50 ng of DNA (14), was utilized. At 28 dpi, parasite burdens in the skeletal and cardiac muscles of WT and Nos2tm1Lau mice were not significantly different (Fig. 2A). In contrast GKO mice had 10- to 40-fold-greater tissue parasite burdens in both types of tissue (P < 0.05). Much-reduced parasite loads in both WT and Nos2tm1Lau mice were also observed in the chronic phase of the infection (day 150) (Fig. 2B), demonstrating that NO is not required for control of T. cruzi in the acute or chronic stages of the infection.
FIG. 2.
Tissue parasite burden of Nos2tm1Lau mice infected with T. cruzi. C57BL/6J (WT), Nos2tm1Lau, and GKO mice were infected with 103 BFT of the Brazil strain of T. cruzi. Tissue parasite burden was determined as stated in Materials and Methods at 28 (A) and 150 (B) dpi. *, P < 0.05.
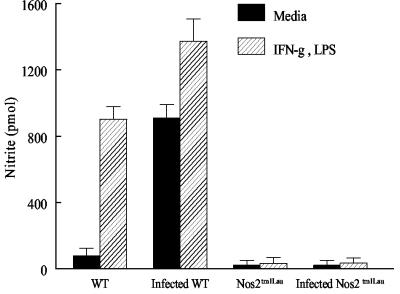
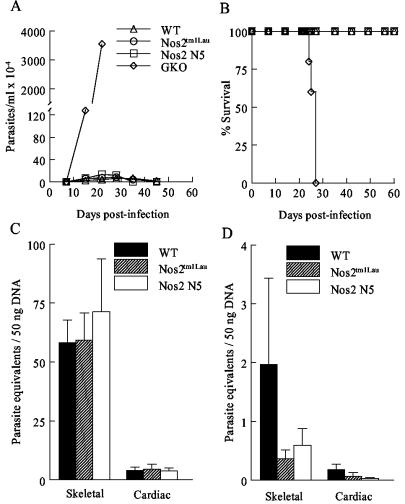
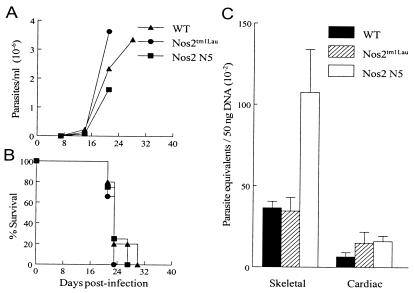
The observed resistance of the Nos2tm1Lau mice to T. cruzi infection is at odds with the hypothesized importance of NO in control of T. cruzi infection and the demonstrated susceptibility of mice treated with iNOS inhibitors (52, 54, 73). In order to further establish that NO was not contributing to the control of T. cruzi in the iNOS-KO mice, we (i) confirmed that inducible NO was not produced in iNOS-KO mice (Fig. 3), (ii) examined survival in a second iNOS-KO strain and with a second parasite strain, and (iii) explored the contribution of other NOS isoforms to control of T. cruzi infection. Infection of an additional iNOS-KO mouse strain, the Nos2 N5 strain, yielded results comparable to those obtained in the Nos2tm1Lau strain (Fig. 4). In this case, infection in the WT, Nos2tm1Lau, Nos2 N5, and GKO mice was initiated with a 10-fold-higher parasite dose, to assess whether NO production might be necessary under this condition. However, as in previous experiments, parasitemia, longevity, and tissue parasite loads were not significantly different between the WT and the two iNOS-KO strains and clearly different from those of the GKO mice (Fig. 4). Infection of mice with a more virulent parasite strain, the Tulahuen strain, also failed to reveal a differential susceptibility of iNOS-KO mice relative to that of WT mice (Fig. 5). In this case, infection with 15 fibroblast-derived trypomastigotes of the Tulahuen strain resulted in high parasitemia and 100% mortality during the acute phase of infection in WT, Nos2tm1Lau, and Nos2 N5 mice (Fig. 5A and B). Neither the skeletal muscle nor cardiac muscle parasite load in the Nos2 N5 mice was significantly greater than that for WT or Nos2tm1Lau mice (Fig. 5C).
FIG. 3.
NO is not produced by stimulated cells from infected iNOS-KO mice. Peritoneal exudate cells from naïve and infected (108 dpi) C57BL/6J (WT) and Nos2tm1Lau mice were cultured in medium alone or medium containing 100 U of IFN-γ/ml and 10 ng of LPS/ml. After 48 h supernatants were collected and assayed for nitrite levels with the Griess reaction.
FIG. 4.
Response of Nos2 N5 mice to T. cruzi infection. C57BL/6J (WT), Nos2tm1Lau, Nos2 N5, and GKO mice were infected with 104 BFT of the Brazil strain of T. cruzi, and the parasitemia (A) and survival (B) were observed. Tissue parasite burden was determined as stated in Materials and Methods at 28 (C) and 150 (D) dpi. *, P < 0.05.
FIG. 5.
Response of iNOS-KO mice to the Tulahuen strain of T. cruzi. C57BL/6J (WT), Nos2tm1Lau, and Nos2 N5 mice were infected with 15 BFT of the Tulahuen strain of T. cruzi, and parasitemia (A) and survival (B) were observed. Tissue parasite burden (C) was determined as stated in Materials and Methods at 21 dpi.
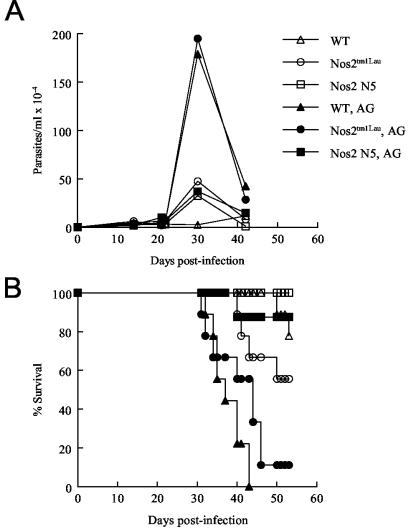
These results solidly establish that mice lacking the ability to produce iNOS are nevertheless similar to WT mice in their ability to control (or not control) T. cruzi infection. However, studies using iNOS inhibitors to treat mice infected with T. cruzi have arrived at the opposite conclusion, i.e., that NO is a critical factor in the control of T. cruzi infection (9, 17, 44, 52, 54, 73). One explanation for this apparent contradiction is that the iNOS inhibitors used in these studies lacked specificity and therefore have activities that extend beyond inhibition of iNOS. To determine if a nonspecific NOS inhibitor alters the response to T. cruzi in mice lacking iNOS, T. cruzi-infected WT and iNOS-KO mice were treated with AG. WT, Nos2tm1Lau, and Nos2 N5 mice were infected with 103 BFT of the Brazil strain and treated with 1% AG after 2 days of infection. Treatment with AG resulted in increased parasitemia in WT and Nos2tm1Lau mice, while the parasitemias of treated Nos2 N5 mice were similar to those of infected mice receiving normal drinking water (Fig. 6A). One hundred percent of WT mice and the majority of Nos2tm1Lau mice treated with AG died during the acute phase of the infection, while the majority of Nos2 N5 mice treated with AG survived infection in two replicate experiments (Fig. 6B and data not shown). These results demonstrate that AG has effects on T. cruzi infection beyond the inhibition of NO production and that the response of iNOS-KO mice to AG differs depending on the specific KO strain.
FIG. 6.
Treatment of infected iNOS-KO mice with an NOS inhibitor. C57BL/6J (WT), Nos2tm1Lau, and Nos2 N5 mice were infected with 103 BFT of the Brazil strain of T. cruzi. Two days after infection drinking water with 1% AG (filled symbols) was administered. Parasitemia (A) and survival (B) were monitored.
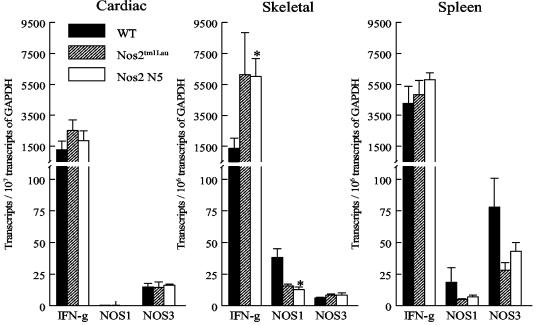
We considered the possibility that iNOS-KO mice may compensate for the lack of iNOS by increased expression of other NO-producing enzymes, NOS1 and NOS3. Recent studies have shown that enhanced expression of NOS1 and NOS3 can be induced by infection or by cytokine exposure (5, 29). Additionally, NOS1 and NOS3 expression has been reported in cardiac and skeletal muscle cells (16, 28, 78). To investigate if NOS1 or NOS3 expression is upregulated in the absence of NOS2, WT, Nos2tm1Lau, and Nos2 N5 mice were infected with 103 BFT of the Brazil strain, and at 21 dpi RNA was extracted from cardiac and skeletal muscle as well as from splenocytes and the mRNA levels for NOS1 and NOS3 were determined by real-time PCR. The levels of transcription of NOS1 or NOS3 were not significantly different among WT, Nos2tm1Lau, and Nos2 N5 mice in the cardiac muscle or spleen (Fig. 7). In the skeletal muscle, transcription of IFN-γ by Nos2 N5 mice was significantly greater than that in WT mice, while transcription of NOS1 was significantly less than that of WT mice (Fig. 7). Thus, the Nos2tm1Lau mice do not compensate for the lack of iNOS by increased expression of NOS1 or NOS3.
FIG. 7.
iNOS-KO mice do not compensate by increasing NOS1 or NOS3 expression. C57BL/6J (WT), Nos2tm1Lau, and Nos2 N5 mice were infected with 103 BFT of the Brazil strain of T. cruzi. At 21 dpi, RNAs from cardiac muscle, skeletal muscle, and spleen were extracted, and first-strand cDNA was synthesized. IFN-γ, nNOS, eNOS, and GAPDH transcript levels were quantified by real-time PCR. *, P < 0.05.
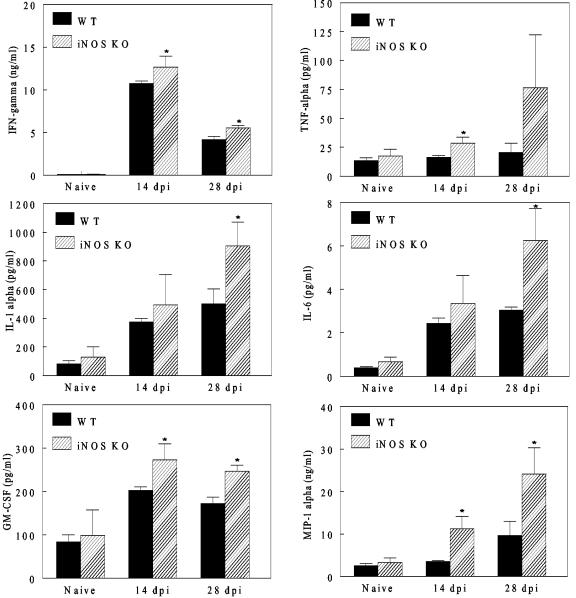
The increased expression of IFN-γ mRNA in iNOS-KO mice suggests that, in the absence of iNOS, cytokine production may be enhanced following T. cruzi infection. To test this hypothesis, WT and Nos2tm1Lau mice were infected with 103 BFT of T. cruzi strain Brazil, and at 14 and 28 dpi splenocytes from these mice were assayed for their in vitro cytokine response. Splenocytes were cultured with medium alone, PMA and calcium ionophore, a pool of T. cruzi peptides that are targets of T. cruzi-specific CD8+ T cells, or a T. cruzi lysate. The levels of cytokines released in culture supernatants were then evaluated using an 18-plex cytokine assay. The cytokines measured included those involved in hematopoiesis (IL-3, GM-CSF, and G-CSF), cellular trafficking (KC, MIP-1α, and RANTES), and innate (TNF-α, IL-1α, IL-1β, IL-6, IL-10, IL-12p40, and IL-12p70) and adaptive (IL-2, IL-4, IL-5, IL-17, and IFN-γ) immune responses. As a positive control, cells were stimulated with PMA and calcium ionophore, resulting in production of all 18 cytokines by cells from both WT and Nos2tm1Lau mice (data not shown). The levels of KC, RANTES, IL-2, IL-4, IL-10, IL-12p40, and IL-12p70 production by Nos2tm1Lau cells in response to T. cruzi peptides or lysate were not significantly different from those of WT cells (data not shown). However, upon stimulation with T. cruzi lysate, production of IFN-γ, TNF-α, IL-1α, IL-1β, IL-6, IL-17, GM-CSF, G-CSF, and MIP-1α was significantly increased in cells from iNOS-KO mice compared to that in WT cells (selected cytokines are represented in Fig. 8). These results show that cytokine production in Nos2tm1Lau mice is significantly upregulated compared to that of WT mice.
FIG. 8.
Increased cytokine production by cells from iNOS-KO mice upon stimulation with T. cruzi lysate. C57BL/6J (WT) and Nos2tm1Lau mice were infected with 103 BFT of the Brazil strain of T. cruzi. At 14 and 28 dpi splenocytes were harvested and stimulated with medium, T. cruzi-specific peptides, or T. cruzi lysate. Culture supernatants were assayed in triplicate for cytokines with a Bio-Plex cytokine assay. * (P < 0.05) designates a significant increase in cytokine production by Nos2tm1Lau cells relative to WT cells.
DISCUSSION
This study sought to clarify the contribution of NO to the control of T. cruzi infection. NO has a number of important roles in immunity, including the induction and suppression of apoptosis, and microbicidal activity (reviewed in references 1, 11, and 30). NO is reported to be crucial for the control of numerous intracellular pathogens, including influenza virus A, Listeria monocytogenes, Leishmania spp., and T. gondii (23, 45, 63). Substantial data have also been presented supporting NO as the mechanism by which IFN-γ controls T. cruzi infection (17, 39, 43, 44, 48, 61, 67, 73). The enhanced susceptibility of T. cruzi-infected IFN-γ- and IFN-γ receptor-deficient mice has been attributed to significantly reduced NO production (38, 59). Additionally, T. cruzi infection results in elevated iNOS levels in plasma (73), and a study of iNOS localization in T. cruzi-infected mice revealed iNOS in cellular infiltrates and infected tissues (59). T. cruzi-infected mice administered iNOS inhibitors exhibit higher parasite levels and greater mortality than do untreated mice (52, 54, 73).
However, the specificity of iNOS inhibitors has recently come into question (reviewed in references 3 and 8). For example, the inhibitor AG, previously regarded as iNOS specific, inhibits all NOS isoforms, with selectivity for NOS1 and NOS2 being greater than that for NOS3 (75, 76). Additionally, AG has various other activities, including the generation of hydrogen peroxide by inhibition of catalase (34, 50; reviewed in reference 71), the reduction of advanced glycosylation end products in diabetes (reviewed in reference 10), the inhibition of oxidative modification of low-density lipoproteins (56), the inhibition of histamine metabolism (6), and the inhibition of polyamine catabolism (65). The lack of specificity of iNOS inhibitors to assess the contribution of NO to control of T. cruzi infection brings into question the absolute necessity of iNOS for control of infection. In order to address this issue more directly, iNOS-KO mice were utilized in this study.
Two iNOS-KO mouse strains were used to determine the necessity of iNOS for control of T. cruzi infection. The Nos2tm1Lau strain (C57BL/6 background) lacks the calmodulin binding domain, which is required for iNOS activity (32), and the Nos2 N5 strain (C57BL/6 background) lacks the first four exons, including the translational start site, and therefore produces no iNOS protein (36). In both KO mouse strains parasitemia, tissue parasite load, and mortality during infection with the Brazil strain or the Tulahuen strain of T. cruzi were similar to those of WT mice. These results are in stark contrast to the effect of IFN-γ on T. cruzi infection. As previously reported (72), IFN-γ-deficient mice exhibit high parasitemia and greatly accelerated death rates, as well as massive tissue parasite burdens, upon infection with T. cruzi. Thus, this study clearly indicates that iNOS is not essential for control of T. cruzi infection in mice and contradicts the previous assertion that NO induction by IFN-γ is the major mechanism of control of T. cruzi infection.
One possible reason that iNOS-KO mice are able to resist T. cruzi infection is that they compensate for the absence of iNOS by enhancement of other immune effector mechanisms. In iNOS-KO mice, control of T. cruzi infection does not appear to correlate with increased expression of the other NOS isoforms. However, the enhanced production of parasite-antigen-induced cytokines including IFN-γ, TNF-α, IL-1α, GM-CSF, and MIP-1α may help compensate for the absence of NO. IFN-γ has a broad range of activities, many of which could contribute to immune control of T. cruzi (reviewed in references 64 and 66). Additionally, TNF-α and GM-CSF have been reported to have significant cytolytic and infection-inhibitory activity on T. cruzi in the absence of NO production (49). MIP-1α is involved in cellular recruitment to tissues during acute T. cruzi infection (53), and increased production in iNOS-KO mice would be expected to enhance trafficking of effector cells to sites of infection. Thus, the altered cytokine response by iNOS-KO mice may explain the survival of T. cruzi infection in the absence of iNOS.
This work adds to a growing literature on the role of NO in T. cruzi infection. Studies utilizing treatment with inhibitors of iNOS (9, 17, 39, 43, 44, 48, 59, 62, 67, 73) have demonstrated enhanced in vitro and in vivo survival and growth of T. cruzi under conditions of inhibited NO production, and we confirm these in vivo findings using AG treatment of mice in this study. However, we failed to confirm the increased susceptibility of iNOS-KO mice as reported previously (21, 40). There are a number of possible reasons for the results differing between these studies and our own, including variation in the genetic background and/or housing conditions of mice and the use of parasite strains that differ in virulence. A similar variance in results of studies by different groups has been reported with respect to T. cruzi infection in perforin- and granzyme-KO mice (31, 42, 47). Huang et al. (22) did not report parasite load or survival data in their studies using iNOS-KO mice and the Brazil strain of T. cruzi (the same combination used in the present study), but they report pathology data on iNOS mice surviving for at least 180 days, thus confirming that in this mouse-parasite strain combination, iNOS is not crucial for survival. It is likely that in some infection conditions (e.g., a host genetic background with naturally higher susceptibility infected with a highly virulent strain [or high-infecting dose]) the absence of a more minor effector mechanism like induction of iNOS results in a lethal infection while under infection conditions that are less taxing to the host (higher natural resistance or lower virulence of the infecting parasites) the absence of this mechanism can be tolerated or compensated for by other immune effector mechanisms. In addition to the difference in survival of iNOS-KO mice observed by us and by Holscher et al. (21), we also observed heightened expression of cytokines and chemokine mRNAs in iNOS-KO mice, while in the studies by Holscher et al. the iNOS-KO mice did not produce heightened levels of IL-1-α, TNF-α, or IFN-γ relative to those of WT mice (21). In spite of the different outcomes reported in these studies, one point that is supported by all studies is that the phenotypes of iNOS-KO and the GKO mouse strains are distinct following T. cruzi infection. In all cases, parasitemia and time to death are significantly higher and shorter, respectively, in GKO mice than in iNOS-KO mice. These results thus support the conclusion that the effects of IFN-γ on the control of infection extend well beyond simply the induction of NO production.
Although NO clearly can have significant adverse effects on T. cruzi in vitro and in some cases is an important mechanism of parasite control in vivo, this study conclusively shows that iNOS is not required for survival in murine T. cruzi infection and is thus not the primary means by which IFN-γ mediates protection in this infection. Other data supporting this conclusion include the finding that, unlike the case in infections with other intracellular pathogens, treatment of T. cruzi-infected mice with iNOS inhibitors after the early acute phase of infection does not compromise the ability of mice to control the infection (62). Additionally, no correlation has been detected between NOS promoter polymorphisms and the severity of Chagas' disease in humans (12). Although NO is considered the primary means of IFN-γ-induced protection in T. cruzi infection, it is perhaps not surprising that the situation is not this simple in vivo. IFN-γ induces transcription of hundreds of genes and mediates activities ranging from enhanced antigen presentation to cellular activation (7, 66). For example, we (K. L. Cummings and R. L. Tarleton, unpublished results) and others have found that chemokine and chemokine receptors important in the trafficking of effector cells to sites of infection in T. cruzi are delayed and reduced in expression in the absence of IFN-γ (4). Effective antigen processing and presentation are also likely to be adversely effected in IFN-γ-KO mice. Thus, NO production is likely to be just one of many factors that collectively contribute to IFN-γ-induced protection in T. cruzi infection.
Acknowledgments
This work was supported by grants AI33106 and AI22070 from the National Institutes of Health.
We recognize the assistance of Tamara Rosario McBreyer, Mark Heiges, and Diana Martin.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Abrahamsohn, I. A., and R. L. Coffman. 1995. Cytokine and nitric oxide regulation of the immunosuppression in Trypanosoma cruzi infection. J. Immunol. 155:3955-3963. [PubMed] [Google Scholar]
- 2.Adams, L. B., J. B. Hibbs, Jr., R. R. Taintor, and J. L. Krahenbuhl. 1990. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from l-arginine. J. Immunol. 144:2725-2729. [PubMed] [Google Scholar]
- 3.Alderton, W. K., C. E. Cooper, and R. G. Knowles. 2001. Nitric oxide synthases: structure, function and inhibition. Biochem. J. 357:593-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aliberti, J. C., J. T. Souto, A. P. Marino, J. Lannes-Vieira, M. M. Teixeira, J. Farber, R. T. Gazzinelli, and J. S. Silva. 2001. Modulation of chemokine production and inflammatory responses in interferon-gamma- and tumor necrosis factor-R1-deficient mice during Trypanosoma cruzi infection. Am. J. Pathol. 158:1433-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barna, M., T. Komatsu, and C. S. Reiss. 1996. Activation of type III nitric oxide synthase in astrocytes following a neurotropic viral infection. Virology 223:331-343. [DOI] [PubMed] [Google Scholar]
- 6.Bieganski, T., J. Kusche, W. Lorenz, R. Hesterberg, C. D. Stahlknecht, and K. D. Feussner. 1983. Distribution and properties of human intestinal diamine oxidase and its relevance for the histamine catabolism. Biochim. Biophys. Acta 756:196-203. [DOI] [PubMed] [Google Scholar]
- 7.Boehm, U., T. Klamp, M. Groot, and J. C. Howard. 1997. Cellular responses to interferon-gamma. Annu. Rev. Immunol. 15:749-795. [DOI] [PubMed] [Google Scholar]
- 8.Boer, R., W. R. Ulrich, T. Klein, B. Mirau, S. Haas, and I. Baur. 2000. The inhibitory potency and selectivity of arginine substrate site nitric-oxide synthase inhibitors is solely determined by their affinity toward the different isoenzymes. Mol. Pharmacol. 58:1026-1034. [PubMed] [Google Scholar]
- 9.Bourguignon, S. C., C. R. Alves, and S. Giovanni-De Simone. 1997. Detrimental effect of nitric oxide on Trypanosoma cruzi and Leishmania major-like cells. Acta Trop. 66:109-118. [DOI] [PubMed] [Google Scholar]
- 10.Brownlee, M., A. Cerami, and H. Vlassara. 1988. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N. Engl. J. Med. 318:1315-1321. [DOI] [PubMed] [Google Scholar]
- 11.Brunet, L. R. 2001. Nitric oxide in parasitic infections. Int. Immunopharmacol. 1:1457-1467. [DOI] [PubMed] [Google Scholar]
- 12.Calzada, J. E., M. A. Lopez-Nevot, Y. Beraun, and J. Martin. 2002. No evidence for association of the inducible nitric oxide synthase promoter polymorphism with Trypanosoma cruzi infection. Tissue Antigens 59:316-319. [DOI] [PubMed] [Google Scholar]
- 13.Camargo, M. M., A. C. Andrade, I. C. Almeida, L. R. Travassos, and R. T. Gazzinelli. 1997. Glycoconjugates isolated from Trypanosoma cruzi but not from Leishmania species membranes trigger nitric oxide synthesis as well as microbicidal activity in IFN-gamma-primed macrophages. J. Immunol. 159:6131-6139. [PubMed] [Google Scholar]
- 14.Cummings, K. L., and R. L. Tarleton. 2003. Rapid quantitation of Trypanosoma cruzi in host tissue by real-time PCR. Mol. Biochem. Parasitol. 129:53-59. [DOI] [PubMed] [Google Scholar]
- 15.Ding, A. H., C. F. Nathan, and D. J. Stuehr. 1988. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 141:2407-2412. [PubMed] [Google Scholar]
- 16.Feron, O., C. Dessy, D. J. Opel, M. A. Arstall, R. A. Kelly, and T. Michel. 1998. Modulation of the endothelial nitric-oxide synthase-caveolin interaction in cardiac myocytes. Implications for the autonomic regulation of heart rate. J. Biol. Chem. 273:30249-30254. [DOI] [PubMed] [Google Scholar]
- 17.Gazzinelli, R. T., I. P. Oswald, S. Hieny, S. L. James, and A. Sher. 1992. The microbicidal activity of interferon-gamma-treated macrophages against Trypanosoma cruzi involves an l-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-beta. Eur. J. Immunol. 22:2501-2506. [DOI] [PubMed] [Google Scholar]
- 18.Green, S. J., M. S. Meltzer, J. B. Hibbs, Jr., and C. A. Nacy. 1990. Activated macrophages destroy intracellular Leishmania major amastigotes by an l-arginine-dependent killing mechanism. J. Immunol. 144:278-283. [PubMed] [Google Scholar]
- 19.Hibbs, J. B., Jr., R. R. Taintor, and Z. Vavrin. 1987. Macrophage cytotoxicity: role for l-arginine deiminase and imino nitrogen oxidation to nitrite. Science 235:473-476. [DOI] [PubMed] [Google Scholar]
- 20.Hibbs, J. B., Jr., Z. Vavrin, and R. R. Taintor. 1987. l-Arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J. Immunol. 138:550-565. [PubMed] [Google Scholar]
- 21.Holscher, C., G. Kohler, U. Muller, H. Mossmann, G. A. Schaub, and F. Brombacher. 1998. Defective nitric oxide effector functions lead to extreme susceptibility of Trypanosoma cruzi-infected mice deficient in gamma interferon receptor or inducible nitric oxide synthase. Infect. Immun. 66:1208-1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang, H., J. Chan, M. Wittner, L. A. Jelicks, S. A. Morris, S. M. Factor, L. M. Weiss, V. L. Braunstein, C. J. Bacchi, N. Yarlett, M. Chandra, J. Shirani, and H. B. Tanowitz. 1999. Expression of cardiac cytokines and inducible form of nitric oxide synthase (NOS2) in Trypanosoma cruzi-infected mice. J. Mol. Cell. Cardiol. 31:75-88. [DOI] [PubMed] [Google Scholar]
- 23.Karupiah, G., J. H. Chen, S. Mahalingam, C. F. Nathan, and J. D. MacMicking. 1998. Rapid interferon gamma-dependent clearance of influenza A virus and protection from consolidating pneumonitis in nitric oxide synthase 2-deficient mice. J. Exp. Med. 188:1541-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan, I. A., J. D. Schwartzman, T. Matsuura, and L. H. Kasper. 1997. A dichotomous role for nitric oxide during acute Toxoplasma gondii infection in mice. Proc. Natl. Acad. Sci. USA 94:13955-13960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kilbourn, R. G., and P. Belloni. 1990. Endothelial cell production of nitrogen oxides in response to interferon gamma in combination with tumor necrosis factor, interleukin-1, or endotoxin. J. Natl. Cancer Inst. 82:772-776. [DOI] [PubMed] [Google Scholar]
- 26.Kilbourn, R. G., S. S. Gross, R. F. Lodato, J. Adams, R. Levi, L. L. Miller, L. B. Lachman, and O. W. Griffith. 1992. Inhibition of interleukin-1-alpha-induced nitric oxide synthase in vascular smooth muscle and full reversal of interleukin-1-alpha-induced hypotension by N omega-amino-l-arginine. J. Natl. Cancer Inst. 84:1008-1016. [DOI] [PubMed] [Google Scholar]
- 27.Kirchhoff, L. V., J. R. Votava, D. E. Ochs, and D. R. Moser. 1996. Comparison of PCR and microscopic methods for detecting Trypanosoma cruzi. J. Clin. Microbiol. 34:1171-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobzik, L., B. Stringer, J. L. Balligand, M. B. Reid, and J. S. Stamler. 1995. Endothelial type nitric oxide synthase in skeletal muscle fibers: mitochondrial relationships. Biochem. Biophys. Res. Commun. 211:375-381. [DOI] [PubMed] [Google Scholar]
- 29.Komatsu, T., Z. Bi, and C. S. Reiss. 1996. Interferon-gamma induced type I nitric oxide synthase activity inhibits viral replication in neurons. J. Neuroimmunol. 68:101-108. [DOI] [PubMed] [Google Scholar]
- 30.Kroncke, K. D., K. Fehsel, C. Suschek, and V. Kolb-Bachofen. 2001. Inducible nitric oxide synthase-derived nitric oxide in gene regulation, cell death and cell survival. Int. Immunopharmacol. 1:1407-1420. [DOI] [PubMed] [Google Scholar]
- 31.Kumar, S., and R. L. Tarleton. 1998. The relative contribution of antibody production and CD8+ T cell function to immune control of Trypanosoma cruzi. Parasite Immunol. 20:207-216. [DOI] [PubMed] [Google Scholar]
- 32.Laubach, V. E., E. G. Shesely, O. Smithies, and P. A. Sherman. 1995. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc. Natl. Acad. Sci. USA 92:10688-10692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liew, F. Y., S. Millott, C. Parkinson, R. M. Palmer, and S. Moncada. 1990. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from l-arginine. J. Immunol. 144:4794-4797. [PubMed] [Google Scholar]
- 34.Lopez-Belmonte, J., and B. J. Whittle. 1995. Aminoguanidine-provoked leukocyte adherence to rat mesenteric venules: role of constitutive nitric oxide synthase inhibition. Br. J. Pharmacol. 116:2710-2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Low, H. P., M. A. Santos, B. Wizel, and R. L. Tarleton. 1998. Amastigote surface proteins of Trypanosoma cruzi are targets for CD8+ CTL. J. Immunol. 160:1817-1823. [PubMed] [Google Scholar]
- 36.MacMicking, J. D., C. Nathan, G. Hom, N. Chartrain, D. S. Fletcher, M. Trumbauer, K. Stevens, Q. W. Xie, K. Sokol, N. Hutchinson, et al. 1995. Altered responses to bacterial infection and endotoxic shock in mice lacking inducible nitric oxide synthase. Cell 81:641-650. (Erratum, 81:1171.) [DOI] [PubMed] [Google Scholar]
- 37.Martin, E., C. Nathan, and Q. W. Xie. 1994. Role of interferon regulatory factor 1 in induction of nitric oxide synthase. J. Exp. Med. 180:977-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martins, G. A., L. Q. Vieira, F. Q. Cunha, and J. S. Silva. 1999. Gamma interferon modulates CD95 (Fas) and CD95 ligand (Fas-L) expression and nitric oxide-induced apoptosis during the acute phase of Trypanosoma cruzi infection: a possible role in immune response control. Infect. Immun. 67:3864-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Metz, G., Y. Carlier, and B. Vray. 1993. Trypanosoma cruzi upregulates nitric oxide release by IFN-gamma-preactivated macrophages, limiting cell infection independently of the respiratory burst. Parasite Immunol. 15:693-699. [DOI] [PubMed] [Google Scholar]
- 40.Michailowsky, V., N. M. Silva, C. D. Rocha, L. Q. Vieira, J. Lannes-Vieira, and R. T. Gazzinelli. 2001. Pivotal role of interleukin-12 and interferon-gamma axis in controlling tissue parasitism and inflammation in the heart and central nervous system during Trypanosoma cruzi infection. Am. J. Pathol. 159:1723-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moser, D. R., L. V. Kirchhoff, and J. E. Donelson. 1989. Detection of Trypanosoma cruzi by DNA amplification using the polymerase chain reaction. J. Clin. Microbiol. 27:1477-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muller, U., V. Sobek, S. Balkow, C. Holscher, A. Mullbacher, C. Museteanu, H. Mossmann, and M. M. Simon. 2003. Concerted action of perforin and granzymes is critical for the elimination of Trypanosoma cruzi from mouse tissues, but prevention of early host death is in addition dependent on the FasL/Fas pathway. Eur. J. Immunol. 33:70-78. [DOI] [PubMed] [Google Scholar]
- 43.Munoz-Fernandez, M. A., M. A. Fernandez, and M. Fresno. 1992. Activation of human macrophages for the killing of intracellular Trypanosoma cruzi by TNF-alpha and IFN-gamma through a nitric oxide-dependent mechanism. Immunol. Lett. 33:35-40. [DOI] [PubMed] [Google Scholar]
- 44.Munoz-Fernandez, M. A., M. A. Fernandez, and M. Fresno. 1992. Synergism between tumor necrosis factor-alpha and interferon-gamma on macrophage activation for the killing of intracellular Trypanosoma cruzi through a nitric oxide-dependent mechanism. Eur. J. Immunol. 22:301-307. [DOI] [PubMed] [Google Scholar]
- 45.Murray, H. W., and C. F. Nathan. 1999. Macrophage microbicidal mechanisms in vivo: reactive nitrogen versus oxygen intermediates in the killing of intracellular visceral Leishmania donovani. J. Exp. Med. 189:741-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nabors, G. S., and R. L. Tarleton. 1991. Differential control of IFN-gamma and IL-2 production during Trypanosoma cruzi infection. J. Immunol. 146:3591-3598. [PubMed] [Google Scholar]
- 47.Nickell, S. P., and D. Sharma. 2000. Trypanosoma cruzi: roles for perforin-dependent and perforin-independent immune mechanisms in acute resistance. Exp. Parasitol. 94:207-216. [DOI] [PubMed] [Google Scholar]
- 48.Norris, K. A., J. E. Schrimpf, J. L. Flynn, and S. M. Morris, Jr. 1995. Enhancement of macrophage microbicidal activity: supplemental arginine and citrulline augment nitric oxide production in murine peritoneal macrophages and promote intracellular killing of Trypanosoma cruzi. Infect. Immun. 63:2793-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olivares Fontt, E. O., P. De Baetselier, C. Heirman, K. Thielemans, R. Lucas, and B. Vray. 1998. Effects of granulocyte-macrophage colony-stimulating factor and tumor necrosis factor alpha on Trypanosoma cruzi trypomastigotes. Infect. Immun. 66:2722-2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ou, P., and S. P. Wolff. 1993. Aminoguanidine: a drug proposed for prophylaxis in diabetes inhibits catalase and generates hydrogen peroxide in vitro. Biochem. Pharmacol. 46:1139-1144. [DOI] [PubMed] [Google Scholar]
- 51.Overbergh, L., D. Valckx, M. Waer, and C. Mathieu. 1999. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine 11:305-312. [DOI] [PubMed] [Google Scholar]
- 52.Petray, P., E. Castanos-Velez, S. Grinstein, A. Orn, and M. E. Rottenberg. 1995. Role of nitric oxide in resistance and histopathology during experimental infection with Trypanosoma cruzi. Immunol. Lett. 47:121-126. [DOI] [PubMed] [Google Scholar]
- 53.Petray, P., R. Corral, P. Meckert, and R. Laguens. 2002. Role of macrophage inflammatory protein-1α (MIP-1α) in macrophage homing in the spleen and heart pathology during experimental infection with Trypanosoma cruzi. Acta Trop. 83:205-211. [DOI] [PubMed] [Google Scholar]
- 54.Petray, P., M. E. Rottenberg, S. Grinstein, and A. Orn. 1994. Release of nitric oxide during the experimental infection with Trypanosoma cruzi. Parasite Immunol. 16:193-199. [DOI] [PubMed] [Google Scholar]
- 55.Petray, P. B., M. E. Rottenberg, G. Bertot, R. S. Corral, A. Diaz, A. Orn, and S. Grinstein. 1993. Effect of anti-gamma-interferon and anti-interleukin-4 administration on the resistance of mice against infection with reticulotropic and myotropic strains of Trypanosoma cruzi. Immunol. Lett. 35:77-80. [DOI] [PubMed] [Google Scholar]
- 56.Picard, S., S. Parthasarathy, J. Fruebis, and J. L. Witztum. 1992. Aminoguanidine inhibits oxidative modification of low density lipoprotein protein and the subsequent increase in uptake by macrophage scavenger receptors. Proc. Natl. Acad. Sci. USA 89:6876-6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reed, S. G. 1988. In vivo administration of recombinant IFN-gamma induces macrophage activation, and prevents acute disease, immune suppression, and death in experimental Trypanosoma cruzi infections. J. Immunol. 140:4342-4347. [PubMed] [Google Scholar]
- 58.Rottenberg, M. E., M. Bakhiet, T. Olsson, K. Kristensson, T. Mak, H. Wigzell, and A. Orn. 1993. Differential susceptibilities of mice genomically deleted of CD4 and CD8 to infections with Trypanosoma cruzi or Trypanosoma brucei. Infect. Immun. 61:5129-5133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rottenberg, M. E., E. Castanos-Velez, R. de Mesquita, O. G. Laguardia, P. Biberfeld, and A. Orn. 1996. Intracellular co-localization of Trypanosoma cruzi and inducible nitric oxide synthase (iNOS): evidence for dual pathway of iNOS induction. Eur. J. Immunol. 26:3203-3213. [DOI] [PubMed] [Google Scholar]
- 60.Rottenberg, M. E., A. Riarte, L. Sporrong, J. Altcheh, P. Petray, A. M. Ruiz, H. Wigzell, and A. Orn. 1995. Outcome of infection with different strains of Trypanosoma cruzi in mice lacking CD4 and/or CD8. Immunol. Lett. 45:53-60. [DOI] [PubMed] [Google Scholar]
- 61.Rottenberg, M. E., L. Sporrong, I. Persson, H. Wigzell, and A. Orn. 1995. Cytokine gene expression during infection of mice lacking CD4 and/or CD8 with Trypanosoma cruzi. Scand. J. Immunol. 41:164-170. [DOI] [PubMed] [Google Scholar]
- 62.Saeftel, M., B. Fleischer, and A. Hoerauf. 2001. Stage-dependent role of nitric oxide in control of Trypanosoma cruzi infection. Infect. Immun. 69:2252-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Scharton-Kersten, T. M., G. Yap, J. Magram, and A. Sher. 1997. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J. Exp. Med. 185:1261-1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schroder, K., P. J. Hertzog, T. Ravasi, and D. A. Hume. 2004. Interferon-gamma: an overview of signals, mechanisms and functions. J. Leukoc. Biol. 75:163-189. [DOI] [PubMed] [Google Scholar]
- 65.Seiler, N., F. N. Bolkenius, and B. Knodgen. 1985. The influence of catabolic reactions on polyamine excretion. Biochem. J. 225:219-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shtrichman, R., and C. E. Samuel. 2001. The role of gamma interferon in antimicrobial immunity. Curr. Opin. Microbiol. 4:251-259. [DOI] [PubMed] [Google Scholar]
- 67.Silva, J. S., G. N. Vespa, M. A. Cardoso, J. C. Aliberti, and F. Q. Cunha. 1995. Tumor necrosis factor alpha mediates resistance to Trypanosoma cruzi infection in mice by inducing nitric oxide production in infected gamma interferon-activated macrophages. Infect. Immun. 63:4862-4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tarleton, R. L. 1990. Depletion of CD8+ T cells increases susceptibility and reverses vaccine-induced immunity in mice infected with Trypanosoma cruzi. J. Immunol. 144:717-724. [PubMed] [Google Scholar]
- 69.Tarleton, R. L., M. J. Grusby, M. Postan, and L. H. Glimcher. 1996. Trypanosoma cruzi infection in MHC-deficient mice: further evidence for the role of both class I- and class II-restricted T cells in immune resistance and disease. Int. Immunol. 8:13-22. [DOI] [PubMed] [Google Scholar]
- 70.Tarleton, R. L., J. Sun, L. Zhang, and M. Postan. 1994. Depletion of T-cell subpopulations results in exacerbation of myocarditis and parasitism in experimental Chagas' disease. Infect. Immun. 62:1820-1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Thiemermann, C. 1997. Nitric oxide and septic shock. Gen. Pharmacol. 29:159-166. [DOI] [PubMed] [Google Scholar]
- 72.Torrico, F., H. Heremans, M. T. Rivera, E. Van Marck, A. Billiau, and Y. Carlier. 1991. Endogenous IFN-gamma is required for resistance to acute Trypanosoma cruzi infection in mice. J. Immunol. 146:3626-3632. [PubMed] [Google Scholar]
- 73.Vespa, G. N., F. Q. Cunha, and J. S. Silva. 1994. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infect. Immun. 62:5177-5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Werner-Felmayer, G., E. R. Werner, D. Fuchs, A. Hausen, G. Reibnegger, and H. Wachter. 1990. Tetrahydrobiopterin-dependent formation of nitrite and nitrate in murine fibroblasts. J. Exp. Med. 172:1599-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wolff, D. J., D. S. Gauld, M. J. Neulander, and G. Southan. 1997. Inactivation of nitric oxide synthase by substituted aminoguanidines and aminoisothioureas. J. Pharmacol. Exp. Ther. 283:265-273. [PubMed] [Google Scholar]
- 76.Wolff, D. J., and A. Lubeskie. 1995. Aminoguanidine is an isoform-selective, mechanism-based inactivator of nitric oxide synthase. Arch. Biochem. Biophys. 316:290-301. [DOI] [PubMed] [Google Scholar]
- 77.World Health Organization. 2000. Chagas disease: multi-governmental initiatives. Tropical Disease Research Fifteenth Programme report. [Online.] http://www.who.int/tdr/research/progress9900/partnerships/chagas.htm.
- 78.Xu, K. Y., D. L. Huso, T. M. Dawson, D. S. Bredt, and L. C. Becker. 1999. Nitric oxide synthase in cardiac sarcoplasmic reticulum. Proc. Natl. Acad. Sci. USA 96:657-662. [DOI] [PMC free article] [PubMed] [Google Scholar]