Abstract
Recent studies have revealed that several Gram-negative species utilize variations of the well-known chemotaxis signaling cascade to switch lifestyles in order to survive environmental stress. Two survival strategies covered in this review are the development of dormant cyst cells and biofilm formation. Both of these two types of structures involve exopolysaccharide-mediated cell-cell interactions which result in multicellular communities that confer resistance to stress conditions such as desiccation and antibiotics. This review is centered on recent advances in the understanding of phosphate flow and novel output signals in chemosensory signaling pathways that are involved in cyst formation and biofilms.
Keywords: Chemotaxis-Like, Signal, Transduction, Systems
Involvement Of Chemotaxis-Like Signal Transduction Cascades In Controlling Cellular Development And Biofilm Formation
Many Gram-negative species are known to undergo cellular and multicellular developmental processes. Soil-inhabiting bacteria such as Myxococcus xanthus, Azospirillum brasilense, and Azotobacter vinelandii are known to produce metabolically dormant resting cysts that are resistant to moderate heat (<60 °C) [1,2], severe desiccation [1,3] and UV-irradiation[1,4]. Some aquatic Gram-negative species such as Rhodospirillum centenum, a thermotolerant photosynthetic member of the Azospirillum clade, and the pathogen Legionella pneumophila also produce dormant cysts [5,6]. In most cases the production of resting cysts has not been well studied beyond microscopic observations of the developmental process, which is tightly coupled with the formation of multicellular communities. Examples include: fruiting body formation of Myxococcus xanthus following the development of myxospores, and cyst formation in Azospirillum sp. that involves the formation of a multicellular floc from which desiccation resistant cysts develop. The development of flocs is not well understood but involves the entanglement of non-motile cells in a fibrillar matrix comprised of exopolysaccharide (EPS) polymers [3]. This is not unlike the development of attached biofilms that involves the formation of multicellular communities comprised of non-motile cells also held together by EPS. Furthermore, under conditions of stress, cells in a biofilm can enter a dormant desiccation resistant stage via the formation of persister cells [4,7].
Recently, genetic and biochemical studies have revealed that several Gram-negative species utilize novel variations of the well-known chemotaxis signaling cascade to control the formation of desiccation resistant cyst cells, flocs, and biofilms. This review is centered on advances in the understanding of phosphate flow and novel output signals encoded by these alternative chemosensory signaling pathways.
ACF Signal Transduction Pathways
Regulatory pathways that utilize chemotaxis like components represent some of the more complex signal transduction systems in prokaryotes. Recent bioinformatic analyses of 450 prokaryotic genomes identified 416 chemosensory systems within 245 species based on the number of putative CheA proteins [8]. Many species contain Che gene clusters that code for proteins with the simplified standard chemotaxis architecture that is present in E. coli (see BOX1 and BOX Figure 1 for an overview of the paradigm E. coli chemotaxis signaling cascade). However, 126 other species contain multiple chemotaxis-like gene clusters many of which contain additional auxiliary proteins and/or multi-domain hybrid components. In recent years it has been shown that many of these more complex additional chemosensorygene clusters regulate processes other than motility.
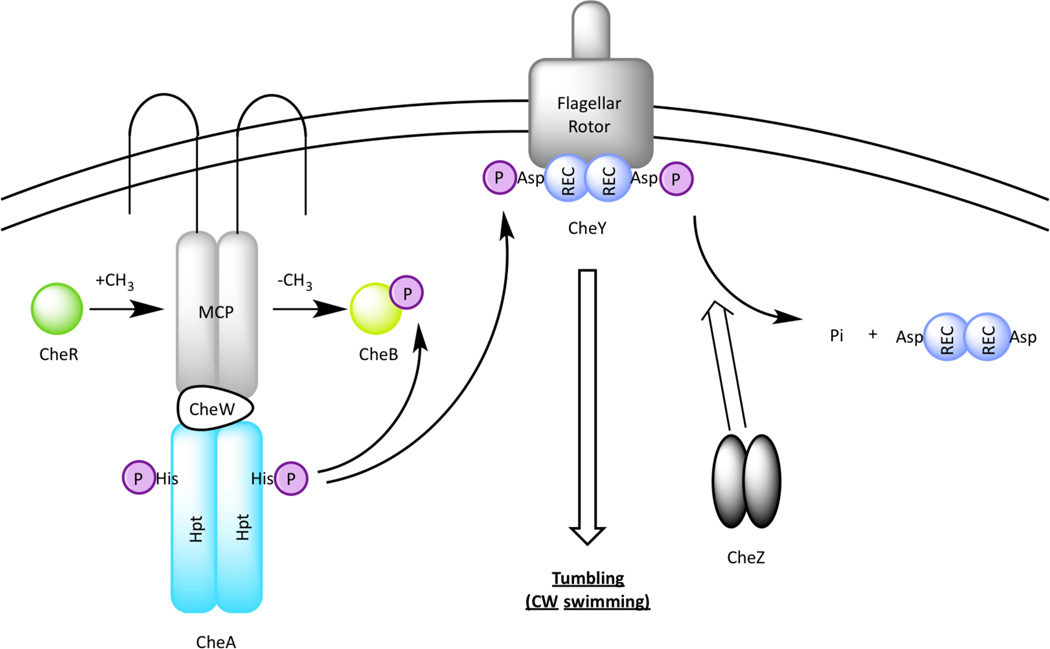
Box1: The E. coli Chemotaxis Signaling Paradigm.
Bacterial chemotaxis is a biased movement towards higher concentrations of life-sustaining nutrients and lower concentrations of toxins. It involves sensing a gradient of chemicals as small as a few molecules [53] and moving in response to these environmental signals to maximize survival.
The well-studied E. coli chemotaxis motility system can be considered an atypical TCS comprised of a HK CheA with no signal input domain, and the RR CheY that has a REC domain with no output module (BOX Figure). The addition of chemoreceptors to the TCS scheme allows for signal amplification (hence enhanced sensitivity) and signal adaptation (hence the ability to sense a chemical gradient) through reversible methylation makes the chemotaxis one of the most intricate sensory systems in prokaryotes [54]. CheA indirectly senses environmental inputs by forming a complex with a methyl-accepting chemoreceptor (MCP) via the scaffolding protein CheW (BOX Figure 1). In E. coli, five membrane spanning MCPs serve as sensors for extracellular chemical stimuli such as amino acids and sugars. These chemoreceptors are homodimers that form trimer and higher order clusters [55]. A decrease in attractant binding to the MCPs activates CheA autophosphorylation and an increase in repellent concentration cause the MCPs to turn off CheA activity [55]. When activated by a bound MCP, CheA autophosphorylates and transfers the phosphoryl group to the REC domain of CheY. Subsequent binding of CheY~P to the flagellar rotor leads to a change in flagellar rotation from counterclockwise to clockwise, causing an E. coli cell to undergo a brief, reorienting tumble.
The ligand binding activity of MCPs are altered by a methyltransferase (CheR) and a methylesterase (CheB) to form an adaptation system. In this process CheR constitutively transfers methyl groups from S-adenosylmethionine to specific glutamate residues on MCPs [56] that CheB subsequently removes after being phosphorylated by CheA. Since ligand binding affinity is controlled by MCP methylation, CheR and CheB thus constitutes a feedback mechanism that constantly resets the MCPs to a pre-stimulus state as the bacterium travels through a ligand gradient [57]. This allows MCPs to monitor changes in a receptor ligand over a wide range of concentrations and to subsequently control the kinase activity of CheA. Finally, the amount of CheY~P in a cell is regulated by its phospho-donor CheA~P as well as a phosphatase CheZ, which accelerates dephosphorylation of CheY~P. For more complex chemotaxis systems, readers are referred to a recent review article [58].
BOX Figure.
Chemotaxis signal transduction in Escherichia coli. CheA forms a tertiary complex with a chemorecepter (MCP) and the scaffolding protein CheW to receive chemical stimuli in the environment. Once a signal is received by an MCP, CheA autokinase is activated which subsequently phosphorylates CheY. Phosphorylated CheY binds to the flagellar rotor, leading to a change in flagellar rotation. Signal is terminated via the phosphatase activity by CheZ. An adaptation mechanism of the MCPs involves a methyltransferase CheR, which constitutively methylates MCPs, and a methylesterase CheB, which demethylates MCPs only when phosphorylated by CheA.
Currently, chemosensory systems are functionally classified into those regulating flagellar motility, type IV pili (TFP)-based motility, and alternative cellular functions (ACF) [8]. Early genetic studies discovered that ACF pathways regulate diverse processes [9] such as cell development [10,11], biofilm formation [12], exopolysaccharide production [13], and flagellum biosynthesis [14]. ACF signaling pathways employ a core structure of the E. coli chemotaxis system but are also diversified in regards to components that are phosphorylated downstream of CheA. Of the many complex Che-like ACF systems as revealed by genome sequencing, only a very few have been analyzed for function. Their frequency of occurrence, coupled with the diversity of phosphoryatable components that they encode, suggests that we are just scratching the surface on understanding complexities of Che-like ACF signal transduction.
Recent publications have appeared that address localization, phosphate flow, regulator modification and output functions of ACF systems in Myxococcus xanthus, Rhodospirillum centenum, Azospirillum brasilense, and Pseudomonas aeruginosa. This review will focus on the ACF class of chemosensory pathways in the aforementioned microbes that control cyst and biofilm formation. We will highlight deviation of these signaling pathways from the classical chemotaxis architecture to facilitate the control of processes other than chemotaxis.
Che Regulation of Myxospore Development in Myxococcus xanthus
Myxococcus xanthus is a soil bacterium that upon starvation aggregates to form mounds and subsequently fruiting bodies (Figure 1A), which are comprised of desiccation- and heat-resistant myxospores. During this process, aggregation, fruiting body formation, myxospore development, and motility are highly coordinated. Motility of M. xanthus does not involve flagella and instead uses gliding motility for directed movement. Two independent systems regulate gliding motility in M. xanthus: social (S-) motility, which utilizes type IV pili [15], and adventurous (A-) motility, which utilizes helical motors along the cell body [16].
Figure 1.
Bacterial species with metabolic versatility. (A) Myxococcus xanthus fruiting bodies (yellow), courtesy of Gregory Velicer, ETH Zurich. (B) Encysting Rhodospirillum centenum cysts (brown) among vegetative parent cells (green). (C) Flocculating Azospirillum brasilence (purple). (D) Pseudomonus aeruginosa biofilm (green) on mouse trachea, courtesy of Thomas Moninger, University of Iowa Central Microscopy Research Facilities, Iowa City, IA, USA.
In this species there are a remarkable 8 chemosensory systems coded for in the genome. The Frz (Che1) signaling cascade controls the frequency of cell reversals, which is coupled with the S- and A-motility engines via the response regulator FrzZ. FrzZ is only phosphorylated and localizes to the leading cell pole when cells are gliding on surfaces [17]. The Dif (Che2) signaling cascade controls exopolysaccharide (EPS) biosynthesis [13,18], which is needed for pili retraction during fruiting body formation. The Che3 signaling cascade controls developmental gene expression during sporulation [10]. Additionally, there is crosstalk between Dif and Frz [19] to mediate chemotactic responses to phosphatidylethanolamine lipids, underscoring the interconnectivity and complexity of these signaling networks. Detailed biochemical analysis of these chemosensory systems is ongoing with the Dif and Che3 systems best characterized as described below.
Dif System
The Dif signal transduction pathway (Figure 2A) controls EPS production, which is essential for fruiting body formation and spore formation in response to starvation [20]. This ACF system consists of homologs of MCP (DifA), CheW (DifC), CheA (DifE), and CheY (DifD). Instead of using the CheB/CheR adaptation mechanism, the Dif system is equipped with DifG, a protein homologous to the phosphatase CheC in Bacillus subtilis that is thought to dephosphorylate DifD. Deletion of the MCP-like difA or CheA-like difE abolishes EPS synthesis [13,18] while loss of CheY-like difD or its phosphatase difG causes EPS overproduction [13]. difE null mutations are also epistatic to difD and difG mutations in EPS production [21]. Consequently, it is proposed that DifD functions as a phosphate sink by removal of phosphoryl groups from DifE~P to create DifD~P. DifG subsequently regenerates unphosphorylated DifD by functioning as a DifD~P phosphatase. This model is supported by in vivo experiments which shows an interaction between DifD and DifG in two hybrid experiments and in vitro where biochemically isolated DifG dephosphorylates DifD~P [22]. Furthermore, since the CheY-like DifD functions as a negative regulator of EPS biosynthesis, it has been hypothesized that unidentified mediators exist that relay a signal transduced from DifE to produce an uncharacterized output [21] (Figure 2A).
Figure 2.
Che pathways in Myxococcus xanthus. (A) The Dif signal transduction pathway. The Dif system lacks homologs of CheB and CheR. The CheY homolog DifD serves as a phosphate sink to the CheA homolog DifE, which is proposed to have unidentified downstream partners that control exopolysaccharide production. A CheC homolog DifG functions as a phosphatase of DifD. (B) The Che3 signal transduction pathway. The Che3 system controlling developmental gene expression during fruiting body formation involves two gene clusters, che3 and crdS. CheA3 negatively regulates the CrdS-CrdA TCS by functioning as a phosphatase to the response regulator CrdA. As part of the che3 and crdS gene clusters, uncharacterized peptidylglycan-binding protein CrdB and penicillin-binding protein Pbp1A may provide additional inputs into the Che3 pathway.
Che3 System
The che3 gene cluster that controls myxospore development encodes homologs of two MCPs (MCP3A and MCP3B), CheB (CheB3), CheR (CheR3), CheW (CheW3), a CheA-CheY hybrid (CheA3), and a periplasmic lipoprotein-peptidoglycan binding protein hybrid CrdB (Figure 2B). Unlike the majority of the bacterial chemosensory systems, Che3 lacks a CheY homolog. Interestingly, immediately upstream and divergently transcribed from the che3 lociisa gene encoding a RR CrdA, which was shown to form a TCS with an HK CrdS encoded several kbs away from the che3 cluster (Figure 2B). [23] When cultured on starvation medium, crdS and crdA mutants are both delayed in cell aggregation while the cheA3 mutant aggregates prematurely [23]. CheA3 interacts with CrdA strongly in a yeast two-hybrid assay [10] and reduces the half-life of CrdA~P 6-fold [23]. Since CheA3 fails to autophosphorylate in vitro [23] it is proposed to function as a CrdA phosphatase. CrdB is thought to transmit envelope stress to MCP3A/3B to control the phosphatase activity of CheA3 (Figure 2B). Another periplasmic peptidoglycan binding protein Pbp1A is encoded by the crdS cluster that may feed input into the CrdS-CrdA TCS by interacting with CrdS to regulate its ability to phosphorylate CrdA and thus development.
The Che3 system therefore represents an interesting interaction between a chemosensory-like signaling system with a more typical TCS that is not part of the che3 gene cluster (Figure 2B). This highlights the versatility of ACF systems through the interaction with different signal transduction pathways.
Che Regulation of Cyst Cell Development in Rhodospirillum centenum
Rhodospirillum centenum is a photosynthetic member of the Azospirillum clade that possesses three chemotaxis-like pathways: the Che1 cascade that controls chemotaxis [24], the Che2 signaling cascade that regulates flagella biosynthesis [14], and the Che3 signaling pathway that regulates cyst formation [11,25]. Cyst formation is a unique survival mechanism employed by several nitrogen-fixing bacteria [26,27] that promotes transition into a dormant state upon starvation. Cyst cell development involves dramatic morphological changes in cells: shedding of flagella, cell rounding, formation of polyhydroxybutyrate granules as energy storage, formation of the exine layer consisting of lipoproteins and exopolysaccharides, and cell clustering [5]. Mature cysts (Figure 1B) are resistant to a variety of environmental stresses including heat and desiccation [5], and can germinate to regenerate vegetative cells as nutrients become available [5].
Che3 System
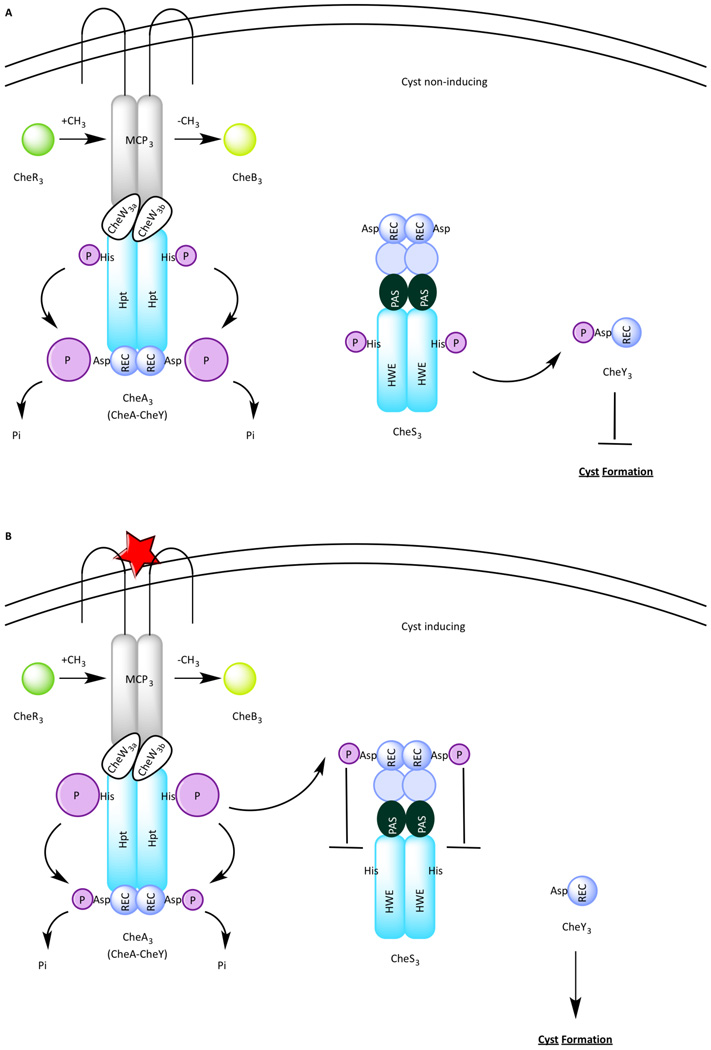
The Che3 signal transduction cascade (Figure 3) represents a unique deviation from the E. coli paradigm. This signaling cascade contains a MCP (MCP3), CheB (CheB3), CheR (CheR3) two CheWs (CheW3a and CheW3b), CheY (CheY3) and a second histidine kinase (CheS3). Strains deleted of mcp3, cheW3a, cheW3b, cheR3, and cheA3 exhibit a disruption of cyst formation [11] and are thus called hypo-cyst strains. In contrast, in-frame deletions of cheS3, cheY3, and cheB3 produce a hyper-cyst phenotype characterized by premature formation of cysts [11]. CheY3 is phosphorylated not by CheA3 as occurs in the E. coli paradigm, but is instead phosphorylated by CheS3 [25] (Figure 3A). The incorporation of a separate sensor kinase to pair with CheY resembles the aforementioned Che3 system in M. xanthus. However unlike Che3 system, CheA3 in R. centenum serves a function other than a phosphatase. Genetic and biochemical studies indicate that under cyst inducing growth conditions, CheA3 functions as a negative regulator of the flow of phosphoryl groups from CheS3 to CheY3 [25] (Figure 3B). This occurs through phosphorylation of a regulatory REC domain of CheS3 by CheA3 [25]. Once the CheS3 REC domain is phosphorylated the ability of CheS3 to phosphorylate CheY3 is impeded (Figure 3B).
Figure 3.
The Che3 pathway in Rhodospirillum centenum. (A) Under cyst non-inducing growth conditions, CheS3 phosphorylates CheY3 to repress cyst formation. (B) Under cyst inducing growth conditions, MCP3 receives a signal thus activating CheA3, which in turn phosphorylates a receiver domain of CheS3, leading to inhibition of the CheS3-CheY3 TCS.
Che Regulation of Cell Clumping in Azospirillum brasilense
Azospirillum brasilense is a soil bacterium that closely associates with plant roots [28] and promotes root hair growth by synthesis of several plant hormones [29]. As a microaerophile and diazotrophile, A. brasilense exhibits strong aerotactic behaviors that guide the cells to a low oxygen niche that is optimal for nitrogen fixation [30]. A striking feature of A. brasilense physiology is that under persisting high aeration, mobile cells with single polar flagella form transient clumps at the non-flagellated pole [31] followed by flocculation, which is characterized by massive aggregation of desiccation-resistant cyst cells [3] (Figure 1C). One of the chemosensory clusters in A. brasilenseche3, which is orthologous to the che3 cluster in closely related R. centenum [32], is predicted to control flocculation and cyst formation in A. brasilense. Additionally, another chemotaxis cluster che1 was shown to regulate EPS production, which was hypothesized to promote clumping and flocculation in A. brasilense [3].
Che1 System
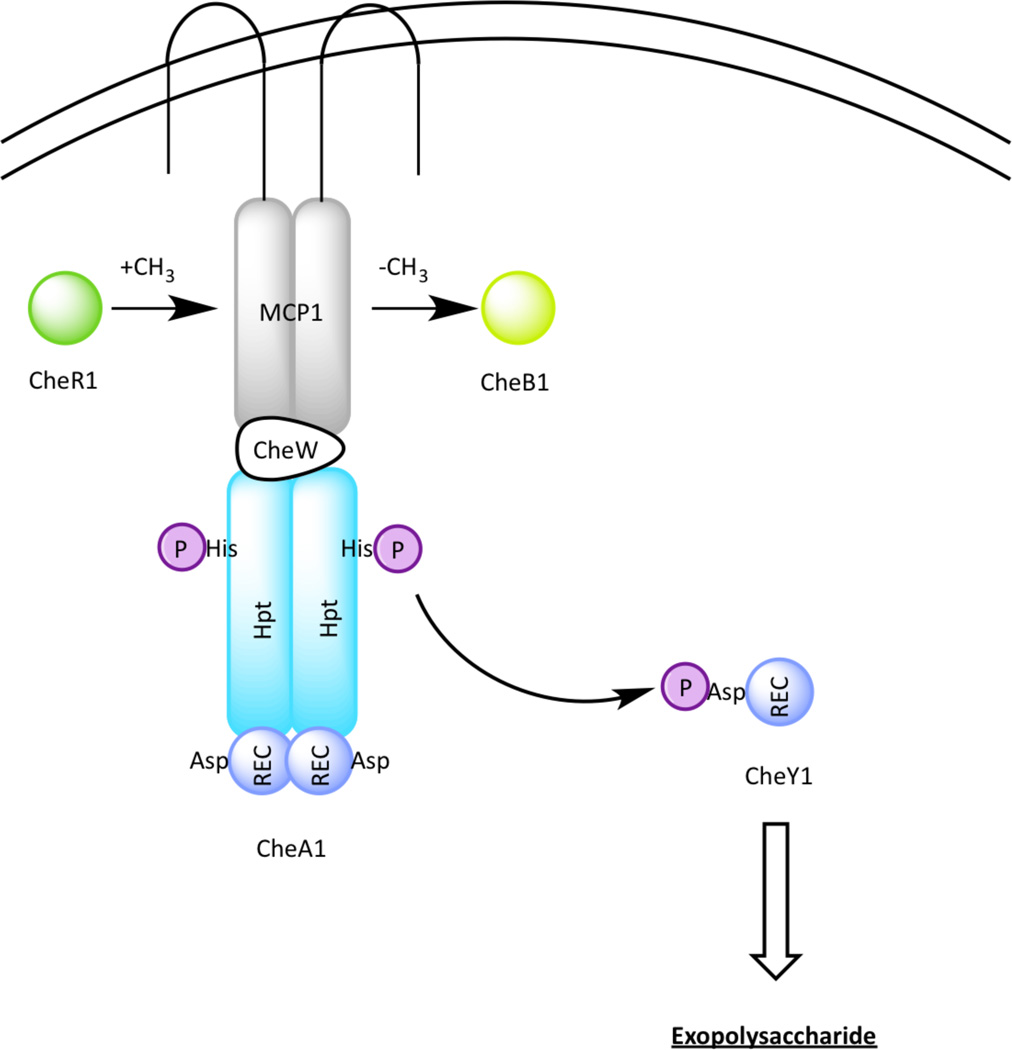
The che1 genes were first identified because of their ability to complement motile but non-chemotactic mutants [33]. However, strains lacking cheA1 or cheY1 displayed reduced chemotaxis whereas those lacking the che1 operon showed increased chemotaxis [34]. These observations suggest that the Che1 signal transduction cascade (Figure 4) has a minor and likely indirect role in chemotactic behavior. Supporting this hypothesis, subsequent studies have shown that che1 mutants exhibited defects in EPS production when grown under flocculation-inducing conditions [34]. Strains containing deletions in genes cheA1 or cheY1 bind significantly more Congo red, an EPS binding dye, while strains deleted of cheB1-cheR1 or che1 bind significantly less to Congo red than wild-type cells[12,34].
Figure 4.
The Che1 pathway in Azospirillum brasilence. The Che1 pathway plays minor roles in chemotaxis and aerotaxis and to modulate production of exopolysaccharide, which is required for clumping and flocculation.
The hypothesis that EPS promotes flocculation is supported by the flocculation defects in che1 mutants. Deletion strains ΔcheA1 and ΔcheY1, which overproduce EPS quantitatively form more aggregation than do wild-type cells [34,35], while EPS-defective ΔcheB1-cheR1 mutant showed virtually no flocculation [31]. Interestingly, ΔcheY1 clumps later and flocculates earlier than ΔcheA1 [31], which indicate that more signal transduction pathways may be integrated into Che1. An explanation to why EPS is a prerequisite to flocculation was provided by Bible et al, who demonstrated a positive correlation between clumping and swimming velocity [31]. They proposed that EPS synthesis on the cell surface might modulate clumping by adjusting adhesive cell surface properties. More insights on the signaling events that relay within the Che1 pathway would necessitate biochemical characterization of the individual protein components.
Che Regulation of Biofilm Formation in Pseudomonas aeruginosa
Pseudomonas aeruginosa is metabolically versatile and opportunistic pathogen that infects insects, plants, animals and immunocompromised humans, and has become a significant health risk as a hospital-acquired infection. To support growth in a wide variety of natural habitats, this species possesses a large pool of TCS proteins [36] and in addition contains four chemosensory pathways that mediate chemotaxis [37–39], swarming motility [40], type IV pili (TFP) synthesis, TFP-dependent twitching motility [41,42], virulence gene expression [42,43], and biofilm formation [12,40]. Biofilms are implicated in many chronic infectious diseases and are technically challenging to eradicate due to a highly heterogeneous and protective architecture. Therefore, P. aeruginosa is an ideal model organism for studying pathogenicity in general and for biofilm formation (Figure 1D). Extensive studies conducted on P. aeruginosa have shown that Wsp and Chp constitute ACF signaling pathways that regulate biofilm formation and virulence, respectively.
Wsp System
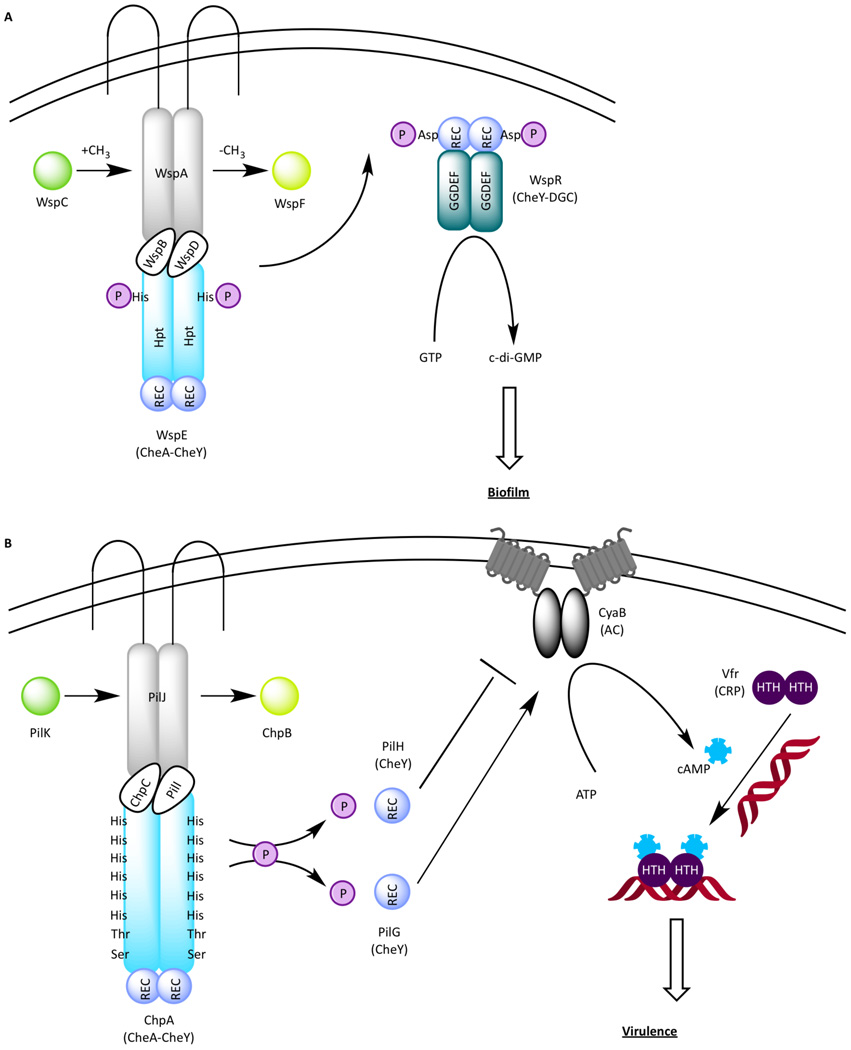
The Wsp chemosensory system (Figure 5A) consists of seven chemotaxis proteins comprised of homologs of MCP (WspA), CheR (WspC), CheB (WspF), two CheWs (WspD and WspB), CheA-CheY hybrid (WspE), and a novel response regulator WspR. Interestingly, WspR has a REC domain at its N-terminus followed by a diguanylate cyclase (DGC) GGDEF domain that produces cyclic di-guanosine monophosphate (c-di-GMP) [12]. C-di-GMP is a small signaling molecule that coordinates the life style transition from the planktonic motile state to the sessile “cooperative living” in a biofilm community in many bacterial species [44–48]. The planktonic and sessile lifestyles of P. aeruginosa also correlate with acute and chronic infections in humans. When phosphorylated by WspE, a yellow fluorescent protein (YFP)-tagged WspR (WspR-YFP~P) forms bright clusters in vivo [49]. These clusters contain an estimated 20 WspR-YFP~P tetramers [50], which are believed to have the highest level of DGC activity [50]. WspA is a membrane-bound MCP homolog that forms clusters at both polar and lateral locations [51]. Although the exact signal sensed by WspA is currently unknown, WspR-YFP clusters form in surface-grown cells, suggesting that the Wsp pathway responds to solid surfaces [49]. WspF is a predicted CheB-like methylesterase that by analogy to CheB in E. coli removes methyl groups from WspA, thereby resetting the Wsp system to a pre-stimulus state. A null wspF mutant, in which WspA is presumably permanently methylated leading to increased phosphorylation of WspR by WspE, exhibits increased WspR-YFP clustering [12,49], elevated c-di-GMP production [12], reduced swarming [49], and premature biofilm formation [12], as manifested by wrinkled colony morphology [49]. Collectively, these data suggest that the Wsp signal transduction pathway controls biofilm formation by modulating c-di-GMP levels in response to growth on surfaces.
Figure 5.
(A) The Wsp signal transduction pathway. A TCS used by the Wsp system consists of WspE, a CheA-CheY hybrid, and WspR containing a N-terminal REC domain followed by a GGDEF module with diguanylate cyclase activity. WspR produces c-di-GMP, which in turn promotes biofilm formation in P. aeruginosa. (B) The Chp signal transduction pathway. Two CheY homologs PilH and PilG differentially regulate the activity of an adenylate cyclase (AC), which produces cAMP to control virulence via Vfr, a transcription factor belonging to the cAMP receptor protein (CRP) family. The novel kinase ChpA contains nine predicted phosphorylatable sites, including six conserved histidines located in Hpt domains, and another two located in Hpt-like domains where the conserved histidines are substutituted with a threonine and a serine, respectively.
Che Regulation of Other ACF Pathways
As more signaling systems are investigated, the list of diverse cellular processes controlled by ACF systems are undoubtedly expanding well beyond cyst cell and biofilm development. One characterized example is the Chp signal transduction cascade that controls the production of a second messenger cyclic adenosine monophosphate (cAMP), which ultimately modulate gene expression of virulence factors.
Chp System
The Chp chemosensory system (Figure 5B) controls P. aeruginosa virulence by modulating the activity of CyaB, anadenylate cyclase (AC). It consists of eight proteins that are homologs of MCP (PilJ), CheR (PilK), CheB (ChpB), two CheWs (PilI and ChpC), two CheYs (PilG and PilH), and a CheA-CheY hybrid (ChpA). Two additional proteins ChpD (an AraC family transcriptional regulator) and ChpE (a putative integral membrane protein) encoded by open reading frames downstream of chpC may also be part of the pathway [41].
The CheA-like kinase ChpA is a novel 269 kDa CheA-like protein with 9 potential phosphorylation sites: in addition to a C-terminal REC domain, there are 8 histidine phosphotransfer (Hpt) domains each containing a phosphorylatable histidine residue and two Hpt-like domains containing a threonine and a serine substitutions that can also be potentially phosphorylated [41]. Of the 9 predicted phosphorylation domains in ChpA, the REC domain is essential for pilin-based twitching motility, with Hpt domains Hpt2 and Hpt3 having a minor contribution for twitching motility [52]. The MCP homolog pilJ, the CheW homolog chpC, and the two CheY homologs pilG, and pilH were initially thought to regulate twitching motility because mutations in these genes resulted in reduced (for pilJ, chpA, and pilG mutants) or elevated (for pilH mutant) pilin production. However, further studies indicated that the Chp chemonsenosry pathway indirectly impacts type IV pili biosynthesis/twitching motility as a result of a primary defect in cAMP production. In P. aeruginosa, the two adenylate cyclases (ACs) CyaA and CyaB are responsible for converting ATP to the second messenger molecule cAMP with CyaB being the major contributor [42]. The two CheY-like components in the Chp pathway, PilG and PilH, have opposing effects on the enzymatic activity of CyaB with PilG stimulating cAMP production and PilH impeding cAMP production. P. aeruginosa strains with individual chp gene deletions in a cyaA null background have either elevated levels of intracellular cAMP and surface pilin production (pilH) or reduced levels of intracellular cAMP and surface pilin (pilG, pilI, pilJ, and chpA) relative to the parent cyaA strain. Underproduction of pilin in each of these mutants can be complemented by exogenous addition of cAMP [42]. cAMP ultimately affects pilin synthesis by binding to a transcription factor of the cAMP receptor protein (CRP) family called Vfr [43]. Very little is known about how single-domain CheYs that contain no output domain function in organisms other than E. coil where the interaction of CheY with the flagellar switch is well characterized. Thus, future studies of how this pair of CheY homologs (PilG and PilH) differentially affect the AC activity of CyaB are warranted.
Summary
Many genomes contain multiple chemosensory gene clusters that exhibit considerable diversity from the E. coli chemotaxis paradigm. The vast majority of these additional Che-like gene clusters have not been studied so we are just starting to understand the cellular processes that are regulated by these systems as well as the means that they do so. The challenges going forward will be to obtain a much better understanding of the function and molecular details of ACF systems in the many uncharacterized species that contain these signaling cascades.
The recent genetic and biochemical analyses of ACF signaling pathways from a variety of Gram-negative species demonstrate surprising complexity and versatility. The E. coli paradigm seems to be a good model for the MCP-CheW-CheA complex that modifies kinase activity over a wide range of ligand concentrations. However, the E. coli model diverges beyond this point as CheA does not phosphorylate CheY homologs in several ACF systems such as the M. xanthus Che3 and R. centenum Che3 signaling cascades. In these systems described above, the chemosensory components regulate the flow of phosphoryl groups in a companion downstream two-component system. Also discussed are cases where chemosensory components modify downstream proteins that are involved in the production of small signaling metabolites such as cAMP and c-di-GMP. In these cases the Che-like system seems to function as upstream overarching regulator of a more distinct downstream signaling pathway.
One advantage of having ACF Che-like components (specifically MCP-CheW-CheA components) regulate downstream signaling events is the capability of MCP chemoreceptors to be tuned to different ligand concentrations in response to methylation and de-methylation by CheR and CheB homologs, respectively. Furthermore, individual CheW-CheA homologs are also capable of interacting with multiple MCP chemoreceptors providing a wide diversity input signals and a wide diversity of ligand concentrations for the control of ACF signaling pathways. For example, the R. centenum genome has three distinct Che-like gene clusters that code for three CheA homologs as well as 46 annotated MCP receptors that are scattered throughout the genome. The vast majority of these MCPs remain uncharacterized but their presence clearly indicates that these three distinct chemosensory signaling pathways are regulated by a very wide diversity of input signals and input strengths. Clearly the Che3 cascade has evolved to utilize multiple MCPs to ensure that cyst development is only induced under conditions that are unfavorable for vegetative growth. Going forward, an understanding of the involvement of multiple input signals as provided by such MCP diversity will have to be addressed.
Box 2: Outstanding Questions.
What are functions of additional Che clusters that are present in many other species of bacteria that have not yet been analyzed?
What are the signals sensed by organisms with multiple Che systems that contain dozens of membrane-spanning and cytosolic chemoreceptors?
How do single domain CheY homologs that lack output domains execute cellular functions? Do they resemble the E. coli CheY and interact with structural proteins or do they interact with enzymes to alter their functions?
Is there cross talk between chemotaxis and ACF pathways in organisms with multiple Che systems?
Are there additional two-component systems (e.g. photoreceptors) that either feed into or are regulated by chemosensoty systems to achieve complex signal integration?
Acknowledgements
We are grateful to former members of the Bauer laboratory, especially James E. Berleman, Johnathan W. Willett, and Jeremiah N. Marden, for many helpful discussions. Our research is supported by a grant from the National Institutes of Health R01 GM099703 to C.E.B.
References
- 1.Sudo SZ, Dworkin M. Resistance of vegetative cells and microcysts of Myxococcus xanthus. J Bacteriol. 1969;98:883–887. doi: 10.1128/jb.98.3.883-887.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadasivan L, Neyra CA. Cyst production and brown pigment formation in aging cultures of Azospirillum brasilense ATCC 29145. J Bacteriol. 1987;169:1670–1677. doi: 10.1128/jb.169.4.1670-1677.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadasivan L, Neyra CA. Flocculation in Azospirillum brasilense and Azospirillum lipoferum: exopolysaccharides and cyst formation. J Bacteriol. 1985;163:716–723. doi: 10.1128/jb.163.2.716-723.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tal S, Okon Y. Production of the reserve material poly-β-hydroxybutyrate and its function in Azospirillum brasilense Cd. Can J Microbiol. 1985;31:608–613. [Google Scholar]
- 5.Berleman JE, Bauer CE. Characterization of cyst cell formation in the purple photosynthetic bacterium Rhodospirillum centenum. Microbiology (Reading, Engl) 2004;150:383–390. doi: 10.1099/mic.0.26846-0. [DOI] [PubMed] [Google Scholar]
- 6.Garduño RA, et al. Intracellular growth of Legionella pneumophila gives rise to a differentiated form dissimilar to stationary-phase forms. Infection and Immunity. 2002;70:6273–6283. doi: 10.1128/IAI.70.11.6273-6283.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewis K. Persister cells. Annu Rev Microbiol. 2010;64:357–372. doi: 10.1146/annurev.micro.112408.134306. [DOI] [PubMed] [Google Scholar]
- 8.Wuichet K, Zhulin IB. Origins and diversification of a complex signal transduction system in prokaryotes. Sci Signal. 2010;3:ra50. doi: 10.1126/scisignal.2000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirby JR. Chemotaxis-like regulatory systems: unique roles in diverse bacteria. Annu Rev Microbiol. 2009;63:45–59. doi: 10.1146/annurev.micro.091208.073221. [DOI] [PubMed] [Google Scholar]
- 10.Kirby JR, Zusman DR. Chemosensory regulation of developmental gene expression in Myxococcus xanthus. Proc Natl Acad Sci USA. 2003;100:2008–2013. doi: 10.1073/pnas.0330944100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berleman JE, Bauer CE. Involvement of a Che-like signal transduction cascade in regulating cyst cell development in Rhodospirillum centenum. Molecular Microbiology. 2005;56:1457–1466. doi: 10.1111/j.1365-2958.2005.04646.x. [DOI] [PubMed] [Google Scholar]
- 12.Hickman JW, et al. A chemosensory system that regulates biofilm formation through modulation of cyclic diguanylate levels. Proc Natl Acad Sci USA. 2005;102:14422–14427. doi: 10.1073/pnas.0507170102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Black WP, Yang Z. Myxococcus xanthus chemotaxis homologs DifD and DifG negatively regulate fibril polysaccharide production. J Bacteriol. 2004;186:1001–1008. doi: 10.1128/JB.186.4.1001-1008.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berleman JE, Bauer CE. A che-like signal transduction cascade involved in controlling flagella biosynthesis in Rhodospirillum centenum. Molecular Microbiology. 2005;55:1390–1402. doi: 10.1111/j.1365-2958.2005.04489.x. [DOI] [PubMed] [Google Scholar]
- 15.Zusman DR, et al. Chemosensory pathways, motility and development in Myxococcus xanthus. Nat Rev Microbiol. 2007;5:862–872. doi: 10.1038/nrmicro1770. [DOI] [PubMed] [Google Scholar]
- 16.Nan B, et al. Flagella stator homologs function as motors for myxobacterial gliding motility by moving in helical trajectories. Proc Natl Acad Sci USA. 2013;110:E1508–E1513. doi: 10.1073/pnas.1219982110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaimer C, et al. Chemosensory signaling controls motility and subcellular polarity in Myxococcus xanthus. Curr Opin Microbiol. 2012;15:751–757. doi: 10.1016/j.mib.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Z, et al. Myxococcus xanthus dif genes are required for biogenesis of cell surface fibrils essential for social gliding motility. J Bacteriol. 2000;182:5793–5798. doi: 10.1128/jb.182.20.5793-5798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu Q, et al. Independence and interdependence of Dif and Frz chemosensory pathways in Myxococcus xanthus chemotaxis. Molecular Microbiology. 2008;69:714–723. doi: 10.1111/j.1365-2958.2008.06322.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Z, et al. A new set of chemotaxis homologues is essential for Myxococcus xanthus social motility. Molecular Microbiology. 1998;30:1123–1130. doi: 10.1046/j.1365-2958.1998.01160.x. [DOI] [PubMed] [Google Scholar]
- 21.Black WP, et al. Type IV pili function upstream of the Dif chemotaxis pathway in Myxococcus xanthus EPS regulation. Molecular Microbiology. 2006;61:447–456. doi: 10.1111/j.1365-2958.2006.05230.x. [DOI] [PubMed] [Google Scholar]
- 22.Black WP, et al. Phosphorylation and dephosphorylation among Dif chemosensory proteins essential for exopolysaccharide regulation in Myxococcus xanthus. J Bacteriol. 2010;192:4267–4274. doi: 10.1128/JB.00403-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Willett JW, Kirby JR. CrdS and CrdA comprise a two-component system that is cooperatively regulated by the Che3 chemosensory system in Myxococcus xanthus. MBio. 2011;2 doi: 10.1128/mBio.00110-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang ZY, Bauer CE. Analysis of a chemotaxis operon from Rhodospirillum centenum. J Bacteriol. 1997;179:5712–5719. doi: 10.1128/jb.179.18.5712-5719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He K, et al. phosphate flow between hybrid histidine kinases CheA3 and CheS3 controls Rhodospirillum centenum cyst formation. PLoS Genet. 2013;9:e1004002. doi: 10.1371/journal.pgen.1004002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevenson LH, Socolofsky MD. Cyst formation and poly-beta-hydroxybutyric acid accumulation in Azotobacter. J Bacteriol. 1966;91:304–310. doi: 10.1128/jb.91.1.304-310.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Favinger J, et al. Rhodospirillum centenum, sp. nov., a thermotolerant cyst-forming anoxygenic photosynthetic bacterium. Antonie Van Leeuwenhoek. 1989;55:291–296. doi: 10.1007/BF00393857. [DOI] [PubMed] [Google Scholar]
- 28.Steenhoudt O, Vanderleyden J. Azospirillum a free-living nitrogen-fixing bacterium closely associated with grasses: genetic, biochemical and ecological aspects. Fems Microbiol Rev. 2000;24:487–506. doi: 10.1111/j.1574-6976.2000.tb00552.x. [DOI] [PubMed] [Google Scholar]
- 29.Bashan Y, et al. Azospirillum-plant relationships: physiological, molecular, agricultural, and environmental advances (1997–2003) Can J Microbiol. 2004;50:521–577. doi: 10.1139/w04-035. [DOI] [PubMed] [Google Scholar]
- 30.Zhulin IB, et al. Oxygen taxis and proton motive force in Azospirillum brasilense. J Bacteriol. 1996;178:5199–5204. doi: 10.1128/jb.178.17.5199-5204.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bible A, et al. The Azospirillum brasilense Che1 chemotaxis pathway controls swimming velocity, which affects transient cell-to-cell clumping. J Bacteriol. 2012;194:3343–3355. doi: 10.1128/JB.00310-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wisniewski-Dyé F, et al. Azospirillum genomes reveal transition of bacteria from aquatic to terrestrial environments. PLoS Genet. 2011;7:e1002430–e1002430. doi: 10.1371/journal.pgen.1002430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hauwaerts D, et al. A major chemotaxis gene cluster in Azospirillum brasilense and relationships between chemotaxis operons in alpha-proteobacteria. FEMS Microbiol Lett. 2002;208:61–67. doi: 10.1111/j.1574-6968.2002.tb11061.x. [DOI] [PubMed] [Google Scholar]
- 34.Bible AN, et al. Function of a chemotaxis-like signal transduction pathway in modulating motility, cell clumping, and cell length in the alphaproteobacterium Azospirillum brasilense. J Bacteriol. 2008;190:6365–6375. doi: 10.1128/JB.00734-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edwards AN, et al. Characterization of cell surface and extracellular matrix remodeling of Azospirillum brasilense chemotaxis-like 1 signal transduction pathway mutants by atomic force microscopy. FEMS Microbiol Lett. 2011;314:131–139. doi: 10.1111/j.1574-6968.2010.02156.x. [DOI] [PubMed] [Google Scholar]
- 36.Stover CK, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 37.Masduki A, et al. Isolation and characterization of chemotaxis mutants and genes of Pseudomonas aeruginosa. J Bacteriol. 1995;177:948–952. doi: 10.1128/jb.177.4.948-952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kato J, et al. Cloning and characterization of chemotaxis genes in Pseudomonas aeruginosa. Biosci. Biotechnol. Biochem. 1999;63:155–161. doi: 10.1271/bbb.63.155. [DOI] [PubMed] [Google Scholar]
- 39.Ferrández A, et al. Cluster II che genes from Pseudomonas aeruginosa are required for an optimal chemotactic response. J Bacteriol. 2002;184:4374–4383. doi: 10.1128/JB.184.16.4374-4383.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caiazza NC, et al. Inverse regulation of biofilm formation and swarming motility by Pseudomonas aeruginosa PA14. J Bacteriol. 2007;189:3603–3612. doi: 10.1128/JB.01685-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitchurch CB, et al. Characterization of a complex chemosensory signal transduction system which controls twitching motility in Pseudomonas aeruginosa. Molecular Microbiology. 2004;52:873–893. doi: 10.1111/j.1365-2958.2004.04026.x. [DOI] [PubMed] [Google Scholar]
- 42.Fulcher NB, et al. The Pseudomonas aeruginosa Chp chemosensory system regulates intracellular cAMP levels by modulating adenylate cyclase activity. Molecular Microbiology. 2010;76:889–904. doi: 10.1111/j.1365-2958.2010.07135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fuchs EL, et al. The Pseudomonas aeruginosa Vfr regulator controls global virulence factor expression through cyclic AMP-dependent and -independent mechanisms. J Bacteriol. 2010;192:3553–3564. doi: 10.1128/JB.00363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simm R, et al. GGDEF and EAL domains inversely regulate cyclic di-GMP levels and transition from sessility to motility. Molecular Microbiology. 2004;53:1123–1134. doi: 10.1111/j.1365-2958.2004.04206.x. [DOI] [PubMed] [Google Scholar]
- 45.Tischler AD, Camilli A. Cyclic diguanylate (c-di-GMP) regulates Vibrio cholerae biofilm formation. Molecular Microbiology. 2004;53:857–869. doi: 10.1111/j.1365-2958.2004.04155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thormann KM, et al. Control of formation and cellular detachment from Shewanella oneidensis MR-1 biofilms by cyclic di-GMP. J Bacteriol. 2006;188:2681–2691. doi: 10.1128/JB.188.7.2681-2691.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sisti F, et al. Cyclic-di-GMP signalling regulates motility and biofilm formation in Bordetella bronchiseptica. Microbiology (Reading, Engl) 2013;159:869–879. doi: 10.1099/mic.0.064345-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zogaj X, et al. Cyclic di-GMP stimulates biofilm formation and inhibits virulence of Francisella novicida. Infection and Immunity. 2012;80:4239–4247. doi: 10.1128/IAI.00702-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Güvener ZT, Harwood CS. Subcellular location characteristics of the Pseudomonas aeruginosa GGDEF protein, WspR, indicate that it produces cyclic-di-GMP in response to growth on surfaces. Molecular Microbiology. 2007;66:1459–1473. doi: 10.1111/j.1365-2958.2007.06008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huangyutitham V, et al. Subcellular clustering of the phosphorylated WspR response regulator protein stimulates its diguanylate cyclase activity. MBio. 2013;4:e00242–e00313. doi: 10.1128/mBio.00242-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.O'Connor JR, et al. Surface sensing and lateral subcellular localization of WspA, the receptor in a chemosensory-like system leading to c-di-GMP production. Molecular Microbiology. 2012;86:720–729. doi: 10.1111/mmi.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leech AJ, Mattick JS. Effect of site-specific mutations in different phosphotransfer domains of the chemosensory protein ChpA on Pseudomonas aeruginosa motility. J Bacteriol. 2006;188:8479–8486. doi: 10.1128/JB.00157-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wadhams GH, Armitage JP. Making sense of it all: bacterial chemotaxis. Nat. Rev. Mol. Cell Biol. 2004;5:1024–1037. doi: 10.1038/nrm1524. [DOI] [PubMed] [Google Scholar]
- 54.Hazelbauer GL, Lai W-C. Bacterial chemoreceptors: providing enhanced features to two-component signaling. Curr Opin Microbiol. 2010;13:124–132. doi: 10.1016/j.mib.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hazelbauer GL, et al. Bacterial chemoreceptors: high-performance signaling in networked arrays. Trends Biochem. Sci. 2008;33:9–19. doi: 10.1016/j.tibs.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Springer WR, Koshland DE. Identification of a protein methyltransferase as the cheR gene product in the bacterial sensing system. Proc Natl Acad Sci USA. 1977;74:533–537. doi: 10.1073/pnas.74.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Springer MS, et al. Protein methylation in behavioural control mechanisms and in signal transduction. Nature. 1979;280:279–284. doi: 10.1038/280279a0. [DOI] [PubMed] [Google Scholar]
- 58.Porter SL, et al. Signal processing in complex chemotaxis pathways. Nat Rev Microbiol. 2011;9:153–165. doi: 10.1038/nrmicro2505. [DOI] [PubMed] [Google Scholar]