Abstract
The isothiocyanate (ITC) sulforaphane (SFN) was shown at low levels (1–5 µM) to promote cell proliferation to 120–143% of the controls in a number of human cell lines, whilst at high levels (10–40 µM) it inhibited such cell proliferation. Similar dose responses were observed for cell migration, i.e. SFN at 2.5 µM increased cell migration in bladder cancer T24 cells to 128% whilst high levels inhibited cell migration. This hormetic action was also found in an angiogenesis assay where SFN at 2.5 µM promoted endothelial tube formation (118% of the control), whereas at 10–20 µM it caused significant inhibition. The precise mechanism by which SFN influences promotion of cell growth and migration is not known, but probably involves activation of autophagy since an autophagy inhibitor, 3-methyladenine, abolished the effect of SFN on cell migration. Moreover, low doses of SFN offered a protective effect against free-radical mediated cell death, an effect that was enhanced by co-treatment with selenium. These results suggest that SFN may either prevent or promote tumour cell growth depending on the dose and the nature of the target cells. In normal cells, the promotion of cell growth may be of benefit, but in transformed or cancer cells it may be an undesirable risk factor. In summary, ITCs have a biphasic effect on cell growth and migration. The benefits and risks of ITCs are not only determined by the doses, but are affected by interactions with Se and the measured endpoint.
Introduction
The term ‘hormesis’ is often used by toxicologists to refer to a ‘biphasic dose response to an environmental agent characterized by low dose stimulation and by high dose inhibitory or toxic effect’ [1], [2]. The hormesis concept is the most fundamental dose-response relationship in the biomedical, nutrition and toxicological sciences [1]. In a comprehensive review, Calabrese provided evidence that more than a hundred anti-tumour agents enhanced the proliferation of human tumour cells at low doses in a manner fully consistent with the hormetic dose-response relationship [2]. One of the interesting characteristics of such dose-responses was that they occurred in most types of tumour cells and were independent of organ. Recent findings suggest that some phytochemicals exhibit biphasic dose responses in cells with low doses activating signalling pathways that result in increased expression of genes encoding cytoprotective proteins and antioxidant enzymes [3]. The dietary hormetic compounds identified so far include resveratrol, epigallocatechin gallate (EGCG), curcumin, quercetin, allicin, capsaicin, carnosic acid and sulforaphane (SFN) [4]–[8]. From an evolutionary perspective, the noxious properties of phytochemicals have an important protective role in dissuading insects and fungi from damaging plants. However, the relatively small doses of phytochemicals ingested by humans that consume these plants are not toxic and instead induce mild cellular stress responses. This phenomenon has been widely described as ‘hormesis’ or adaptive dose response in the fields of biology and medicine [4], [9], [10].
The isothiocyanate (ITC), SFN (4-methylsulfinylbutylisothiocyanate), was first isolated from the commonly-consumed cruciferous vegetable, broccoli and is one of the most potent naturally-occurring inducers of the Kelch-like ECH-associated protein 1 (Keap1)-nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant response elements (ARE) pathway [11]. The induction of Nrf2 protects normal cells from free-radical mediated oxidative stress via upregulation of chemoprotective genes, and the action of SFN is based on its ability to induce a Nrf2-driven enzyme quinone reductase (NQO1) [12]. In the 20 years subsequent to its discovery, the protective effects of SFN have been demonstrated in various cell culture systems and animal models, with the result that SFN is by far the most extensively studied ITC from cruciferous vegetables. The anti-carcinogenic mechanisms of ITCs have also been well-documented, including up-regulation of phase II detoxification enzymes, anti-inflammation, promotion of cell cycle arrest and apoptosis [13]–[17]. During the last decade, Keap1-Nrf2-ARE has been considered as a critical anti-cancer pathway in chemoprevention [18]-[20]. However, more recently, there have been some deleterious reports of Nrf2, including promotion of tumour cell growth and chemoresistance [21]–[25]. In order to survive, cancer cells may hijack the Nrf2 pathway which upregulates a battery of antioxidant enzymes, thereby maintaining a favourable redox balance in order to acquire malignant properties [26]. Overexpression of Nrf2 could enhance cell proliferation and cause resistance to chemotherapeutic interventions in some types of cancer, including human lung and pancreatic cancers [27], [28]. A few previous investigations have shown that SFN exhibits a dose-dependent effects on cell proliferation in cultured tumour cell lines and normal cells including human mesenchymal stem cells [29]–[31]. In the present study, we showed that SFN exhibited a hormetic dose response on cell growth, migration and angiogenesis. Whether the hormetic effect is beneficial or harmful depends on the selected endpoint and/or the nature of the cells (normal or tumour). Although the term hormesis is employed by toxicologists to describe a bell-shaped dose response, characterized by a beneficial effect at low doses and a toxic (or inhibitory) activity at high doses, this expression of low dose benefit might not be true for the effect of ITCs in cancer chemoprevention. Since hormesis shows little selectivity, the biological effects of ITCs on normal cells and tumour cells will differ. From this perspective, a low dose effect of ITCs in promoting tumour cell proliferation and migration in animal models must be evaluated prudently. Thus, a precise strategy that aims to optimise the beneficial effects and minimise the risk of ITCs should be developed with care in relation to cancer prevention and treatment.
Results
Effects of ITCs on cell growth
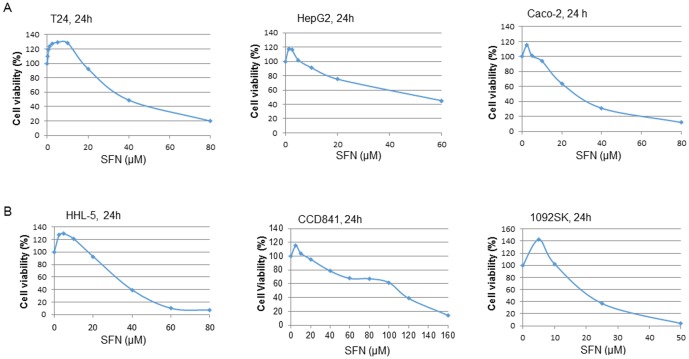
Due to the nature of the hormetic dose response, there is no selectivity of ITCs on cell growth, so it is likely that ITCs can promote tumour cell growth at low doses. In several in vitro cell culture studies, low concentrations of SFN have been shown to promote tumour cell growth, but no detailed discussion or suggestions for follow-up studies to investigate the mechanisms were provided [32]–[34]. At low concentrations, ITCs have been shown to induce proliferation and/or protect cells against a toxic agent, H2O2, in Caco-2 cells [30] and in hepatocytes [29]. Fig. 1A shows the effects of SFN on cell growth, with lower doses (1–5 µM) promoting cell growth (20–43% greater than the control) and high doses (10–40 µM) inhibiting cell growth in a number of tumour cell lines, namely, bladder cancer T24, hepatoma HepG2, and colon cancer Caco-2. Similar dose response effects were found in normal cell lines including immortalised hepatocyte HHL-5, colon epithelial CCD841 and skin fibroblast CCD-1092SK cell lines (Fig. 1B).
Figure 1. Effects of SFN on the proliferation of normal and tumour cells.
When cells grew to 70–80% confluence, a range of doses of SFN (0–160 µM) were added to the cell culture medium for 24–48 h. The control cells were treated with DMSO (0.1%), and cell viability was determined by the MTT cell proliferation assay (CCD-1092SK cell viability was determined by WST-1 assay according to manufacturer's instructions [88]). Each data point represents the mean ± SD of at least 5 replicates. Statistical significance from the control, *p<0.05, or **p<0.01. A: results from bladder cancer T24, hepatoma HepG2, and colon cancer Caco-2 cells. B: Results from immortalised hepatocyte HHL-5, colon epithelial CCD841, and skin fibroblast CCD-1092SK cell lines.
Effects of SFN on cell migration
Fig. 2A shows a bell-shaped dose response of SFN on bladder cancer T24 cell migration. SFN at 2.5 and 3.75 µM increased tumour cell migration to 128 and 133% in comparison with corresponding controls. Such SFN-induced cell migration is associated with the ability of SFN to activate autophagy. When an autophagy inhibitor, 3-methyladenine (3-MA), was used it alleviated SFN (2.5 µM)-induced cell migration from 128 to 26% although it has less inhibitory effect on SFN treatments at 5 or 10 µM (Fig. 2B). Moreover, 3-MA also decreased the migration of non-SFN treated cells to 12% of the control.
Figure 2. Effects of SFN and 3-MA on cell migration.
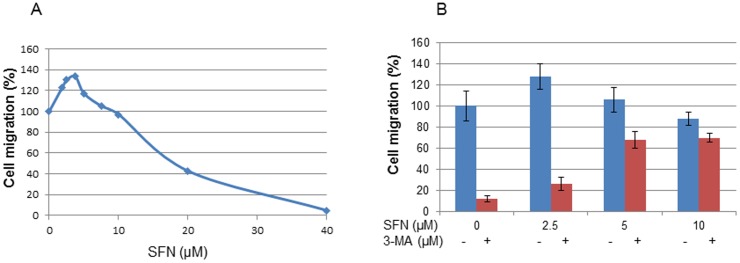
A: After starvation overnight, bladder cancer T24 cells were treated with SFN at the concentrations indicated for 24 h, cell migration was measured by a cell migration assay using the ThinCert cell culture inserts (Greiner Bio-One Ltd.). Each bar represents the mean ± SD of 3 replicates. B: Effect of pre-treatment of 3-MA on cell migration. DMSO (0.1% was used as a control). Statistical significance from the control, *p<0.05, or **p<0.01.
ITCs and activation of Nrf2
SFN is an activator of Nrf2 via which it can up-regulate more than a hundred protective genes, including most antioxidant and chemopreventive enzymes [11], [35]. There is no doubt that up-regulation of Nrf2-ARE pathway is beneficial in normal cells, i.e. activation of Nrf2 and its driven cytoprotective enzymes can be protective against oxidative damage and it has been suggested that activation of the Nrf2 signalling pathway can thus be a promising strategy in cancer prevention [36]. But, ITCs have no selectivity towards either normal or tumour cells with regards to Nrf2 activation. Nrf2 can be hijacked by tumour cells [26], and a recent report suggests that Nrf2 is a protooncogene which modulates tumour cell growth [37]. In transformed cells, Nrf2 may promote cell growth or cause chemoresistance [38]. In this study, SFN (2.5–10 µM) induced similar levels of translocation of Nrf2 into the nucleus of normal human hepatocytes HHL-5 (4.1–7.1 fold), and hepatoma HepG2 (4.1–5.9 fold) cells (Fig. 3).
Figure 3. Effect of SFN on translocation of Nrf2 into cell nucleus.

Nrf2 was detected in nuclear extracts from cells exposed to SFN (0, 2.5, 5 and 10 µM) for 24 h, using a Western blot assay. Control cells were treated with DMSO (0.1%). A: immortalised human hepatocyte HHL-5; B: human heptoma HepG2 cells.
Protective role of low dose ITC treatment against oxidative damage
In the fields of biology and medicine, hormesis is defined as an adaptive response of cells and organisms to a moderate stress. A mild stress induces the activation of signalling pathways such as Nrf2, NF-kB, Sirtuin, FOXO, hypoxia-inducible factor (HIF) thus leading to intrinsic changes (e.g. induction of antioxidant enzymes) that can confer resistance to more severe stress [4], [6]. Fig. 4A and 4B show that pretreatment of HHL-5 and MCF-7 cells with 5 µM SFN offered protection against H2O2-induced cell death, i.e. cell viability increased from 36.6 to 63.9%; and from 50.3 to 83.7% with 400 µM H2O2 treatments, respectively. Moreover, the protective effect of pretreatment with SFN (2 µM) on H2O2-induced cell death could be enhanced by cotreatment with selenium (Se) in HHL-5 cells (Fig. 4C), i.e. H2O2 decreased cell viability to 34.8% in HHL-5 cells but when cells were pre-treated with SFN (2 µM), or Se (0.1 µM) for 24 h, the cell viability increased to 41.7 and 51%, respectively and co-treatment SFN and Se increased cell viability to 65.5%. This protective effect may be involved in either chemoprotection or chemoresistance, depending on the nature of the cells.
Figure 4. Effect of pre-treatment of cells with SFN protect against H2O2-induced cell death.
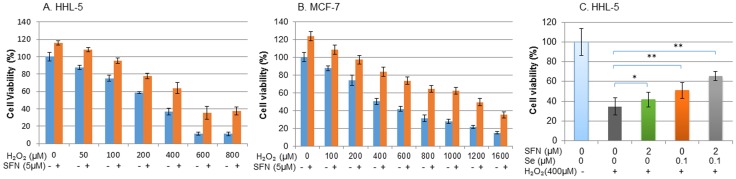
Cells were cultured in 96 well plates. When they reached 70–80% confluence, cells were pre-treated with SFN (5 µM) for 24 h (HHL-5, A) or 48 h (MCF-7, B). The cell culture medium was replaced with H2O2 at the concentrations indicated for a further 24 h. C: HHL-5 cell were pre-treated with SFN (2 µM) and Se (0.1 µM) for 24 h before exposure to H2O2 (400 µM) for a further 24 h. The cell viability was measured using MTT assay. Statistical significance from corresponding controls: *p<0.05; **p<0.01.
Biphasic effects of SFN on angiogenesis
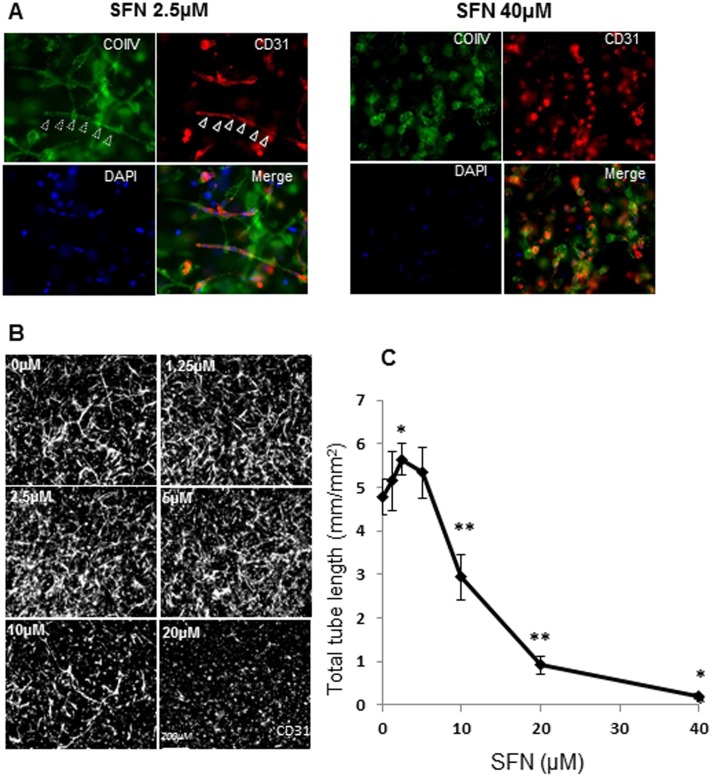
Angiogenesis (new blood vessel growth) is crucial in the development and spread of a variety of human cancers. It is, therefore, important to examine the anti-angiogenic effects of potential anti-cancer agents. In contrast, inadequate blood supply to the heart and other tissues, resulting from insufficient new blood vessel growth, is a feature of many cardiovascular diseases. SFN has been shown to inhibit angiogenesis at high concentrations [39]. In this study, SFN at 2.5 µM promoted tube formation to 118% of the control, i.e. total tube length was 4.78 mm/mm2 in control and 5.65 mm/mm2 in SFN (2.5 µM) treated cells (Fig. 5). SFN at 5 µM showed a less significant promotion (111% relative to the control), whereas 10 and 20 µM SFN inhibited tube formation significantly (decreased to 61 and 20% of the control, respectively). SFN at low dose promoted the formation of a continuous basement membrane around endothelial tubes; whereas at high doses of SFN, fragmented basement membranes were found (Fig. 5A). These data suggest that for anti-angiogenesis a relatively high dose of SFN should be used since a lower dose may promote angiogenesis. However, the stimulating effect of low doses on new blood vessel formation could be beneficial in patients with cardiovascular diseases.
Figure 5. Effect of SFN on endothelial tube formation in a 3-D angiogenesis assay.
Culture medium supplemented with SFN (0–40 µM) was added to the top of 3-D collagen gels and then changed every 24 h with fresh SFN added. 3-D gels were fixed at day 5, immunostained with CD31 (red) and collagen type IV (green), and counterstained with DAPI (blue). (A): Low magnification pictures were taken from five random fields of each sample and calculated for average tube length. (B) Representative pictures are shown in triple staining with higher magnification. Data are expressed as mean ± SD (n = 5) (C). *P<0.05; ** P<0.01 compared to untreated control.
Discussion
Hormetic effect of ITCs on cell growth, migration and angiogenesis
The hormetic zone concentrations (approximately 1–5 µM) of ITCs that are added in cell culture could readily be achieved in human plasma after consumption of a meal rich in cruciferous vegetables, or from extracts or supplements [40]–[44]. Table 1 shows the plasma levels of ITCs measured in several human studies (see also reference [45]). SFN is derived from the action of the endogenous enzyme, myrosinase on the glucosinolate, glucoraphanin which is found in cruciferous vegetables. The glucosinolate contents of common Brassica are available from a database developed by McNaughton and Marks [46]. The highest glucosinolate value was from cress (389 mg/100 g fresh weight) while the lowest value was from Chinese cabbage (20 mg/100 g fresh weight), although cultivar type and growing conditions both influence these figures. Broccoli contains 61.7 mg/100 g (19.3–127.5 mg glucoraphinin/100 g) [46], which is equivalent to 141.3 µmol SFN/100 g (44.2–292.1 µmol/100 g fresh weight) if the conversion is 100% efficient. Food processing and cooking conditions are crucial factors in influencing the activity of myrosinase, and subsequent formation of ITCs [47]. The main influence on the ensuing production of ITCs in vivo is how the brassica vegetables have been cooked [48]. Extensive studies of SFN have provided convincing evidence that SFN is a chemopreventive agent [49], [50]; and the mechanisms of its action involves the induction of phase II enzymes, cell cycle arrest and apoptosis [16], [51].
Table 1. Human studies with plasma levels of dietary ITCs.
| Study type | Subjects (n) | Dose | Plasma conc. (µM) | Refs |
| Metabolisms, pharmacokinetics | 4 | 200 µmol ITCs (largely SFN) | 0.94–2.27 | Ye et al., 2002 [40]. |
| Metabolism | 16 | GST(+): 107 & 345.8 µmol SFN; GST(-): 95 & 342 µmol SFN | 2.2; 7.3 2.3; 7.4 | Gasper et al., 2005 [41]. |
| Metabolism | 4 | 70 or 120 µmol SFN | 0.9 or 2.1 | Cramer et al., 2011 [42]. |
| Bioavailability | 12 | 150 µmol glucoraphanin | 2.2 | Clarke et al., 2011 [43]. |
| Pharmacokinetics | 4 | 100 g watercress | 0.928 (±0.25) | Ji et al., 2003 [44]. |
In general, findings from epidemiological studies on the association between vegetable intake and cancer risk are inconsistent. A high intake of cruciferous vegetables has, however, been shown to decrease the risk of several types of cancer, including those of colon and lung [52], [53]. If the hormetic effects of ITCs are involved in cancer growth, the overall biological impact of cruciferous vegetable on cancer risk becomes much more complicated. However, if a low dose of ITCs promotes cancer cell growth it may help to explain why epidemiological studies do not show a consistent association between cruciferous vegetable intake and the risk of cancer. Therefore, it is crucial to understand the mechanisms of action of the hormetic effects of ITCs. In in vitro cell cultures, the mechanisms by which low doses of SFN promote cell growth may be related to the effect SFN has on the activation of growth promoting molecules (such as HER2, RAS, RAF, MEK, ERK, PI3K, AKT and mTOR), signal transduction pathways such as NF-kB, FOXO, HIF, Nrf2, autophagy and receptors [54]–[56].
Autophagy involves the formation of double-membraned vesicles (autophagosomes), which encapsulate the cytoplasm and organelles and fuse with lysosomes, leading to degradation of the contents of the vesicle [57]. SFN is known to be an inducer of autophagy [58], but it is unclear how induction of autophagy is associated with suppression of cell migration. Other potential targets of SFN may include matrix metalloproteinases (MMPs), microtubules, collagens and integrins, survivin and zinc finger E-box binding homeobox 1 (ZEB1) [59]. A very recent study suggests that activation of autophagy is associated with chemoresistance, and that histone deacetylase (HDAC)10 protects neuroblastoma cells from cytotoxic agents by mediating autophagy [55]. This work indicates that co-treatment with HDAC10 inhibitor and a chemotherapeutic drug (doxorubicin) is a promising way to improve treatment response. Another study suggests that Notch activation is largely dispensable for SFN-mediated inhibition of cell migration in human prostate cancers [60], and this could be a therapeutic advantage as Notch activation is common in human prostate cancers. High constitutive levels of Nrf2 occur in many tumours, whilst overexpression of Nrf2 in cancer cells protects them from the cytotoxic effects of anticancer therapies, resulting in chemoresistance [22], [61]. There are interactions between ITCs and Se in the up-regulation of thioredoxin reductase (TR-1) and glutathione peroxidase 2 (GPx2) [30] and it is clear that ITCs and Se exhibit a plethora of multi-targeted effects in cancer chemoprevention. Interestingly, Se also promotes the migration and invasion of prostate cancer PC3 cells [62].
Assessment of the hormetic effect of ITCs
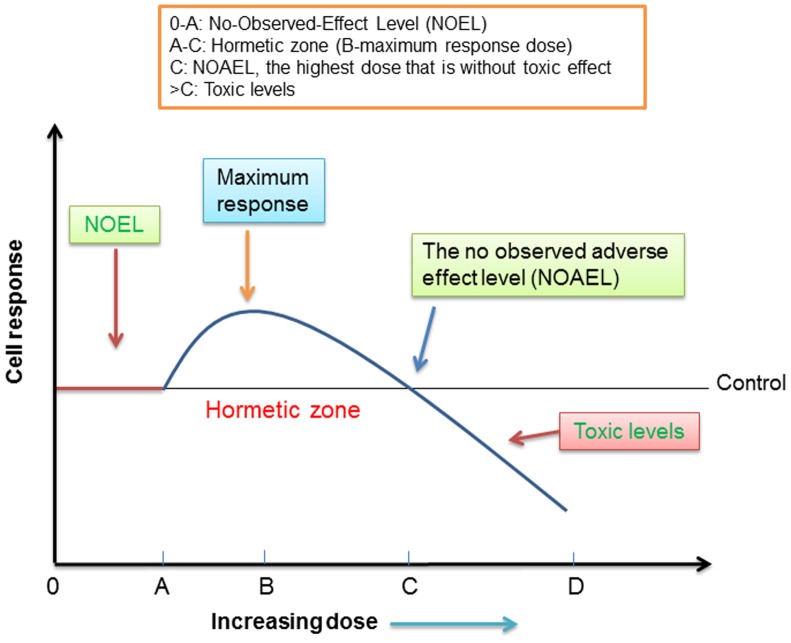
Consumption of cruciferous vegetables would not only provide ITCs but also contribute other nutrients and phytochemicals, including tocopherols, flavonoids, ascorbate and Se. These components could counteract/interact with the prooxidant/antioxidant activities of ITCs. Based on the hormetic nature of ITCs, consumption of a quantity of cruciferous vegetables that provide a hormetic level of ITCs in plasma could be a risk factor for those who have transformed cells in the body. A schematic diagram for analysing the benefits and risks of dietary ITCs is proposed in Fig. 6. For all dietary compounds and toxic substances, “the dose makes the poison” [wording simplified from “all things are poison, and nothing is without poison; only the dose permits something not to be poisonous” (Paracelsus, 1493–1541)]. For dietary ITCs, there should be a “no effect level” prior to the detection of any biological effects. Indeed, the level of ITCs in the plasma of a majority of the population is likely to be much lower than sub-µM and may not exert any biological effects on cells. However, following increased intakes, such as in the trials listed in Table 1 or for individuals taking supplements, the plasma ITC levels could reach the hormetic zone concentrations. Typical characteristics of the hormetic zone (dose A-C) includes low concentrations stimulating and high concentrations inhibiting effects. For SFN, the hormetic zone is found to be 1–5 µM in the in vitro cell culture experiments, although the dose-effects found in vitro experiments should not be directly extrapolated to humans. It is possible that the hormetic zone and the No Observed Adverse Effect Level (NOAEL, dose C) in humans is significantly different. In order to maximise the beneficial effect and minimise the risk, both genetic factors and interactions between dietary components should be considered. For example, genetic polymorphisms of glutathione transferases (GSTs) affect SFN metabolism and the risk of cancer [63]. On the other hand, supplementation with cruciferous vegetables increased GSTA1/2 activity, the effect being most marked in GSTM1-null/GSTT1-null men [64]. Although there are currently few epidemiological studies that employ genotyping, research of this nature will increase in the future and it is likely the nutrigenetics will provide a basis for personalised medicine and nutrition. Interactions between bioactive phytochemicals and nutrients may contribute to the overall benefits and risks of ITCs depending on the health status of the individuals. The inductions of Nrf2 and antioxidant enzymes such as TR-1 could also be of either benefit or risk depending on the nature of the target cells (normal vs tumour).
Figure 6. A schematic diagram on the hormetic effect of ITCs.
For all cell types, dosage range 0-A is safe. In the majority of diets, the intakes of hormetic phytochemicals are likely to fall within this safe range. For normal cells, dose B could be used promote new blood vessel formation or promote wound healing; doses >C are toxic. For tumour cells, doses between A and C should be avoided; and doses >C to D could be used for chemotherapy.
Where are we now? How can we maximise the benefits and minimise the risks?
Thirty years ago, researchers focused on the potential toxic (goitrogenic) properties of glucosinolate breakdown products [65]. In 1992, sulforaphane was isolated from broccoli and anti-carcinogenic studies were based on its potent activity in the induction of phase II enzymes [12], [66]. Over the last decade, many Nrf2 inducers including ITCs, resveratrol, catechin, cucurmin, and quercetin have been reported [67], [68] with both chemopreventive and oncogenic activities [69]–[71]. Recently, two Nrf2 inhibitors, brusatol (from the seeds of Brucea sumatrana) and trigonelline (from coffee) were reported to enhance the efficacy of anticancer therapy [72], [73]. Moreover, Nrf2 knockdown has been shown to inhibit tumour growth, increase the efficacy of chemotherapy in cervical cancer [74], and inhibit the angiogenesis of rat cardiac micro-vascular endothelial cells under hypoxic conditions [75]. Therefore, it is clear that the role of Nrf2 in cancer development is a topic of controversy and Nrf2 activators such as SFN and other ITCs may contribute both benefits and risks in cancer development.
An understanding of the complex plethora and divergent natures of ITCs and other dietary Nrf2 activators and their hormetic dose responses, combined with an accurate diagnosis (stage of cancer), and genetic analysis may, in the not-too-distant future, initiate the significant potential that personalised medicine may have. New diagnostic techniques exploiting gold nanoparticles can spot tumour-like masses as small as 5 mm in the liver [76]. Gold nanoparticles with a polyelectrolyte coating can make even smaller tumours visible through X-ray scatter imaging, thereby enabling earlier diagnosis. Once tumours can be diagnosed at such a very early stage, a potential therapeutic approach could be the nanoencapsulation of cancer-fighting phytochemicals or drugs through monitored and targeted delivery [77]. But, it must be remembered that ITCs at high concentrations are also toxic towards normal cells. Adverse effects have been reported in in vitro studies using 10–30 µM SFN, including induction of DNA, RNA and mitochondrial damage [78]–[80]. Moreover, there was also a case report of liver toxicity in an individual who consumed 800 ml broccoli soup a day for 4 weeks [81]. Low levels of ITCs can generate reactive oxygen species (ROS), and activate Nrf2-ARE to switch on antioxidant enzymes. Although high levels of ROS can damage protein, lipids and DNA in cells, low levels of ROS can play an important role in immune defence, antibacterial action, vascular tone, and signal transduction [82]. Recently, James Watson hypothesised that diabetes, dementias, cardiovascular disease and some cancers are all linked to a failure to generate sufficient ROS [83]. The challenge is to define the balance between the generation of ROS and the antioxidant capacity in each type of cells. For dietary ITCs, it is important to define the optimal range of intakes for promoting health. Nevertheless, further human studies are required to establish the personalised optimal doses, safety and efficacy profiles using more sensitive biomarkers.
Materials and Methods
Materials
Sulforaphane was purchased from Enzo Life Sciences (UK). Sodium selenite, dimethylsulfoxide (DMSO), hydrogen peroxide, Bradford reagent, methylthiazolyldiphenyl-tetrazolium bromide (MTT), phenylmethylsulfonyl fluoride (PMSF), and all other materials and reagents were purchased from Sigma-Aldrich (UK). Rabbit polyclonal primary antibodies to Nrf2, Sam68 and horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG as secondary antibodies were all obtained from Santa Cruz Biotechnology Inc. (Heidelberg, Germany). Anti-collagen IV and anti-human CD31/PECAM-1 were purchased from Millipore and BD Biosciences (UK), respectively. Secondary antibodies conjugated with Cy2 and Cy3 were purchased from Jackson Immuno Research (UK). Mini-complete proteinase inhibitor and WST-1 reagent were purchased from Roche Applied Sciences (UK). Electrophoresis and Western blotting supplies were supplied by Bio-Rad (UK). The enhanced chemiluminescence (ECL) kit was purchased from GE Healthcare (UK).
Cell culture
Immortalised human hepatocytes (defined as HHL-5) were kindly supplied by Dr Arvind Patel, Medical Research Council (MRC) Virology Unit (Glasgow, UK) [84]. All other cell lines were purchased from ATCC. Cells were routinely cultured in DMEM supplemented with foetal bovine serum (10%), 2 mM glutamine, penicillin (100 U/ml) and streptomycin (100 µg/ml) under 5% CO2 in air at 37°C.
Cell proliferation assay
The cell proliferation MTT assay was employed to detect the toxicity of SFN (1–160 µM) on cultured cells. When cells were at approximately 70–80% confluence, cells were exposed to various concentrations of SFN for different times using DMSO (0.1%) as control. After all treatments, the medium was removed, 5 mg/ml MTT was added, and incubated at 37°C for 1 h to allow the MTT to be metabolized. Then the formazan produced was re-suspended in 100 µl DMSO per well. The final absorbance in the wells was recorded using a microplate reader (BMG Labtech Ltd, UK) at a wavelength of 550 nm and a reference wavelength of 650 nm.
Cell migration assay
Cell migration was quantified using a ThinCert cell culture inserts cell migration assay (Greiner Bio-One Ltd.). After overnight starvation in serum free medium, cells were treated with various concentrations of SFN for 24 h, the cells migrating through a PET membrane were labelled fluorescently with Calcein-AM and quantified by microplate reader (BMG Labtech Ltd, UK) with an excitation wavelength of 485 nm and emission wavelength of 525 nm.
Protein extraction and Western blot Analysis
For total protein, HHL-5 cells were washed twice with ice-cold PBS, harvested by scraping in 20 mM Tris-HCl (pH 8), 150 mM NaCl, 2 mM EDTA, 10% glycerol, 1% Nonidet P40 (NP-40) containing mini-complete proteinase inhibitor. The cell suspensions were placed in an ice bath for 20 min and then centrifuged at 12,000 g for 15 min at 4°C. Supernatant was collected and the protein concentration determined by the Bradford Brilliant Blue G dye-binding assay of using BSA as a standard. For the nuclear protein, the extraction was performed by using a Nuclear Extract Kit (Active Motif, UK), following the manufacturer's instructions.
Protein extracts were heated at 95°C for 5 min in loading buffer and loaded onto 10% SDS-polyacrylamide gels together with a molecular weight marker. After routine electrophoresis and transfer, the polyvinylidene difluoride (PVDF) membrane was blocked with 5% fat free milk in PBST (0.05% Tween 20) for 1 h and incubated with a specific primary antibody in 5% milk in PBST for 1 h. The membrane was washed three times for 45 min with PBST and then incubated with the secondary antibody diluted with 5% milk in PBST for 1 h. After three further washes for 45 min with PBST, the antibody binding was determined using an ECL kit (GE Healthcare, UK) and densitometry was measured by Fluor Chem Imager (Alpha Innotech, San Leandro, CA).
Angiogenesis assay - tube formation in a 3-D model
Human umbilical vein endothelial cells (HUVEC) and pericytes (PVC) were co-cultured in collagen type I gel as described previously [85]. SFN (0–40 µM) was added to the medium (top of 3-D collagen gel) and the medium was changed every 24 h with fresh SFN added. At day 5, samples were fixed, immunostained with CD31 and collagen type IV and counterstained with DAPI. Magnification pictures were taken from five random fields of each sample and average tube length measured.
Statistics
Data are represented as the mean ± SD. The differences between the groups were examined using one-way ANOVA test, or student's t-test. A p value <0.05 was considered to be statistically significant. IC50 values of SFN and H2O2 were determined using CalcuSyn Software (Biosoft, UK).
Conclusions and Future Perspectives
Based on findings from the research reported here, greater effort should be expended on the evaluation of the interactive/synergistic effects on the cancer risk of various phytochemicals and phytochemical-rich foods. Risk/benefit assessment of ITCs and other dietary bioactives may be linked to genotype, health status or tumour stage, and of course the dose, all of which must be included in future research priorities. More precise dietary guidelines and policies for cancer prevention could also be developed based on the understanding of these fundamental factors. There are at least five ongoing human trials using SFN or broccoli sprout preparations registered with http://www.clinicaltrials.gov/ and it will be of great interest to study the results in coming years. In the absence of precise knowledge in these areas, it is considered prudent to study the molecular mechanisms of the interactions between ITCs and other bioactives/nutrients in cell cultures and animal models prior to undertaking large, very expensive human trials. In this sense, β-carotene has been a good example. In observational studies, high intake of carotenoids from food has been associated with reduced risk of cancer. However, observational studies are inherently unreliable and it would be a big mistake to conduct human trials without having sufficient information about the mechanisms of action in cells. In intervention trials, β-carotene supplements have not been found to offer any benefits; in fact, when taken in high doses for a long period of time, they slightly increased the risk of some forms of cancer [86]. However, this is an area of activity that is rapidly developing and this assessment may well need to be revisited in the light of emerging scientific data. These results show that low concentrations of ITCs especially SFN may be potentially beneficial or harmful, depending on the endpoint of interest and the cell type, i.e. beneficial to normal angiogenesis and harmful in promotion of cancer cell growth. SFN is important because it is present in our normal diet from cruciferous vegetables and also because of its commercial applications (there are many different brands of broccoli extracts marketed as supplements). Based on the hormetic dose response, nutraceutical producers should carefully consider the efficacy of the application of ITC/SFN-rich products/supplements. On the basis of their biphasic effects on cell growth and migration, there is no doubt that ITCs belong to the so-called hormetic class of phytochemicals.
In summary, low concentrations of ITCs can potentially be either beneficial or harmful. Since there is little selectivity in the hormetic effect, the benefits or risks of ITCs at lower doses could be different in normal and tumour cells. In tumour cells, low doses of SFN could have the capability to increase the risk of tumour development. In contrast, in normal endothelial cells, SFN could be significantly cardio-protective (angiogenetic). This type of conflict between beneficial and harmful effects is not uncommon and may be related to the different biological systems, tissues and chemical agents under investigation [87]. The evidence regarding the hormetic dose response induced by SFN is obvious, but the relevant molecular mechanisms are not fully understood, and thus deserve greater attention in future research. Nutrition scientists and oncologists should be aware of the potential risks of dietary ITCs, especially of the possible role of hormesis if they are used as food supplements. Finally, the majority of the available evidence described above is based on in vitro cell culture experiments. Research is also needed to evaluate the relative risks, as well as benefits, of the hormetic effects in medium- to long-term supplementation with dietary ITCs and other phytochemicals in animal studies and small scale human trials.
Acknowledgments
The authors are grateful to Dr Arvind Patel (MRC Virology Unit, Glasgow) for providing the immortalised human hepatocytes. The authors also wish to thank previous group members and colleagues Mr Jim Bacon and Professor Sue Fairweather-Tait for helpful discussions during the preparation of this manuscript.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This study was supported in part by a grant from the National Natural Science Foundation of China (NSFC No. 81128011) and an award from the Cancer Prevention Research Trust, United Kingdom. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Calabrese EJ, Iavicoli I, Calabrese V (2013) Hormesis: its impact on medicine and health. Hum Exp Toxicol 32:120–152. [DOI] [PubMed] [Google Scholar]
- 2. Calabrese EJ (2005) Cancer biology and hormesis: human tumor cell lines commonly display hormetic (biphasic) dose responses. Crit Rev Toxicol 35:463–582. [DOI] [PubMed] [Google Scholar]
- 3. Mattson MP (2008) Dietary factors, hormesis and health. Ageing Res Rev 7:43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Son TG, Camandola S, Mattson MP (2008) Hormetic dietary phytochemicals. Neuromolecular Med 10:236–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Calabrese EJ, Mattson MP, Calabrese V (2010) Resveratrol commonly displays hormesis: occurrence and biomedical significance. Hum Exp Toxicol 29:980–1015. [DOI] [PubMed] [Google Scholar]
- 6. Speciale A, Chirafisi J, Saija A, Cimino F (2011) Nutritional antioxidants and adaptive cell responses: an update. Curr Mol Med 11:770–789. [DOI] [PubMed] [Google Scholar]
- 7. Pietsch K, Saul N, Chakrabarti S, Sturzenbaum SR, Menzel R, et al. (2011) Hormetins, antioxidants and prooxidants: defining quercetin-, caffeic acid- and rosmarinic acid-mediated life extension in C. elegans. Biogerontology 12:329–347. [DOI] [PubMed] [Google Scholar]
- 8. Vargas AJ, Burd R (2010) Hormesis and synergy: pathways and mechanisms of quercetin in cancer prevention and management. Nutr Rev 68:418–428. [DOI] [PubMed] [Google Scholar]
- 9. Cornelius C, Perrotta R, Graziano A, Calabrese EJ, Calabrese V (2013) Stress responses, vitagenes and hormesis as critical determinants in aging and longevity: Mitochondria as a "chi". Immun Ageing 10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mattson MP (2008) Hormesis defined. Ageing Res Rev 7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, et al. (2002) Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res 62:5196–5203. [PubMed] [Google Scholar]
- 12. Zhang Y, Talalay P, Cho CG, Posner GH (1992) A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci U S A 89:2399–2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheung KL, Kong AN (2010) Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J 12:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jakubikova J, Sedlak J, Bacon J, Goldson A, Bao Y (2005) Effects of MEK1 and PI3K inhibitors on allyl-, benzyl- and phenylethyl-isothiocyanate-induced G2/M arrest and cell death in Caco-2 cells. Int J Oncol 27:1449–1458. [PubMed] [Google Scholar]
- 15.Gupta P, Kim B, Kim SH, Srivastava SK (2014) Molecular targets of isothiocyanates in cancer: Recent advances. Mol Nutr Food Res. [DOI] [PMC free article] [PubMed]
- 16. Gamet-Payrastre L, Li P, Lumeau S, Cassar G, Dupont MA, et al. (2000) Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res 60:1426–1433. [PubMed] [Google Scholar]
- 17. Suppipat K, Park CS, Shen Y, Zhu X, Lacorazza HD (2012) Sulforaphane induces cell cycle arrest and apoptosis in acute lymphoblastic leukemia cells. PLoS One 7:e51251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yu X, Kensler T (2005) Nrf2 as a target for cancer chemoprevention. Mutat Res 591:93–102. [DOI] [PubMed] [Google Scholar]
- 19. Hayes JD, McMahon M, Chowdhry S, Dinkova-Kostova AT (2010) Cancer chemoprevention mechanisms mediated through the Keap1-Nrf2 pathway. Antioxid Redox Signal 13:1713–1748. [DOI] [PubMed] [Google Scholar]
- 20. Kensler TW, Egner PA, Agyeman AS, Visvanathan K, Groopman JD, et al. (2013) Keap1-nrf2 signaling: a target for cancer prevention by sulforaphane. Top Curr Chem 329:163–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lau A, Villeneuve NF, Sun Z, Wong PK, Zhang DD (2008) Dual roles of Nrf2 in cancer. Pharmacol Res 58:262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang XJ, Sun Z, Villeneuve NF, Zhang S, Zhao F, et al. (2008) Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis 29:1235–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. DeNicola GM, Karreth FA, Humpton TJ, Gopinathan A, Wei C, et al. (2011) Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 475:106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang DD (2010) The Nrf2-Keap1-ARE signaling pathway: The regulation and dual function of Nrf2 in cancer. Antioxid Redox Signal 13:1623–1626. [DOI] [PubMed] [Google Scholar]
- 25. Kensler TW, Wakabayashi N (2010) Nrf2: friend or foe for chemoprevention? Carcinogenesis 31:90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mitsuishi Y, Motohashi H, Yamamoto M (2012) The Keap1-Nrf2 system in cancers: stress response and anabolic metabolism. Front Oncol 2:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Homma S, Ishii Y, Morishima Y, Yamadori T, Matsuno Y, et al. (2009) Nrf2 enhances cell proliferation and resistance to anticancer drugs in human lung cancer. Clin Cancer Res 15:3423–3432. [DOI] [PubMed] [Google Scholar]
- 28. Lister A, Nedjadi T, Kitteringham NR, Campbell F, Costello E, et al. (2011) Nrf2 is overexpressed in pancreatic cancer: implications for cell proliferation and therapy. Mol Cancer 10:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li D, Wang W, Shan YJ, Barrera LN, Howie AF, et al. (2012) Synergy between sulforaphane and selenium in the up-regulation of thioredoxin reductase and protection against hydrogen peroxide-induced cell death in human hepatocytes. Food Chemistry 133:300–307. [DOI] [PubMed] [Google Scholar]
- 30. Barrera LN, Cassidy A, Wang W, Wei T, Belshaw NJ, et al. (2012) TrxR1 and GPx2 are potently induced by isothiocyanates and selenium, and mutually cooperate to protect Caco-2 cells against free radical-mediated cell death. Biochim Biophys Acta 1823:1914–1924. [DOI] [PubMed] [Google Scholar]
- 31. Zanichelli F, Capasso S, Cipollaro M, Pagnotta E, Carteni M, et al. (2012) Dose-dependent effects of R-sulforaphane isothiocyanate on the biology of human mesenchymal stem cells, at dietary amounts, it promotes cell proliferation and reduces senescence and apoptosis, while at anti-cancer drug doses, it has a cytotoxic effect. Age (Dordr) 34:281–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Misiewicz I, Skupinska K, Kowalska E, Lubinski J, Kasprzycka-Guttman T (2004) Sulforaphane-mediated induction of a phase 2 detoxifying enzyme NAD(P)H: quinone reductase and apoptosis in human lymphoblastoid cells. Acta Biochim Pol 51:711–721. [PubMed] [Google Scholar]
- 33. Jackson SJ, Singletary KW (2004) Sulforaphane inhibits human MCF-7 mammary cancer cell mitotic progression and tubulin polymerization. J Nutr 134:2229–2236. [DOI] [PubMed] [Google Scholar]
- 34. Melchini A, Needs PW, Mithen RF, Traka MH (2012) Enhanced in vitro biological activity of synthetic 2-(2-pyridyl) ethyl isothiocyanate compared to natural 4-(methylsulfinyl) butyl isothiocyanate. J Med Chem 55:9682–9692. [DOI] [PubMed] [Google Scholar]
- 35. Li Y, Paonessa JD, Zhang Y (2012) Mechanism of chemical activation of Nrf2. PLoS One 7:e35122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Giudice A, Montella M (2006) Activation of the Nrf2-ARE signaling pathway: a promising strategy in cancer prevention. Bioessays 28:169–181. [DOI] [PubMed] [Google Scholar]
- 37. Shelton P, Jaiswal AK (2013) The transcription factor NF-E2-related factor 2 (Nrf2): a protooncogene? FASEB J 27:414–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brigelius-Flohe R, Muller M, Lippmann D, Kipp AP (2012) The yin and yang of nrf2-regulated selenoproteins in carcinogenesis. Int J Cell Biol 2012:486147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nishikawa T, Tsuno NH, Okaji Y, Sunami E, Shuno Y, et al. (2010) The inhibition of autophagy potentiates anti-angiogenic effects of sulforaphane by inducing apoptosis. Angiogenesis 13:227–238. [DOI] [PubMed] [Google Scholar]
- 40. Ye L, Dinkova-Kostova AT, Wade KL, Zhang Y, Shapiro TA, et al. (2002) Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin Chim Acta 316:43–53. [DOI] [PubMed] [Google Scholar]
- 41. Gasper AV, Al-Janobi A, Smith JA, Bacon JR, Fortun P, et al. (2005) Glutathione S-transferase M1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. Am J Clin Nutr 82:1283–1291. [DOI] [PubMed] [Google Scholar]
- 42. Cramer JM, Jeffery EH (2011) Sulforaphane absorption and excretion following ingestion of a semi-purified broccoli powder rich in glucoraphanin and broccoli sprouts in healthy men. Nutr Cancer 63:196–201. [DOI] [PubMed] [Google Scholar]
- 43. Clarke JD, Hsu A, Riedl K, Bella D, Schwartz SJ, et al. (2011) Bioavailability and inter-conversion of sulforaphane and erucin in human subjects consuming broccoli sprouts or broccoli supplement in a cross-over study design. Pharmacol Res 64:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ji Y, Morris ME (2003) Determination of phenethyl isothiocyanate in human plasma and urine by ammonia derivatization and liquid chromatography-tandem mass spectrometry. Anal Biochem 323:39–47. [DOI] [PubMed] [Google Scholar]
- 45. Dinkova-Kostova AT, Kostov RV (2012) Glucosinolates and isothiocyanates in health and disease. Trends Mol Med 18:337–347. [DOI] [PubMed] [Google Scholar]
- 46. McNaughton SA, Marks GC (2003) Development of a food composition database for the estimation of dietary intakes of glucosinolates, the biologically active constituents of cruciferous vegetables. Br J Nutr 90:687–697. [DOI] [PubMed] [Google Scholar]
- 47. Galgano F, Favati F, Caruso M, Pietrafesa A, Natella S (2007) The influence of processing and preservation on the retention of health-promoting compounds in broccoli. J Food Sci 72:S130–135. [DOI] [PubMed] [Google Scholar]
- 48. Rungapamestry V, Duncan AJ, Fuller Z, Ratcliffe B (2007) Effect of meal composition and cooking duration on the fate of sulforaphane following consumption of broccoli by healthy human subjects. Br J Nutr 97:644–652. [DOI] [PubMed] [Google Scholar]
- 49. Keum YS, Jeong WS, Kong AN (2005) Chemopreventive functions of isothiocyanates. Drug News Perspect 18:445–451. [DOI] [PubMed] [Google Scholar]
- 50. Fimognari C, Hrelia P (2007) Sulforaphane as a promising molecule for fighting cancer. Mutat Res 635:90–104. [DOI] [PubMed] [Google Scholar]
- 51. Zhang Y (2004) Cancer-preventive isothiocyanates: measurement of human exposure and mechanism of action. Mutat Res 555:173–190. [DOI] [PubMed] [Google Scholar]
- 52. Miller PE, Snyder DC (2012) Phytochemicals and cancer risk: a review of the epidemiological evidence. Nutr Clin Pract 27:599–612. [DOI] [PubMed] [Google Scholar]
- 53. Michaud DS, Spiegelman D, Clinton SK, Rimm EB, Willett WC, et al. (1999) Fruit and vegetable intake and incidence of bladder cancer in a male prospective cohort. J Natl Cancer Inst 91:605–613. [DOI] [PubMed] [Google Scholar]
- 54. Watson J (2013) Oxidants, antioxidants and the current incurability of metastatic cancers. Open Biol 3:120144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Oehme I, Linke JP, Bock BC, Milde T, Lodrini M, et al. (2013) Histone deacetylase 10 promotes autophagy-mediated cell survival. Proc Natl Acad Sci U S A 110:E2592–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Calabrese EJ (2013) Hormetic mechanisms. Crit Rev Toxicol 43:580–606. [DOI] [PubMed] [Google Scholar]
- 57. Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, et al. (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441:885–889. [DOI] [PubMed] [Google Scholar]
- 58. Herman-Antosiewicz A, Johnson DE, Singh SV (2006) Sulforaphane causes autophagy to inhibit release of cytochrome C and apoptosis in human prostate cancer cells. Cancer Res 66:5828–5835. [DOI] [PubMed] [Google Scholar]
- 59. Shan Y, Zhang L, Bao Y, Li B, He C, et al. (2013) Epithelial-mesenchymal transition, a novel target of sulforaphane via COX-2/MMP2, 9/Snail, ZEB1 and miR-200c/ZEB1 pathways in human bladder cancer cells. J Nutr Biochem 24:1062–1069. [DOI] [PubMed] [Google Scholar]
- 60. Hahm ER, Chandra-Kuntal K, Desai D, Amin S, Singh SV (2012) Notch activation is dispensable for D, L-sulforaphane-mediated inhibition of human prostate cancer cell migration. PLoS One 7:e44957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Xu T, Ren D, Sun X, Yang G (2012) Dual roles of sulforaphane in cancer treatment. Anticancer Agents Med Chem 12:1132–1142. [DOI] [PubMed] [Google Scholar]
- 62. Hendrickx W, Decock J, Mulholland F, Bao Y, Fairweather-Tait S (2013) Selenium Biomarkers in Prostate Cancer Cell Lines and Influence of Selenium on Invasive Potential of PC3 Cells. Front Oncol 3:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Moy KA, Yuan JM, Chung FL, Wang XL, Van Den Berg D, et al. (2009) Isothiocyanates, glutathione S-transferase M1 and T1 polymorphisms and gastric cancer risk: a prospective study of men in Shanghai, China. Int J Cancer 125:2652–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fenwick GR, Heaney RK, Mullin WJ (1983) Glucosinolates and their breakdown products in food and food plants. Crit Rev Food Sci Nutr 18:123–201. [DOI] [PubMed] [Google Scholar]
- 66. Zhang Y, Tang L (2007) Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta Pharmacol Sin 28:1343–1354. [DOI] [PubMed] [Google Scholar]
- 67. Zhao CR, Gao ZH, Qu XJ (2010) Nrf2-ARE signaling pathway and natural products for cancer chemoprevention. Cancer Epidemiol 34:523–533. [DOI] [PubMed] [Google Scholar]
- 68. Surh YJ, Kundu JK, Na HK (2008) Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med 74:1526–1539. [DOI] [PubMed] [Google Scholar]
- 69. Sporn MB, Liby KT (2012) NRF2 and cancer: the good, the bad and the importance of context. Nat Rev Cancer 12:564–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Na HK, Surh YJ (2014) Oncogenic potential of Nrf2 and its principal target protein heme oxygenase-1. Free Radic Biol Med 67:353–365. [DOI] [PubMed] [Google Scholar]
- 71. McMahon M, Itoh K, Yamamoto M, Hayes JD (2003) Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem 278:21592–21600. [DOI] [PubMed] [Google Scholar]
- 72. Ren D, Villeneuve NF, Jiang T, Wu T, Lau A, et al. (2011) Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc Natl Acad Sci U S A 108:1433–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Arlt A, Sebens S, Krebs S, Geismann C, Grossmann M, et al. (2013) Inhibition of the Nrf2 transcription factor by the alkaloid trigonelline renders pancreatic cancer cells more susceptible to apoptosis through decreased proteasomal gene expression and proteasome activity. Oncogene 32:4825–4835. [DOI] [PubMed] [Google Scholar]
- 74. Ma X, Zhang J, Liu S, Huang Y, Chen B, et al. (2012) Nrf2 knockdown by shRNA inhibits tumor growth and increases efficacy of chemotherapy in cervical cancer. Cancer Chemother Pharmacol 69:485–494. [DOI] [PubMed] [Google Scholar]
- 75. Kuang L, Feng J, He G, Jing T (2013) Knockdown of Nrf2 inhibits the angiogenesis of rat cardiac micro-vascular endothelial cells under hypoxic conditions. Int J Biol Sci 9:656–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Rand D, Ortiz V, Liu Y, Derdak Z, Wands JR, et al. (2011) Nanomaterials for X-ray imaging: gold nanoparticle enhancement of X-ray scatter imaging of hepatocellular carcinoma. Nano Lett 11:2678–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Santos IS, Ponte BM, Boonme P, Silva AM, Souto EB (2013) Nanoencapsulation of polyphenols for protective effect against colon-rectal cancer. Biotechnol Adv 31:514–523. [DOI] [PubMed] [Google Scholar]
- 78. Zhang Y, Li J, Tang L (2005) Cancer-preventive isothiocyanates: dichotomous modulators of oxidative stress. Free Radic Biol Med 38:70–77. [DOI] [PubMed] [Google Scholar]
- 79. Sestili P, Paolillo M, Lenzi M, Colombo E, Vallorani L, et al. (2010) Sulforaphane induces DNA single strand breaks in cultured human cells. Mutat Res 689:65–73. [DOI] [PubMed] [Google Scholar]
- 80. Fimognari C, Lenzi M, Sestili P, Turrini E, Ferruzzi L, et al. (2012) Sulforaphane potentiates RNA damage induced by different xenobiotics. PLoS One 7:e35267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ekiz F, Yuksel I, Kertmen N, Yuksel O (2010) Liver toxicity due to broccoli juice. Eur J Gastroenterol Hepatol 22:898. [DOI] [PubMed] [Google Scholar]
- 82. Alfadda AA, Sallam RM (2012) Reactive oxygen species in health and disease. J Biomed Biotechnol 2012:936486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Watson JD (2014) Type 2 diabetes as a redox disease. Lancet 383:841–843. [DOI] [PubMed] [Google Scholar]
- 84. Clayton RF, Rinaldi A, Kandyba EE, Edward M, Willberg C, et al. (2005) Liver cell lines for the study of hepatocyte functions and immunological response. Liver Int 25:389–402. [DOI] [PubMed] [Google Scholar]
- 85. Cooley LS, Handsley MM, Zhou Z, Lafleur MA, Pennington CJ, et al. (2010) Reversible transdifferentiation of blood vascular endothelial cells to a lymphatic-like phenotype in vitro. J Cell Sci 123:3808–3816. [DOI] [PubMed] [Google Scholar]
- 86. Omenn GS, Goodman GE, Thornquist MD, Balmes J, Cullen MR, et al. (1996) Effects of a combination of beta carotene and vitamin A on lung cancer and cardiovascular disease. N Engl J Med 334:1150–1155. [DOI] [PubMed] [Google Scholar]
- 87. Calabrese V, Cornelius C, Leso V, Trovato-Salinaro A, Ventimiglia B, et al. (2012) Oxidative stress, glutathione status, sirtuin and cellular stress response in type 2 diabetes. Biochim Biophys Acta 1822:729–736. [DOI] [PubMed] [Google Scholar]
- 88. Warwick E, Cassidy A, Hanley B, Jouni ZE, Bao Y (2012) Effect of phytochemicals on phase II enzyme expression in infant human primary skin fibroblast cells. Br J Nutr 108:2158–2165. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.