Abstract
Upregulation of IL-17 immunity and detrimental effects of IL-17 on human islets have been implicated in human type 1 diabetes. In animal models, the plasticity of Th1/Th17 cells contributes to the development of autoimmune diabetes. In this study, we demonstrate that the upregulation of the IL-17 pathway and Th1/Th17 plasticity in peripheral blood are markers of advanced β cell autoimmunity and impaired β cell function in human type 1 diabetes. Activated Th17 immunity was observed in the late stage of preclinical diabetes in children with β cell autoimmunity and impaired glucose tolerance, but not in children with early β cell autoimmunity. We found an increased ratio of IFN-γ/IL-17 expression in Th17 cells in children with advanced β cell autoimmunity, which correlated with HbA1c and plasma glucose concentrations in an oral glucose tolerance test, and thus impaired β cell function. Low expression of Helios was seen in Th17 cells, suggesting that Th1/Th17 cells are not converted thymus-derived regulatory T cells. Our results suggest that the development of Th1/Th17 plasticity may serve as a biomarker of disease progression from β cell autoantibody positivity to type 1 diabetes. These data in human type 1 diabetes emphasize the role of Th1/Th17 plasticity as a potential contributor to tissue destruction in autoimmune conditions.
Introduction
Type 1 diabetes is an autoimmune disease caused by T cell–mediated destruction of the pancreatic β cells. As the first marker of disease development, autoantibodies against β cell Ags appear into the peripheral blood. During this prediabetic phase, multiple diabetes-associated autoantibodies emerge, such as islet cell Abs, insulin autoantibodies (IAA), glutamic acid decarboxylase Abs (GADA), insulinoma-associated-2 Abs (IA-2A), and zinc transporter 8 Abs (ZnT8A) (1, 2). Although individuals at risk for type 1 diabetes are recognized by screening for HLA-associated risk genotypes and β cell autoantibodies, there is a lack of biomarkers for progression to clinical type 1 diabetes in autoantibody-positive individuals.
Type 1 diabetes is mediated by IFN-γ–producing Th1 cells (3, 4), but recently also the role of IL-17–secreting Th17 cells has been implicated. Th17 immunity is upregulated in the course of insulitis in spontaneous autoimmune diabetes in the NOD mouse, and the neutralization of IL-17 has been observed to prevent diabetes (5). We have previously reported upregulation of Th17 immunity in stimulated PBMCs and in circulating memory T helper cells in children with type 1 diabetes (6). Marwaha et al. (7) showed a significant increase in the proportion of IL-17–secreting CD4+ but also CD8+ cells in patients with type 1 diabetes. Arif et al. (8) found upregulation of the IL-17 response in PBMCs stimulated by islet Ags, and a more recent study demonstrated increased IL-17 immunity in the pancreatic lymph nodes in patients with type 1 diabetes (9). Elevated plasma levels of IL-17 have also been observed in autoantibody-positive children when compared with autoantibody-negative children (10).
IL-17 in combination with IL-1β and IFN-γ reportedly mediates detrimental effects on human pancreatic islets and β cells in vitro. IL-17 increased β cell apoptosis and upregulated the expression of stress response genes and proinflammatory chemokines in β cells (6, 8, 11). Accordingly, the upregulation of Th17 immunity could contribute to the destruction of β cells and the development of type 1 diabetes.
Animal studies suggest that plasticity of Th17 cells, and the development of IFN-γ and IL-17 coproducers in particular, is associated with autoimmunity. Th17 cells from BDC2.5 mice induced autoimmune diabetes in healthy recipients after their conversion into Th1 cells in vivo. The expression of IL-17 was downregulated and IFN-γ was upregulated in vivo in purified BDC2.5 Th17 cells, which infiltrated the islets and transferred diabetes (12, 13). Neutralization of IFN-γ with Abs inhibited diabetes (12, 13), suggesting that the development of a Th1-type response in Th17 cells was essential for the initiation of β cell destruction. In humans, the conversion of Th17 cells into Th17/Th1-type cells has been reported in the synovial fluid of children with juvenile arthritis (14), and in patients with Crohn’s disease IFN-γ–expressing Th17 cells have been demonstrated in the gut (15). These results suggest that the plasticity of Th17 cells is promoted by the inflammatory cytokine milieu in the target tissue in autoimmune conditions.
There is some evidence of T cell plasticity in human type 1 diabetes. Marwaha et al. (7) reported that Th17 cells in type 1 diabetes also expressed FOXP3, which might imply regulatory activity. Beriou et al. (16) found that subjects with type 1 diabetes had a higher frequency of memory CD4+ cells with the capacity to transition into Th17 cells positive for IL-9. Additionally, plasticity of regulatory T cells (Tregs) has been observed in diabetic patients. Purified FOXP3+ Tregs producing IFN-γ showed, however, low expression of Helios, suggesting that the origin of the Tregs with characteristics of a Th1 effector phenotype was probably not from thymus but from the periphery (17).
In this study, we aimed to examine the timing of the upregulation of Th17 immunity and assess the degree of plasticity of Th17 cells in the development of type 1 diabetes, and accordingly we investigated samples from children in various phases of diabetes-associated autoimmunity up to clinical disease. Upregulation of IFN-γ, IL-9, and IL-17 and plasticity of Th17 cells were only seen in children with advanced β cell autoimmunity and impaired glucose tolerance or clinical type 1 diabetes. Interestingly, the IFN-γ/IL-17 mRNA ratio in Th17 cells correlated with glycosylated hemoglobin (HbA1c) and plasma glucose concentrations in an oral glucose tolerance test performed in the children with advanced β cell autoimmunity, suggesting that the increased degree of Th1/Th17 type of plasticity could reflect impaired β cell function and the degree of β cell destruction. Our results show that the upregulation of Th1/Th17 immunity is a late phenomenon in the preclinical phase and could serve as a biomarker for the development of clinical type 1 diabetes in children with diabetes-associated autoantibodies.
Materials and Methods
Study subjects
Altogether we studied 159 children: 28 Finnish children with type 1 diabetes, 90 children (57 Finnish and 33 Estonian children) from the DIABIMMUNE study (18), and 41 children from the Finnish Type 1 Diabetes Prediction and Prevention study (19).
Upregulation of Th17 immunity in PBMCs was studied in 29 children with early β cell autoimmunity (autoantibody [AAb]+, average age 5.7 ± 3.1 y, 13 males), 9 children with advanced long-term β cell autoimmunity and impaired glucose tolerance demonstrated in at least once in an oral glucose tolerance test (OGTT) (impaired glucose tolerance [IGT], average age 7.7 ± 4.3 y, 7 males), 80 children negative for β cell autoantibodies (AAb−, average age 4.8 ± 2.3 y, 30 males), and 15 children with type 1 diabetes (average disease duration 289 ± 586 d, median disease duration 31, average age 8.3 ± 5.5 y, 9 males).
Nine of the 29 children (31%) with early β cell autoimmunity had one autoantibody at the study visit, 7 children (24%) tested positive for two autoantibodies, 6 children (21%) were positive for three autoantibodies, and 7 children were positive for four autoantibodies. One of nine children with advanced β cell autoimmunity and IGT tested positive for one biochemical autoantibody (11%), two children were positive for two autoantibodies (22%), four children were positive for three autoantibodies (44%), and two children were positive for four biochemical autoantibodies (22%) at the time of assessing the activation of Th17 immunity.
Plasticity of Th17 cells was studied in 8 autoantibody-negative children (AAb−, average age 5.5 ± 2.3 y, three males), 5 healthy children with early β cell autoimmunity (AAb+, average age 5.8 ± 3.5 y, four males), 6 children with advanced long-term β cell autoimmunity (average age 9.2 ± 4.5 y, six males), and 13 children with type 1 diabetes (average disease duration 508 ± 1364 d, median disease duration 63 d, average age 10.2 ± 5.1 y, 7 males).
Eighty-seven of the studied children (55%) carried risk-associated HLA-DR/DQ genotypes (14 strongly, 52 moderately, and 21 slightly increased risk) and 66 (42%) carried genotypes conferring neutral or decreased risk for type 1 diabetes. Risk groups were defined as described earlier in Hekkala et al. (20). The risk-associated HLA genotype of five children (3.0%) with type 1 diabetes was not known at the time of analysis. Detailed characteristics of the study subjects are presented in Supplemental Table 1.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the local Ethical Committees. The parents and/or study subjects gave their written informed voluntary consent prior to blood sample collection.
HLA genotyping and analysis of diabetes-associated autoantibodies
HLA genotyping of major type 1 diabetes risk DR-DQ haplotypes of the study subjects was performed with a PCR-based lanthanide-labeled hybridization method using time-resolved fluorometry for detection as described before (21).
The diabetes-associated Abs IAA, GADA, IA-2A, and ZnT8A were quantified with specific radiobinding assays as previously described (22). The cutoff level was 2.80 relative units for IAA, 5.36 for GADA, 0.78 for IA-2A, and 0.61 relative units for ZnT8A, representing the 99th percentiles in >350 Finnish nondiabetic children. Assays were performed at least at two time points to ensure persistent autoantibody formation. Islet cell Abs were detected with the use of indirect immunofluorescence with a cutoff limit for positivity of 2.5 Juvenile Diabetes Foundation units.
T cell preparations and cell cultures
Plasma was separated from the heparinized blood samples by centrifugation, and PBMCs were isolated by Ficoll isogradient centrifugation (GE Healthcare, Uppsala, Sweden). PBMCs were washed three times with PBS (Lonza, Verviers, Belgium) and resuspended in serum-free X-VIVO 15 culture medium (Lonza). For studying the activation of Th17 immunity, PBMCs were cultured at the density of 2 × 105 cells/200 μl/well in triplicates for 40 h in 96-well round-bottom culture plates (Thermo Scientific, Waltham, MA). The cells were stimulated with plate-bound anti-CD3 (precoating with anti-CD3 at 5 μg/ml in PBS, BD Pharmingen, San Diego, CA) and soluble anti-CD28 (1 μg/ml, BD Pharmingen). Stimulated cells were lyzed in Qiagen RLT lysis buffer (Qiagen, Hilden, Germany) for reverse transcription–quantitative PCR (RT-qPCR).
Sorting of activated Th17 cells
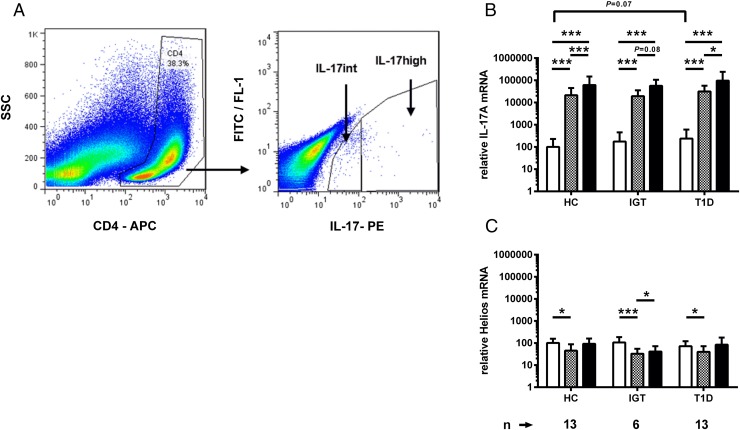
For studying plasticity of activated Th17 cells, PBMCs were stimulated for 72 h as described in the previous section. Activated Th17 cells were labeled with the IL-17 secretion assay kit (Miltenyi Biotec, Gladbach, Germany) with optional staining with anti–CD4-allophycocyanin. Th17 cells were sorted with FACSAria II (BD Biosciences). CD4+ cells with high or intermediate expression of IL-17 (Th17high and Th17int) were collected (Fig. 2) and lysed in RLT buffer (Qiagen) for RT-qPCR analysis.
FIGURE 2.
(A) Gating strategy for FACS sorting of Th17 cells. IL-17–secreting CD4+ cells were stained with a cytokine capture assay and identified based on the IL-17–PE intensity of the CD4+ gated lymphocyte population and sorted into Th17high and Th17int cells. Dead autofluorescent cells were excluded from the sorting based on autofluorescence signal detected on FITC/FL-1 channel. (B) IL-17A mRNA expression is remarkably higher in Th17int (gray bars) and Th17high (black bars) cell fractions in comparison with anti-CD3– and anti-CD28–stimulated PBMCs (white bars). (C) mRNA of Helios, the marker of Tregs, was not enriched in sorted Th17 cells. Bars represent mean and whiskers SD. Relative gene expression of the markers was calculated by comparing ΔCt values of IL-17A and Helios to the average ΔCt value of IL-17A in the stimulated PBMCs of healthy controls. Comparison of different cell populations within the study group was performed with the Student t test for paired samples. Differences between the study groups within a cell population were compared with the Student t test for unpaired samples. *p < 0.05, ***p < 0.001.
RT-qPCR
Total RNA from stimulated PBMCs was isolated with the Qiagen RNeasy Mini kit (Qiagen). An RNeasy Plus Micro kit (Qiagen) was used to isolate total RNA from FACS-purified Th17 cells. cDNA was synthesized using the random hexamer priming of a high-capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). qPCR was performed with TaqMan Fast Master Mix and a StepOne Plus instrument (Applied Biosystems). TaqMan gene expression assays (Applied Biosystems) were used for real-time PCR. Ribosomal 18S was used as an endogenous reference. Relative expression was calculated by the 2−ΔΔCt method.
Analysis method of IFN-γ transcripts in FACS-sorted IFN-γ and IL-17–secreting CD4+ cells
To evaluate the correlation between the expression of IFN-γ mRNA and protein we sorted IFN-γ and/or IL-17A–secreting CD4+ cells from two autoantibody-negative children, three children with advanced β cell autoimmunity, and two children with type 1 diabetes. Briefly, frozen PBMCs were thawed and allowed to recover in X-VIVO 15 culture medium for 4 h in a CO2 incubator at 37°C. Then, the cells were stimulated for 72 h as freshly prepared PBMCs. Stimulated PBMCs were labeled with IL-17–allophycocyanin and IFN-γ–PE secretion assay kits (two-color cytokine secretion assays, Miltenyi Biotec) with CD4-FITC and 7-aminoactinomycin D staining. Viable CD4+ T cells were analyzed, and double-positive and IL-17 or IFN-γ single-positive cells were sorted and subjected to RT-qPCR analysis.
Statistical analyses
Comparisons between groups were performed for log10-transformed data with one-way ANOVA and unpaired or paired Student t tests. Correlations were analyzed with the nonparametric Spearman test. The two-tailed Fisher exact test was used for the comparison of dichotomic data. A p value <0.05 was considered as significant. Statistical analyses were performed with the GraphPad Prism 6.0 software (GraphPad Software, La Jolla, CA), except for the correlation analyses, which were performed with the SPSS 20 software (IBM, Chicago, IL).
Results
Upregulation of the IL-17 pathway in anti-CD3– and anti-CD28–stimulated PBMCs in type 1 diabetes and in advanced β cell autoimmunity
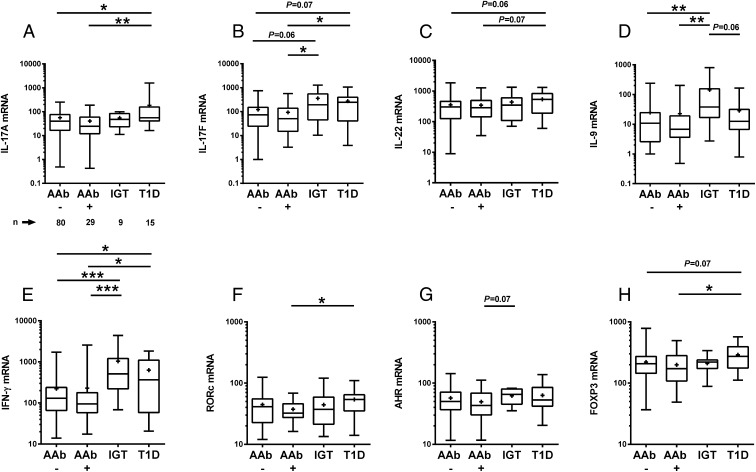
Gene expression of IL-17A, IL-17F, IL-22, IFN-γ, and FOXP3 was higher in children with type 1 diabetes in comparison with autoantibody-negative children and to the children with early β cell autoimmunity (except for IL-22) (Fig. 1). The children with advanced β cell autoimmunity (IGT) showed an increased expression of IL-9 and IFN-γ when compared with autoantibody-negative children (Fig. 1D and 1E, respectively) and enhanced IL-17F levels when compared with the autoantibody-negative children and children with early β cell autoimmunity (Fig. 1B). No differences in the expression of IL-17A, IL-17F, IL-22, IL-9, IFN-γ, or FOXP3 mRNA in activated PBMCs were observed between the children with early β cell autoimmunity (AAb+) and children without any signs for β cell autoimmunity (AAb−) (Fig. 1). IL-22 expression correlated positively with age in children with advanced β cell autoimmunity (r = 0.80, p < 0.05, Spearman test). Age correlated with IL-17A, IL-17F, IL-22, IFN-γ, IL-9, and RORc in children with type 1 diabetes only. Correlations of expression levels of genes in stimulated PBMCs in different study groups are presented in Supplemental Table II. No differences were seen in the gene expression between children from Finland and Estonia (data not shown).
FIGURE 1.
Gene expression levels in anti-CD3– and anti-CD28–stimulated PBMCs in the autoantibody-negative children (AAb−, n = 80), the children with early β cell autoimmunity (AAb+, n = 29), children with advanced β cell autoimmunity (IGT, n = 9), and in children with type 1 diabetes (n = 15). Relative mRNA levels of IL-17A (A), IL-17F (B), IL-22 (C), IL-9 (D), IFN-γ (E), RORc (F), AHR (G), and FOXP3 (H). The boxes represent lower and higher quartiles. Horizontal line in the box represents the median value and whiskers represent minimum and maximum values. Plus sign in the box represents the mean value of the group. The p values were calculated with the Student t test for unpaired samples. Gene expression levels were analyzed with RT-qPCR. Relative gene expression was assessed for each marker by comparing ΔCt value of each sample to the ΔCt value of the calibrator. *p < 0.05, **p < 0.01, ***p < 0.001.
Detection of plasticity of Th17 cells in type 1 diabetes and in advanced β cell autoimmunity
We sorted IL-17–secreting T helper cells from the in vitro–stimulated PBMCs with FACS and evaluated the plasticity of Th17 cells by detecting the expression level of IL-17, IFN-γ, FOXP3, and Helios transcripts in these cells. Gating strategy for sorting of Th17 cells for plasticity analysis is presented in Fig. 2A. Dead cells were excluded from the analysis based on autofluorescence detected on the FITC/FL-1 channel. Remarkable enrichment of IL-17A transcripts was evident in FACS-sorted Th17 cells with intermediate and high expression of the IL-17A protein (Fig. 2B). In contrast, enrichment of Helios was not seen in Th17int or Th17high cells (Fig. 2C).
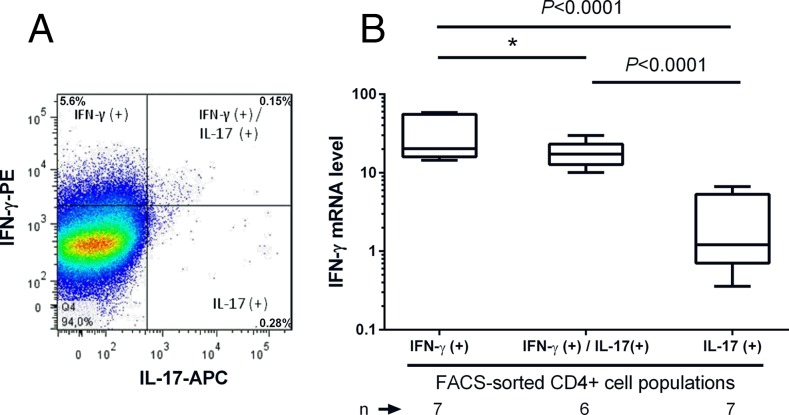
For the method validation, we investigated the relationship between the secreted protein and its mRNA expression, that is, IFN-γ in the FACS-sorted CD4+ cells secreting IFN-γ only, cosecreting IFN-γ and IL-17, and secreting IL-17 alone (Fig. 3). Dead cells were excluded from the analysis by 7-aminoactinomycin D staining of the dual-labeled cells. We observed the highest expression level of IFN-γ transcripts in the FACS-sorted CD4+ cells secreting IFN-γ only whereas the levels were lower in IFN-γ and IL-17 cosecreting cells and lowest in the cells secreting IL-17 alone (Fig. 3), which confirms that the expression level of cytokine-specific transcripts correlates with the secretion of the corresponding cytokine in FACS-sorted cells.
FIGURE 3.
IFN-γ mRNA expression level in the IFN-γ– and/or IL-17–secreting CD4+ cells sorted from anti-CD3– and anti-CD28–stimulated PBMCs. (A) Frozen PBMCs were thawed and stimulated with anti-CD3 and anti-CD28 for 72 h and sorted with FACS into CD4+IFN-γ+, CD4+IFN-γ+IL-17+, and CD4+IL-17+ cells. IFN-γ mRNA expression in sorted cell fractions was analyzed with RT-qPCR. A representative FACS plot of IFN-γ and IL-17 protein expression in CD4+ cells of a child with advanced β cell autoimmunity and IGT is shown. (B) The IFN-γ mRNA expression was highest in the FACS-sorted CD4+ cells secreting IFN-γ alone (n = 7), being somewhat lower in CD4+ cells cosecreting of IFN-γ and IL-17 (n = 6) and lowest in the CD4+ cells secreting IL-17 alone (n = 7). Dead cells were excluded from the analysis by 7-aminoactinomycin D staining of the dual-labeled cells. The p values were calculated with the Student t test for paired samples. *p < 0.05.
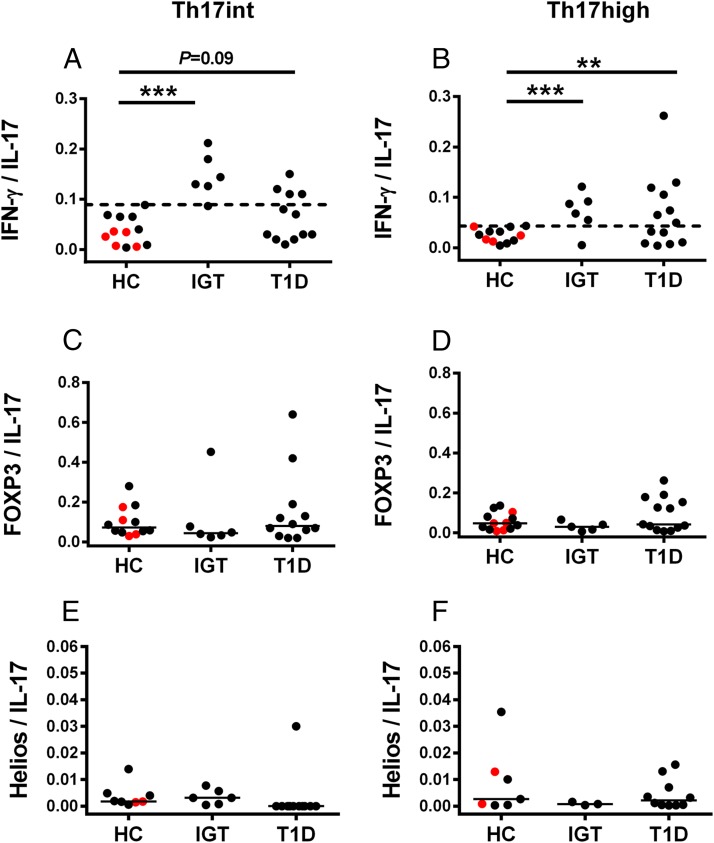
We then compared the plasticity in Th17int and Th17high cells between the study groups. To assess the plasticity of Th17 cells, we calculated the ratio of IFN-γ, FOXP3, and Helios gene expression to IL-17 expression. When the highest IFN-γ/IL-17 mRNA ratio in Th17int and Th17high cells from the healthy children was used as a cutoff value, the children with advanced β cell autoimmunity showed a significantly increased IFN-γ/IL-17 mRNA ratio in Th17int and Th17high cells (Fig. 4). Both FOXP3/IL-17 and Helios/IL-17 ratios were comparable between the study groups in both Th17int and Th17high cells.
FIGURE 4.
The plasticity of sorted Th17 cells was evaluated by calculating the ratio of IFN-γ/IL-17A, FOXP3/IL-17A, and Helios/IL-17A mRNA in Th17int (left column) and Th17high cells (right column) in healthy children (HC), children with advanced β cell autoimmunity and IGT, and in children with type 1 diabetes. Red symbols in the HC group denote the subjects with early β cell autoimmunity. (A and B) The highest IFN-γ/IL-17 ratio in Th17 cells of the healthy children was used as a cutoff for increased Th17 plasticity (dashed line). An increased ratio of IFN-γ/IL-17 in Th17high and Th17int cells was seen in the children with advanced β cell autoimmunity and in the children with type 1 diabetes in comparison with HC. (C–F) FOXP3/IL-17 and Helios/IL-17 ratios in sorted Th17 cells were comparable between the study groups. Statistical significance was calculated with the Fisher exact test. Horizontal lines represent the median ratio. **p < 0.01, ***p < 0.001.
Correlations of mRNA levels in Th17 cells in different study groups are shown in Supplemental Table III. In contrast to stimulated PBMCs, only a few statistically significant correlations were observed in FACS-sorted Th17 cells. In healthy children, IL-17A and FOXP3 mRNA levels correlated significantly in Th17int cells, whereas in Th17high cells IL-17A levels correlated with IFN-γ and FOXP3. In children with advanced β cell autoimmunity (IGT), IL-17A and IFN-γ correlated in Th17int cells, whereas in Th17high cells a significant correlation was found between IL-17A and FOXP3. In patients with type 1 diabetes, IL-17A and IFN-γ showed a significant correlation in Th17high cells. Calendar age correlated with IFN-γ expression in Th17int cells in healthy children and with IFN-γ expression in Th17high cells in children with type 1 diabetes. IFN-γ expression did not correlate with age in Th17int or Th17high cells in the children with advanced β cell autoimmunity.
Increased plasticity of Th17 cells correlated with impaired glucose control
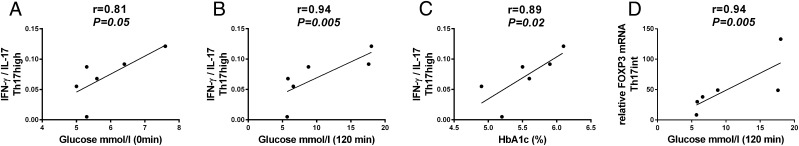
In children with advanced β cell autoimmunity and impaired glucose tolerance, we observed a strong correlation between plasma glucose concentrations and IFN-γ/IL-17 ratio in Th17high cells in the fasting state (Fig. 5A) and at 120 min in the OGTT (Fig. 5B). Additionally, HbA1c and the IFN-γ/IL-17 ratio in Th17high cells correlated strongly in children with advanced β cell autoimmunity (Fig. 5C). No correlation was seen between the IFN-γ/IL-17 ratio and the glucose concentrations or HbA1c levels in Th17int cells in children with advanced β cell autoimmunity. A statistically significant correlation between FOXP3 expression and the 120 min plasma glucose concentration was observed in the OGTT in Th17int cells in children with advanced β cell autoimmunity (Fig. 5D). The FOXP3/IL-17A or Helios/IL-17A ratio did not show any association with HbA1c or plasma glucose concentrations in the fasting state or after 120 min of glucose challenge in the OGTT in children with advanced β cell autoimmunity.
FIGURE 5.
Children with advanced β cell autoimmunity and IGT have an increased IFN-γ/IL-17 ratio in Th17‑ cells, which correlates with the parameters reflecting β cell function (A–C). (A) Fasting plasma glucose concentration correlated with IFN-γ/IL-17 ratio in Th17high cells in children with advanced β cell autoimmunity. (B) IFN-γ/IL-17 ratio in Th17high cells correlated with plasma glucose concentrations at 120 min in the OGTT in children with advanced β cell autoimmunity. (C) HbA1c levels correlated with the IFN-γ/IL-17 ratio in Th17high cells in children with advanced β cell autoimmunity. (D) Statistically significant correlation between the expression of FOXP3 mRNA in Th17int cells and the 120 min glucose concentration in the OGTT was observed in children with advanced β cell autoimmunity. Correlations were calculated with a nonparametric Spearman’s test.
Discussion
We evaluated the upregulation of IL-17 immunity in children at different stages of preclinical diabetes and in children with type 1 diabetes, and observed that the IL-17 pathway is upregulated in peripheral blood in children in the late phase of preclinical diabetes characterized by impaired glucose tolerance, as well as in children with clinical type 1 diabetes. We found no evidence of activated IL-17 immunity in the early stages of the disease process when β cell autoantibodies appear. Most interestingly, sorted Th17 cells showed plasticity characterized by increased expression of IFN-γ, and the degree of Th1/Th17 plasticity correlated with impaired glucose control in children with advanced β cell autoimmunity. These findings suggest that the development of Th17 cells secreting both IFN-γ and IL-17 seems to associate with impaired β cell function and progression to overt type 1 diabetes in autoantibody-positive individuals.
The β cell destruction process leading to clinical type 1 diabetes is relatively slow and stretches often over several years from the appearance of the first marker of disease, namely β cell autoantibodies. The process is not linear but it accelerates close to the diagnosis (23). The detection of β cell autoantibodies, positivity for multiple autoantibodies in particular, is strongly predictive of type 1 diabetes, but there are no biomarkers for the prediction of the rate of progression from autoantibody positivity to clinical type 1 diabetes. Our results demonstrating a correlation between Th1/Th17 plasticity and IGT in children with advanced β cell autoimmunity suggest that the degree of Th1/Th17 plasticity could be a novel biomarker for disease progression and accelerated destruction of the insulin-producing cells in children with β cell autoimmunity. Our study is limited by the cross-sectional design, and these findings should be evaluated in a longitudinal observational study of children with β cell autoimmunity in whom β cell function and the degree of Th17 plasticity are monitored in parallel. The detection of accelerated impairment in the β cell function in autoantibody-positive individuals would facilitate the identification of likely progressors suitable for secondary intervention trials. It might also help in the timing of immune interventions at a stage when the endogenous insulin production is higher than that seen at the time of diagnosis when <30% of functioning β cells are left (24). There is also a need for a biomarker for monitoring the effects of interventions and helping in the assessment of residual β cell function and glycemic control both in children at increased risk for type 1 diabetes and in those with recently diagnosed type 1 diabetes.
Increased Th17 immunity in the peripheral blood has firmly been associated with type 1 diabetes in patients with clinical disease (6–8, 16). To our knowledge, this is the first study demonstrating that Th17 immunity appears in the late phase of preclinical disease in human type 1 diabetes, as earlier suggested in animal models of type 1 diabetes (12, 13, 25). We further demonstrate that Th17 cells express IFN-γ in human type 1 diabetes, and this seems to be associated with the loss of β cell function as also indicated by studies in animal models of autoimmune diabetes (12, 13, 25). Th17 cells purified from diabetic mice and transferred to healthy recipients induced autoimmune diabetes in the recipients, but only when the conversion of Th17 cells into coproducers of IFN-γ and IL-17 took place in vivo (12, 13). Th17 cells are known to show greater instability than other Th cell subsets. In experimental autoimmune encephalomyelitis, most pathogenic IFN-γ–producing cells were derived from Th17 cells in chronic inflammatory settings (26). In the present study, we observed a remarkable increase in the IFN-γ/IL-17 ratio in highly purified Th17 cells from children with advanced β cell autoimmunity and clinical type 1 diabetes, suggesting a similar shift from the Th17 phenotype toward the Th1/Th17 phenotype. The strong positive correlations of the IFN-γ/IL-17 ratio in Th17high cells with the plasma glucose concentrations after the glucose challenge and with the HbA1c values in children with advanced β cell autoimmunity further support the association of Th1/Th17 cells and impaired β cell function in human type 1 diabetes. It is possible that Th17 cells cosecreting IFN-γ and IL-17 are detrimental to β cells and mediate aggressive β cell destruction as indicated by in vitro studies showing an additive effect of IFN-γ and IL-17 on β cell apoptosis (6, 8, 11).
In our approach, we did not detect autoreactivity but we detected emerging plasticity of IL-17-immunity, which reflects phenotype changes in IL-17–secreting T cells in the peripheral blood. Based on animal studies and research on several autoimmune diseases, this kind of change from a well-differentiated T cell phenotype to Th1/Th17 plasticity takes place in autoimmune conditions and could be related to the destruction of the target tissue (7–13, 25, 27, 28). Thus, we do not necessarily need to detect autoreactivity of T cells, as the Th1/Th17 plasticity can be used as a biomarker for the pathogenic β cell autoimmunity when the risk of type 1 diabetes is first identified through an HLA risk genotype and autoantibody positivity.
We cannot exclude the possibility that the upregulation of Th17 immunity and its plasticity are secondary to the subtle elevations in plasma glucose concentrations in children with advanced β cell autoimmunity and type 1 diabetes. Glucose may stimulate β cells to secrete IL-1β, a cytokine important in the activation of Th17 cells (29, 30). Metabolic pathways may affect T cell function and differentiation, and it is of special interest that optimal IFN-γ induction in CD8+ T cells has been shown to be glucose-dependent (31–33). The HbA1c values and fasting glucose concentrations were, however, in the normal range in most of the children with advanced β cell autoimmunity. Nevertheless, the role of glucose needs to be addressed in future studies.
In the present study, the children with type 1 diabetes and the children with advanced β cell autoimmunity had a higher proportion of IL-17–secreting CD4+ cells in stimulated PBMCs than did healthy children (median, 0.2%; range, 0.025–0.45 and 0.14%; 0.11–0.36 versus 0.10%, 0.043–0.3%). Comparable proportions of peripheral blood Th17 cells have been reported in the patients with type 1 diabetes by others. Marwaha et al. (7) demonstrated that the proportion of CD4+IL-17+ cells was 0.47% in children with type 1 diabetes and 0.18% in healthy children based on the intracellular cytokine staining of anti-CD3– and anti-CD28–stimulated and PMA plus ionomycin–restimulated PBMCs. Ferraro et al. (9) reported a slightly higher proportion of CD4+IL-17+ cells using intracellular cytokine staining of PBMCs stimulated with 2-O-tetradecanoylphorbol-13-acetate/ionomycin, that is, ∼1.3 and 0.6% of CD4+IL-17+ cells in adult patients and healthy subjects, respectively. Annunziato et al. (15) observed consistently <1% of Th17 cells among the PMA plus ionomycin–stimulated PBMCs in healthy control subjects.
Tregs can reportedly shift their phenotype and acquire an effector phenotype, characterized by production of IFN-γ or IL-17 (17, 34, 35). We did not find any evidence that Th17 cells in type 1 diabetes would originate from thymic-derived Tregs because the expression of Helios, a marker of thymic-derived Tregs, was low in sorted Th17 cells and did not differ between the children with type 1 diabetes and healthy children. Also, the expression of FOXP3 was similar in Th17 cells from children with type 1 diabetes and in healthy children. However, we observed a strong correlation between FOXP3 expression in Th17int cells and plasma glucose concentrations at 120 min in OGTT in children with advanced β cell autoimmunity. FOXP3 is a transcription factor of Tregs, but it is also upregulated upon TCR-mediated activation of T cells, and thus upregulation of FOXP3 in Th17int cells might reflect the degree of activation of these cells. Accordingly, FOXP3 expression in Th17 cells may reflect the activation of Th17 immunity and show an association with β cell function.
Li and colleagues have demonstrated upregulation of IFN-γ in IL-17-producing invariant NKT cells upon IL-1β in vitro stimulation of PBMCs from patients with type 1 diabetes (36). Because some of the invariant NKT cells express CD4+ cell surface Ag, these cells might be involved in the plasticity observed in our study.
Interestingly, we observed upregulation of IL-17F, IFN-γ, and IL-9 in the activated peripheral blood T cells in children with advanced β cell autoimmunity. This might indicate that the upregulation of IFN-γ and IL-9 precedes the full-blown activation of the Th17 pathway. IL-9 is considered to be a driver for Ag-specific Th17 cells (37). It is also known that IL-17F is expressed earlier than IL-17A during Th17 cell development (38). A recent study showed that IL-9 signaling was associated with type 1 diabetes in two of monozygotic quadruplets (39). Increased numbers of TGF-β1–induced IL-9–secreting Th17 cells have been reported in type 1 diabetes, too (16). Accordingly, IL-9 upregulation could be considered as a marker of Th17 cells also in children with advanced β cell autoimmunity.
In conclusion, upregulation of IFN-γ, IL-9, and IL-17 and plasticity of Th17 cells characterized by coexpression of IFN-γ was only seen in the late phase of the disease process resulting in overt type 1 diabetes. More importantly, the IFN-γ/IL-17 ratio in Th17high cells correlated strongly with plasma glucose concentrations in OGTT and HbA1c levels in children with advanced β cell autoimmunity. Our data suggest that increased plasticity of Th17 cells is a major immunological alteration associated with accelerated β cell destruction preceding the manifestation of type 1 diabetes. Our findings do not support the view that Th17 cells would originate from Tregs in patients with β cell autoimmunity or type 1 diabetes. Th1/Th17 plasticity may provide a novel biomarker for rapid loss of β cell function and progression from β cell autoimmunity to clinical type 1 diabetes.
Supplementary Material
Acknowledgments
We thank The DIABIMMUNE Study Group. We are grateful to Berta Davydova, Sinikka Helander, Juho Hämäläinen, Mevlida Kararic, Markku Latva-Koivisto, Tiina Nurmi, and Iiris Ollila (University of Helsinki) for excellent technical assistance with the autoantibody assays. Additionally, we thank Matti Koski (University of Helsinki) for assistance in database work. We are deeply thankful to Maria Kiikeri, Leena Saarinen, and Anneli Suomela (National Institute for Health and Welfare) for skillful technical assistance. We also thank all of the local study nurses and participating families for commitment to the study.
M.K. and O.V. shared senior coauthorship.
All authors and their affiliations appear at the end of this article.
This work was supported by the European Union Seventh Framework Program (FP7/2007-2013) under Grant 202063 and the Academy of Finland (to O.V. and Centre of Excellence in Molecular Systems Immunology and Physiology Research [2012–2017], Decision 250114 to M.K.), as well as by the Sigrid Juselius Foundation and the Finnish Diabetes Research Foundation.
The online version of this article contains supplemental material.
- AAb
- autoantibody
- GADA
- glutamic acid decarboxylase Ab
- IAA
- insulin autoantibody
- IA-2A
- insulinoma-associated-2 Ab
- IGT
- impaired glucose tolerance
- OGTT
- oral glucose tolerance test
- RT-qPCR
- reverse transcription–quantitative PCR
- Treg
- regulatory T cell
- ZnT8A
- zinc transporter 8 Ab.
DIABIMMUNE Study Group
Mikael Knip, Taina Härkönen, and Samppa Ryhänen (Children’s Hospital, University of Helsinki, 00281 Helsinki, Finland)
Katriina Koski, Matti Koski, and Outi Vaarala (Institute of Clinical Medicine, University of Helsinki, 00140 Helsinki, Finland)
Anu-Maaria Hämäläinen (Jorvi Hospital, Helsinki University Central Hospital, 02740 Espoo, Finland)
Anne Ormisson (Children’s Clinic, Tartu University Hospital, 51014 Tartu, Finland)
Aleksandr Peet and Vallo Tillmann (Department of Pediatrics, Tartu University Hospital, 51014 Tartu, Finland)
Valentina Ulich, Elena Kuzmicheva, and Sergei Mokurov (Ministry of Health and Social Development, Karelian Republic of the Russian Federation, Petrozavodsk 185910, Russia)
Svetlana Markova and Svetlana Pylova (Children’s Republic Hospital, Karelian Republic of the Russian Federation, Petrozavodsk 185002, Russia)
Marina Isakova and Elena Shakurova (Perinatal Center, Karelian Republic of the Russian Federation, Petrozavodsk 185910, Russia)
Vladimir Petrov (Maternity Hospital No. 1, Petrozavodsk 185910, Russia)
Natalya V. Dorshakova, Tatyana Karapetyan, and Tatyana Varlamova (Petrozavodsk State University, Petrozavodsk 185910, Russia)
Jorma Ilonen and Minna Kiviniemi (Immunogenetics Laboratory, University of Turku, 20520 Turku, Finland)
Jorma Ilonen (Department of Clinical Microbiology, University of Eastern Finland, 70211 Kuopio, Finland)
Kristi Alnek, Helis Janson, and Raivo Uibo (Department of Immunology, University of Tartu, 50090 Tartu, Estonia)
Tiit Salum (OÜ Immunotron, 51014 Tartu, Estonia)
Erika von Mutius and Juliane Weber (Children’s Hospital, Ludwig Maximilians University, 80337 Munich, Germany)
Helena Ahlfors, Henna Kallionpää, Essi Laajala, Riitta Lahesmaa, Harri Lähdesmäki, and Robert Moulder (Turku Centre of Biotechnology, University of Turku and Åbo Akademi University, 20520 Turku, Finland)
Janne Nieminen and Terhi Ruohtula (Department of Vaccination and Immune Protection, National Institute for Health and Welfare, 00271 Helsinki, Finland)
Hanna Honkanen, Heikki Hyöty, Anita Kondrashova, and Sami Oikarinen (Department of Virology, University of Tampere, 33014 Tampere, Finland)
Heikki Hyöty (Tampere University Hospital, 33521 Tampere, Finland)
Hermie J. M. Harmsen, Marcus C. De Goffau, and Gjalt Welling (University Medical Center Groningen, 9700 RB Groningen, The Netherlands)
Kirsi Alahuhta and Suvi M. Virtanen (Department for Welfare and Health Promotion, National Institute for Health and Welfare, 00271 Helsinki, Finland)
Disclosures
O.V. is an employee of AstraZeneca as of August 1, 2014. The other authors have no financial conflicts of interest.
References
- 1.Bottazzo G. F., Florin-Christensen A., Doniach D. 1974. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet 304: 1279–1283. [DOI] [PubMed] [Google Scholar]
- 2.MacCuish A. C., Irvine W. J., Barnes E. W., Duncan L. J. 1974. Antibodies to pancreatic islet cells in insulin-dependent diabetics with coexistent autoimmune disease. Lancet 304: 1529–1531. [DOI] [PubMed] [Google Scholar]
- 3.Foulis A. K., McGill M., Farquharson M. A. 1991. Insulitis in type 1 (insulin-dependent) diabetes mellitus in man—macrophages, lymphocytes, and interferon-gamma containing cells. J. Pathol. 165: 97–103. [DOI] [PubMed] [Google Scholar]
- 4.Kallmann B. A., Hüther M., Tubes M., Feldkamp J., Bertrams J., Gries F. A., Lampeter E. F., Kolb H. 1997. Systemic bias of cytokine production toward cell-mediated immune regulation in IDDM and toward humoral immunity in Graves’ disease. Diabetes 46: 237–243. [DOI] [PubMed] [Google Scholar]
- 5.Emamaullee J. A., Davis J., Merani S., Toso C., Elliott J. F., Thiesen A., Shapiro A. M. 2009. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes 58: 1302–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honkanen J., Nieminen J. K., Gao R., Luopajarvi K., Salo H. M., Ilonen J., Knip M., Otonkoski T., Vaarala O. 2010. IL-17 immunity in human type 1 diabetes. J. Immunol. 185: 1959–1967. [DOI] [PubMed] [Google Scholar]
- 7.Marwaha A. K., Crome S. Q., Panagiotopoulos C., Berg K. B., Qin H., Ouyang Q., Xu L., Priatel J. J., Levings M. K., Tan R. 2010. Cutting edge: increased IL-17-secreting T cells in children with new-onset type 1 diabetes. J. Immunol. 185: 3814–3818. [DOI] [PubMed] [Google Scholar]
- 8.Arif S., Moore F., Marks K., Bouckenooghe T., Dayan C. M., Planas R., Vives-Pi M., Powrie J., Tree T., Marchetti P., et al. 2011. Peripheral and islet interleukin-17 pathway activation characterizes human autoimmune diabetes and promotes cytokine-mediated β-cell death. Diabetes 60: 2112–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferraro A., Socci C., Stabilini A., Valle A., Monti P., Piemonti L., Nano R., Olek S., Maffi P., Scavini M., et al. 2011. Expansion of Th17 cells and functional defects in T regulatory cells are key features of the pancreatic lymph nodes in patients with type 1 diabetes. Diabetes 60: 2903–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeung W. C., Al-Shabeeb A., Pang C. N., Wilkins M. R., Catteau J., Howard N. J., Rawlinson W. D., Craig M. E. 2012. Children with islet autoimmunity and enterovirus infection demonstrate a distinct cytokine profile. Diabetes 61: 1500–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grieco F. A., Moore F., Vigneron F., Santin I., Villate O., Marselli L., Rondas D., Korf H., Overbergh L., Dotta F., et al. 2014. 1L-17A increases the expression of proinflammatory chemokines in human pancreatic islets. Diabetologia 57: 502–511. [DOI] [PubMed] [Google Scholar]
- 12.Bending D., De La Pena H., Veldhoen M., Phillips J. M., Uyttenhove C., Stockinger B., Cooke A. 2009. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J. Clin. Invest. 119: 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin-Orozco N., Chung Y., Chang S. H., Wang Y. H., Dong C. 2009. Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur. J. Immunol. 39: 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nistala K., Adams S., Cambrook H., Ursu S., Olivito B., de Jager W., Evans J. G., Cimaz R., Bajaj-Elliott M., Wedderburn L. R. 2010. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc. Natl. Acad. Sci. USA 107: 14751–14756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Annunziato F., Cosmi L., Santarlasci V., Maggi L., Liotta F., Mazzinghi B., Parente E., Filì L., Ferri S., Frosali F., et al. 2007. Phenotypic and functional features of human Th17 cells. J. Exp. Med. 204: 1849–1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beriou G., Bradshaw E. M., Lozano E., Costantino C. M., Hastings W. D., Orban T., Elyaman W., Khoury S. J., Kuchroo V. K., Baecher-Allan C., Hafler D. A. 2010. TGF-β induces IL-9 production from human Th17 cells. J. Immunol. 185: 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McClymont S. A., Putnam A. L., Lee M. R., Esensten J. H., Liu W., Hulme M. A., Hoffmüller U., Baron U., Olek S., Bluestone J. A., Brusko T. M. 2011. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J. Immunol. 186: 3918–3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peet A., Kool P., Ilonen J., Knip M., Tillmann V., DIABIMMUNE Study Group 2012. Birth weight in newborn infants with different diabetes-associated HLA genotypes in three neighbouring countries: Finland, Estonia and Russian Karelia. Diabetes Metab. Res. Rev. 28: 455–461. [DOI] [PubMed] [Google Scholar]
- 19.Kupila A., Muona P., Simell T., Arvilommi P., Savolainen H., Hämäläinen A. M., Korhonen S., Kimpimäki T., Sjöroos M., Ilonen J., et al. Juvenile Diabetes Research Foundation Centre for the Prevention of Type I Diabetes in Finland 2001. Feasibility of genetic and immunological prediction of type I diabetes in a population-based birth cohort. Diabetologia 44: 290–297. [DOI] [PubMed] [Google Scholar]
- 20.Hekkala A., Ilonen J., Knip M., Veijola R., Finnish Paediatric Diabetes Register 2011. Family history of diabetes and distribution of class II HLA genotypes in children with newly diagnosed type 1 diabetes: effect on diabetic ketoacidosis. Eur. J. Endocrinol. 165: 813–817. [DOI] [PubMed] [Google Scholar]
- 21.Mikk M. L., Kiviniemi M., Laine A. P., Härkönen T., Veijola R., Simell O., Knip M., Ilonen J., Finnish Paediatric Diabetes Register 2014. The HLA-B*39 allele increases type 1 diabetes risk conferred by HLA-DRB1*04:04-DQB1*03:02 and HLA-DRB1*08-DQB1*04 class II haplotypes. Hum. Immunol. 75: 65–70. [DOI] [PubMed] [Google Scholar]
- 22.Knip M., Virtanen S. M., Seppä K., Ilonen J., Savilahti E., Vaarala O., Reunanen A., Teramo K., Hämäläinen A. M., Paronen J., et al. Finnish TRIGR Study Group 2010. Dietary intervention in infancy and later signs of β-cell autoimmunity. N. Engl. J. Med. 363: 1900–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akirav E. M., Lebastchi J., Galvan E. M., Henegariu O., Akirav M., Ablamunits V., Lizardi P. M., Herold K. C. 2011. Detection of β cell death in diabetes using differentially methylated circulating DNA. Proc. Natl. Acad. Sci. USA 108: 19018–19023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schölin A., Nyström L., Arnqvist H., Bolinder J., Björk E., Berne C., Karlsson F. A., Diabetes Incidence Study Group in Sweden (DISS) 2011. Proinsulin/C-peptide ratio, glucagon and remission in new-onset type 1 diabetes mellitus in young adults. Diabet. Med. 28: 156–161. [DOI] [PubMed] [Google Scholar]
- 25.Li C. R., Mueller E. E., Bradley L. M. 2014. Islet antigen-specific Th17 cells can induce TNF-α-dependent autoimmune diabetes. J. Immunol. 192: 1425–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirota K., Duarte J. H., Veldhoen M., Hornsby E., Li Y., Cua D. J., Ahlfors H., Wilhelm C., Tolaini M., Menzel U., et al. 2011. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat. Immunol. 12: 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bending D., Newland S., Krejcí A., Phillips J. M., Bray S., Cooke A. 2011. Epigenetic changes at Il12rb2 and Tbx21 in relation to plasticity behavior of Th17 cells. J. Immunol. 186: 3373–3382. [DOI] [PubMed] [Google Scholar]
- 28.Lexberg M. H., Taubner A., Albrecht I., Lepenies I., Richter A., Kamradt T., Radbruch A., Chang H. D. 2010. IFN-γ and IL-12 synergize to convert in vivo generated Th17 into Th1/Th17 cells. Eur. J. Immunol. 40: 3017–3027. [DOI] [PubMed] [Google Scholar]
- 29.Maedler K., Sergeev P., Ris F., Oberholzer J., Joller-Jemelka H. I., Spinas G. A., Kaiser N., Halban P. A., Donath M. Y. 2002. Glucose-induced β cell production of IL-1β contributes to glucotoxicity in human pancreatic islets. J. Clin. Invest. 110: 851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandrup-Poulsen T., Spinas G. A., Prowse S. J., Hansen B. S., Jørgensen D. W., Bendtzen K., Nielsen J. H., Nerup J. 1987. Islet cytotoxicity of interleukin 1. Influence of culture conditions and islet donor characteristics. Diabetes 36: 641–647. [DOI] [PubMed] [Google Scholar]
- 31.Cham C. M., Driessens G., O’Keefe J. P., Gajewski T. F. 2008. Glucose deprivation inhibits multiple key gene expression events and effector functions in CD8+ T cells. Eur. J. Immunol. 38: 2438–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cham C. M., Gajewski T. F. 2005. Glucose availability regulates IFN-γ production and p70S6 kinase activation in CD8+ effector T cells. J. Immunol. 174: 4670–4677. [DOI] [PubMed] [Google Scholar]
- 33.Gerriets V. A., Rathmell J. C. 2012. Metabolic pathways in T cell fate and function. Trends Immunol. 33: 168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou X., Bailey-Bucktrout S., Jeker L. T., Bluestone J. A. 2009. Plasticity of CD4+ FoxP3+ T cells. Curr. Opin. Immunol. 21: 281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou X., Bailey-Bucktrout S. L., Jeker L. T., Penaranda C., Martínez-Llordella M., Ashby M., Nakayama M., Rosenthal W., Bluestone J. A. 2009. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 10: 1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li S., Joseph C., Becourt C., Klibi J., Luce S., Dubois-Laforgue D., Larger E., Boitard C., Benlagha K. 2014. Potential role of IL-17-producing iNKT cells in type 1 diabetes. PLoS ONE 9: e96151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elyaman W., Bradshaw E. M., Uyttenhove C., Dardalhon V., Awasthi A., Imitola J., Bettelli E., Oukka M., van Snick J., Renauld J. C., et al. 2009. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc. Natl. Acad. Sci. USA 106: 12885–12890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee Y. K., Turner H., Maynard C. L., Oliver J. R., Chen D., Elson C. O., Weaver C. T. 2009. Late developmental plasticity in the T helper 17 lineage. Immunity 30: 92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stechova K., Halbhuber Z., Hubackova M., Kayserova J., Petruzelkova L., Vcelakova J., Kolouskova S., Ulmannova T., Faresjö M., Neuwirth A., et al. 2012. Case report: type 1 diabetes in monozygotic quadruplets. Eur. J. Hum. Genet. 20: 457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.