Abstract
The social environment plays a critical role in smoking initiation as well as relapse. We previously reported that rats acquired nicotine self-administration with an olfactogustatory cue only when another rat consuming the same cue was present during self-administration. Because carbon disulfide (CS2) mediates social learning of food preference in rodents, we hypothesized that socially acquired nicotine self-administration is also mediated by CS2. We tested this hypothesis by placing female adolescent Sprague-Dawley rats in operant chambers equipped with two lickometers. Licking on the active spout meeting a fixed-ratio 10 schedule triggered the concurrent delivery of an i.v. infusion (saline, or 30 µg/kg nicotine, free base) and an appetitive olfactogustatory cue containing CS2 (0–500 ppm). Rats that self-administered nicotine with the olfactogustatory cue alone licked less on the active spout than on the inactive spout. Adding CS2 to the olfactogustatory cue reversed the preference for the spouts. The group that received 500 ppm CS2 and the olfactogustatory cue obtained a significantly greater number of nicotine infusions than other groups. After extinction training, the original self-administration context reinstated nicotine-seeking behavior in all nicotine groups. In addition, in rats that received the olfactogustatory cue and 500 ppm CS2 during SA, a social environment where the nicotine-associated olfactory cue is present, induced much stronger drug-seeking behavior compared to a social environment lacking the olfactogustatory cue. These data established that CS2 is a critical signal that mediates social learning of nicotine self-administration with olfactogustatory cues in rodents. Additionally, these data showed that the social context can further enhance the drug-seeking behavior induced by the drug-taking environment.
Introduction
Among the many factors that promote smoking in teenagers, social environment is arguably one of the most critical [1], [2]. Both longitudinal [3] and cross-sectional [4] studies have identified peer smoking as a significant predictor for nicotine dependence. Intriguingly, White, et al. [5], reported that the high concordance in smoking found among monozygotic twins can be sufficiently explained by exposure to similar social environments. Furthermore, social environment also was found to have great impact on smoking cessation [6]. However, the mechanisms underlying the critical influence of social environment are mostly unknown.
Using olfactogustatory stimuli as the contingent sensory cue for i.v. nicotine delivery, we showed the inducing role of social learning in nicotine self-administration (SA) in adolescent rats [7]. In this model, each rat self-administering nicotine was accompanied by a demonstrator rat. These two rats were separated by a divider that allowed orofacial interaction. Licking was used as the operant behavior to deliver an oral olfactogustatory cue contingent with each i.v. nicotine infusion. We found that rats developed conditioned taste aversion to nicotine when the olfactogustatory cue was withheld from the demonstrator rat. In contrast, stable nicotine SA was established when the demonstrator rat had access to the olfactogustatory cue. Because neither the olfactogustatory cue alone nor the demonstrator alone supported nicotine SA, the contingent presentations of the olfactogustatory cue and a signal produced by the demonstrator rat (i.e., a social signal) is required in this model of nicotine SA. The social nature of this model dictated that the behavior of the demonstrator rat affect nicotine intake of the SA rat, which introduces a potential confounding variable should we use this model to study the genetics of nicotine SA [8]. On the other hand, identifying the chemical nature of the social signal could allow us to standardize the social signal across all experimental subjects.
Our nicotine SA protocol parallels the well-established social transmission of food preference paradigm, where interaction with a rat that just consumed flavored food enhanced the preference for that food in naive rats [9]. Galef, et al., established that carbon disulfide (CS2), a volatile compound contained in the exhaled breath of rodents was sufficient for social learning of food preference [10]. Because only orofacial interaction is allowed in our model, a volatile compound in exhaled breath is a strong candidate for the social signal. Additionally, the social signal in our model is likely detected at the same time as the olfactogustatory cue from the demonstrator rats. Thus, we tested the hypothesis that contingent presentations of CS2 and the olfactogustatory cue supports socially-acquired nicotine SA in the absence of demonstrator rats. Furthermore, because the presence of other smokers in the environment predicted higher likelihood of relapse [11], we further hypothesized that socially-transmitted nicotine cue can enhance the context-induced reinstatement of drug-seeking behavior. Our data supported both hypotheses.
Materials and Methods
Animals
Female adolescent Sprague-Dawley rats used for nicotine SA and adult rats used as demonstrators were purchased from Harlan Laboratories (Madison, WI). Adolescent rats were used because the great majority of tobacco use begins during adolescence [12]. Females were used because we found, in one study [8], that female rats were more sensitive to social signals than males. We also reported that adolescent rats acquired nicotine SA more rapidly and attained higher levels of drug intake than adults [13]. Similar to our previous studies [13], [14], rats arrived at our animal facility on approximately postnatal day 31 and received surgery on approximately postnatal day 38. The definition of adolescence in rodents is controversial. According to a conservative perspective in rodents [15], prototypical adolescent changes occur approximately during postnatal day 28–42. Some developmental changes specific to adolescence do persist through PN 55 [15]. Thus, the present experiments were performed within the broadly defined age range of adolescence. Upon arrival, rats were given five to seven days of acclimation to a reversed 12:12 h light–dark cycle (lights off at 9:00 a.m.). Standard rat chow and water were provided ad libitum. All rats were group housed with two to four peers throughout the experiments to avoid social isolation. All procedures were conducted in accordance with the NIH Guidelines concerning the Care and Use of Laboratory Animals, and approved by the Institutional Animal Care and Use Committee of the University of Tennessee Health Science Center.
Experimental treatments
We used 0.4% saccharin and 0.1% unsweetened grape-flavored Kool-Aid (prepared in water) as the olfactogustatory cue. Different concentrations of CS2 (10, 100, and 500 ppm, Sigma, St. Louis, MO) were prepared by diluting CS2 in the olfactogustatory cue. No solvent was used. The effect of the CS2-containing olfactogustatory cue on nicotine SA was tested in the absence of demonstrator rats. The control groups self-administered i.v. nicotine with only the olfactogustatory cue, or only 500 ppm CS2 (in water). A third group of control rats self-administered i.v. saline with 500 ppm CS2. Contextual cue induced reinstatement was tested in all groups following extinction training.
Nicotine self-administration
Nicotine SA was conducted according to our published protocol [7] with some modifications. Rats were implanted with jugular catheters constructed using Micro-Renathane tubing (Braintree Scientific Inc., Braintree, MA) under isoflurane anesthesia. The tubing exited from the back of the rat. Ketoprofen (2 mg/kg, s.c.) was given immediately after surgery for postoperative analgesia. After three days of recovery, rats were given access to nicotine SA 3 h per day for 12 days in the dark-phase of the light cycle. The operant chambers were located in sound attenuating chambers and each contained two drinking spouts fitted on the same wall. Two syringe pumps were placed outside of each sound attenuating chamber, one delivered i.v. nicotine through a swivel located on top of the chamber, and the other delivered the olfactogustatory cue to the active spout. The inactive spout contained no solution. Each spout was connected to a contact lickometer controller allowing the number and the timing of licks to be recorded. SA was conducted using a fixed-ratio 10 schedule with 20 s timeout period (FR10TO20). Thus, 10 licks on the active spout activated the simultaneous delivery of a 60 µl olfactogustatory cue, and an i.v. infusion (nicotine free base, 30 µg/kg or saline). The olfactogustatory solution contained saccharin (0.4%) and unsweetened grape Kool-Aid (0.1%) as well as different concentrations of CS2 (0–500 ppm). A previous report [16] showed that oral CS2 exposure at 100–253 mg/kg/day caused no adverse effects on systemic, neurological and developmental systems in rats. Licks on the inactive spout had no programmed consequence. Licks during the timeout period had no consequences but were recorded. No audio or visual cue was used. Rats were not food or water deprived. Nor did rats receive operant training or priming nicotine injections prior to the initiation of the SA sessions. The patency of the jugular catheters was tested using a fast acting anesthetic, methohexital (0.2 ml, 10 mg/ml), at the end of the 12 SA sessions for each rat. Rats without functional catheters were excluded from the analysis.
Extinction and context-induced reinstatement
Context extinction training was conducted after 12 SA sessions. The context extinction chambers were different from the SA operant chamber in many aspects, including the floor, distinct audiovisual cues and novel odor [17]. There were two clean dry spouts in the chamber. The number of licks were recorded but had no programmed consequence. Because most of the licking during extinction training occurs early during the session, we reduced the time of extinction to one hour per session. Extinction sessions were conducted daily until the number of licks on the “active” spout was reduced to less than 50 for two consecutive daily sessions.
Two reinstatement tests (one session per day, each session was one hour) were conducted once the extinction criterion was met to examine whether the presence of socially-transmitted nicotine cue could enhance context-induced nicotine seeking behavior. During these tests, rats were placed in the original SA chambers but licking on the spouts had no consequence. Because it is likely appetitive, CS2 was not delivered by the licking spout (Fig. 1a). Instead, a randomly-selected, unfamiliar demonstrator rat was used to provide the social environment. A perforated divider was used to separate the demonstrator and the SA rat. In one of the sessions the demonstrator rat had access to the olfactogustatory cue, thus providing an inducing social environment (ISE). In the other session, the demonstrator rat did not have access to the olfactogustatory cue, providing a neutral social environment (NSE). The order of social environment was counterbalanced between rats within the same treatment group.
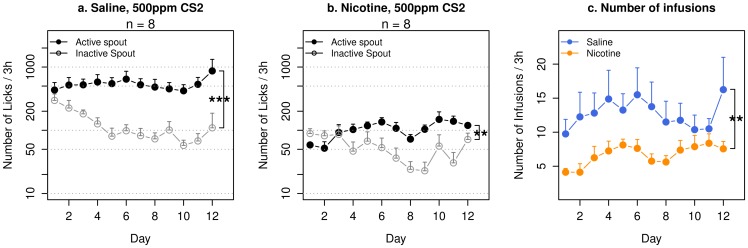
Figure 1. Nicotine self-administration with CS2 in adolescent rats.
Adolescent female Sprague-Dawley rats implanted with jugular catheters were placed in operant chambers equipped with two lickometers. Licking on the active spout that met a fixed-ratio 10 reinforcement schedule triggered contingent delivery of oral CS2 (500 ppm, dissolved in water) and i.v. saline (a) or i.v. nicotine (b). No olfactogustatory cue was used. Both groups showed preference for the active spout. The average number of infusions (c) was 12.9±1.9 for rats that self-administered saline, and 6.7±0.4 for rats that self-administered nicotine. **: p<0.01; ***: p<0.001, repeated measures ANOVA.
Statistical analysis
The number of licks were converted to log scale so that the data fit a normal distribution. The effects of CS2 and nicotine on the number of licks and infusions were analyzed using repeated measures ANOVA. Post-hoc tests were conducted using the Tukey HSD procedure when necessary. Spout and session were treated as within-subject variables, while CS2 concentration and i.v. treatment were between subject variables. Data were presented as mean ± SEM. Statistical significance was assigned when p<0.05. All statistical analyses were conducted using the R statistical language.
Results
Saline or nicotine self-administration with CS2 as the cue
Rats that received contingent oral CS2 (500 ppm) with i.v. saline (Fig. 1a) showed a preference for the active spout (F1,7 = 102.4, p<0.001). The number of licks on the active spout did not change significantly across the sessions (F11,77 = 0.5, p>0.05), neither did the number of infusions change (Fig. 1c. F11,77 = 1.4, p>0.05). There was a significant interaction between spout and session (F11,77 = 2.4, p<0.05). On average, 12.9±1.9 infusions were obtained. These data suggested that CS2 is appetitive.
Rats that received contingent oral CS2 (500 ppm) with i.v. nicotine (Fig. 1b) showed a preference for the active spout (F1,7 = 16.4, p<0.01). The number of licks on the active spout increased significantly across the sessions (F11,77 = 2.4, p<0.05). However, the number of infusions did not change significantly (Fig. 1c. F11,77 = 1.4, p>0.05). There was a significant interaction between spout and session (F11,77 = 9.0, p<0.01). On average, 6.7±0.4 infusions were obtained. These data suggested that CS2 supported nicotine SA.
Rats that received i.v. saline emitted significantly more licks on the active spout compared to rats that received i.v. nicotine (Fig. 1a, 1b, F1,13 = 40.0, p<0.001), and obtained significantly more infusions (Fig. 1c. F1,13 = 14.8, p<0.01), suggesting that nicotine is potentially aversive.
Nicotine self-administration with an olfactogustatory cue and different CS2 concentrations
As expected based on our previous studies [7], rats that self-administered i.v. nicotine with a contingent oral olfactogustatory cue without CS2 (Fig. 2a) emitted fewer licks on the active spout compared to the inactive spout (F1,7 = 7.0, p<0.05), suggesting that contingent olfactogustatory cue was associated with the aversive effect of nicotine. The number of licks did not change over the sessions (F11,77 = 0.4, p>0.05), suggesting extending the training did not change the overall subjective value of the stimuli (i.e., nicotine and the olfactogustatory cue).
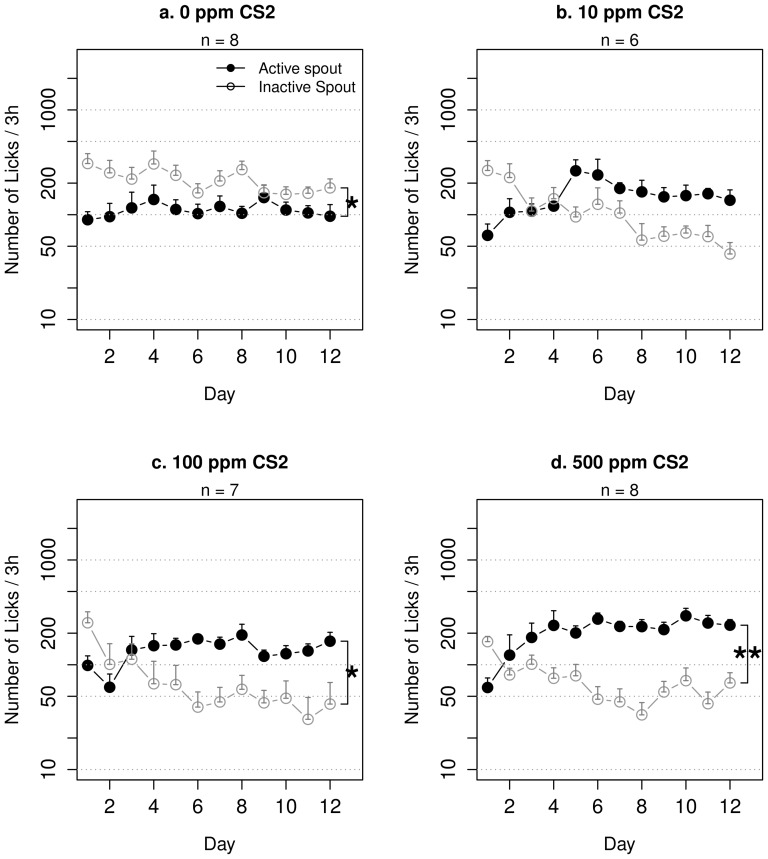
Figure 2. Nicotine self-administration with an olfactogustatory cue and CS2 in adolescent rats.
Adolescent rats self-administered i.v. nicotine with contingent olfactogustatory cue containing different concentrations of CS2. Rats that received only the olfactogustatory cue (a) licked less on the active spout. With increasing concentration of CS2 included in the olfactogustatory cue, the number of licks on the active spout took fewer sessions to surpass that on the inactive spouts (10 ppm: 4 sessions, 100 ppm: 3 sessions, 500 ppm: 1 session). *: p<0.05; **: p<0.01, repeated measures ANOVA.
Rats that received nicotine SA with contingent olfactogustatory cue and 10 ppm CS2 (Fig. 2b) did not show preference for either spout (F1,5 = 3.2, p>0.05). However, the number of licks on the active spout significantly increased across the sessions (F11,55 = 2.4, p<0.05). There was a significant interaction between the effect of spout and session (F11,55 = 7.5, p<0.001). The average number of licks on the active spout exceeded that of the inactive spout starting from day 5 and remained greater throughout the rest of the training sessions, suggesting that the inclusion of CS2 gradually changed the affective value of the stimuli (i.e., nicotine, the olfactogustatory cue, and CS2)
Rats that received nicotine SA with contingent olfactogustatory cue and 100 ppm CS2 (Fig. 2c) showed significant preference for the active spout (F1,6 = 9.5, p<0.05), suggesting that the overall stimuli was appetitive. The interaction between spout and session was statistically significant (F11,66 = 5.3, p<0.001) The number of licks on the active spout increased significantly across the sessions (F11,66 = 2.8, p<0.01). The average number of licks on the active spout exceeded that of the inactive spout starting from day 4 and remained greater throughout the rest of the training sessions.
Rats that received nicotine SA with contingent olfactogustatory cue and 500 ppm CS2 (Fig. 2d) showed significant preference for the active spout (F1,7 = 27.4, p<0.01). The number of licks on the active spout increased significantly across the sessions (F11,77 = 5.2, p<0.001). The interaction between spout and session was significant (F11,77 = 10.4, p<0.001). The average number of licks on the active spout exceeded that of the inactive spout starting from day 2 and remained greater throughout the rest of the training sessions.
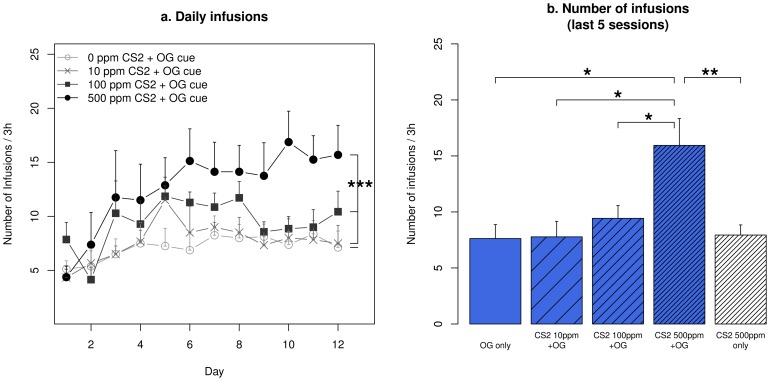
In Fig. 3a. we compare the number of nicotine infusions obtained by rats receiving nicotine SA with olfactogustatory cue and different concentrations of CS2. Repeated measures ANOVA found that the number of infusions increased significantly for all groups (F11,319 = 7.1, p<0.001). Tukey HSD post-hoc test showed rats that received 500 ppm CS2 obtained significantly more infusions compared to all three other groups (p<0.001, p<0.001, and p<0.01 for 0, 10, and 100 ppm CS2, respectively). Fig. 3b summarizes the number of infusions for the last five days, when nicotine infusion was stable. Rats that received 500 ppm CS2 and olfactogustatory cue obtained significantly more infusions compared to those that received the olfactogustatory cue with lower CS2 concentrations or no CS2 (p<0.05 for all), or 500 ppm CS2 without olfactogustatory cue (p<0.01).
Figure 3. Number of infusions obtained by rats self-administering nicotine with an olfactogustatory cue and different concentrations of CS2.
(a) The number of daily infusions obtained by rats that received 500 ppm CS2 was significantly higher than all other groups. (b) The average number of infusions obtained during the last five sessions was compared between different oral cues. * p<0.05, Tukey HSD; ** p<0.01, Tukey HSD; ***: p<0.001, Repeated measures ANOVA.
Context-induced drug-seeking behavior
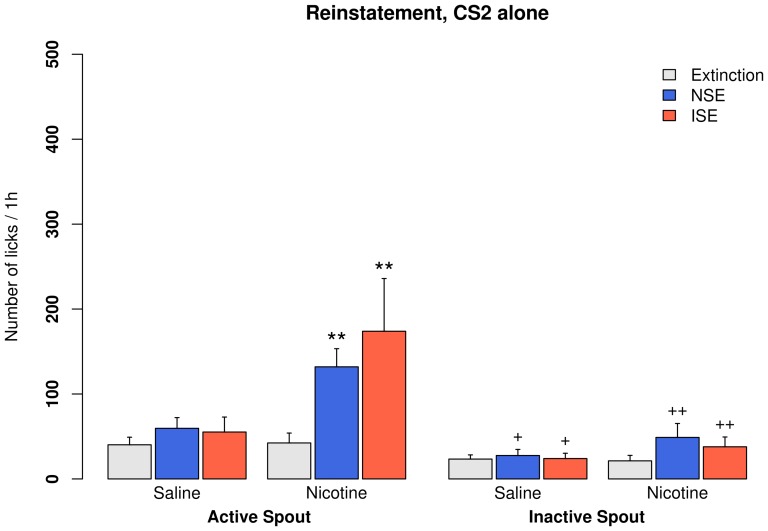
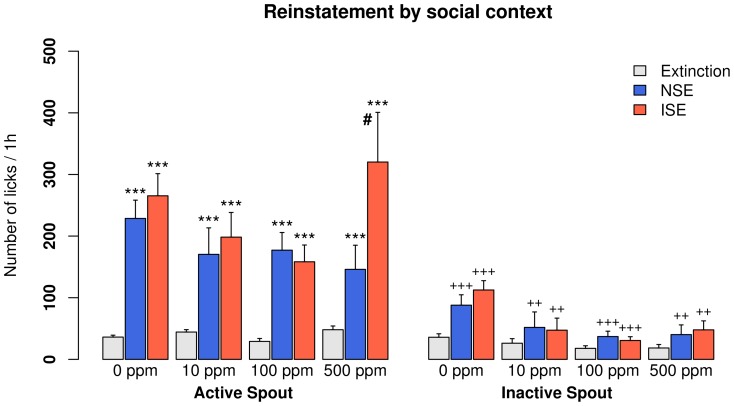
Most rats reached the extinction criteria in less than five sessions. The main effects of re-exposing to the SA context, the types of social environment (ISE vs. NSE), and the spout (active vs. inactive) are shown in Table 1. No statistically significant interaction was found. Overall, the original SA context did not change the number of active licks during reinstatement tests in rats that received i.v. saline with a CS2 cue (Fig. 4). In contrast, context-induced drug-seeking behavior was seen in all groups that received i.v. nicotine, regardless of the sensory cues they received (Figs. 4 and 5). The effect of social context was only significant in the group that received 500 ppm CS2, where the number of active licks was 320±80.7 in the ISE and 146±39.2 in the NSE.
Table 1. Statistical results for context-induced reinstatement.
| OG cue | CS2 (ppm) | i.v. Drug | Reinstatement | Social context | Spout |
| No | 500 | Saline | F2,16 = 0.9, p>0.05 | p>0.05 | F1,6 = 18.5, p<0.05 |
| No | 500 | Nicotine | F2,16 = 2.2, p<0.01 | p>0.05 | F1,6 = 33.1, p<0.01 |
| Yes | 0 | Nicotine | F2,19 = 53.3, p<0.001 | p>0.05 | F1,8 = 55.0, p<0.001 |
| Yes | 10 | Nicotine | F2,13 = 18.5, p<0.001 | p>0.05 | F1,5 = 24.5, p<0.01 |
| Yes | 100 | Nicotine | F2,16 = 48.2, p<0.001 | p>0.05 | F1,7 = 79.5, p<0.001 |
| Yes | 500 | Nicotine | F2,16 = 20.1, p<0.001 | p<0.05 | F1,6 = 16.7, p<0.01 |
Two reinstatement tests were conducted consecutively in the presence of demonstrator rats that provided either a neutral social environment or an inducing social environment. Repeated measures ANOVA was used to compare the number of licks on the active spout between extinction and reinstatement, the number of licks on the active spout between the two social contexts, and the number of licks on the active vs. the inactive spout during reinstatement tests.
Figure 4. Context-induced drug-seeking in rats self-administering saline or nicotine with CS2.
Extinction training was conducted in operant chambers different from those used in the self-administration sessions. Two reinstatement tests were conducted consecutively in the presence of a demonstrator rat. The demonstrator rat either provided a neutral social environment (NSE, i.e., did not have access to the olfactogustatory cue), or an inducing social environment (ISE, i.e., consuming olfactogustatory olfactogustatory cue). The sequence of tests were counterbalanced between rats. **: p<0.01, compared to extinction; +: p<0.05, compared to the active spouts; ++: p<0.01, compared to the active spouts.
Figure 5. Context-induced drug-seeking in rats self-administering nicotine with an olfactogustatory cue and different concentrations of CS2.
The nicotine self-administration environment induced strong drug-seeking behavior in all nicotine groups. In rats that received 500 ppm CS2 during self-administration, the inducing social environment induced a significantly greater amount of drug-seeking behavior compared to the neutral social environment. ***: p<0.001 compared to extinction; ++: p<0.01 compared to the active spouts; +++: p<0.001 compared to the active spouts; #: p<0.05 compared to NSE. NSE: the neutral social environment; ISE: the inducing social environment.
Discussion
We showed that a contingent olfactogustatory cue was associated with the aversive effect of self-administered nicotine. However, the addition of CS2 to the olfactogustatory cue supported nicotine SA. Among the three groups of rats that self-administered nicotine with an olfactogustatory cue and CS2, both the 100 and 500 ppm CS2 groups showed preference for the active spout. After extinction training, the original SA context reinstated nicotine-seeking behavior in all groups that self-administered nicotine. Further, in rats that self-administered nicotine with 500 ppm CS2 and an olfactogustatory cue, the socially-transmitted nicotine-associated odor cue increased drug-seeking behavior above that induced by a neutral social environment.
Many clinical studies have shown that nicotine has few positive affective effects. Rather, aversive effects, such as nausea, dizziness, coughing, and headache are much more common [12], [18]. In spite of these negative effects, approximately 40% of teenagers that experiment with cigarettes become regular smokers [19]. One critical factor that has large influence on smoking initiation is the social environment [5], [20], [21]. We previously reported a model of nicotine SA in adolescent rats, where the presence of a demonstrator rat that carried the nicotine-associated olfactogustatory cue was a determining factor for the acquisition of SA [7]. The data presented here identified CS2 as the social signal that mediated the effect of the demonstrator rats.
CS2 is produced by respiratory tract [22] and gut [23] bacteria and is present in the breath of rodents and humans [24]. A recent study showed that a specialized type of olfactory sensory neuron that expresses the receptor guanylyl cyclase type D (GC-D) is required for the detection of CS2. Mice with mutations in the genes of this pathway are deficient in social learning [25]. However, it is unlikely that CS2 is a social signal that facilitates smoking among teenagers, because social learning in primates and humans requires much more complex cognitive functions [26]. On the other hand, we argue that despite the vast difference in the signals involved in social learning, the central processing of such signals is, to some extent, conserved. One example is the neuropeptide oxytocin, which mediates a variety of social behaviors in both humans and rodents, including social learning [27], [28]. In a separate study, we have begun looking at the role of this neuropeptide in nicotine SA using our model. Therefore, our model has the potential to reveal mechanisms of social learning critical for smoking in humans. One practical advantage of using CS2 is that it allows the amount of social signal to be standardized. Therefore, it eliminates the variation caused by the different behaviors among demonstrators [8].
One key finding from these experiments is that CS2 reversed the preference for the spouts in rats self-administering nicotine. We have found that the ratio of licks on the active/inactive spout has a strong correlation (r = 0.8, p<0.001) with the size of lick clusters (Wang and coworkers, manuscript under review). The size of lick clusters reflects the subjective value of oral stimuli [29], with appetitive stimuli producing larger lick clusters. Therefore, a greater number of licks on the active spout than on the inactive spout indicates that a stimulus is appetitive. Fig. 1a showed that rats preferred the active spout when CS2 alone was used as the sensory cue for i.v. saline, which suggested that CS2 per se was appetitive. This was in agreement with findings that social environments are rewarding [30]. Fig. 1b showed that rats that self-administered nicotine with CS2 alone also preferred the active spout, suggesting that the combined stimuli provided by CS2 and nicotine was appetitive.
In contrast to Fig 1b, our previous study (see Fig. 6 in [7]) found that rats that self-administered nicotine accompanied by demonstrators without access to the odor cue did not prefer the active spout. These two experiments were similar in that rats self-administered nicotine in an environment where CS2 was present (contained in the olfactogustatory cue in this experiment and from live companion rats in our previous experiment). However, there are two major experimental design differences: 1) Rats in Fig. 1b did not receive contingent olfactogustatory cue, while the SA rats in our previous study received contingent taste cue; 2) Rats in Fig. 1b received CS2 as a discrete cue contingent with the delivery of nicotine, while in our previous study the demonstrator was part of the context. Therefore, a likely explanation for these data is that nicotine produces both rewarding and aversive effects. The overall subjective effect of nicotine is determined by the sensory stimuli contingently presented with nicotine. Odor and taste cues are associated with nicotine induced aversion, even when the cues are highly appetitive [7]. Social cues however, are associated with nicotine reward (Fig. 1b). Lastly, the odor cue needed to be socially-transmitted [7], or presented together with CS2 (Fig. 2) to overcome the conditioned taste and odor aversion produced by nicotine.
Fig. 2 showed that the number of inactive licks were higher than the active ones throughout the 12 sessions in the group that received nicotine SA with an olfactogustatory cue but not CS2. However, with increasing CS2 concentrations, the number of active licks took progressively fewer sessions to surpass that of the inactive licks. Because CS2 per se is appetitive (Fig. 1a), the behavioral changes could result from the enhanced reward value of CS2, the interaction between the reward values of nicotine and CS2, or a weakened association between the aversive effect of nicotine and the olfactogustatory cue.. Our current data cannot differentiate between these alternative mechanisms. Although it is difficult to equate the CS2 concentrations with live rats, the data shown in Fig. 3 indicated that rats that received 10–100 ppm obtained approximately the same amounts of nicotine as those accompanied by randomly selected unfamiliar demonstrators [7]. We also reported that familiar demonstrators facilitated an enhanced nicotine intake [7], which was similar to those obtained by rats that received 500 ppm CS2 in this study.
Clinical studies have found that exposure to a smoking-related environment elicits a robust craving to smoke [31]. The presence of other smokers in the environment predicted a higher likelihood of relapse [11], [32]. Therefore, we tested whether socially-transmitted nicotine cue can enhance context-induced reinstatement of drug-seeking behavior. We have previously shown [8] that re-exposing rats that acquired nicotine SA in a social setting to the nicotine-taking environment induced strong reinstatement behavior. Additionally, the amount of social interaction during reinstatement was a significant predictor of reinstatement. However, because reinstatement behavior was only tested in an environment containing demonstrators actively consuming the olfactogustatory cue in that study, the role of socially-transmitted signal was not dissociable from the overall environment. In the current study, we clarified the role of socially-transmitted odor cue during the reinstatement test. We did not contingently present CS2 during the reinstatement tests because CS2 is likely rewarding (Fig. 1a). Instead, we provided demonstrator rats in the nicotine-taking environment during two consecutive reinstatement tests, where the demonstrators either did or did not have access to the olfactogustatory cue. Figs. 4 and 5 showed that the SA environment induced drug-seeking behavior in all groups that self-administered nicotine. Furthermore, in rats that received the olfactogustatory cue and 500 ppm CS2 during SA, a social environment where nicotine associated olfactory cue is socially-transmitted (i.e., ISE) induced much stronger drug-seeking behavior compared to a social environment lacking that olfactogustatory cue (NSE). Therefore, these data showed that socially-transmitted nicotine-associated odor cue enhanced drug-seeking behavior.
In summary, we have established a nicotine SA model in adolescent rats where stable nicotine intake is enabled by contingent presentations of CS2 and an olfactogustatory cue. In addition, socially-transmitted nicotine-associated odor cue enhanced drug-seeking behavior induced by the drug-taking context.
Acknowledgments
The authors thank Dr. Wenyan Han for her general assistance and criticisms of this study.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work.
References
- 1. Urberg KA, Değirmencioğlu SM, Pilgrim C (1997) Close friend and group influence on adolescent cigarette smoking and alcohol use. Dev Psychol 33:834–844. [DOI] [PubMed] [Google Scholar]
- 2. Powell LM, Tauras Ja, Ross H (2005) The importance of peer effects, cigarette prices and tobacco control policies for youth smoking behavior. J Health Econ 24:950–968. [DOI] [PubMed] [Google Scholar]
- 3. Racicot S, McGrath JJ, Karp I, O'Loughlin J (2012) Predictors of nicotine dependence symptoms among never-smoking adolescents: a longitudinal analysis from the Nicotine Dependence in Teens Study. Drug Alcohol Depend 130:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greenlund KJ, Johnson CC, Webber LS, Berenson GS (1997) Cigarette smoking attitudes and first use among third- through sixth-grade students: the Bogalusa Heart Study. Am J Public Health 87:1345–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. White VM, Byrnes GB, Webster B, Hopper JL (2008) Does smoking among friends explain apparent genetic effects on current smoking in adolescence and young adulthood? Br J Cancer 98:1475–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Christakis NA, Fowler JH (2008) The collective dynamics of smoking in a large social network. N Engl J Med 358:2249–2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen H, Sharp BM, Matta SG, Wu Q (2011) Social interaction promotes nicotine self-administration with olfactogustatory cues in adolescent rats. Neuropsychopharmacology 36:2629–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wang T, Han W, Wang B, Jiang Q, Solberg-Woods LC, et al. (2014) Propensity for social interaction predicts nicotine-reinforced behaviors in outbred rats. Genes Brain Behav 13:202–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galef BG (2011) A case study in behavioral analysis, synthesis and attention to detail: social learning of food preferences. Behav Brain Res 231:266–271. [DOI] [PubMed] [Google Scholar]
- 10. Galef BG Jr, Mason JR, Preti G, Bean NJ (1988) Carbon disulfide: a semiochemical mediating socially-induced diet choice in rats. Physiol Behav 42:119–124. [DOI] [PubMed] [Google Scholar]
- 11. Zhou X, Nonnemaker J, Sherrill B, Gilsenan AW, Coste F, et al. (2008) Attempts to quit smoking and relapse: factors associated with success or failure from the ATTEMPT cohort study. Addict Behav 34:365–373. [DOI] [PubMed] [Google Scholar]
- 12. Eissenberg T, Balster RL (2000) Initial tobacco use episodes in children and adolescents: current knowledge, future directions. Drug Alcohol Depend 59 Suppl 1S41–60. [DOI] [PubMed] [Google Scholar]
- 13. Chen H, Matta SG, Sharp BM (2006) Acquisition of nicotine self-administration in adolescent rats given prolonged access to the drug. Neuropsychopharmacology 32:700–709. [DOI] [PubMed] [Google Scholar]
- 14. Chen H, Hiler KA, Tolley EA, Matta SG, Sharp BM (2012) Genetic factors control nicotine self-administration in isogenic adolescent rat strains. PLoS One 7:e44234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Spear L (2000) Modeling adolescent development and alcohol use in animals. Alcohol Res Health 24:115–123. [PMC free article] [PubMed] [Google Scholar]
- 16.Jones-Price C, Kimmel CA, Marr MC, Wolkowski-Tyl R (1984) Teratologic evaluation of carbon disulfide (CAS No. 75-15-0) administered to CD rats on gestational days 6 through 15. NITS Technical Report PB84-192343.
- 17. Wells AM, Lasseter HC, Xie X, Cowhey KE, Reittinger AM, et al. (2011) Interaction between the basolateral amygdala and dorsal hippocampus is critical for cocaine memory reconsolidation and subsequent drug context-induced cocaine-seeking behavior in rats. Learn Mem 18:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hahn G, Charlin VL, Sussman S, Dent CW, Manzi J, et al. (1990) Adolescents' first and most recent use situations of smokeless tobacco and cigarettes: similarities and differences. Addict Behav 15:439–448. [DOI] [PubMed] [Google Scholar]
- 19. Hofstetter CR, Hovell MF, Jung K-R, Raman R, Irvin V, et al. (2007) The first puff: forces in smoking initiation among Californians of Korean descent. Nicotine Tob Res 9:1277–1286. [DOI] [PubMed] [Google Scholar]
- 20. Schepis TS, Rao U (2005) Epidemiology and etiology of adolescent smoking. Curr Opin Pediatr 17:607–612. [DOI] [PubMed] [Google Scholar]
- 21. Hu M-C, Davies M, Kandel DB (2005) Epidemiology and correlates of daily smoking and nicotine dependence among young adults in the United States. Am J Public Health 96:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kamboures MA, Blake DR, Cooper DM, Newcomb RL, Barker M, et al. (2005) Breath sulfides and pulmonary function in cystic fibrosis. Proc Natl Acad Sci U S A 102:15762–15767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Umber BJ, Shin H-W, Meinardi S, Leu S-Y, Zaldivar F, et al. (2013) Gas signatures from Escherichia coli and Escherichia coli-inoculated human whole blood. Clin Transl Med 2:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Phillips M, Sabas M, Greenberg J (1993) Increased pentane and carbon disulfide in the breath of patients with schizophrenia. J Clin Pathol 46:861–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Munger SD, Leinders-Zufall T, McDougall LM, Cockerham RE, Schmid A, et al. (2010) An olfactory subsystem that detects carbon disulfide and mediates food-related social learning. Curr Biol 20:1438–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zentall TR (2011) Perspectives on observational learning in animals. J Comp Psychol 126:114–128. [DOI] [PubMed] [Google Scholar]
- 27. Popik P, Van Ree JM (1993) Social transmission of flavored tea preferences: facilitation by a vasopressin analog and oxytocin. Behav Neural Biol 59:63–68. [DOI] [PubMed] [Google Scholar]
- 28. Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, et al. (2010) Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci 30:4999–5007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Davis JD, Smith GP (1992) Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behav Neurosci 106:217–228. [PubMed] [Google Scholar]
- 30. Thiel KJ, Sanabria F, Neisewander JL (2009) Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology 204:391–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Conklin CA, Robin N, Perkins KA, Salkeld RP, McClernon FJ (2008) Proximal versus distal cues to smoke: the effects of environments on smokers' cue-reactivity. Exp Clin Psychopharmacol 16:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stöffelmayr B, Wadland WC, Pan W (2003) An examination of the process of relapse prevention therapy designed to aid smoking cessation. Addict Behav 28:1351–1358. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.