Abstract
Background
Gastric electrical stimulation (GES) is a therapeutic option for intractable symptoms of gastroparesis (GP). Idiopathic GP (ID-GP) represents a subset of GP. AIMS: A prospective, multicenter, double-blinded, randomized, crossover study to evaluate the safety and efficacy of Enterra GES in the treatment of chronic vomiting in ID-GP.
Methods
Thirty-two ID-GP subjects (mean age 39; 81% F, mean 7.7 years of GP) were implanted with GES. The stimulator was turned ON for 1½ months followed by double-blind randomization to consecutive 3-month crossover periods with the device either ON or OFF. ON stimulation was followed in unblinded fashion for another 4.5 months. Twenty-five subjects completed the crossover phase and 21 finished 1 year of follow-up.
Key Results
During the unblinded ON period, there was a reduction in weekly vomiting frequency (WVF) from baseline (61.2%, P < 0.001). There was a non-significant reduction in WVF between ON vs OFF periods (the primary outcome) with median reduction of 17% (P > 0.10). Seventy-five percent of patients preferred the ON vs OFF period (P = 0.021). At 1 year, WVF remained decreased (median reduction = 87%, P < 0.001), accompanied by improvements in GP symptoms, gastric emptying and days of hospitalization (P < 0.05).
Conclusions & Inferences
(i) In this prospective study of Enterra GES for ID-GP, there was a reduction in vomiting during the initial ON period; (ii) The double-blind 3-month periods showed a non-significant reduction in vomiting in the ON vs OFF period, the primary outcome variable; (iii) At 12 months with ON stimulation, there was a sustained decrease in vomiting and days of hospitalizations.
Keywords: gastric stimulation, gastroparesis, idiopathic gastroparesis, nausea, vomiting
Key Messages
Gastric electrical stimulation (GES) is a therapeutic option for intractable symptoms of gastroparesis (GP). The primary objective of this study was to demonstrate an improvement in weekly vomiting frequency (WVF) when the device was turned ON, relative to when the device was turned OFF during a blinded, 3 month, crossover phases. The secondary goal was to demonstrate a reduction in symptom scores and to assess changes in quality of life, gastric emptying, number of days in hospital, and BMI in our ID-GP cohort when receiving active stimulation for up to 12 months.
32 patients with GP of idiopathic origin, majority young women (81%), were implanted with GES. The stimulator was turned ON for 1½ months followed by double-blind randomization to consecutive 3 month crossover periods with the device either ON or OFF. ON stimulation was followed in unblinded fashion for another 4.5 months. During the unblinded ON period, there was a significant reduction WVF from baseline (61.2%, P < 0.001), and it was followed with median reduction of WVF by 17% (P > 0.10) between ON and OFF phase of the study. At 1 year, the mean WVF remained decreased by 87%, (P < 0.001), and it was accompanied by improvements in GP symptoms, gastric emptying and days of hospitalization (P < 0.05).
Importance of the study
The first massage is that initiation of GES for 6 weeks caused a rapid and significant reduction of symptoms which was able to be sustained despite a period of up to 3 months with the device OFF.
Even though, the double blind 3 month periods showed a non-significant reduction in vomiting in the ON vs. OFF period, at 12 months with ON stimulation, there was a continuous decrease in vomiting symptoms and days of hospitalizations.
Future placebo-controlled research trials must be initiated at the time of surgery, with ON and OFF phases being designed in be parallel but not cross-over fashion.
Introduction
Gastroparesis (GP) describes a chronic gastric motility disorder with delayed gastric emptying and symptoms, which include early satiety, nausea and vomiting.1–4 Gastroparesis has many causes with diabetic GP being the classic disorder. However, approximately one third of GP patients are ‘idiopathic’ (ID) meaning that the pathogenic basis of the GP condition is mostly unknown. This unknown aetiology creates a challenge to their clinical and therapeutic management.5
In the treatment of severe symptoms of drug-refractory GP, there are not many therapeutic options.6,7 Initial therapies include nutritional modifications, medications to stimulate gastric emptying and medications to reduce symptoms of nausea and vomiting. Botulinum toxin injection into the pylorus may provide short-term reduction in symptoms, but placebo-controlled studies have not been favorable.8,9 When medications fail to control symptoms, interventional measures to support nutritional status may be required. Some GP patients refractory to medical treatment are candidates for gastric electrical stimulation (GES). The Enterra System (Medtronic Inc, Minneapolis, MN, USA) utilizes high frequency (14 Hz; 12 cpm) short pulse width (330 μs) and low-energy stimulation. This neurostimulation approach with the Enterra System is FDA approved and has been available under a Humanitarian Device Exemption (HDE) program since March 2000 for use in the treatment of chronic, intractable (drug-refractory) nausea and vomiting secondary to GP of diabetic or ID etiology.10
Over the last 11 years, Enterra GES has been used for treatment of some refractory patients based on its safety and efficacy profile.11 The improvement of nausea and vomiting shown in many open-labelled clinical trials indicates that reduction in GP symptoms can be achieved.12 The first placebo-controlled multicenter Worldwide Anti-Vomiting Electrical Stimulation Study reported a significant decrease in vomiting during the double-blind period of 1-month ON compared with the 1-month OFF period following implantation, particularly with diabetic GP.13 With continued ON stimulation, there was improved quality of life (QOL) and a modest improvement in gastric retention at 6 and 12 months of GES. Other non-placebo-controlled publications have confirmed similar outcomes with a sustained decrease in GP symptoms, nutritional support, days of hospitalization, which was associated with improvements in body mass index (BMI) and, in diabetic GP, better glycemic control.14–18
Due to the paucity of double-blind placebo-controlled data focused on Enterra GES therapy, this clinical trial was initiated to evaluate the efficacy and safety of gastric neurostimulation therapy for severe gastroparetic subjects with ID etiology. The purpose of this clinical evaluation was to demonstrate the safety and efficacy of Enterra® Therapy in the treatment of chronic, intractable (drug-refractory) nausea and vomiting secondary to GP of ID etiology. The primary objective was to demonstrate that there was a reduction in weekly vomiting frequency (WVF) when the device was turned ON, relative to when the device was turned OFF during a blinded crossover phase. The secondary objectives were as follows: (i) to demonstrate a reduction in symptom scores when the device is turned ON relative to when the device was turned OFF, and (ii) to demonstrate a long-term reduction in WVF at 12 months relative to baseline. Additional goals included assessment of the safety of Enterra Therapy, evaluation of the 12-month responder rate, 12-month change in symptom score, 12-month change in QOL, gastric emptying, number of days in hospital and BMI.
Materials and Methods
This was a prospective, multicenter, double-blind, randomized, controlled, two-period crossover study conducted at eight centres in the United States under Institutional Review Board approval. The study design is presented in Fig.1. The device was turned ON for the first 1½ months after implant to allow for full recovery from surgery prior to randomization. At 1½ months, each subject was randomized in a masked fashion to one of two treatment arms: three OFF followed by 3 months of stimulation ON. The subject, physician and study coordinator were blinded to the stimulation status during the crossover phase. At the end of the crossover period, the subjects were programmed ON and evaluated at a 12-month follow-up visit and annually thereafter until study closure (Fig.1 – detailed study design).
Figure 1.
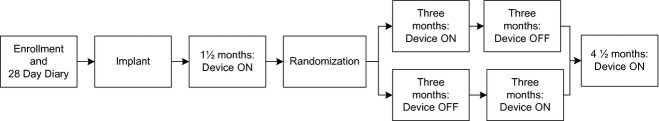
Study design.
Study subjects
All subjects signed a written informed consent prior to enrollment in the study. To be eligible for the study, subjects were required to meet the following inclusion criteria: at least 18 years of age; symptoms of nausea and vomiting requiring treatment for greater than 1 year associated with GP of ID etiology unresponsive or intolerant to prokinetic and antiemetic drug classes tried over a minimum of 1 month, and on a stable dose of prokinetics for a minimum of 30 days prior to baseline, unless contraindicated. Subjects were required to undergo a gastric emptying test (GET) using the standardized isotope-labelled low-fat egg substitute meal with imaging out to 4 h19,20 and were considered eligible if results showed greater than 10% retention at 4 h and/or greater than 60% at 2 h. All subjects had to be symptomatic and experience at least seven episodes of vomiting during a seven consecutive day period as captured on a 28-day baseline diary.
Exclusion criteria included the following: mechanical obstruction; diabetes mellitus (DM); pseudo-obstruction; scleroderma; amyloidosis; Parkinson's disease; Muscular Sclerosis; paraneoplastic syndromes; current parathyroid and adrenal disorders; prior gastric surgery for gastric resection, bariatric surgery, fundoplication or vagotomy; current primary disorders such as psychogenic vomiting, eating disorders or swallowing disorders; chemical dependency; peritoneal dialysis or unstable haemodialysis; and current or planned pregnancy.
Implantation technique
Study subjects were implanted with the Enterra® Therapy system (Model 7425G or Model 3116; Medtronic Inc.). Two intramuscular leads (Model 4351; Medtronic Inc.) were inserted into the muscularis propria of the stomach using either laparoscopy or laparotomy as previously described.21 The two leads were placed 10 cm from the pylorus on the greater curvature of the stomach and 1 cm apart and were connected to the neurostimulator device placed subcutaneously in the abdominal wall. The device was programmed to standardized parameters (5 mA, 14 Hz, 330 μs, cycle on 0.1 s, cycle off 5 s) using a programmer (Model 7432 or Model 8840; Medtronic). During the first 7.5 months, the programming parameters were not changed, with the exception that the voltage was adjusted based on impedance to maintain a 5 mA current. Furthermore, the voltage was set to 0 during the OFF period. After the 7.5 month visit, programming parameters could be adjusted at the investigator's discretion based on the assessment of the subject's symptoms status.
Outcome measures
Subjects were required to record daily vomiting episodes in a 28-day diary to assess WVF prior to each visit. The frequency and severity of GP symptoms (vomiting, nausea, early satiety, bloating, postprandial fullness, epigastric pain, and epigastric burning) were assessed using a 5-point symptom interview questionnaire at baseline and each follow-up visit. The frequency symptom scores were rated by the patient as 0, absent; 1, rare (1 per week); 2, occasional (2–3 per week); 3, frequent (4–6 per week); 4, extremely frequent (≥7 per week). The severity symptom scores were rated as 0, absent; 1, mild (not influencing the normal activities); 2, moderate (diverting from, but not urging modifications, of usual activities); 3, severe (influencing usual activities, severely enough to urge modifications); 4, extremely severe (requiring bed rest). The sums of the frequency or severity ratings of the seven symptoms were used as an overall frequency or severity total symptom score (TSS).
Health-related QOL was assessed at baseline and follow-up visits using the previously validated short form-36 (SF-36) Health Status Survey questionnaire, version 1.22 Gastric emptying was evaluated of a solid meal at baseline and 12 months using a standardized scintigraphy method and a low-fat test meal.20 Type of nutritional support (oral, J-tube, G-tube, TPN) and whether or not it was continuous or intermittent was collected at baseline and on follow-up visits.
Number of days in the hospital for treatment of GP was collected at baseline and visits up to 12 months.
Adverse events were monitored throughout the study. All events were classified using the Medical Dictionary for Regulatory Activities (MedDRA). Cause and severity of each adverse event was assessed by the principal investigator and adjudicated by an Adverse Events Committee. The cause of the adverse event was classified as being ‘device-related’ (the event is caused by a suspected device malfunction), ‘therapy-related’ (the event is directly or indirectly caused by the surgical implantation procedure; or is associated with the presence and/or use of the device) or ‘patient-related’ (the event is associated with the subject's underlying diagnosis or a new diagnosis, unrelated to the device). Serious adverse events were considered when they resulted in death, were life threatening, required inpatient hospitalization or prolongation of existing hospitalization, resulted in persistent or significant disability or incapacity, or resulted in a congenital anomaly/birth defect.
Sample size, randomization and blinding
A 25% reduction in WVF when device was ON relative to when device was OFF was considered to be clinically significant. Based on 80% power to detect this significant difference with a standard deviation of 50% (α = 0.05, two-sided), 32 subjects were required for analysis. To compensate for non-evaluable subjects, a maximum of 75 subjects were allowed to be implanted. Subjects were randomized by Sponsor at 1 : 1 ratio stratified by center in a block size of four to have therapy turned ON or OFF at the beginning of the crossover periods. Randomization assignments were generated centrally, put into sealed envelopes and sent to authorized unblinded personnel at the study site prior to the randomization visit. The subjects, the investigators and the study coordinators were blinded to the device settings during the crossover period. Authorized unblinded personnel checked the device status and programmed the device at follow-up visits during the crossover period. The record of such an activity was kept in a separate binder not accessible to the other study-site personnel.
Statistical analysis
The primary objective was assessed by the within subject percent (%) reduction in WVF during ON period relative to OFF period. The secondary objective of WVF was assessed by the percent reduction in WVF at 12 months relative to baseline. Both objectives used completed cases and were analysed using a Wilcoxon Signed Rank test. Probability values were deemed significant at a level of 0.05. Subjects with a 50% or greater reduction in WVF at 12 months were defined as responders. A one-sided binomial test with a significance level of 0.025 was used to test whether the responder rate at 12-months was >50%.
Symptom scores (individual and TSS) and SF-36 (eight sub-scores, Physical Component Summary [PCS], and Mental Component Summary [MCS]) were analysed using either a paired t-test or Wilcoxon Signed Rank test. Probability values were deemed significant at a level of 0.05. No adjustments were made for multiple hypothesis testing.
A Wilcoxon Signed Rank test was used and a significance level of 0.05 was applied for analyses of additional study measurements. Statistical analysis was performed using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline demographics
Thirty-two subjects (81% women) with a mean age of 39 years (range 22–64) and mean BMI of 25.1 kg m−2 (range 14–39) from six sites underwent implant of Enterra® Therapy between October 2002 and December 2008. Two additional study sites did not enroll subjects. Subjects had symptoms of GP for a mean of 7.7 years (range 1.5–28) prior to enrolment and a median vomiting frequency of 17.3 episodes per week. All subjects had delayed gastric emptying with a median gastric retention of 69% at 2 h and 31% at 4 h. In total, 10 subjects were receiving nutritional support, five (16%) enteral (J-tube), four (13%) oral, and one (3%) parenteral. Two of the five patients with J-tubes discontinued early from the study (one lost to follow-up and one subject withdrew consent before implantation), and they were not part of the evaluation of the study results. Two of the remaining patients were able to discontinue them during the trial.
For the subjects who were randomized, there were no significant differences in baseline characteristics between the two randomized groups.
Subject disposition
Among the 32 subjects enrolled and implanted, five were not randomized. There were 25 subjects who completed the crossover phase and 21 subjects who completed the 12-month visit (Fig.2 – full details on subject flow).
Figure 2.
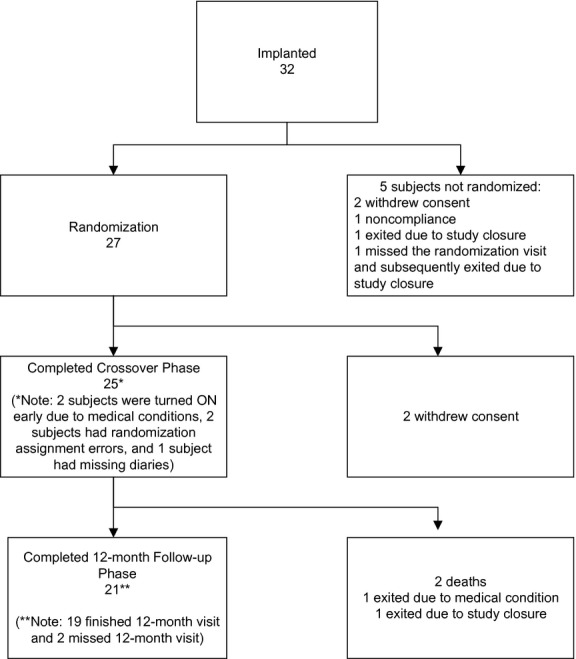
Subject disposition.
Initial 1½-month results
To assess the impact of the initial ON period prior to randomization, a post hoc analysis of WVF at 1½ month compared with baseline was completed for subjects who provided diary data at 1½-month visit (n = 25), regardless of their subsequent follow-up status of this 1½-month visit. The median reduction in WVF of 61.2% was statistically significant (P < 0.001) at 1½ months compared with baseline with a median WVF of 17.3 episodes at baseline and 5.5 episodes at 1½ months (Fig.3). Among these 25 subjects, only eight did not respond with greater than 25% reduction in WVF during the 6-week initial active stimulation. The mean TSS for frequency was also statistically significantly decreased (14.6%, P < 0.001) during this period of time from 21.4 to 16.1 points.
Figure 3.
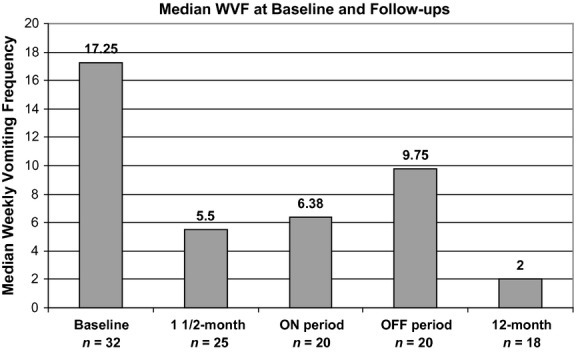
Weekly vomiting frequency at baseline and follow-ups.
Crossover phase
Of those subjects who completed the crossover phase, 20 provided diary data to assess the WVF during ON and OFF states. Among these 20 subjects, 12 were randomized to ON–OFF and eight to OFF–ON sequence of the GES stimulation. The data analyses are provided in Table1 and Fig.3. As already presented, most subjects showed a large reduction in WVF from baseline to 1½ months at which time they were randomized to be either ON or OFF for period of 3 months each. During the double-blind crossover phase, the median WVF of 6.4 episodes during the ON state was less than the 9.8 episodes during the OFF state (Table1). The within-patient median reduction in WVF from ON to OFF during this crossover phase, the primary outcome variable, was 17.3% (P = 1.0). Of interest is that 15 of 20 (75%) of the study patients were females and they had an 18.4% greater median reduction in vomiting during ON vs OFF, whereas five males were 37.5% worse during ON vs OFF. The frequency and severity of the TSS also did not show statistical differences between ON and OFF states (P = 0.933 and 0.556 respectively).
Table 1.
Results during crossover phase
| Variable | N | ON state | OFF state | P-value |
|---|---|---|---|---|
| WVF*median (interquartile range) | 20 | 6.4 (2.8–17.3) | 9.8 (3.6–25.6) | 1.000 |
| Frequency symptom score† mean ± SD | ||||
| Vomiting | 21 | 2.38 ± 1.24 | 2.71 ± 1.19 | 0.823 |
| Nausea | 21 | 3.29 ± 1.06 | 3.48 ± 0.87 | 0.910 |
| Early satiety | 21 | 2.76 ± 1.37 | 2.62 ± 1.50 | 0.352 |
| Bloating | 21 | 2.33 ± 1.59 | 2.29 ± 1.62 | 0.555 |
| Postprandial fullness | 21 | 2.10 ± 1.26 | 2.38 ± 1.53 | 0.230 |
| Epigastric pain | 21 | 2.00 ± 1.38 | 2.33 ± 1.46 | 0.969 |
| Epigastric burning | 21 | 1.14 ± 1.42 | 1.38 ± 1.66 | 0.031 |
| Total symptom score (TSS) | 21 | 16.0 ± 6.29 | 17.19 ± 6.98 | 0.932 |
| Severity symptom score‡ mean ± SD | ||||
| Vomiting | 21 | 2.10 ± 1.26 | 2.29 ± 1.15 | 0.838 |
| Nausea | 21 | 2.38 ± 1.12 | 2.71 ± 1.15 | 0.936 |
| Early satiety | 21 | 1.95 ± 1.20 | 1.95 ± 1.36 | 0.609 |
| Bloating | 21 | 1.71 ± 1.38 | 1.86 ± 1.53 | 0.539 |
| Postprandial fullness | 21 | 1.52 ± 1.03 | 2.00 ± 1.48 | 0.176 |
| Epigastric pain | 21 | 1.62 ± 1.28 | 1.90 ± 1.34 | 0.840 |
| Epigastric burning | 21 | 0.81 ± 1.08 | 1.10 ± 1.37 | 0.063 |
| Total symptom score (TSS) | 21 | 12.10 ± 5.83 | 13.81 ± 6.95 | 0.556 |
WVF, Weekly Vomiting Frequency.
For each individual symptom frequency score, 0 = absent and 4 = extremely frequent (≥7 per week), total symptom frequency score is the sum of all the individual symptom scores.
For each individual symptom severity score, 0 = absent and 4 = extremely severe (requiring bed rest), total symptom severity score is the sum of all the individual symptom scores.
At the end of the crossover phase, subjects were surveyed as to which state they preferred. Both the patients and the investigators were blinded to the treatment sequence. Of the 20 subjects, 15 (75%) preferred the ON state and five (25%) preferred the OFF state. The proportion of subjects that preferred the ON state (75%) was statistically significantly different from 50% as expected by chance (binomial exact test, one-sided P = 0.021).
12-month follow-up phase
There were 19 subjects who finished the 12-month follow-up visit and provided required study data. One of the 19 subjects had missing diary data for WVF. The results of analysis are presented in Tables2 and 3.
Table 2.
Results of weekly vomiting frequency at 12-month follow-up
| Analysis method | N | Baseline | 12-Month | Median per cent reduction | P-value |
|---|---|---|---|---|---|
| Completed case | 18 | 17.3 (10–36.8) | 2 (0.3–8.5) | 87.1% (−80.4–100%) | <0.001 |
| Per-protocol | 19 | 17 (6–36.8) | 2.3 (0.3–8.5) | 85.3% (−80.4–100%) | <0.001 |
| ITT | 27 | 21.8 (9.5–36.8) | 4 (1.5–23) | 80.9% (−102.6–100%) | 0.003 |
Results are presented as median (interquartile range).
Table 3.
Other study results at 12-month follow-up
| Variable | N | Baseline | 12-Month | P-value |
|---|---|---|---|---|
| Frequency symptom score, mean ± SD | ||||
| Vomiting | 19 | 3.32 ± 0.95 | 1.68 ± 1.42 | 0.001 |
| Nausea | 19 | 3.79 ± 0.54 | 2.68 ± 1.29 | 0.005 |
| Early satiety | 19 | 3.26 ± 0.81 | 2.00 ± 1.37 | 0.001 |
| Bloating | 19 | 3.05 ± 1.27 | 1.79 ± 1.81 | 0.005 |
| Postprandial fullness | 19 | 3.42 ± 0.90 | 1.84 ± 1.54 | 0.001 |
| Epigastric pain | 19 | 2.84 ± 1.30 | 1.63 ± 1.67 | 0.002 |
| Epigastric burning | 19 | 2.05 ± 1.75 | 1.37 ± 1.64 | 0.154 |
| TSS | 19 | 21.74 ± 5.16 | 13.00 ± 7.92 | <0.001 |
| Severity symptom score, mean ± SD | ||||
| Vomiting | 19 | 2.95 ± 0.85 | 1.37 ± 1.07 | <0.001 |
| Nausea | 19 | 3.21 ± 0.79 | 1.68 ± 0.89 | <0.001 |
| Early satiety | 19 | 2.58 ± 0.90 | 1.58 ± 1.26 | 0.001 |
| Bloating | 19 | 2.21 ± 1.08 | 1.53 ± 1.58 | 0.044 |
| Postprandial fullness | 19 | 2.89 ± 0.99 | 1.47 ± 1.22 | <0.001 |
| Epigastric pain | 19 | 2.37 ± 1.21 | 1.47 ± 1.50 | 0.011 |
| Epigastric burning | 19 | 1.84 ± 1.61 | 1.16 ± 1.42 | 0.114 |
| TSS | 19 | 18.05 ± 6.34 | 10.26 ± 7.09 | <0.001 |
| SF-36 health survey, mean ± SD | ||||
| PF | 19 | 36.61 ± 12.16 | 42.79 ± 13.31 | 0.032 |
| RP | 19 | 29.81 ± 5.19 | 37.63 ± 12.3 | 0.006 |
| BP | 19 | 32.84 ± 7.76 | 37.37 ± 15.25 | 0.150 |
| GH | 19 | 32.58 ± 9.89 | 34.21 ± 10.31 | 0.520 |
| VT | 19 | 30.37 ± 9.03 | 38.47 ± 11.82 | 0.003 |
| SF | 19 | 26.57 ± 11.62 | 38.85 ± 12.82 | <0.001 |
| RE | 19 | 38.15 ± 12.76 | 44.25 ± 12.89 | 0.069 |
| MH | 19 | 34.90 ± 14.15 | 39.32 ± 11.33 | 0.020 |
| PCS | 19 | 32.66 ± 8.8 | 37.86 ± 13.28 | 0.043 |
| MCS | 19 | 34.11 ± 11.67 | 41.27 ± 12.29 | 0.001 |
| % Gastric retention, median (interquartile range) | ||||
| @ 2 h | 16 | 63.5 (56.5–74%) | 49 (40.5–63.5%) | 0.016 |
| @ 4 h | 16 | 26 (16.5–37%) | 16.5 (4.8–37.5%) | 0.236 |
| Days in hospital, median (interquartile range) | 19 | 2 (0-9) | 0 (0–0) | 0.006 |
| BMI, median (interquartile range) | 19 | 26.96 (19.05–31.92) | 24.74 (22.25–31.61) | 0.768 |
TSS, Total symptom score; PF, Physical functioning; RP, Role physical; BP, Bodily pain; GH, General health; VT, Vitality; SF, Social functioning; RE, Role emotional; MH, Mental health; PCS, Physical component summary; MCS, Mental component summary.
We have observed that the WVF at 12 months decreased significantly when compared with baseline, with a median reduction of 87.1% (P < 0.001). The median WVF was 17.3 episodes at baseline and two episodes at 12 months. In this period, a responder was defined as having a 50% or greater reduction in WVF from baseline to 12 months, and there were 17 responders (94.4%, P < 0.001) in our cohort.
Two sensitivity analyses, intent-to-treat (ITT) and per-protocol (PP) were performed to address the missing WVF data at 12 months. The ITT analysis included all the subjects who were randomized. Per-protocol analysis included all subjects who finished 12 months of follow-up, including one subject with missing diary data. The imputation method of last-observation-carried-forward was applied to adjust for the missing data for the PP and ITT analyses. Only those observations made while the device was turned ON were carried forward. Per-protocol and ITT analyses revealed a median reduction of 85.3% (P < 0.001) and 80.9% (P = 0.003), respectively, from baseline to 12 months.
The mean TSS frequency and severity scores were significantly decreased from baseline to 12 months (P < 0.001). Six individual symptom scores, specifically vomiting, nausea, early satiety, bloating, postprandial fullness and epigastric pain, were also decreased significantly from baseline to 12 months for both frequency and severity symptom scores (P < 0.05). There was no significant reduction in the frequency or severity symptom scores of epigastric burning at 12 months (P = 0.154 and 0.114 respectively).
Quality of life scores at 12 months was also improved from baseline. Statistically significant improvements were observed in the PCS and MCS scores (P = 0.043 and P < 0.001 respectively). Increased values in sub-scores were observed in all eight domains of the SF-36 survey, with statistically significant improvements in the physical functioning, role physical, vitality, social functioning and mental health domains (P < 0.05).
Annualized median days in the hospital decreased from a median of 2 days at baseline to 0 days at 12 months (P = 0.006).
There were three patients with J-tubes and one other patient on parenteral feeding, who continued in the study and they all had improvement in either WVF or symptom scores or both and their results were included in the final analysis. Two of the three patients were able to have their J-tubes removed while the parenteral nutrition-dependent patient had less frequent supplements.
BMI and weight measurements were stable over the follow-up period (Table3).
Gastric emptying overall improved at 12 months with stimulation. Two-hour scintigraphy gastric emptying (n = 16) was significantly improved at 12 months with a median retention at 2 h of 49.0% (interquartile rage 40.5–63.5%) compared with 63.5% (interquartile rage 56–74%) at baseline (P = 0.016). There was a numerical improvement in 4-h gastric emptying with a median retention at 4 h of 16.5% (interquartile rage 4.8–37.5%) compared with 26.0% (interquartile rage 16.5–37%) at baseline (P = 0.236). Overall 10 subjects (62%) had an improved rate of emptying, with six subjects (38%) normalizing their gastric emptying (<10% retention at 4 h) at 12-month visit. There was a non-significant difference in the grading of symptoms between the group of patients whose gastric emptying showed mild retention at 4 h (11%) vs those with profound delays in emptying (>40%) at the 12 month follow-up.
After the crossover phase, from 7½ month to 12 months of follow-up, stimulation parameters could be adjusted by the investigators: Pulse width remained at 330 μs, except for two patients (10%) who were programmed at a 450 μs. The Pulse Rate remained at 14 Hz in all patients; Voltage is calculated from current and impedance. For the patients whose programming data were available at both 7½- and 12-month visits, the mean current was 6.2 mA at 7½-month visit and there was a mean increase of 1.3 mA at the 12-month visit, reflecting these small adjustments.
Adverse events
A total of 170 adverse events were collected in the study. Of these, 145 (85.3%) were patient-related events. There were 24 (14.1%) therapy- or device-related events, of which three were serious. Among the three serious events, there was one paresthaesia, one lead migration/dislodgement and one migration of neurostimulator. The serious event of paresthaesia was described as the subject experiencing a midline ‘jolting’ sensation every 15 min, which was effectively resolved with device re-programming, and no residual effects. Overall, two of 32 subjects (6.3%) required surgical intervention for the previously mentioned lead migration/dislodgment and the neurostimulator migration. There was one ID subject who died of unknown cause. As the cause of death is unknown, the relatedness could not be determined by the investigator or the Adverse Event Committee. None of the implanted subjects developed infection of the leads and/or pocket housing the implantable pulse generator. Moreover, no explants of the Enterra System were reported during 12 months of observation.
Among the 145 patient-related events, 70 were serious events. Signs and symptoms of GP, such as nausea and vomiting, were considered an adverse event only when they resulted in hospitalization for more than 23 h. Gastroparesis-related hospitalizations (coded as ‘impaired gastric emptying’ in MedDRA) occurred 41 times in 11 subjects, comprising 58.6% of all serious patient-related adverse events. Other serious patient-related adverse events reported more than once were related to hypertension,3 infection or complication of the feeding tube2 and headache.2
There was a mortality rate of 6.3% (two of 32 subjects) at 1 year. The cause of death for one of the ID subjects was sudden cardiac arrest. The cause of death for the other subject remains undetermined, as the clinical-site personnel had been unable to obtain any elucidating information from public records or the family. Therefore, there is no information to indicate whether this death was device- or therapy-related.
Discussion
The primary objective of this study of gastric electric stimulation in patients with refractory nausea and vomiting from ID-GP was to demonstrate an improvement in WVF when the device was turned ON, relative to when the device was turned OFF during a blinded crossover phase. Our primary outcome measure showed only a non-significant trend (17% reduction) in the improvement in WVF during the ON compared with the OFF double-blind period. Of interest, the reduction in WVF, which occurred in the first 1½ months after initiation of gastric stimulation therapy, was sustained throughout the crossover period and subsequent follow-up evaluations with more than 80% improvement in frequency of vomiting being observed at 12 months.
One implication of our observations is that the rapid and significant induction of symptom improvement in the first 6 weeks was able to be sustained, despite a period of up to 3 months with the device OFF. Subjects reported that when the device was ON, they experienced a median of 17.3% less episodes of vomiting per week than when device was OFF. This positive effect from being ON compared with OFF was very prominent in female patients who represented 75% of the study participants while not pronounced in male subjects. In addition, there was a statistically significant personal preference for the ON vs OFF state of gastric stimulation with 75% of patients favoring the symptoms response while being in the ON state.
This study revealed that the initial reduction in vomiting frequency and overall GP symptoms with gastric electric stimulation was able to be sustained over the year follow-up period. This major favorable effect was associated with improvements in QOL and a significant reduction in hospitalization days, thus secondary outcome goals were met in this ID group as was the case in the diabetic GP trial and also reported in the literature.23,24 Mortality rate at 12 months in this idiopathic gastroparesis (ID-GP) study was lower than in the corresponding diabetic GP trial, 6.3% vs 12.7% respectively.25 Obviously, the clinical complications of ongoing DM provide different and more serious comorbidity and challenges.
Gastric emptying was also numerically improved over the 1-year study with GES, with 38% of patients normalizing GET results, which was higher than previously observed in diabetic GP where 25% returned to normal.13,25 This outcome could be consistent with the hypothesis that time, and adequate treatment, may regenerate gastric tissue and nerves, and reverse the injury/damage secondary to a viral or bacterial gastroenteritis that is suspected as the etiology in many ID-GP patients.26,27
There were many challenges and factors, which influenced the recruitment, execution and outcome of this study, resulting in a severely underpowered data analysis limiting the interpretation of the results. The major contributing factors, which hampered the recruitment of ID cohort of GP patients were as follows: (i) the lengthy insurance approval process for the preauthorization of therapy; (ii) availabilities of Enterra Therapy through FDA approved HDE application, allowing patients to have access to the therapy without participating in the double-blind research study; (iii) A small number of centres6 were willing to conduct the protocol, compared to many (>90) other centres where Enterra was available through HDE application.
We believe that lack of a ‘washout period’ between any of the ON and OFF phases compromised the data we obtained and conceivably masked the GES effects. In addition, the question concerning ‘placebo effect’ and the presence of ‘cell/tissue memory phenomenon’ or ‘carry over effect’ induced by continuous electrical stimulation for the first 1½ months, and in half the subjects up to 4½ months, remains a confounding aspect of this trial as was the case in the similarly designed trial in diabetic GP.25 The influence of placebo or regression to the mean in this electrical stimulation trial is a possibility to explain the long-term improvement. However, the patients studied were chronically symptomatic and refractory to all other treatments. These patients were recruited at six different academic centers and historically required many admissions to their respective Emergency Departments or hospitals in the months preceding their entry into this research trial, thus providing evidence against ‘a placebo’ effect that might be sustained for 1 year. The memory or imprinting effect of 6 or 18 weeks of continuous stimulation suggests that any future trials require randomization from the time of surgical implant using a parallel study design without a crossover arm. Another option would be to have an initial single-blind OFF period after surgery of 1–2 months followed by randomization to either ON or OFF and no crossover phase.
One another suggestion for a future study is also to consider establishing more precise scales, indices and questionnaires to appreciate changes in symptoms of enrolled subjects. The newer condition-specific, validated tools and scales such as PAGI-SYM, PAGI-QOL and GCSI have been incorporated into GP research in the last few years, but they were not available at the time the double-blind Enterra studies were being conducted.28,29 The 5-point (0–4) symptom interview questionnaire utilized in this study was not refined enough to distinguish differences; for example, as related to frequency of GP symptoms. The highest rating of four points on the 5-point scale was marked as an extremely frequent – meaning ≥7 episodes of vomiting/week. However, subjects could be vomiting anywhere from 8 to >100 times per week and would grade those events equally as extremely severe and extremely frequent by marking ‘4 points’ regardless. We believe that this scale may have camouflaged many important observations and made interpretation of the results more difficult.
In conclusion, although the double-blinded ON/OFF GES treatment did not achieve its primary outcome objective, the 12-month clinical outcome data from this clinical trial of Enterra GES support the efficacy and safety of Enterra therapy for subjects diagnosed with severe, medication-unresponsive GP of ID etiology. Improvements in study design and symptoms assessment are suggested for future randomized controlled clinical trials with Enterra Therapy in GP, so that questions of clinical efficacy can be accurately addressed.
Acknowledgments
The authors especially thank Lisa Ruehlow for overall study management and Ye Tan for statistical analysis, as well as Kristine Westgard, Kellie Berg and their colleagues at Medtronic Neuromodulation. A special acknowledgement is given to all investigators, research coordinators and support staff at all contributing centres for their dedication and effort.
Glossary
- DM
diabetes mellitus
- GES
gastric electrical stimulation
- GET
gastric emptying test
- GP
gastroparesis
- ID-GP
idiopathic gastroparesis
- ID
idiopathic
- ITT
intent to treat
- PP
per protocol
- QOL
quality of life
- SF-36
short form-36
- TSS
total symptoms score
- WAVESS
Worldwide Anti-vomiting Electrical Stimulation Study
- WVF
weekly vomiting frequency
Funding
This clinical study was sponsored by Medtronic Inc.
Conflicts of Interest
The authors disclose the following: All authors received funding from Medtronic for the completion of this research. Medtronic was involved in the study design in conjunction with the study investigators. Data were collected at investigational sites and compiled by Medtronic. Medtronic conducted the statistical analysis. Data interpretation was completed by the study investigators in conjunction with Medtronic statistician and data managers. The authors had complete access to the data that support this publication. Potential conflicts of interest have been disclosed to study participants.
RM: Current Research Grant support from the NIH- NIDDK, also from Tranzyme Pharma; Salix Pharma; Rhythm Pharma; Novartis Pharma.; Red Hill Pharma; and Novartis Pharma. Dr. McCallum is on the Advisory Board of Prostrakan Pharma, a Consultant for Evoke Pharma, and a speaker for Ironwood- Forest Pharma. The NIH Grant support was in place during part of the time that the Enterra trial was being conducted. The other relationships and research activities were not actually in place during the conduct of this Research trial that is the subject of this publication. Dr. McCallum's financial relationship with Medtronic, the sponsor of this trial, during the time that the trial was being conducted involved occasional Speaker presentations at GI meetings in the USA and occasional telephone conferences as an Advisor.
IS: Current Research Grant support from the NIH- NIDDK; NIH- Diabetes Complication Consortium. And TAKEDA Pharmaceuticals North America, Inc –Investigator Initiated –Sponsored Trial.
HP: Evoke Pharma: consultant and grant, Tranzyme Pharma: consultant and grant, GSK: consultant and grant, ProStakan: consultant and grant, SmartPill: consultant and grant.
WS: No competing interests.
WS: No competing interests.
FB: Medtronic: Honorarium to provide implant training.
JW: Medtronic: Lecturer at symposium.
TN: No competing interests.
Author Contribution
RM study concept and design, acquisition of data, interpretation of data, drafting of the manuscript, critical review of manuscript, critical review and final approval of manuscript; IS acquisition of data, interpretation of data, drafting of the manuscript, critical review of manuscript, critical review and final approval of manuscript; HP study concept and design, acquisition of data, interpretation of data, drafting of the manuscript, critical review and final approval of manuscript; WS acquisition of data, interpretation of data, critical review and final approval of manuscript; FB acquisition of data, interpretation of data, critical review and final approval of manuscript; JW study concept and design, acquisition of data, interpretation of data, critical review and final approval of manuscript; TN study concept and design, acquisition of data, interpretation of data, critical review and final approval of manuscript.
References
- 1.Soykan I, Sivri B, Sarosiek I, McCallum RW. Demography, clinical characteristics, psychological and abuse profiles, treatment, and long-term follow-up of patients with gastroparesis. Dig Dis Sci. 1998;43:2398–404. doi: 10.1023/a:1026665728213. [DOI] [PubMed] [Google Scholar]
- 2.Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association medical position statement: diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:2589–91. doi: 10.1053/j.gastro.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 3.Hasler WL. Gastroparesis: symptoms, evaluation, and treatment. Gastroenterol Clin N Am. 2007;36:619–47. doi: 10.1016/j.gtc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Kong M-F, Horowitz M, Jones KL, et al. Natural history of Diabetic Gastroparesis. Diabetes Care. 1999;22:503–7. doi: 10.2337/diacare.22.3.503. [DOI] [PubMed] [Google Scholar]
- 5.Hasler WL. Gastroparesis-current concepts and considerations. Medscape J Med. 2008;10:16. [PMC free article] [PubMed] [Google Scholar]
- 6.McCallum RW, Soffer E, Pasricha J, et al. Treatment of gastroparesis: a multidisciplinary clinical review. Neurogastroenterol Motil. 2006;18:263–83. doi: 10.1111/j.1365-2982.2006.00760.x. [DOI] [PubMed] [Google Scholar]
- 7.Reddymasu S, McCallum RW. Pharmacotherapy of gastroparesis. Expert Opin Pharmacother. 2009;10:1–16. doi: 10.1517/14656560902722505. [DOI] [PubMed] [Google Scholar]
- 8.Arts J, van Gool S, Caenepeel P, Verbeke K, Janssens J, Tack J. Influence of intrapyloric botulinum toxin injection on gastric emptying and meal-related symptoms in gastroparesis patients. Aliment Pharmacol Ther. 2006;24:661–7. doi: 10.1111/j.1365-2036.2006.03019.x. [DOI] [PubMed] [Google Scholar]
- 9.Bromer MQ, Friedenberg F, Miller LS, Fisher RS, Swartz K, Parkman HP. Endoscopic pyloric injection of botulinum toxin A for the treatment of refractory gastroparesis. Gastrointest Endosc. 2005;61:833–9. doi: 10.1016/s0016-5107(05)00328-7. [DOI] [PubMed] [Google Scholar]
- 10.US Food and Drug Administration. p. 47. H990014—EnterraTM therapy system (formerly named gastric electrical stimulation (GES) system). Available at: http://www.fda.gov/cdrh/ode/H990014sum.html. Accessed March 31, 2000.
- 11.McCallum RW, Lin Z, Forster J, Roeser K, Hou Q, Sarosiek I. Gastric electrical stimulation improves outcome of patients with gastroparesis for up to 10 years. Clin Gastroenterol Hepatol. 2011;9:314–9. doi: 10.1016/j.cgh.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 12.McCallum R, Lin Z, Wetzel P, Sarosiek I, Forster J. Clinical response to gastric electrical stimulation in patients with postsurgical gastroparesis. Clin Gastroenterol Hepatol. 2005;3:49–54. doi: 10.1016/s1542-3565(04)00605-6. [DOI] [PubMed] [Google Scholar]
- 13.Abell T, McCallum RW, Hocking M, et al. Gastric electrical stimulation for medical refractory gastroparesis. Gastroenterology. 2003;125:421–8. doi: 10.1016/s0016-5085(03)00878-3. [DOI] [PubMed] [Google Scholar]
- 14.Abell T, Lou J, Tabbaa M, et al. Gastric electrical stimulation for gastroparesis improves nutritional parameters at short, intermediate, and long-term follow-up. J Parenter Enteral Nutr. 2003;27:277–81. doi: 10.1177/0148607103027004277. [DOI] [PubMed] [Google Scholar]
- 15.Lin Z, Sarosiek I, Forster J, McCallum RW. Symptom responses, long-term outcomes and adverse events beyond 3 years of high-frequency gastric electrical stimulation for gastroparesis. Neurogastroenterol Motil. 2006;18:18–27. doi: 10.1111/j.1365-2982.2005.00732.x. [DOI] [PubMed] [Google Scholar]
- 16.McCallum RW, Pasricha PJ. Gastroparesis and gastric electrical stimulation: a useful option for gastroparesis. AGA Perspectives. 2006;2:5. [Google Scholar]
- 17.Soffer E, Abell T, Lin Z, et al. Review article: gastric electrical stimulation for gastroparesis – physiological foundations, technical aspects and clinical implications. Aliment Pharmacol Ther. 2009;30:681–94. doi: 10.1111/j.1365-2036.2009.04082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Namin F, Lin Z, Sarosiek I, McCallum RW. Gastric electrical stimulation: who are the best candidates and what are the results. Pract Gastroenterol. 2005;8:28–47. [Google Scholar]
- 19.House A, Champion MC, Chamberlain M. National survey of radionuclide gastric emptying studies. Can J Gastroenterol. 1997;11:317–21. doi: 10.1155/1997/959482. [DOI] [PubMed] [Google Scholar]
- 20.Tougas G, Eaker EY, Abell TL, et al. Assessment of gastric emptying using a low fat meal: establishment of international control values. Am J Gastroenterol. 2000;95:1456–62. doi: 10.1111/j.1572-0241.2000.02076.x. [DOI] [PubMed] [Google Scholar]
- 21.Forster J, Sarosiek I, Delcore R, et al. Gastric pacing is a new surgical treatment for gastroparesis. Am J Surg. 2001;182:676–81. doi: 10.1016/s0002-9610(01)00802-9. [DOI] [PubMed] [Google Scholar]
- 22.Ware JE, Kosinski M. QualityMetric. Lincoln, RI: QualityMetric Incorporated; 2001. SF-36 physical & mental health summary scales: a manual for users of version 1. In: 2nd edn. [Google Scholar]
- 23.Cutts TF, Luo J, Starkebaum W, et al. Is gastric electrical stimulation superior to standard pharmacological therapy in improving GI symptoms, healthcare resources, and long-term health care benefits? Neurogastroenterol Motil. 2005;17:35–43. doi: 10.1111/j.1365-2982.2004.00609.x. [DOI] [PubMed] [Google Scholar]
- 24.Lin Z, McElhinney C, Sarosiek I, Forster J, McCallum RW. Chronic gastric electrical stimulation for gastroparesis reduces the use of prokinetic and/or antiemetic medications and the need for hospitalization. Dig Dis Sci. 2005;50:1328–34. doi: 10.1007/s10620-005-2782-7. [DOI] [PubMed] [Google Scholar]
- 25.McCallum RW, Snape W, Brody F, Wo J, Parkman HP. Gastric electrical stimulation with Enterra therapy improves symptoms from diabetic gastroparesis in a prospective study. Clin Gastroenterol Hepatol. 2010;8:947–54. doi: 10.1016/j.cgh.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 26.Bityutskiy L, Soykan I, McCAllum RW. Viral gastroparesis: a subgroup of idiopathic gastroparesis, clinical characteristics and long-term outcome. Am J Gastroenterol. 1997;92:1501–4. [PubMed] [Google Scholar]
- 27.Pande H, Lacy BE, Crowell MD. Inflammatory causes of gastroparesis: report of five cases. Dig Dis Sci. 2002;47:2664–8. doi: 10.1023/a:1021036601462. [DOI] [PubMed] [Google Scholar]
- 28.Revicki DA, Rentz AM, Dubois D, et al. Development and validation of a patient-assessed gastroparesis symptom severity measure: the Gastroparesis Cardinal Symptom Index. Aliment Pharmacol Ther. 2003;18:141–50. doi: 10.1046/j.1365-2036.2003.01612.x. [DOI] [PubMed] [Google Scholar]
- 29.Rentz AM, Kahrilas P, Stanghellini V, et al. Development and psychometric evaluation of the patient assessment of upper gastrointestinal symptom severity index (PAGI-SYM) in patients with upper gastrointestinal disorders. Qual Life Res. 2004;13:1737–49. doi: 10.1007/s11136-004-9567-x. [DOI] [PubMed] [Google Scholar]


