Abstract
Purpose
To determine whether statin use at time of surgery is associated with survival following nephrectomy or partial nephrectomy for renal cell carcinoma (RCC). Statins are thought to exhibit a protective effect on cancer incidence and possibly cancer survival in a number of malignancies; to date, no studies have shown an independent association between statin use and mortality in RCC.
Methods
A retrospective cohort study of 916 patients who underwent radical or partial nephrectomy for RCC from 2000–2010 at a single institution was performed. Primary outcomes were overall (OS) and disease-specific survival (DSS). Univariable survival analyses were performed using the Kaplan-Meier and log-rank methods. Multivariable analysis was performed using a Cox proportional hazards model. The predictive discrimination of the models was assessed with Harrell’s c-index.
Results
Median follow-up of the entire cohort was 42.5 months. The three-year OS estimate was 83.1% (95%CI, 77.6–87.3%) for statin users and 77.3% (95%CI, 73.7–80.6%) for non-statin users (p=0.53). Three-year DSS was 90.9% (95%CI, 86.3–94.0%) for statin users and 83.5% (95%CI, 80.1–86.3%) for non-statin users (p=0.015). After controlling for age, ASA class, pT stage, pN stage, metastatic status, pre-operative anemia and corrected hypercalcemia, and blood type, statin use at time of surgery was independently associated with improved OS (HR 0.62, 95%CI 0.43–0.90; p=0.011) and DSS (HR 0.48, 95%CI 0.28–0.83; p=0.009). The multivariable model for DSS had excellent predictive discrimination with a c-index of 0.91.
Conclusions
These data suggest that statin usage at time of surgery is independently associated with improved OS and DSS in patients undergoing surgery for RCC.
Keywords: Statins, nephrectomy, partial nephrectomy, renal cell carcinoma
Introduction
Nearly 65,000 new cases of renal cell carcinoma (RCC) are diagnosed each year in the United States, and it is expected to account for almost 13,500 deaths in 2012 [1]. Surgery, by nephron-sparing approaches or radical nephrectomy, is the mainstay of curative treatment for RCC [2]. Several predictors of mortality after nephrectomy for locoregional RCC have been identified, including age, race, gender, stage, grade, tumor size, nutritional status, performance status, and ABO blood type [3–6]. While these risk factors may provide important prognostic information, with exception to nutritional status, most provide little potential for intervention to alter the course of the disease.
Supported by a number of epidemiologic risk studies, there has been increasing interest in the anti-neoplastic properties of statins in recent years [7–13]. Statins are widely used cholesterol-lowering medications which act by inhibiting 3-hydroxy-3-methyl glutaryl-coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme of the mevalonate pathway. Disruption of this pathway is thought to inhibit cancer growth and metastasis by affecting critical cellular functions, including cell proliferation, maintenance of membrane integrity, cell signaling, protein synthesis, and cell-cycle progression [14].
Despite plausible mechanistic links for a protective role of statins in the development of cancer, epidemiological studies evaluating the association between statin use and cancer risk have been controversial [14–18]. While earlier studies had suggested an increased risk of cancer associated with statin use, other studies have reported a neutral effect, and the remaining have described protective effects for some cancers of up to a 50% relative risk reduction in cancer incidence [7, 8, 10, 18, 19]. Results from the evaluation of statin use and cancer-related mortality have been mixed as well, although a recent, well-performed nationwide study of patients with cancer in the Danish population found a 15% reduction in cancer-related mortality associated with statin use [13, 18, 20].
A limited number of studies have evaluated statin use and the risk of developing RCC, with conflicting results [7, 11, 12, 21, 22]. A nested case-control study of almost 500,000 veteran patients in the south-central United States found a 48% risk reduction of RCC associated with statin use [11]. More recently, a smaller, prospective population-based study of two cohorts of United States health professionals confirmed this protective effect of statins on the risk of developing RCC, but only in women without a history of hypertension [12]. In regards to statins and prognosis after surgery for RCC, a recent single-institution study suggested a significant association between statin use at time of surgery and RCC progression (defined as development of metastases or RCC-specific death), although this result was not statistically significant when modeling statin usage as a time-dependent post-operative variable and when accounting for those who initiated statin therapy after surgery for RCC [23]. Furthermore, statin use at time of surgery for RCC was not independently associated with overall survival and no independent analysis of disease-specific survival was reported. Therefore, while statin use has been associated with reduced cancer-related mortality in other malignancies, its impact on RCC prognosis following surgical treatment has not been definitively established [13, 23]. We therefore sought to evaluate whether statin use is associated with survival following surgery for RCC. Given the evidence supporting a protective effect of statins in cancer as well as the presumed metabolic nature of RCC, we hypothesized that current statin use at time of surgery would be associated with improved OS and DSS in patients undergoing radical or partial nephrectomy for RCC [24].
Patients and Methods
We performed a retrospective cohort study of 916 consecutive patients with information on statin use who underwent radical or partial nephrectomy for RCC of all stages from 2000–2010. All RCC histologic subtypes were included. All surgeries were performed at Vanderbilt University Medical Center, with pre-operative evaluation and post-operative care standardized to institutional protocol. Follow-up was at the discretion of the treating physician. Cause of death was determined by the treating physicians, death certificate, and/or chart review.
A staff surgical pathologist evaluated all surgical specimens. Stage and grade were assigned according to the 2010 American Joint Committee on Cancer guidelines and Fuhrman grading system, respectively. Clinical, pathological, and survival data were collected prospectively and were supplemented by chart review. Institutional Review Board approval was obtained for the creation of a prospective database and for retrospective analysis of this cohort.
The primary outcome measures in this study were overall (OS) and disease-specific survival (DSS). Duration of follow-up was the time from surgery to the date of death or last clinic visit. Patients who were alive at last follow-up were censored for OS and DSS analysis; those who died of causes other than RCC were censored for DSS.
We evaluated clinical and pathologic variables including age, gender, race (white vs. non-white), American Society of Anesthesiology (ASA) physical status classification system, body mass index (BMI), pre-operative anemia (hematocrit <41% for men and <36% for women), pre-operative hypoalbuminemia (albumin<3.5 g/dL), pre-operative corrected hypercalcemia ([pre-operative calcium – 0.707*(pre-operative albumin-3.4)] >10 mg/dL), Fuhrman nuclear grade (I–II vs. III–IV), pathologic T stage, node status, presence of metastasis, tumor histology (clear cell vs. non-clear cell), procedure performed (radical vs. partial nephrectomy), red blood cell transfusion status, ABO blood group (type O vs. non-O), and statin use at time of surgery. Patients without radiographic or palpable evidence of lymphadenopathy generally did not undergo lymphadenectomy (Nx) and were grouped with pathologic N0 patients for analysis.
Statistical analysis
The relationship between statin use and clinicopathologic variables was assessed using chi-squared tests. Univariable survival analyses were performed using the Kaplan-Meier and log-rank methods. Cox proportional hazards models for OS and DSS were constructed for the multivariable survival analyses. Based on the univariable analyses as well as prior analysis of this institutional data set, covariates were age, ASA score, pT stage, Fuhrman grade, node status, metastatic status, ABO blood group (type O vs. non-O), pre-operative anemia and pre-operative hypercalcemia [6]. Age was omitted from the multivariable model for DSS. Charlson comorbidity index was only available for the most recent subset of patients, therefore ASA score was used as an indicator of overall comorbidity. A total of 666 patients had complete information for all variables, and these patients were included in the multivariable survival analyses. Harrell’s c-index was calculated as a measure of the predictive discrimination of the multivariable models. Exploratory multivariable analyses using Cox proportional hazards models were performed on the cohort of patients with only locoregional disease to further evaluate the survival impact of statins on this cohort. All analyses were conducted with STATA data analysis software (College Station, TX, version 12).
Results
The median age of the cohort was 60.8 years (interquartile range [IQR] 51.3–69.3 years). Median follow-up of the entire cohort was 42.5 months (IQR 19.1–67.1 months). The median follow-up of patients alive at last follow up was 52.1 months (IQR 27.1–74.2 months). Table 1 gives the distribution of clinicopathologic variables by statin use. Statin use was associated with increased age, male sex, higher ASA class, less pre-operative hypoalbuminemia and hypercalcemia, partial nephrectomy, and N stage. There were 164 overall deaths and 98 disease-specific deaths among the 666 patients with complete data who were eligible for inclusion in the multivariable models.
Table 1.
Distribution of patients by clinical and pathological variables according to statin use
| Characteristic | All | Statin Use | p* | ||||
|---|---|---|---|---|---|---|---|
| No
|
Yes
|
||||||
| n | % | n | % | n | % | ||
| All | 916 | 100 | 646 | 71% | 270 | 29% | |
| Age (years) | |||||||
| ≤50 | 197 | 22% | 176 | 27% | 21 | 8% | |
| 51–60 | 237 | 26% | 168 | 26% | 69 | 26% | |
| 61–70 | 266 | 29% | 170 | 26% | 96 | 36% | |
| 71–80 | 179 | 20% | 109 | 17% | 70 | 26% | |
| >80 | 37 | 4% | 23 | 4% | 14 | 5% | <0.001 |
| Sex | |||||||
| Female | 322 | 35% | 245 | 38% | 77 | 29% | |
| Male | 594 | 65% | 401 | 62% | 193 | 71% | 0.007 |
| Race | |||||||
| White | 828 | 90% | 580 | 90% | 248 | 92% | |
| Non-white | 85 | 9% | 64 | 10% | 21 | 8% | 0.312 |
| ASA class | |||||||
| 1–2 | 283 | 31% | 236 | 37% | 47 | 17% | |
| 3–4 | 591 | 65% | 374 | 58% | 217 | 80% | <0.001 |
| BMI (kg/m2) | |||||||
| <18.5 | 6 | 1% | 5 | 1% | 1 | 0% | |
| 18.5–25 | 177 | 19% | 128 | 20% | 49 | 18% | |
| 25–30 | 275 | 30% | 184 | 28% | 91 | 34% | |
| >30 | 301 | 33% | 196 | 30% | 105 | 39% | 0.336 |
| Anemia | |||||||
| No | 641 | 70% | 460 | 71% | 181 | 67% | |
| Yes | 268 | 29% | 180 | 28% | 88 | 33% | 0.166 |
| Hypercalcemia | |||||||
| No | 678 | 74% | 457 | 71% | 221 | 82% | |
| Yes | 25 | 3% | 22 | 3% | 3 | 1% | 0.03 |
| Hypoalbuminemia | |||||||
| No | 647 | 71% | 429 | 66% | 218 | 81% | |
| Yes | 57 | 6% | 51 | 8% | 6 | 2% | <0.001 |
| pTstage | |||||||
| T1 | 538 | 59% | 371 | 57% | 167 | 62% | |
| T2 | 102 | 11% | 70 | 11% | 32 | 12% | |
| T3 | 253 | 28% | 186 | 29% | 67 | 25% | |
| T4 | 23 | 3% | 19 | 3% | 4 | 1% | 0.319 |
| N stage | |||||||
| N0 | 862 | 94% | 600 | 93% | 262 | 97% | |
| N+ | 54 | 6% | 46 | 7% | 8 | 3% | 0.015 |
| M stage | |||||||
| M0 | 811 | 89% | 567 | 88% | 244 | 90% | |
| M1 | 105 | 11% | 79 | 12% | 26 | 10% | 0.284 |
| Grade | |||||||
| I–II | 566 | 62% | 388 | 60% | 178 | 66% | |
| III–IV | 316 | 34% | 229 | 35% | 87 | 32% | 0.224 |
| Histology | |||||||
| Clear cell | 665 | 73% | 471 | 73% | 194 | 72% | |
| Non-clear cell | 251 | 27% | 175 | 27% | 76 | 28% | 0.743 |
| Transfused | |||||||
| Yes | 170 | 19% | 129 | 20% | 41 | 15% | |
| No | 743 | 81% | 516 | 80% | 227 | 84% | 0.093 |
| Blood type | |||||||
| O | 432 | 47% | 313 | 48% | 119 | 44% | |
| non-O | 450 | 49% | 305 | 47% | 145 | 54% | 0.13 |
| Nephrectomy | |||||||
| Radical | 584 | 64% | 425 | 66% | 159 | 59% | |
| Partial | 332 | 36% | 221 | 34% | 111 | 41% | 0.048 |
All p values from chi-squared test.
Percentages may not add up to 100% due to missing data.
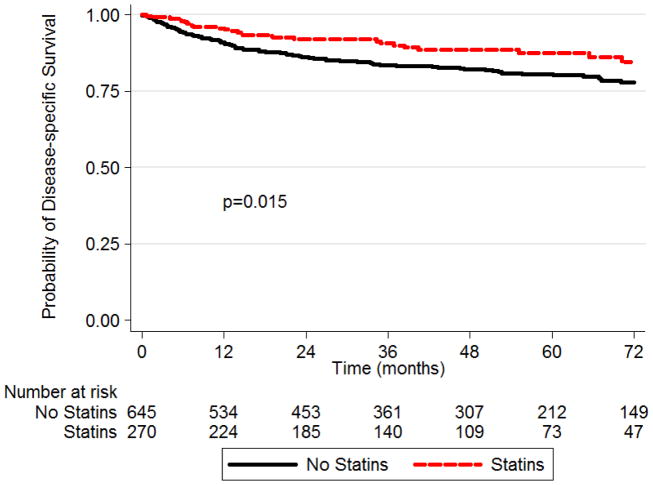
Actuarial three-year OS for our entire cohort of 916 patients was 79.0% (95% confidence interval [CI] 76.0–81.6%). The three-year OS estimate was 83.1% (95%CI 77.6–87.3%) for statin users and 77.3% (95%CI 73.7–80.6%) for non-statin users (p=0.53). Three-year DSS was 90.9% (95%CI 86.3–94.0%) for statin users and 83.5% (95%CI 80.1–86.3%) for non-statin users (p=0.015). The Kaplan-Meier survival analysis for DSS by statin usage at time of surgery is shown in the Figure.
Figure 1.
Kaplan-Meier survival analysis for disease-specific survival by statin usage at time of surgery (log rank p=0.015)
In the univariable analyses, age, ASA class, pT stage, node status, grade, metastatic status, blood group, pre-operative anemia and corrected hypercalcemia were all significantly associated with OS (Table 2). Statin usage was not statistically significant in the univariable analysis for OS (hazard ratio [HR] 0.91 for statin users vs. non-users, 95%CI 0.68–1.21; p=0.527) (Table 2).
Table 2.
Univariable and multivariable Cox proportional hazards regression for overall survival
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | CI | p | HR | CI | p | |
| Age | 1.03 | 1.02–1.04 | <0.001 | 1.02 | 1.01–1.04 | 0.001 |
| ASA class | ||||||
| 1–2 | referent | referent | ||||
| 3–4 | 2.30 | 1.65–3.21 | <0.001 | 1.71 | 1.11–2.65 | 0.015 |
| pT stage | ||||||
| T1 | referent | referent | ||||
| T2 | 1.52 | 0.97–2.39 | 0.07 | 1.43 | 0.79–2.57 | 0.235 |
| T3 | 3.59 | 2.71–4.74 | <0.001 | 1.35 | 0.92–1.99 | 0.121 |
| T4 | 12.30 | 7.36–20.56 | <0.001 | 4.99 | 2.61–9.54 | <0.001 |
| Node positive | 5.52 | 3.92–7.77 | <0.001 | 1.96 | 1.20–3.20 | 0.007 |
| Metastatic disease | 5.63 | 4.29–7.39 | <0.001 | 2.36 | 1.59–3.52 | <0.001 |
| Grade | ||||||
| I–II | referent | referent | ||||
| III–IV | 3.61 | 2.78–4.69 | <0.001 | 1.72 | 1.21–2.46 | 0.003 |
| Corrected Hypercalcemia | 4.87 | 2.94–8.05 | <0.001 | 2.91 | 1.69–5.00 | <0.001 |
| Anemia | 3.46 | 2.68–4.46 | <0.001 | 2.34 | 1.67–3.28 | <0.001 |
| Blood group (non-O vs. O) | 1.37 | 1.07–1.77 | 0.014 | 1.66 | 1.21–2.29 | 0.002 |
| Statin Use | 0.91 | 0.68–1.21 | 0.527 | 0.62 | 0.43–0.90 | 0.011 |
In the multivariable analysis for OS, age, higher ASA class, higher stage and grade, presence of metastatic disease, node positivity, pre-operative anemia and corrected hypercalcemia, and non-O blood group were all significantly associated with decreased OS (Table 2). Additionally, statin usage at time of surgery was independently associated with a decreased risk of overall mortality (HR 0.62, 95%CI 0.43–0.90; p=0.011). The c-index of this model was 0.81, indicating good predictive discrimination.
In the univariable analyses for DSS, statin use at time of surgery, ASA class, pT stage, node status, grade, presence of metastatic disease, pre-operative anemia and corrected hypercalcemia were all significantly associated with DSS (Table 3). In the multivariable analysis for DSS, higher ASA class, higher stage and grade, presence of metastatic disease, node positivity, pre-operative anemia and corrected hypercalcemia, and non-O blood group were all significantly associated with decreased DSS (Table 3). Similar to the multivariable analysis for OS, statin usage at time of surgery was independently associated with a decreased risk of disease-specific mortality in the multivariable analysis (HR 0.48, 95%CI 0.28–0.83; p=0.009) (Table 3). Predictive discrimination for this model was excellent, with a c-index of 0.91.
Table 3.
Univariable and multivariable Cox proportional hazards regression for disease-specific survival
| Univariable | Multivariable | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | CI | p | HR | CI | p | |
| ASA class | ||||||
| 1–2 | referent | referent | ||||
| 3–4 | 2.02 | 1.33–3.06 | 0.001 | 1.63 | 0.94–2.83 | 0.081 |
| pT stage | ||||||
| T1 | referent | referent | ||||
| T2 | 4.24 | 2.16–8.35 | <0.001 | 3.40 | 1.40–8.27 | 0.007 |
| T3 | 13.02 | 7.96–21.31 | <0.001 | 3.68 | 1.95–6.93 | <0.001 |
| T4 | 46.62 | 24.07–90.31 | <0.001 | 9.56 | 4.08–22.41 | <0.001 |
| Node positive | 8.59 | 5.89–12.52 | <0.001 | 1.57 | 0.94–2.62 | 0.082 |
| Metastatic disease | 10.20 | 7.35–14.15 | <0.001 | 2.88 | 1.79–4.65 | <0.001 |
| Grade | ||||||
| I–II | referent | referent | ||||
| III–IV | 9.05 | 6.05–13.55 | <0.001 | 4.80 | 2.61–8.83 | <0.001 |
| Corrected Hypercalcemia | 7.46 | 4.30–12.96 | <0.001 | 3.14 | 1.69–5.83 | <0.001 |
| Anemia | 4.59 | 3.28–6.41 | <0.001 | 2.38 | 1.52–3.74 | <0.001 |
| Blood group (non-O vs. O) | 1.38 | 0.99–1.91 | 0.056 | 1.87 | 1.22–2.87 | 0.004 |
| Statin Use | 0.60 | 0.40–0.91 | 0.016 | 0.48 | 0.28–0.83 | 0.009 |
Exploratory multivariable survival analyses for OS and DSS were performed for patients with locoregional disease only (n=811). 582 had complete information and were included in the multivariable model. With a median follow-up of 42.8 months (IQR 20.8–63), there were 104 overall deaths and 45 disease-specific deaths. After accounting for pT stage, node status, grade, and pre-operative anemia (as well as age in the OS model), statins remained independently associated with improved DSS (HR 0.31, 95%CI 0.13–0.73; p=0.007) and OS (HR 0.63, 95%CI 0.40–0.97; p=0.037) (complete model not shown).
Discussion
While there is increasing evidence suggesting beneficial effects of statins on cancer risk and cancer mortality, there has yet to be a randomized controlled trial evaluating statin use for the specific purpose of reducing cancer-specific mortality [10–13]. We showed that statin use at time of surgery is independently associated with improved OS and DSS in a large retrospective cohort of patients undergoing radical or partial nephrectomy for RCC of all stages. Specifically, there was a 38% reduction in the risk of overall mortality and a 52% reduction in the risk of disease-specific mortality. Our results remained consistent in an exploratory analysis of patients with locoregional disease only.
Although data on the effect of statins on RCC is limited, there exist several large observational studies which indicate a possible reduction in RCC incidence for patients on statins [11, 12]. In regards to statins and RCC mortality, a large Danish population-based study evaluated 5,942 patients with kidney cancer with 2,717 deaths [13]. After controlling for tumor size, nodal status, metastatic status, age, sex, presence of cardiovascular disease and diabetes, and a number of other clinical and demographic factors, there was a trend towards improved disease-specific survival (HR 0.85, 95% CI 0.72–1.01; p=0.07). This was not a strictly post-surgical cohort of patients and detailed information on tumor stage, grade, follow-up, statin use, and pre-operative laboratory values were not available, which may have limited their results specific to kidney cancer. Regardless, a 15% overall cancer-specific mortality reduction was observed across the entire Danish population for those who were on statins compared to those who were not. A recent, well-performed retrospective study evaluated statin utilization and prognosis after surgery for RCC in a large cohort of patients at a single institution [23]. A statistically significant 33% reduction in the risk of progression (defined as development of metastases or RCC-specific death) was noted with those on statins at time of surgery, although this result was not statistically significant when modeling post-operative statin usage as a time-dependent variable. DSS was not independently reported on and statin use at time of surgery was not associated with OS except for when modeling post-operative statin use as a time-dependent variable. Additionally, no association was found between statin dose or type and the risk of progression after surgery.
There are numerous potential mechanisms that may explain the observed association between statins and decreased mortality after surgery for RCC. Cholesterols are an essential part of the cell membrane and it has been suggested that the simple reduction in endogenously-produced plasma cholesterols afforded by statins may inhibit cancer growth [13]. Both in vivo and in vitro models have also suggested that the potential anti-tumor effects of statins are achieved through induction of apoptosis, inhibition of angiogenesis, and modulation of protein prenylation to alter inflammatory and immune responses [15, 17, 25]. In addition to the anti-inflammatory and anti-neoplastic effects of statins, a few studies have attempted to clarify the biologic effects of statins on RCC as well as to further elucidate a mechanism for the apparent anti-neoplastic activity of statins in RCC [16, 26, 27].
One study has identified the Akt/mammalian target of rapamycin (mTOR) signaling cascade as a potential target of statins in RCC cell lines [16]. Fluvastatin was shown to induce apoptosis in two different RCC cell lines at physiologic concentrations via phosphorylation of Akt and down-regulation of mTOR. The tumor suppressor protein, programmed cell death 4 (PDCD4), was shown to be induced as a downstream effector of the fluvastatin-dependent inhibition of the mTor pathway, which corresponds to the increased apoptosis in RCC cells exposed to fluvastatin. The Akt/mTOR pathway plays a crucial role in cell growth and survival of RCC cells, and mTOR inhibitors, such as temsirolimus, have proven efficacy in the treatment of RCC [28, 29]. In other RCC experimental studies, statins were found to inhibit angiogenesis in vitro and reduce RCC metastases in a mouse model, and also potentiate the cytotoxic and cytostatic effects of sorafenib, a drug used to treat advanced RCC [26, 27]. These studies elucidate a plausible mechanism which provides RCC-specific evidence that may explain the observed survival benefit of patients on statins in our study.
Due in part to its single-institution retrospective nature, this study has important limitations. Complete data were only available for 666 of the 916 patient original cohort. The majority (n=213) were missing albumin (required to calculate corrected serum calcium), raising the possibility of selection bias. However this was mitigated by separate exploratory multivariable analyses performed either by excluding calcium from the model or using uncorrected serum calcium, yielding 811 patients (88.5%) with complete information in which statins remained an independent predictor of improved OS and DSS (data not shown). An additional limitation is that we did not have information on duration or dosage of statin use, although other studies have indicated that there is not a dose response for the protective effects of statins towards cancer [13, 23]. While it is possible that patients who were not on statins at time of surgery had previously been on statins or started on statins after surgery, this would most likely bias our results towards the null. Statin use could be a proxy for improved access to healthcare or better health habits, although given the observed effect on disease-specific survival, this seems less likely. The association between survival and statin use may also simply represent an association with a related condition or medication, such as aspirin use or hyperlipidemia. Since almost all patients with a diagnosis of hyperlipidemia were on a statin in our cohort, we are unable to separate these two entities. Furthermore, there was no association with statin use at time of surgery and pT stage or metastatic status. Given the generally short time between diagnosis and surgery, it would be unlikely that a patient would not be prescribed a statin or would cease taking a statin pre-operatively due to unfavorable disease characteristics (i.e. healthy user bias).
Conclusions
We found a significant and independent association between statin use at time of surgery and improved OS and DSS in patients undergoing radical or partial nephrectomy for RCC of all stages. This finding was highly robust as the association persisted in a number of additional exploratory analyses. While statin use has previously been identified to be associated with RCC incidence and also has been shown to be associated with RCC progression, statin use has not been previously identified as an independent predictor of survival in patients undergoing surgery for RCC. Our work adds to the increasing volume of literature indicating a potential non-cardiovascular cancer-specific survival benefit in patients on statins. In the future it will be important to assess in a prospective fashion whether this association translates into a survival benefit in patients undergoing surgery for RCC.
Acknowledgments
The project described was supported in part by Award Numbers K08 CA113452 (PEC) from the National Institutes of Health, by the Vanderbilt Medical Scholars Program and the NIH CTSA grant TL1 TR000447 (OLT).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Ljungberg B, Cowan NC, Hanbury DC, Hora M, Kuczyk MA, Merseburger AS, et al. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol. 2010;58:398–406. doi: 10.1016/j.eururo.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 3.Karakiewicz PI, Suardi N, Capitanio U, Jeldres C, Ficarra V, Cindolo L, et al. A preoperative prognostic model for patients treated with nephrectomy for renal cell carcinoma. Eur Urol. 2009;55:287–95. doi: 10.1016/j.eururo.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 4.Kutikov A, Egleston BL, Wong YN, Uzzo RG. Evaluating overall survival and competing risks of death in patients with localized renal cell carcinoma using a comprehensive nomogram. J Clin Oncol. 2010;28:311–7. doi: 10.1200/JCO.2009.22.4816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan TM, Tang D, Stratton KL, Barocas DA, Anderson CB, Gregg JR, et al. Preoperative nutritional status is an important predictor of survival in patients undergoing surgery for renal cell carcinoma. Eur Urol. 2011;59:923–8. doi: 10.1016/j.eururo.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaffenberger SD, Morgan TM, Stratton KL, Boachie AM, Barocas DA, Chang SS, et al. ABO blood group is a predictor of survival in patients undergoing surgery for renal cell carcinoma. BJU Int. 2012;110:E641–6. doi: 10.1111/j.1464-410X.2012.11366.x. [DOI] [PubMed] [Google Scholar]
- 7.Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar HJ. The risk of cancer in users of statins. J Clin Oncol. 2004;22:2388–94. doi: 10.1200/JCO.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 8.Poynter JN, Gruber SB, Higgins PD, Almog R, Bonner JD, Rennert HS, et al. Statins and the risk of colorectal cancer. N Engl J Med. 2005;352:2184–92. doi: 10.1056/NEJMoa043792. [DOI] [PubMed] [Google Scholar]
- 9.Cauley JA, McTiernan A, Rodabough RJ, LaCroix A, Bauer DC, Margolis KL, et al. Statin use and breast cancer: Prospective results from the Women’s Health Initiative. J Natl Cancer I. 2006;98:700–7. doi: 10.1093/jnci/djj188. [DOI] [PubMed] [Google Scholar]
- 10.Platz EA, Leitzmann MF, Fisvanathan K, Rimm EB, Stampfer MJ, Willett WC, et al. Statin drugs and risk of advanced prostate cancer. J Natl Cancer I. 2006;98:1819–25. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 11.Khurana V, Caldito G, Ankem M. Statins might reduce risk of renal cell carcinoma in humans: case-control study of 500,000 veterans. Urology. 2008;71:118–22. doi: 10.1016/j.urology.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 12.Liu W, Choueiri TK, Cho E. Statin use and the risk of renal cell carcinoma in 2 prospective US cohorts. Cancer-Am Cancer Soc. 2012;118:797–803. doi: 10.1002/cncr.26338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367:1792–802. doi: 10.1056/NEJMoa1201735. [DOI] [PubMed] [Google Scholar]
- 14.Chan KK, Oza AM, Siu LL. The statins as anticancer agents. Clin Cancer Res. 2003;9:10–9. [PubMed] [Google Scholar]
- 15.Wong WW, Dimitroulakos J, Minden MD, Penn LZ. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia. 2002;16:508–19. doi: 10.1038/sj.leu.2402476. [DOI] [PubMed] [Google Scholar]
- 16.Woodard J, Sassano A, Hay N, Platanias LC. Statin-dependent suppression of the Akt/mammalian target of rapamycin signaling cascade and programmed cell death 4 up-regulation in renal cell carcinoma. Clin Cancer Res. 2008;14:4640–9. doi: 10.1158/1078-0432.CCR-07-5232. [DOI] [PubMed] [Google Scholar]
- 17.Thurnher M, Nussbaumer O, Gruenbacher G. Novel aspects of mevalonate pathway inhibitors as antitumor agents. Clin Cancer Res. 2012;18:3524–31. doi: 10.1158/1078-0432.CCR-12-0489. [DOI] [PubMed] [Google Scholar]
- 18.Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta-analysis. JAMA. 2006;295:74–80. doi: 10.1001/jama.295.1.74. [DOI] [PubMed] [Google Scholar]
- 19.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335:1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 20.Ng K, Ogino S, Meyerhardt JA, Chan JA, Chan AT, Niedzwiecki D, et al. Relationship between statin use and colon cancer recurrence and survival: results from CALGB 89803. J Natl Cancer Inst. 2011;103:1540–51. doi: 10.1093/jnci/djr307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedman GD, Flick ED, Udaltsova N, Chan J, Quesenberry CP, Jr, Habel LA. Screening statins for possible carcinogenic risk: up to 9 years of follow-up of 361,859 recipients. Pharmacoepidemiol Drug Saf. 2008;17:27–36. doi: 10.1002/pds.1507. [DOI] [PubMed] [Google Scholar]
- 22.Chiu HF, Kuo CC, Kuo HW, Lee IM, Lee CT, Yang CY. Statin use and the risk of kidney cancer: a population-based case-control study. Expert Opin Drug Saf. 2012;11:543–9. doi: 10.1517/14740338.2012.678831. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton RJ, Morilla D, Cabrera F, Leapman M, Chen LY, Bernstein M, et al. The association between statin medication and progression after surgery for localized renal cell carcinoma. The Journal of urology. 2014;191:914–9. doi: 10.1016/j.juro.2013.10.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Linehan WM, Srinivasan R, Schmidt LS. The genetic basis of kidney cancer: a metabolic disease. Nat Rev Urol. 2010;7:277–85. doi: 10.1038/nrurol.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paraskevas KI, Tzovaras AA, Briana DD, Mikhailidis DP. Emerging indications for statins: a pluripotent family of agents with several potential applications. Curr Pharm Des. 2007;13:3622–36. doi: 10.2174/138161207782794194. [DOI] [PubMed] [Google Scholar]
- 26.Bil J, Zapala L, Nowis D, Jakobisiak M, Golab J. Statins potentiate cytostatic/cytotoxic activity of sorafenib but not sunitinib against tumor cell lines in vitro. Cancer Lett. 2010;288:57–67. doi: 10.1016/j.canlet.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 27.Horiguchi A, Sumitomo M, Asakuma J, Asano T, Hayakawa M. 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitor, fluvastatin, as a novel agent for prophylaxis of renal cancer metastasis. Clin Cancer Res. 2004;10:8648–55. doi: 10.1158/1078-0432.CCR-04-1568. [DOI] [PubMed] [Google Scholar]
- 28.Cho D, Signoretti S, Regan M, Mier JW, Atkins MB. The role of mammalian target of rapamycin inhibitors in the treatment of advanced renal cancer. Clin Cancer Res. 2007;13:758s–63s. doi: 10.1158/1078-0432.CCR-06-1986. [DOI] [PubMed] [Google Scholar]
- 29.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]