Abstract
Dietary fat was recognized as a good source of energy and fat-soluble vitamins by the first part of the 20th century, but fatty acids were not considered to be essential nutrients because they could be synthesized from dietary carbohydrate. This well-established view was challenged in 1929 by George and Mildred Burr who reported that dietary fatty acid was required to prevent a deficiency disease that occurred in rats fed a fat-free diet. They concluded that fatty acids were essential nutrients and showed that linoleic acid prevented the disease and is an essential fatty acid. The Burrs surmised that other unsaturated fatty acids were essential and subsequently demonstrated that linolenic acid, the omega-3 fatty acid analog of linoleic acid, is also an essential fatty acid. The discovery of essential fatty acids was a paradigm-changing finding, and it is now considered to be one of the landmark discoveries in lipid research.
Keywords: linoleic acid, α-linolenic acid, arachidonic acid, eicosapentaenoic acid, docosahexaenoic acid, prostaglandins, fat-soluble vitamins
In this era of genomics, proteomics, and lipidomics, it is sobering to remember that a major question in biochemistry 100 years ago was whether fat is an essential dietary component. Fat was known to be an excellent source of energy, and studies done in the first quarter of the 20th century demonstrated that lipoids2 contained in dietary fat were necessary for growth and normal physiological function. However, the opinion of the leading experts was that the fatty acids were not essential nutrients. In 1929, a young, comparatively unknown assistant professor of plant physiology at the University of Minnesota, George Oswald Burr (Fig. 1), reported that the deficiency disease observed in rats fed a fat-free diet was caused by the absence of dietary fatty acids, not by the lack of a lipoid contained in the fat, and he concluded that fat was an essential dietary component (1). Burr then demonstrated that the addition of a small amount of linoleic acid, the 18-carbon ω-6 polyunsaturated fatty acid containing two double bonds (18:2ω-6), cured this deficiency disease and, therefore, was an essential fatty acid (2). These two seminal papers are now regarded as classics in biochemistry (3), but they initially met with considerable skepticism (4). To understand why, one must appreciate the paradigm-changing nature of the discovery and the stature of the experts whose views concerning dietary fat were being challenged by Burr’s findings.
Fig. 1.
George Oswald Burr. Reprinted from Smithsonian Institution Archives (SIA2008-0081) (http://siarchives.si.edu) with permission.
VIEWS ON THE ROLE OF DIETARY FAT IN THE EARLY 20th CENTURY
Proteins and carbohydrates were known to be indispensable dietary components by the first decade of the 20th century. However, dietary fat was not considered to be essential because fatty acids were known to be synthesized from carbohydrates. The evidence concerning fat was not definitive due to the inability to completely extract fat from the other dietary components using the methods available in the early 1900s, and the experimental fat-free diets of that era contained traces of residual fat.
Two of the most prominent physiological chemists of the early 20th century, Thomas B. Osborne of the Connecticut Agricultural Experiment Station and Lafayette B. Mendel of the Sheffield Scientific School at Yale University, began their studies on the role of dietary fat in 1912. Osborne and Mendel were working collaboratively in New Haven and were already recognized world-wide for their pioneering studies on dietary proteins. Their initial findings indicated that rats gained weight normally when fed a fat-free food mixture, and they concluded that “true fats” are not required for growth (5). However, Osborne and Mendel were aware of the work of Wilhelm Stepp in Strasbourg, who found that a lipoid present in egg yolk was an essential nutrient for mice (6, 7). MacArthur and Luckett at the University of Illinois also reported that a lipoid extracted from egg yolk was necessary for optimum growth of mice (8).
Osborne and Mendel realized that the fat-free diet used in their studies may have contained an essential lipoid because it had not been extracted with hot alcohol (5). They explored this issue and in 1913 found that the growth of rats actually was reduced by a fat-free diet but was restored when an ether-extract of protein-free milk was added to the food mixture (9). The necessary factor was shown to be present in milk fat, butter fat, egg yolk, and cod liver oil (10, 11), and extremely small quantities of this “accessory substance” supported growth. Although Osborne and Mendel determined that the substance was not an amine, they suggested that it was similar to the vital amines, then called “vitamines”, that were known to be essential dietary components. Elmer McCollum, who had done a year of postdoctoral study with Osborne and Mendel, but by this time was working independently at the University of Wisconsin, also reported that an ether-soluble substance contained in egg or butter fat restored the growth of rats consuming a fat-extracted diet (12). He concluded that the growth-promoting effect was due to an indispensible organic complex “in the nature of lipins”, or some substance accompanying lipins, which is an “essential accessory article in foodstuffs” (13, 14). The substance discovered by Osborne and Mendel, and independently by McCollum, was initially called the “growth-promoting fat-soluble vitamin” and was subsequently designated as vitamin A. Both groups reported that the failure of the rats to grow was not due to the absence of dietary fat, lecithin, or cholesterol (10, 12), findings that diverted attention away from the possibility that fatty acids might be essential nutrients.
The question of the essentiality of dietary fat was rekindled between 1918 and 1920 by Hans Aron in Breslau, who reported that fats had a specific nutrient value that could not be replaced by other foodstuffs and was not accounted for by caloric value alone (15, 16). Osborne and Mendel argued that these findings were not convincing because they were obtained with butter, which contained other vital nutrients besides fat (17). Because of the uncertainty raised by Aron’s findings and the confusion between lack of fats and deficiency of fat-soluble vitamins, Osborne and Mendel decided to reexamine the question of whether “true fat” was an essential dietary component.
DIETARY FAT STUDIES IN THE EARLY 1920s
Osborne and Mendel fed young rats diets exceedingly low in true fats, which they defined as compounds soluble in ether (17). The diets contained adequate amounts of fat-soluble vitamins from dried alfalfa and water soluble vitamins from dried yeast. To reduce the fat content as much as possible, the dried meat present in the food mixture was extracted five times with ether containing alcohol. The rats fed this lipid-extracted diet grew as well as those fed diets with liberal portions of butter fat or lard, and Osborne and Mendel concluded:
“If true fats are essential for nutrition during growth the minimum necessary must be exceedingly small.” (ref. 17; p. 152)
While this statement equivocates to some degree, the research community of the 1920s interpreted it as a definitive statement that dietary fat was not essential. Negative results also were reported in 1921 by Jack C. Drummond in London (18). Drummond fed young rats a diet lacking neutral fat from weaning to maturity and found that they developed normally and exhibited normal behavior. He concluded that neutral fats are not required in the diet provided that the vitamins associated with fat are supplied adequately, and he stated that the real value of fat is that it is a convenient source of energy. Based on the findings of these leading experts, there was generally agreement that true fats, that is, glycerides and their fatty acid moieties, were not essential nutrients.
These results and conclusions of Osborne and Mendel, and of Drummond, had a powerful influence on nutritional science in the 1920s. George Burr explained why at the Golden Jubilee International Congress on Essential Fatty Acids and Prostaglandins in 1980. The Congress, organized by Ralph Holman, was held to honor Burr for the discovery of essential fatty acids (19), and also Ulf von Euler for his part in the discovery of prostaglandins (PGs) (20). In remarks delivered at the Congress banquet, Burr said that:
“We had been told on high authority that fats per se were not required in the diet, and our minds were closed.” (ref. 21; p. xxv)
Considering the stature of the individuals who concluded that fat was not an essential nutrient, it is easy to understand why Burr considered this as coming from high authority.
Thomas B. Osborne was internationally renowned for his work on dietary proteins and was one of the most prominent American biochemists of the early 20th century (22). He was a member of the National Academy of Sciences and an Honorary Fellow of the Chemical Society (London). Osborne served as the fourth President of the American Society of Biological Chemists,3 was awarded a gold medal by the Paris Exposition of 1900, and received an honorary degree from Yale University. Osborne’s collaborator, Lafayette B. Mendel, was an equally prominent leader of American biochemistry and a founder of the science of nutrition (23). Mendel was head of the Department of Physiological Chemistry at the Sheffield Scientific School. This renowned department was founded by Russell H. Chittenden, considered the dean of American biochemistry (24), and it was the first scientific department devoted specifically to biochemical studies in the United States. Mendel also was the Sterling Professor of Physiological Chemistry at Yale University, was elected to the National Academy of Sciences, served as the fifth President of the American Society of Biological Chemists and the first President of the American Institute of Nutrition, and received honorary degrees from Michigan, Rutgers, and Western Reserve Universities. Jack C. Drummond was a well-recognized nutritional biochemist who had a large laboratory in London in the 1920s. Drummond was appointed the first Professor of Biochemistry at University College London in 1922, became Dean of the Faculty of Medical Science, and was subsequently elected a Fellow of the Royal Society (25). A young, relatively unknown investigator had to be mature, self-confident, and willing to take chances to challenge such high authority, and George Burr was such an individual.
GEORGE OSWALD BURR
Burr was born in 1896 in Conway, Arkansas, played cornet in the Conway Juvenile Band, and harvested wheat in Kansas during summer vacations (4). He received a BA degree from Hendrix College in 1916, where he was a member of the football team. Burr had a variety of experiences between 1916 and the end of 1918. He was Principal of a high school in Crossett, Arkansas, Professor of Science at Kentucky Wesleyan College, attended summer school at the University of Chicago, worked for General Electric in Erie, Pennsylvania, and served in the United States Army Signal Corps.
In 1919, Burr was appointed Chief Chemist of the Arkansas Feed and Fertilizer Inspectors. He resigned shortly thereafter and formed the Little Rock Oil Company to drill for oil, but the company disbanded after hitting a dry well. Burr then obtained a MS degree in chemistry and mathematics at the University of Arkansas and began working for the Missouri Pacific Railroad. He resigned in 1920 after winning a scholarship to the University of Illinois to work on the synthesis of organic arsenicals. In 1921, Burr accepted a job as a science teacher at the Wichita, Kansas high school, but resigned when he was awarded a Fellowship from the Department of Biochemistry at the University of Minnesota to join the laboratory of Professor Ross Gortner. While a graduate student, Burr again showed his entrepreneurial spirit by opening a mill in Wells, Minnesota to produce sugar from corn. Burr also worked for two summers on plant distribution in the Utah and Arizona deserts with Professor J. Arthur Harris, head of the Department of Botany at the University of Minnesota. Although this summer job was unrelated to his thesis project, Burr’s association with Professor Harris had a pivotal influence on his future career. In 1924 at the age of 28, Burr received a PhD in Biochemistry and Chemistry from the University of Minnesota. His thesis characterized condensation products formed during protein hydrolysis called humins.
Burr’s experiences were much more extensive and varied than the average newly minted PhD. He had taught in public schools, studied at four universities, worked in industry and State government, did field work on plants, and had military service. His moves to new locations and ventures in drilling for oil and milling corn indicate a degree of self-confidence and willingness to take chances. These traits would serve him well in his subsequent research studies.
Postdoctoral studies at the University of California, Berkeley
Burr was awarded a National Research Council Fellowship to work with Herbert M. Evans at the University of California, and he headed for Berkeley after receiving his PhD degree. Evans was an anatomist and physiologist who, with Katherine Scott Bishop, had recently discovered a dietary factor essential for reproduction, subsequently called vitamin E (26, 27). Evans, who directed a large well-funded laboratory, needed a biochemist to isolate and characterize the anti-sterility factor that he and Bishop had discovered, and Burr had the necessary expertise (19). Burr progressively purified vitamin E from wheat germ, first isolating it to the oil extract and then to the nonsteroid fraction of the nonsaponifiable lipids (28). Burr stated that by chance the Evans group was having trouble with reproducibility of their vitamin E experiments which they attributed to the presence of variable amounts of lipid containing vitamin E in the basal diet used to produce the sterile female rats for testing (21).
To investigate this possibility, Burr set up a separate colony of rats that were fed a fat-free diet that he prepared, consisting of sucrose recrystallized from alcohol, purified and reprecipitated casein, salts, and vitamin supplements. The rats fed this diet developed a disease that was different from vitamin E deficiency. Evans and Burr reported this new dietary deficiency, initially only emphasizing the potential usefulness of the experimental diet without speculating on the cause of the deficiency (29). Burr’s further work demonstrated that, unlike the known fat-soluble vitamins that were present in the nonsaponifiable lipid fraction, the substance which prevented the disease was present in the fatty acid fraction of the lipid extract. Based on this finding, Evans and Burr hypothesized that the active factor was a new vitamin-like substance present in the fatty acid fraction of fat and tentatively designated it as vitamin F (30). Burr, in his written comments in 1980, stated:
“Over a period of 4 years of work and 3 published papers, it never occurred to us that the deficiency was the lack of a well-known fatty acid.” (ref. 21; p. xxv)
A personal event occurred during Burr’s tenure in Berkeley that turned out to be an important factor in his subsequent discovery of essential fatty acids. Burr married Mildred Lawson, an assistant in the Evans laboratory who was in charge of the rat colony (4). Mildred’s expertise with laboratory rats was vital for Burr’s subsequent studies on fatty acid deficiency, and she was the coauthor of the two classic papers on the discovery of essential fatty acids (1, 2). Mildred Burr was also a coauthor of the 1932 paper reporting the essentiality of α-linolenic acid (18:3ω-3), the ω-3 analog of linoleic acid that is the parent of the ω-3 family of polyunsaturated fatty acids (31).
Faculty appointment at the University of Minnesota
The University of Minnesota completed a new Botany Building with adequate space in 1926, and Professor Harris, with whom Burr had worked during summers on plant distribution in the desert, was given new positions to expand the Botany faculty. Harris recognized Burr’s talent and succeeded in recruiting him as an Assistant Professor of Plant Physiology. Burr left for Minneapolis in September, 1928, stating:
“With deep sorrow and high hopes, the Burr’s left Berkeley in their Model T Ford roadster with two cages of Long-Evans rats…. On cold fall nights, our pets were smuggled into hotel rooms under long overcoats.” (ref. 4; p. 536)
Although Professor Harris hired Burr as a plant physiologist, he told Burr that he didn’t care what type of research he did as long as it was good work (19). Burr decided to continue his fat nutrition studies, so Harris arranged space for a rat colony in the attic of the Anatomy Building. The attic room was equipped with air conditioning and the finest individual metabolic cages, and Burr set up a small rat colony with the cooperation of C. M. Jackson, Professor of Anatomy. Burr received support from the University of Minnesota Research Fund and a grant from the Graduate College (1), but funding still was very limited. He states that because of the shortage of research funds, Mildred Burr pitched in and made some of the special observations, including the effects of the fat-free diet on the estrus cycle and fertility (4). Thus, the paradigm-changing studies on essential fatty acids had their beginning, and the resulting papers were published with Mildred Burr as coauthor (4).
The classic papers of 1929 and 1930
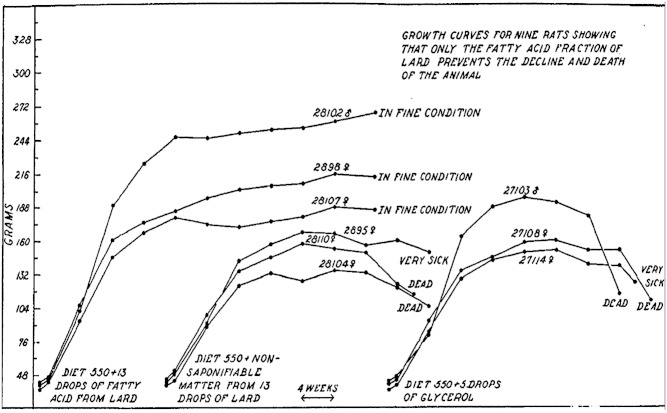
Burr realized that to make further progress, he had to rigidly exclude fat from the diet and describe the new deficiency symptoms in quantitative terms so that the relative curative value of additives could be measured (19). The paper published in the May 1929 issue of the Journal of Biological Chemistry describes the purification of the fat-free diet in great detail and contains a much more complete description of the deficiency disease than the prior Evans and Burr publications (29, 30). The results proved that dietary fat was required to stimulate growth and prevent disease in rats fed the fat-free diet (1). The key finding, shown in Fig. 2 which is reprinted from Burr’s 1929 paper, was that the component of the fat that stimulated growth and prevented disease was the fatty acid fraction, not the nonsaponifiable lipids or the glycerol backbone of the glycerides. Burr concluded that:
“The data presented here definitely settle the uncertainty as to the necessity for fats in the diet (of the rat) and prove not only that ingested fats have a beneficial effect upon the animal but that under certain experimental conditions outlined in this paper they are essential constituents of the diet.”4 (ref. 1; p. 345)
Fig. 2.
Data from Burr’s 1929 paper demonstrating the essentiality of fatty acid. The saponifiable (fatty acid) fraction of lard stimulated growth of rats when added to a fat-free diet (left set of data), but the nonsaponifiable lipids (middle set), and glycerol (right set) did not. Each line represents data from a single rat, and the time interval between data points is 4 weeks. Reprinted with permission from (1).
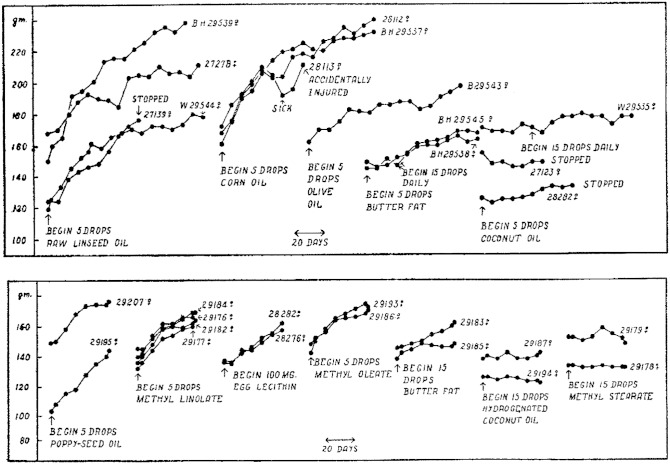
The second paper, published in 1930, describes additional abnormalities that occurred in the rats fed a fat-free diet, including effects on water exchange and ovulation, and investigates the nature of the essential fatty acid (2). Because the preparation of pure unsaturated fatty acids was problematic at that time due to isomerization of the double bonds, the studies were done primarily with oils containing different combinations of fatty acids, and also with fatty acid esters. Burr found that oils containing linoleic acid and methyl linoleate were effective, and he stated that:
“We were driven to the conclusion that the only thing that could be missing from the diet was linoleic acid. So, in March or April 1930, we wrote a paper announcing linoleic acid as an essential fatty acid, and that term was born.” (ref. 21; p. xxv)
Figure 3 contains key data reprinted from the 1930 paper demonstrating the essentiality of linoleic acid. It shows that lipids containing linoleic acid, especially linseed oil, corn oil, and poppy seed oil that have a high content of linoleic acid, as well as methyl linoleate, stimulated growth and prevented essential fatty acid deficiency in rats fed the fat-free basal diet. Egg lecithin, butter fat, and olive oil, which contain lesser amounts of linoleate, were somewhat effective, whereas coconut oil, which is highly saturated, and methyl stearate were ineffective. While these results indicated that methyl oleate also stimulated growth, this finding was not confirmed in Burr’s subsequent studies (31).
Fig. 3.
Data from Burr’s 1930 paper demonstrating that linoleic acid is an essential fatty acid. Lipids that contain linoleic acid, especially linseed oil, corn oil and poppy seed oil which have a high percentage of linoleic acid, and methyl linoleate, stimulated the growth of rats when added to the fat-free diet, whereas coconut oil which is highly saturated and methyl stearate did not. While methyl oleate also stimulated growth in this study, subsequent work did not substantiate this finding (see Fig. 4). Each line represents data from a single rat, and the time interval in days is indicated by the size of the double-headed arrow at the bottom of each figure. Reprinted with permission from (2).
Two additional important insights are contained in the 1930 paper (2). Burr reasoned that because the quantity of dietary linoleic acid required to prevent the deficiency disease was very small, linoleic acid is not synthesized by animals. Furthermore, he stated that in addition to linoleic acid, some of the more highly unsaturated fatty acids present in phospholipids are also probably essential. This was largely ignored, and the commonly held interpretation was that linoleic acid is “the” essential fatty acid.
THE CONTROVERSY AND ITS RESOLUTION
Ralph Holman states that the subject of the essentiality of polyunsaturated fatty acids was born into controversy because the finding was too revolutionary for many. He quotes Burr as saying:
“In my opinion the most striking aspect about the discovery of EFA [essential fatty acids] was the complete surprise with which it struck the nutrition researchers. The belief was deeply rooted that, except as carriers of fat-soluble vitamins, fats were merely a concentrated source of calories easily stored in plants and animals.” (ref. 4; p. 536)
Herbert Evans, Burr’s postdoctoral mentor, who by then had been elected to the National Academy of Sciences and was internationally recognized for the discovery of vitamin E (27), wrote a letter of condolence chiding Burr for having stuck his neck out and made such an error (4, 19). Burr states that this criticism was especially disturbing because he was well aware of the difficulties in that era of establishing the purity of unsaturated fatty acids (4).
Ironically, a paper from the Lafayette Mendel’s laboratory describing the effects of a fat-free diet on the growth of rats was also published in the May 1929 issue of the Journal of Biological Chemistry (32). Mendel’s group observed that rats fed a fat-free diet grew poorly and exhibited the same symptoms as described by Burr. They found that the best growth occurred in the rats that received a small amount of fat in the diet, and they stated that their findings strengthened the argument that dietary fat may have a beneficial effect. However, in contrast with Burr’s definitive statement, their overall conclusion was equivocal:
“Whether this apparent beneficial effect of a small amount of fat is due to its content of vitamin A or other vitamins, or to its action as a vehicle for the fat soluble vitamins, or whether fat per se is essential, is not conclusively demonstrated.” (ref. 32; p. 262)
This paper is often discussed in a context that implies that the Mendel group challenged Burr’s conclusion regarding the essentiality of fatty acid (3, 4, 19). However, the two papers were received by the Journal of Biological Chemistry 8 days apart in February 1929 and were published in May, and there is no evidence of any communication between Burr and the Mendel group. Therefore, the “not conclusively demonstrated” statement regarding the essentiality of fat almost certainly was meant to apply to Mendel’s results, not to Burr’s results. When considered in this light, the paper from the Mendel group is far less confrontational than is often implied, although it undoubtedly was disconcerting for Burr to see the “not conclusively demonstrated” conclusion of this world-famous laboratory in the same issue as his own paper.
Burr states that Evans put his laboratory to work to prove him wrong (4). However, in studies published between 1932 and 1934, the Evans group reproduced Burr’s findings and credits him with the original observations (33–35), clearly stating in a 1934 paper that they had extended Burr’s work (35). Burr also stated in 1980 that Sir Jack Drummond5 said that the essential fatty acid conclusion was wrong and set his laboratory to work to find the correct answer (4). However, there appears to be no publication from Drummond’s laboratory that refutes Burr’s results.
In 1931, Hume and Smith in London confirmed that rats on a fat-free diet develop a scaly tail, but they attributed this to a deficiency of a B vitamin present in yeast, not to the absence of fat (36). However, in further studies, Hume et al. (37) reproduced more of the essential fatty acid deficiency syndrome in rats and demonstrated that methyl linoleate cured the disease, thus confirming Burr’s results.
A detailed review in 1937 by Anderson, a coauthor of the 1929 paper from Mendel’s laboratory (32), listed many papers that confirmed Burr’s findings and stated that:
“Burr and Burr.... presented for serious consideration a hitherto unsuspected possible role of certain specific fatty acids in the animal organism.” (ref. 38; p. 341)
Burr considered this as a “note of skepticism” (4), and the caution implicit in this statement probably reflected a lingering doubt by the remaining members of the Yale group.6 However, some skepticism probably was justified, even as late as 1937, because proof that linoleic acid cannot be synthesized by animals was not obtained until isotopes became available for metabolic studies at the end of the 1930s (39, 40).
In retrospect, it is easy to understand why Burr’s findings were greeted initially with considerable skepticism. These were not trivial findings about some esoteric problem; rather, they dealt with a central question intimately related to the high-visibility vitamin research of that era, and some of the most prominent figures in biochemistry and nutrition had a stake in the outcome. The ingredients for controversy were there, a relatively young and inexperienced investigator who was disputing the long-accepted findings of the experts (4, 19). While Burr admitted that he was disturbed by the initial doubt, his results were confirmed and generally accepted within a few years, and requests for reprints came to Burr from around the world (4).
THE ESSENTIALITY OF LINOLEIC ACID
In 1931, Burr reported that linoleic acid was not synthesized from carbohydrates in the rat (41), and Green and Hilditch (42) subsequently found that the cow also did not synthesize linoleic acid from dietary components. These nutritional observations were confirmed in the late 1930s by Schoenheimer and Rittenberg in their metabolic studies with deuterated water in mice. They found that saturated and monounsaturated fatty acids were labeled with deuterium, but linoleic acid was not, proving that linoleic acid was derived from the diet (39, 40).
Investigators concentrated on the effects of linoleic acid after Burr’s findings were confirmed, and disregarded the possibility that products synthesized from it might have essential functions. By the late 1930s, however, nutritional studies demonstrated a linkage between linoleic acid and arachidonic acid (20:4ω-6). Nunn and Smedley-Maclean (43), in London, found that arachidonic acid increased in the liver when rats on a fat-free diet were fed methyl linoleate. These findings were confirmed and extended in the 1940s by Holman and Burr (44), who developed the alkali isomerization spectrophotometric method to measure individual polyunsaturated fatty acids in a fatty acid mixture. They found that tissue levels of arachidonic acid decreased when rats were fed a fat-free diet and increased when corn oil was added to the diet (44, 45). Further evidence that arachidonic acid was synthesized from linoleic acid was obtained in rats fed pure linoleic acid (46).
Fatty acid chain elongation and desaturation were not known in the 1940s, and Burr, Holman, and their associates puzzled as to the how two additional double bonds could be added to convert linoleic acid to arachidonic acid (4). The answer was provided between 1953 and 1960 by an elegant series of studies with radiolabeled compounds carried out by Jim Mead and colleagues at the University of California, Los Angeles (47). Mead and coworkers initially found that [1-14C]acetate was incorporated into arachidonic acid, but not linoleic acid, and concluded that arachidonic acid was formed by elongation of linoleic acid (48). They confirmed this by showing that [1-14C]linoleic acid condensed with acetate to form radiolabeled arachidonic acid (49). They subsequently demonstrated that γ-linolenic acid (18:3ω-6) was an intermediate in the conversion of linoleic to arachidonic acid (50), and that dihomo-γ-linolenic acid (20:3ω-6) was the intermediate in the conversion of γ-linolenic to arachidonic acid (51). The pathway determined by Mead and coworkers:
was subsequently extended by showing that arachidonic acid can be converted to two 22-carbon fatty acid products, 22:4ω-6 and 22:5ω-6 (52).
Nutritional studies done in the late 1930s and early 1940s indicated that arachidonic acid, like linoleic acid, was an essential fatty acid. Methyl arachidonate was found to be more effective than methyl linoleate in promoting weight gain in rats fed a fat-free diet (53). Furthermore, the skin lesions in the essential fatty acid-deficient rats were cured by feeding methyl arachidonate (54), and the reproductive abnormalities were cured by ethyl arachidonate (55).
MECHANISTIC BASIS FOR THE ESSENTIALITY OF LINOLEIC ACID
Essentiality in the first half of the 20th century was based on the maintenance of normal physiological function and the prevention of disease (56). While Burr did not determine the biochemical basis for the essentiality of linoleic acid, he stated in 1929:
“If fatty acids are responsible for cures, we must assign them a function far more subtle than the production of 9 Calories/gram.” (ref. 1; p. 366)
Burr did not specify what this more subtle function might be, but it seems likely from this statement that he envisioned biochemical effectors similar to the lipid mediators that were eventually shown to be synthesized from essential fatty acids. The mechanistic breakthrough came when Burr was no longer active in fatty acid research. In 1964, Van Dorp’s group at the Unilever Research Laboratories in Vlaardingen and Bergström’s group at the Karolinska Institute in Stockholm independently showed that arachidonic acid was converted to PGE2 (57, 58). Van Dorp stated:
“It may even be possible that the actual function of essential fatty acids is to act as precursors of prostaglandins.” (ref. 57; p. 207)
Similarly, Bergström stated:
“...the symptoms of essential fatty acid deficiency at least partly are due to an inadequate biosynthesis of the various members of the prostaglandin hormone system.” (ref. 58; p. 210)
Bergström’s group also demonstrated that dihomo-γ-linolenic acid was converted to PGE1 (59), indicating a further linkage between linoleic acid, its ω-6 fatty acid elongation and desaturation products, and the formation of the PG biomediators.
In addition to serving as the substrate for the production of homo-γ-linolenic and arachidonic acids, linoleic acid is required to generate the ω-hydroxyceramides that covalently attach to epidermal proteins to form a barrier that prevents water loss through the skin (60).
ESSENTIAL FATTY ACIDS IN HUMANS
Burr and his student, Arild Hansen, participated in a 1938 study to determine whether linoleic acid was essential for humans (61). They observed 40% decreases in serum linoleic and arachidonic acids in a healthy adult male after 6 months on a nearly fat-free diet, decreases that were similar to those seen in the essential fatty acid-deficient rat. Although no harmful effects occurred, except for gradual weight loss, it was concluded that clinical symptoms of essential fatty acid deficiency would have developed if the diet had been continued over a prolonged period.
For the next 20 years, the evidence that humans require essential fatty acids remained indirect and was based entirely on the fact that the serum fatty acid changes in poorly nourished humans were similar to those that occurred in experimental animals (62). However, more convincing human evidence was obtained in 1958, when skin abnormalities were observed in infants fed a low-fat diet (63). Addition of saturated fat was not helpful, but the skin symptoms were cured when linoleic acid was added to the diet in the form of trilinolein at 2% of calories. Addition of linoleic acid also reversed the low diene and tetraene content of the serum.7 5,8,11-Eicosatrienoic acid, the triene that accumulates in essential fatty acid-deficient rats (64), also decreased in the serum of the infants when linoleic acid was added to the diet (63). Further studies indicated that the minimum requirement for dietary linoleic acid in the young infant was 1% of calories, and the optimum amount was 4% (65).
However, some skepticism regarding whether essential fatty acids were required by humans existed until long-term parenteral nutrition was introduced in 1968 (66). Essential fatty acids were not included in the parenteral nutrition solutions that were used initially, and cases of human essential fatty acid deficiency occurred. An adult male who was treated in 1971 with a fat-free intravenous solution developed the plasma phospholipid fatty acid signs of essential fatty acid deficiency and a skin rash. These abnormalities were reversed when a soybean emulsion containing 86 g/l linoleic acid was added to the parenteral solution (67). Likewise, essential fatty acid deficiency that developed in infants treated with parenteral nutrition was cured when Intralipid®, which is rich in linoleic acid, was added to the parenteral solution (68–70). Thus, it became obvious during the 1970s that linoleic acid was also an essential nutrient for humans.
ESSENTIALITY OF ω-3 FATTY ACIDS
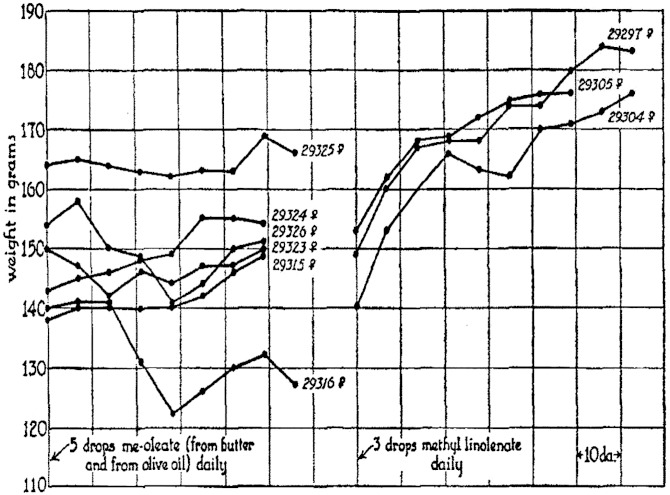
In 1931, Wesson and Burr (41) reported that like linoleic acid, its 18-carbon ω-3 analog α-linolenic acid (18:3ω-3), was not synthesized in the rat. This finding was confirmed at the end of the decade by Schoenheimer and Rittenberg’s (40) metabolic studies of deuterium incorporation into lipids. Furthermore, as shown in Fig. 4, which is reprinted from Burr’s 1932 paper, α-linolenic acid was effective in stimulating weight gain in rats on an essential fatty acid-deficient diet (31). Based on these results, Burr concluded that α-linolenic acid is also an essential fatty acid. However, other investigators found that methyl linolenate was only one-sixth as effective as methyl linoleate in promoting weight gain, and that ethyl linolenate did not cure the skin lesions or facilitate reproduction in essential fatty acid-deficient rats (36, 37). Furthermore, α-linolenic acid competitively inhibited the effectiveness of linoleic acid in preventing the symptoms of essential fatty acid deficiency (37). These findings created uncertainty as to whether α-linolenic acid and ω-3 fatty acids were essential, and it took almost 50 years and the work of many other investigators to overcome these doubts (56).
Fig. 4.
Data from Burr’s 1932 paper demonstrating that α-linolenic acid is an essential fatty acid. Methyl linolenate stimulated the growth of rats on a fat-free diet (right side), while methyl oleate did not (left side). Each line represents data taken from a single rat, and the time interval of each horizontal segment of the grid is 10 days. Reprinted with permission from (31).
Evidence that α-linolenic acid is an essential nutrient became convincing only after EPA (20:5ω-3) and DHA (22:6ω-3), which are synthesized from α-linolenic acid, were shown to have important functional effects. Nutritional experiments done in rats and chicks in the late 1930s and 1940s indicated that α-linolenic acid was converted to more highly unsaturated fatty acids that contained five and six double bonds (45, 46, 71, 72). Klenk and Bongard (73), in Cologne, reported that a 22-carbon fatty acid containing six double bonds was enriched in brain phosphatides in 1952; Hammond and Lundberg (74), at the University of Minnesota, purified the DHA from hog brain phosphatides and determined its structure in 1953; and Klenk (75) determined the metabolic pathway for the conversion of α-linolenic acid to DHA8 in 1960.
In 1961, Biran and Bartley (76), in Oxford, showed that DHA was contained in membrane-bound organelles isolated from brain, and Fred Snyder’s group, in Oak Ridge, extended these findings by showing that DHA was highly enriched in synaptic membrane phospholipids (77). This suggestion that DHA might play a role in neurotransmission was confirmed by Gene Anderson and colleagues, in Houston, who showed in 1973 that DHA, which is abundant in retinal phospholipids, facilitated the electrical response to visual excitation (78, 79). These striking findings demonstrated that ω-3 fatty acids are functionally important. However, the prevalent opinion in the 1960s and early 1970s was that ω-3 fatty acids had no unique or essential function and were present in the body simply because they were contained in the diet. Because of the general lack of interest in ω-3 fatty acids at the time, these seminal results had very little impact when they were initially reported.
In 1964, Bergström’s group demonstrated that EPA was converted to PGE3 by a sheep vesicular gland homogenate (59), and they subsequently detected PGE3 in human seminal plasma (80). However, investigators were focused on PGE2 and PGE1 at that time, and these findings were hardly noticed. In 1976, Phil Needleman and colleagues in St. Louis found that PGG3/PGH3, the endoperoxides synthesized from EPA by sheep seminal vesicles, were converted to thromboxane (TX)A3, and that unlike TXA2, the corresponding arachidonic acid product, TXA3, did not aggregate platelets (81). Needleman’s laboratory also showed that either PGG3/PGH3 or a PG synthesized from them by coronary arteries had vasoactive properties (82). Again, because there was little interest in ω-3 fatty acids, investigators focused on results with the corresponding arachidonic acid products that also were reported in these papers and overlooked these striking EPA findings.
The widespread perception that ω-3 fatty acids had no important functions changed abruptly in 1978 when Jørn Dyerberg and H. O. Bang in Aalborg reported that the incidence of myocardial infarction was very low in Greenland Eskimos whose diet consisted of marine lipids rich in EPA and DHA (83). They found that the plasma of the Greenland Eskimos contained large amounts of ω-3 fatty acids as compared with plasma of Danish Caucasians, and the plasma phospholipids of the Eskimos contained high levels of EPA but very little arachidonic acid. Furthermore, the Eskimos had low levels of plasma cholesterol, triglycerides, and atherogenic lipoproteins, their high density lipoproteins were elevated, and they had an increased bleeding tendency. Dyerberg and Bang (84) found that EPA inhibited platelet aggregation, and they showed that the cyclooxygenase in vascular tissue produced an anti-aggregatory PG from EPA. They concluded that the incidence of myocardial infarction was low in the Greenland Eskimos because EPA protected against thrombosis by inhibiting platelet aggregation by competitively inhibiting the conversion of arachidonic acid to TXA2, being converted to TXA3, or being converted to an inhibitory PG by the vasculature. Thus, the findings of Dyerberg, Bang, and their colleagues established a connection between EPA and PGs, plasma lipoproteins, thrombosis, and atherosclerosis, topics at the forefront of vascular biology and lipid research in the 1970s and 1980s, and the biomedical world suddenly realized that ω-3 fatty acids were important.
Interest initially was centered on EPA because of the eicosanoid and thrombosis connections. However, DHA ordinarily is the most abundant ω-3 fatty acid present in the tissues, particularly in the retina and brain, and emphasis gradually shifted to DHA as results indicating that DHA had a vital role in the nervous system accumulated (85). It became apparent by the end of the 1990s that the requirement for DHA probably is the primary reason why ω-3 fatty acids are essential (86). This tentative conclusion was supported by subsequent findings indicating that DHA enhances cognition (87) and synaptic function (88), and that it is converted to lipid mediators that facilitate the resolution of acute inflammation (89), provide neuroprotection (90), and promote hippocampal development (91).
Evidence for the essentiality of α-linolenic acid in humans was obtained in 1982 by Ralph Holman and colleagues (92). They observed that a 6-year-old female who had a 300 cm intestinal excision developed neurological symptoms during treatment with a parenteral nutrition emulsion containing 76% linoleic acid, but only 0.66% α-linolenic acid. When the α-linolenic acid content of the emulsion was increased to 6.9%, the DHA in the serum phospholipids increased from 1.54 to 4.35%, and the neurological symptoms were alleviated. The implication that α-linolenic acid was the essential factor was questioned, and it was suggested that the neurological abnormalities were caused by a deficiency of the elongation and desaturation products of α-linolenic acid (93). In response, Holman, Johnson, and Hatch agreed and stated:
“We do recognize that probably the essentiality of linolenic acid resides in the polyunsaturated fatty acids formed from it, just as is the case for the linoleic family of polyunsaturated fatty acids. ...We therefore suggest that linolenic acid is a required dietary nutrient for humans and that ω3 polyunsaturated fatty acids are required for normal nerve function.” (ref. 94; p. 1253)
Additional clinical studies demonstrated that DHA increased visual acuity and cognitive function in human infants (95, 96), providing further evidence that the requirement for DHA in the nervous system is a major reason for the essentiality of ω-3 fatty acids.
THE AFTERMATH
The discovery of essential fatty acids is one of the great advances in lipid research. It reversed the commonly held belief that dietary fat was merely a source of energy and fat-soluble vitamins, and established fatty acids as essential dietary components. The impact of this discovery is indicated by the more than 46,000 publications currently listed in PubMed under the heading of essential fatty acid, the importance of essential fatty acids in membrane properties and signal transduction, and the potent effects of the lipid mediators produced from arachidonic acid, EPA, and DHA. While George Burr received some recognition for his paradigm-changing discovery (4), it was modest compared with the honors received by his contemporaries who made major discoveries related to dietary fat (22, 23, 25, 27).
Several factors contributed to Burr’s failure to achieve a level of prominence equivalent to that of his contemporaries. New findings about fatty acids were not considered newsworthy because fatty acids were commonplace, whereas discoveries about vitamins attracted widespread attention because vitamins were at the forefront of biomedical research in the first half of the 20th century. A contributing factor was that Burr kept working quietly to accumulate the evidence needed to strengthen his discovery and did not seek the limelight (4). Furthermore, Burr decided to pursue his interest in plant biochemistry in 1956 and moved to Hawaii and then to Taiwan, and he was no longer involved in lipid research during the 1960s and 1970s when the PG and ω-3 fatty acid discoveries stimulated widespread interest in essential fatty acids.
Burr was appointed as a consultant to the Royal Swedish Institute for Scientific and Engineering Research in 1946, and he was invited by the Nobel Foundation to submit a nomination for the Nobel Prize in Physiology or Medicine. This obviously pleased him greatly, because it is one of the few honors that he mentioned in his autobiographical material (4). The 1982 Nobel Prize in Physiology or Medicine was awarded to Sune Bergström, Bengt Samuelsson, and Sir John Vane for their discoveries concerning PGs, and while Burr was not included, it must have pleased him to know that his landmark discovery of essential fatty acids would eventually lead to findings worthy of this ultimate honor.
REFERENCES
- 1.Burr G. O., Burr M. M. 1929. A new deficiency disease produced by the rigid exclusion of fat from the diet. J. Biol. Chem. 82: 345–367. [DOI] [PubMed] [Google Scholar]
- 2.Burr G. O., Burr M. M. 1930. On the nature and role of the fatty acids essential in nutrition. J. Biol. Chem. 86: 587–621. [Google Scholar]
- 3.Classics, 2012. Essential fatty acids: the work of George and Mildred Burr. J. Biol. Chem. 287: 35439–35444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holman R. T. 1988. George O. Burr and the discovery of essential fatty acids. J. Nutr. 118: 535–540. [DOI] [PubMed] [Google Scholar]
- 5.Osborne T. B., Mendel L. B. 1912. Feeding experiments with fat-free food mixtures. J. Biol. Chem. 12: 81–89. [Google Scholar]
- 6.Stepp W. 1909. Versuche über Fütterung mit lipoidfreier Nahrung. Biochem. Z. 22: 453–460. [Google Scholar]
- 7.Wolf G., Carpenter K. J. 1997. Early research into the vitamins: The work of Wilhelm Strepp. J. Nutr. 127: 1255–1259. [DOI] [PubMed] [Google Scholar]
- 8.MacArthur C. G., Luckett C. L. 1915. Lipins in nutrition. J. Biol. Chem. 20: 161–174. [Google Scholar]
- 9.Osborne T. B., Mendel L. B. 1913. The relation of growth to the chemical constituents of the diet. J. Biol. Chem. 15: 311–326. [Google Scholar]
- 10.Osborne T. B., Mendel L. B. 1914. The influence of butter fat on growth. J. Biol. Chem. 16: 423–437. [Google Scholar]
- 11.Osborne T. B., Mendel L. B. 1914. The influence of cod liver oil and some other fats on growth. J. Biol. Chem. 17: 401–408. [Google Scholar]
- 12.McCollum E. V., Davis M. 1913. The necessity of certain lipins in the diet during growth. J. Biol. Chem. 15: 167–175. [Google Scholar]
- 13.McCollum E. V., Davis M. 1914. Observations on the isolation of the substance in butter fat which exerts a stimulating influence on growth. J. Biol. Chem. 19: 245–250. [Google Scholar]
- 14.McCollum E. V., Davis M. 1915. Nutrition with purified food substances. J. Biol. Chem. 20: 641–658. [Google Scholar]
- 15.Aron H. 1918. Über den Nahrwerte. Biochem. Z. 92: 207–221. [Google Scholar]
- 16.Aron H. 1920. Nahrungswert und die Bedeutung von nahrenden Fett. Biochem. Z. 103: 172–177. [Google Scholar]
- 17.Osborne T. B., Mendel L. B. 1920. Growth on diets poor in true fat. J. Biol. Chem. 45: 145–152. [Google Scholar]
- 18.Drummond J. C., Coward K. H. 1921. Nutrition and growth on diets devoid of true fats. Lancet. 198: 698–700. [Google Scholar]
- 19.Burr G. O. 1981. The essential fatty acids fifty years ago. Prog. Lipid Res. 20: xxvii–xxix. [DOI] [PubMed] [Google Scholar]
- 20.von Euler US. 1981. Prostaglandin, historical remarks. Prog. Lipid Res. 20: xxxi–xxxv. [DOI] [PubMed] [Google Scholar]
- 21.Burr G. O. 1981. Presentations at the Golden Jubilee Banquet: if, by chance. Prog. Lipid Res. 20: xxv–xxvi. [Google Scholar]
- 22.Vickery H. B. 1931. Biographical memoir of Thomas Burr Osborne, 1859–1929. In Biographical Memoirs. Vol. xiv. National Academy of Sciences, Washington, DC. 261–304 .
- 23.Chittenden R. H. 1936. Lafayette Benedict Mendel, 1872–1935. In Biographical Memoirs. Vol. xvii. National Academy of Sciences, Washington, DC. 123–155.
- 24.Vickery H. B. 1944. Biographical memoir of Russell Henry Chittenden, 1856–1943. In Biographical Memoirs. Vol. xxiv. National Academy of Sciences, Washington, DC. 59–104.
- 25.Hollingsworth D. F., Wright N. C. 1954. Sir Jack Cecil Drummond, 1891–1952. Br. J. Nutr. 8: 319–324. [DOI] [PubMed] [Google Scholar]
- 26.Evans H. M., Bishop K. S. 1922. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science. 56: 650–651. [DOI] [PubMed] [Google Scholar]
- 27.Corner G. W. 1974. Herbert McLean Evans, 1882–1971. In Biographical Memoirs. National Academy of Sciences, Washington, DC. 153–192. [PubMed] [Google Scholar]
- 28.Evans H. M., Burr G. O. 1927. The antisterility fat soluble vitamin E. Memoirs of the University of California. Vol. 8. University of California, Berkeley, CA. 1–176. [Google Scholar]
- 29.Evans H. M., Burr G. O. 1927. A new dietary deficiency with highly purified diets. Proc. Soc. Exp. Biol. Med. 24: 740–743. [Google Scholar]
- 30.Evans H. M., Burr G. O. 1928. A new dietary deficiency with highly purified diets. III. The beneficial effect of fat in the diet. Proc. Soc. Exp. Biol. Med. 25: 390–397. [Google Scholar]
- 31.Burr G. O., Burr M. M., Miller E. S. 1932. On the fatty acids essential in nutrition. III. J. Biol. Chem. 97: 1–9. [Google Scholar]
- 32.McAmis A. J., Anderson W. E., Mendel L. B. 1929. Growth of rats on “fat-free” diets. J. Biol. Chem. 82: 247–262. [Google Scholar]
- 33.Evans H. M., Lepkovsky S. 1932. Vital need of the body for certain unsaturated fatty acids: III. Inability of the rat organism to synthesize the essential unsaturated fatty acids. J. Biol. Chem. 99: 231–234. [Google Scholar]
- 34.Evans H. M., Lepkovsky S., Murphy E. A. 1934. Vital need of the body for certain unsaturated fatty acids: IV. Reproduction and lactation upon fat-free diets. J. Biol. Chem. 106: 431–440. [Google Scholar]
- 35.Evans H. M., Lepkovsky S., Murphy E. A. 1934. Vital need of the body for certain unsaturated fatty acids: VI. Male sterility on fat-free diets. J. Biol. Chem. 106: 445–450. [Google Scholar]
- 36.Hume E. M., Smith H. H. 1931. XXXVII. The relation of a fat-free diet to the scaly tail condition in rats described by Burr and Burr. Biochem. J. 25: 300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hume E. M., Nunn L. C. A., Smedley-MacLean I., Smith H. H. 1938. CCLXXXI. Studies of the essential unsaturated fatty acids in their relation to the fat-deficiency disease of rats. Biochem. J. 32: 2162–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson W. E., Williams H. H. 1937. The role of fat in the diet. Physiol. Rev. 17: 335–372. [Google Scholar]
- 39.Bernhard K., Schoenheimer R. 1940. The inertia of highly unsaturated fatty acids in the animal, investigated with deuterium. J. Biol. Chem. 133: 707–712. [Google Scholar]
- 40.Schoenheimer R., Rittenberg D. 1940. The study of intermediary metabolism of animals with the aid of isotopes. Physiol. Rev. 20: 218–248. [Google Scholar]
- 41.Wesson L. G., Burr G. O. 1931. The metabolic rate and respiratory quotients of rats on a fat-deficient diet. J. Biol. Chem. 91: 525–539. [Google Scholar]
- 42.Green T. G., Hilditch T. P. 1935. Some further observations on the occurrence of an octadecadienoic acid in cow butter fat. Biochem. J. 29: 1564–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nunn L. C. A., Smedley-Maclean I. 1938. The nature of the fatty acids stored by the liver in the fat-deficient disease of rats. Biochem. J. 32: 2178–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holman R. T., Burr G. O. 1948. Alkali conjugation of the unsaturated fatty acids. Arch. Biochem. 19: 474–482. [PubMed] [Google Scholar]
- 45.Rieckenhoff I. G., Holman R. T., Burr G. O. 1949. Polyethenoid fatty acid metabolism. Effect of dietary fat on polyethenoid fatty acids of rat tissues. Arch. Biochem. 20: 331–340. [PubMed] [Google Scholar]
- 46.Widmer C., Holman R. T. 1950. Polyethenoid fatty acid metabolism. II. Deposition of polyunsaturated fatty acids in fat-deficient rats upon single fatty acid supplementation. Arch. Biochem. 25: 1–12. [PubMed] [Google Scholar]
- 47.Mead J. F. 1981. The essential fatty acids: past, present and future. Prog. Lipid Res. 20: 1–6. [DOI] [PubMed] [Google Scholar]
- 48.Mead J. F., Steinberg G., Howton D. R. 1953. Metabolism of essential fatty acids: Incorporation of acetate into arachidonic acid. J. Biol. Chem. 205: 683–689. [PubMed] [Google Scholar]
- 49.Steinberg G., Slaton W. H., Jr, Howton D. R., Mead J. F. 1956. Metabolism of essential fatty acids: IV. Incorporation of linoleate into arachidonic acid. J. Biol. Chem. 220: 257–264. [PubMed] [Google Scholar]
- 50.Mead J. F., Howton D. R. 1957. Metabolism of essential fatty acids: VII. Conversion of γ-linoleic acid to arachidonic acid. J. Biol. Chem. 229: 575–582. [PubMed] [Google Scholar]
- 51.Howton D. R., Mead J. F. 1960. Metabolism of essential fatty acids: X. Conversion of 8,11,14-eicosatrienoic acid to arachidonic acid in the rat. J. Biol. Chem. 235: 3385–3386. [PubMed] [Google Scholar]
- 52.Marcel Y. L., Christiansen K., Holman R. T. 1968. The preferred metabolic pathway from linoleic to arachidonic acid in vitro. Biochim. Biophys. Acta. 164: 25–34. [DOI] [PubMed] [Google Scholar]
- 53.Turpeinen O. 1938. Further studies on the unsaturated fatty acids in nutrition. J. Nutr. 15: 351–366. [Google Scholar]
- 54.Hume E. M., Nunn L. C. A., Smedley-Maclean I., Smith H. H. 1940. Fat deficiency disease in rats. The relative curative potencies of methyl linoleate and methyl arachidonate with a note on the action of the methyl esters of fatty acids from cod liver oil. Biochem. J. 34: 879–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quackenbush F. W., Kummerow F. A., Steenbock H. 1942. The effectiveness of linoleic, arachidonic and linolenic acids in reproduction and lactation. J. Nutr. 24: 213–224. [DOI] [PubMed] [Google Scholar]
- 56.Holman R. T. 1998. The slow discovery of the importance of ω3 essential fatty acids in human health. J. Nutr. 128: 427S–433S. [DOI] [PubMed] [Google Scholar]
- 57.Van Dorp D. A., Beerthius R. K., Nugteren D. H., Vonkeman H. 1964. The biosynthesis of prostaglandins. Biochim. Biophys. Acta. 90: 204–207. [DOI] [PubMed] [Google Scholar]
- 58.Bergström S., Danielsson H., Samuelsson B. 1964. The enzymatic formation of prostaglandin E2 from arachidonic acid. Prostaglandins and related factors 32. Biochim. Biophys. Acta. 90: 207–210. [DOI] [PubMed] [Google Scholar]
- 59.Bergström S., Danielsson H., Klenberg D., Samuelsson B. 1964. The enzymatic conversion of essential fatty acids to prostaglandins: Prostaglandins and related factors 34. J. Biol. Chem. 239: PC4006–PC4008. [PubMed] [Google Scholar]
- 60.Zheng Y., Yin H., Boeglin W. E., Elias P. M., Crumrine D., Beier D. R., Brash A. R. 2011. Lipoxygenases mediate the effect of essential fatty acid in skin barrier formation. A proposed role in releasing omega-hydroxyceramide for construction of the corneocyte lipid envelope. J. Biol. Chem. 286: 24046–24056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown W. R., Hansen A. E., Burr G. O., McQuarrie I. 1938. Effects of prolonged use of extremely low-fat diet on an adult human subject. J. Nutr. 16: 511–524. [Google Scholar]
- 62.Hansen A. E., Burr G. O. 1946. Essential fatty acids and human nutrition. J. Am. Med. Assoc. 132: 855–859. [PubMed] [Google Scholar]
- 63.Hansen A. E., Haggard M. E., Boelsche A. N., Adam D. J. D., Wiese H. F. 1958. Essential fatty acids in infant nutrition. III. Clinical manifestations of linoleic acid deficiency. J. Nutr. 66: 565–576. [DOI] [PubMed] [Google Scholar]
- 64.Mead J. F., Slaton W. H., Jr 1956. Metabolism of essential fatty acids. III. Isolation of 5,8,11-eicosatrienoic acid from fat-deficient rats. J. Biol. Chem. 219: 705–709. [PubMed] [Google Scholar]
- 65.Wiese H. F., Hansen A. E., Adam D. J. D. 1958. Essential fatty acids in infant nutrition. I. Linoleic acid requirement in terms of serum di-, tri- and tetraenoic acid levels. J. Nutr. 66: 345–360. [DOI] [PubMed] [Google Scholar]
- 66.Yamanaka W. K., Ceemans G. W., Hutchinson M. L. 1980. Essential fatty acid deficiency in humans. Prog. Lipid Res. 19: 187–215. [DOI] [PubMed] [Google Scholar]
- 67.Collins F. D., Sinclair A. J., Royle J. P., Coats D. A., Maynard A. T., Leonard R. F. 1971. Plasma lipids in human linoleic acid deficiency. Nutr. Metab. 13: 150–167. [DOI] [PubMed] [Google Scholar]
- 68.Caldwell M. D., Jonsson H. T., Othersen H. B., Jr 1972. Essential fatty acid deficiency in an infant receiving prolonged parenteral alimentation. J. Pediatr. 81: 894–898. [DOI] [PubMed] [Google Scholar]
- 69.Tashiro T., Ogata H., Yokoyama H., Mashima Y., Itoh K. 1976. The effect of fat emulsion IIntralipid) on essential fatty acid deficiency in infants receiving intravenous alimentation. J. Pediatr. Surg. 11: 505–515. [DOI] [PubMed] [Google Scholar]
- 70.Postuma R., Pease P. W. B., Watts R., Taylor S., McEvoy F. A. 1978. Essential fatty acid deficiency in infants receiving parenteral nutrition. J. Pediatr. Surg. 13: 393–398. [DOI] [PubMed] [Google Scholar]
- 71.Klenk E., Montag W. 1958. Uber die C22-Polyensauren der Glycerinphosphatides des Gehirns: The C22-polyenoic acids of the phosphatides of the brain. J. Neurochem. 2: 233–242. [DOI] [PubMed] [Google Scholar]
- 72.Reiser R., Gilson B. 1950. The metabolism of fatty acids in growing chicks. J. Nutr. 42: 325–336. [DOI] [PubMed] [Google Scholar]
- 73.Klenk E., Bongard W. 1952. Die constitution der ungesättigten C20- und C22-fettsäuren der glycerinphosphatide des gehirns [Article in German]. Hoppe Seylers Z. Physiol. Chem. 291: 104–118. [PubMed] [Google Scholar]
- 74.Hammond E. G., Lundberg W. O. 1953. A methyl docosahaenoate: Its isolation and characterization. J. Am. Oil Chem. Soc. 30: 438–441. [Google Scholar]
- 75.Klenk E., Mohrhauer H. 1960. Metabolism of polyenoic acids in the rat. Hoppe Seylers Z. Physiol. Chem. 320: 218–232. [DOI] [PubMed] [Google Scholar]
- 76.Biran L. A., Bartley W. 1961. Distribution of fatty acids in lipids of rat brain, brain mitochondria and microsomes. Biochem. J. 79: 159–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cotman C., Blank M. L., Moehl A., Snyder F. 1969. Lipid composition of synaptic plasma membranes isolated from rat brain by zonal centrifugation. Biochemistry. 8: 4606–4612. [DOI] [PubMed] [Google Scholar]
- 78.Benolken R. M., Anderson R. E., Wheeler T. G. 1973. Membrane fatty acids associated with the electrical response in visual excitation. Science. 182: 1253–1254. [DOI] [PubMed] [Google Scholar]
- 79.Anderson R. E., Benolken R. M., Dudley P. A., Landis D. J., Wheeler T. G. 1974. Polyunsaturated fatty acids of photoreceptor membranes. Exp. Eye Res. 18: 205–213. [DOI] [PubMed] [Google Scholar]
- 80.Bergström S., Carlson L. A., Weeks J. R. 1968. The prostaglandins: a family of biologically active lipids. Pharmacol. Rev. 20: 1–48. [PubMed] [Google Scholar]
- 81.Needleman P., Minkes M., Raz A. 1976. Thromboxanes: Selective biosynthesis and distinct biological properties. Science. 193: 163–165. [DOI] [PubMed] [Google Scholar]
- 82.Needleman P., Kulkarni P. S., Raz A. 1977. Coronary tone modulation: Formation and actions of prostaglandins, endoperoxides and thromboxanes. Science. 195: 409–412. [DOI] [PubMed] [Google Scholar]
- 83.Dyerberg J., Bang H. O. 1978. Dietary fat and thrombosis. Lancet. 1: 152. [DOI] [PubMed] [Google Scholar]
- 84.Dyerberg J., Bang H. O., Stoffersen E., Moncada J., Vane J. R. 1978. Eicosapentaenoic acid and the prevention of thrombosis and atherosclerosis. Lancet. 2: 117–119. [DOI] [PubMed] [Google Scholar]
- 85.Salem N., Jr, Litman B., Kim H-Y., Gawrisch K. 2001. Mechanism of action of docosahexaenoic acid in the nervous system. Lipids. 36: 945–959. [DOI] [PubMed] [Google Scholar]
- 86.Spector A. A. 1999. Essentiality of fatty acids. Lipids. 34: S1–S3. [DOI] [PubMed] [Google Scholar]
- 87.Catalan J., Moriguchi T., Slotnick B., Murphy M., Greiner R. S., Salem N., Jr 2002. Cognitive deficits in docosahexaenoic acid-deficient rats. Behav. Neurosci. 116: 1022–1031. [DOI] [PubMed] [Google Scholar]
- 88.Cao D., Kavela K., Kim J., Moon H-S., Jin S. B., Lovinger D., Kim H-Y. 2009. Docosahexaenoic acid promotes neuronal development and synaptic function. J. Neurochem. 111: 510–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hong S., Gronert K., Devchand P. R., Moussignac R-L., Serhan C. N. 2003. Novel docosatrienes and 17S-resolvins generated from docosahexaenoic acid in murine brain, human blood and glial cells. Autacoids in anti-inflammation. J. Biol. Chem. 278: 14677–14687. [DOI] [PubMed] [Google Scholar]
- 90.Lukiw W. J., Cui J-C., Marcheselli V. L., Bodker M., Botkjaer A., Gotlinger K., Serhan C. N., Bazan N. G. 2005. A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J. Clin. Invest. 115: 2774–2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim H-Y., Moon H-S., Cao D., Lee J., Kevala K., Jun S. B., Lovinger D. M., Akbar M., Huang B. X. 2011. N-docosahexaenoylamide promotes development of hippocampal neurons. Biochem. J. 435: 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Holman R. T., Johnson S. B., Hatch T. F. 1982. A case of human linolenic acid deficiency involving neurological abnormalities. Am. J. Clin. Nutr. 35: 617–623. [DOI] [PubMed] [Google Scholar]
- 93.Bozian R. C., Moussavian S. N. 1982. Human linolenic acid deficiency. Am. J. Clin. Nutr. 36: 1253–1255. [DOI] [PubMed] [Google Scholar]
- 94.Holman R. T, Johnson S. B, Hatch T. F. 1982. Reply to letter by Bozian and Moussavian. Am. J. Clin. Nutr. 36: 1254–1255. [Google Scholar]
- 95.Uauy R., Hoffman D. R., Periano P., Birch D. G., Birch E. E. 2001. Essential fatty acids in visual and brain development. Lipids. 36: 885–895. [DOI] [PubMed] [Google Scholar]
- 96.Carlson S. E., Werkman S. H., Peeples J. M., Wilson W. H. 1994. Long-chain fatty acids and early visual and cognitive development in preterm infants. Eur. J. Clin. Nutr. 48 (Suppl 2): S27–S30. [PubMed] [Google Scholar]