Abstract
Lactosylceramide [LacCer; β-Gal-(1-4)-β-Glc-(1-1)-Cer] has been shown to contain very long fatty acids that specifically modulate neutrophil properties. The interactions between LacCer and proteins and their role in cell signaling processes were assessed by synthesizing two molecular species of azide-photoactivable tritium-labeled LacCer having acyl chains of different lengths. The lengths of the two acyl chains corresponded to those of a short/medium and very long fatty acid, comparable to the lengths of stearic and lignoceric acids, respectively. These derivatives, designated C18-[3H]LacCer-(N3) and C24-[3H]LacCer-(N3), were incorporated into the lipid rafts of plasma membranes of neutrophilic differentiated HL-60 (D-HL-60) cells. C24-[3H]LacCer-(N3), but not C18-[3H]LacCer-(N3), induced the phosphorylation of Lyn and promoted phagocytosis. Incorporation of C24-[3H]LacCer-(N3) into plasma membranes, followed by illumination, resulted in the formation of several tritium-labeled LacCer-protein complexes, including the LacCer-Lyn complex, into plasma membrane lipid rafts. Administration of C18-[3H]LacCer-(N3) to cells, however, did not result in the formation of the LacCer-Lyn complex. These results suggest that LacCer derivatives mimic the biological properties of natural LacCer species and can be utilized as tools to study LacCer-protein interactions, and confirm a specific direct interaction between LacCer species containing very long fatty acids, and Lyn protein, associated with the cytoplasmic layer via myristic/palmitic chains.
Keywords: lactosylceramide, photoactivable sphingolipids, C24 fatty acid chain, interdigitation
Glycosphingolipids (GSLs) are amphiphilic components of the outer layer of plasma membranes that participate in the transduction of information across the membrane by determining the lateral organization of cellular membranes and/or by modulating the function of several classes of membrane-associated proteins (1). More than 400 different oligosaccharide chains associated with GSLs have been identified, but the number of existing GSL molecular species is at least 10-fold higher, due to the heterogeneity in their ceramide moiety (2, 3). These physicochemical properties suggest that the molecular varieties and expression patterns of GSLs reflect their biological functions in each organism.
Although GSL-enriched lipid rafts have been implicated in a number of important membrane events (4–6), the molecular mechanisms by which GSLs mediate cell functions remain unclear. One major issue is the association of GSLs with signal transducer molecules localized to the cytosolic side of the plasma membrane. Over the past several years, we have attempted to clarify this issue using cells of the human neutrophilic lineage (7). Lactosylceramide [LacCer; β-Gal-(1-4)-β-Glc-(1-1)-Cer] species containing C24:0 and C24:1 fatty acids, or C24-LacCer, are components of plasma membrane lipid rafts in human neutrophils and act as pattern recognition receptors responsible for chemotaxis, phagocytosis, and superoxide generation (5, 7, 8). These functions are highly dependent on the Src family kinase, Lyn. The interaction of microorganisms with LacCer activates Lyn, resulting in its phosphorylation, a reaction considered the first step in the Lyn-mediated immunological functions of human neutrophils (9). These events can be easily reproduced experimentally by treating neutrophils with anti-LacCer antibodies.
The LacCer contents in lipid rafts of human promyelocytic leukemia HL-60 cells induced to differentiate into neutrophilic cells by DMSO are similar to those of human neutrophils (7). However, the LacCer on plasma membranes of these DMSO-treated human promyelocytic leukemia cells (D-HL-60 cells) is more enriched in shorter fatty acids than the LacCer of neutrophils (7). Treatment of D-HL-60 cells with formyl peptide (fMLP; N-formylmethionine-leucine-phenylalanine), but not anti-LacCer monoclonal antibody, was found to induce Lyn phosphorylation and chemotactic and superoxide-generating activities. Nevertheless, treatment of D-HL-60 cells, which consisted mainly of C16:0-LacCer and had quite low contents of C24-fatty acid chains, with anti-LacCer antibodies induced chemotactic and superoxide generating activities following cell loading with exogenous C24-LacCer (7). Lyn knockdown by siRNA completely abolished the effect of C24:1-LacCer loading on the LacCer-mediated functions of D-HL-60 cells. These observations suggested that C24-LacCer is specifically required for neutrophil properties and that long hydrophobic chains on LacCer may be responsible for their direct interaction with Lyn, via interdigitation with the acyl chains that allow the association of Lyn with the cytosolic membrane layer.
Photolabeling experiments have been performed using radiolabeled and photoactivable lipids (10). A GM1 ganglioside containing tritium-labeled sialic acid and with an azide at the end of the ceramide moiety was the first radiolabeled ganglioside (11), followed by the synthesis of other photoactivable gangliosides (12). Upon addition to cultured cells, these exogenous ganglioside derivatives become associated with the cells and undergo metabolic processing in ways that closely resemble those observed for natural compounds, suggesting that they may be utilized as tools to study the interactions between GSLs and proteins. Photolabeling with photoactivable GM3 provided evidence for the specific role of GM3 in reducing insulin receptor-mediated processes in adipocytes (13) and showed that, following its interaction with CD9, GM3 downregulated tumor cell motility and malignancy (14). GM1 was found to interact with several membrane and cytosolic proteins (15–18), two of which were characterized as caveolin-1 (16) and tubulin (17), and that GM3, GM1, and GD1b interacted with TAG-1 in the plasma membrane lipid rafts of neuronal cells (19, 20).
This study was designed to show a direct interaction between C24-LacCer and Lyn. Cells were photolabeled with tritium-labeled analogs of LacCer having the photoactivable nitrophenylazide group at the end of the acyl chain. Under illumination, nitrophenylazide generates the highly reactive nitrene moiety, which can covalently bind to nearby molecules.
MATERIALS AND METHODS
Mouse anti-LacCer monoclonal IgMs T5A7 (5) and Huly-m13 were purchased from Ancell (Bayport, MN). Rabbit anti-phospho-Lyn IgG (Y396) was from Abcam (Cambridge, UK), anti-Lyn IgG was from Santa Cruz Biotechnology (Santa Cruz, CA), and anti-Gαi IgG was from Cell Signaling (Beverley, MA). Phycoerythrin-conjugated anti-human CD11b and mouse IgGIK were from eBioscience (San Diego, CA). All other monoclonal antibodies were from BD Biosciences (San Jose, CA). DMSO, BSA, and fMLP were purchased from Sigma-Aldrich Japan (Tokyo, Japan). LacCer species with homogeneous fatty acid moieties were synthesized as described (7).
HL-60 cells were maintained in culture in RPMI-1640 medium supplemented with 10% FBS. To induce differentiation into D-HL-60 cells, HL-60 cells were cultured for 8 days in the presence of 1.3% DMSO, with differentiation confirmed by CD11b expression using flow cytometry analysis (21, 22).
Synthesis of tritium-labeled photoactivable LacCer, [3H]LacCer(N3)
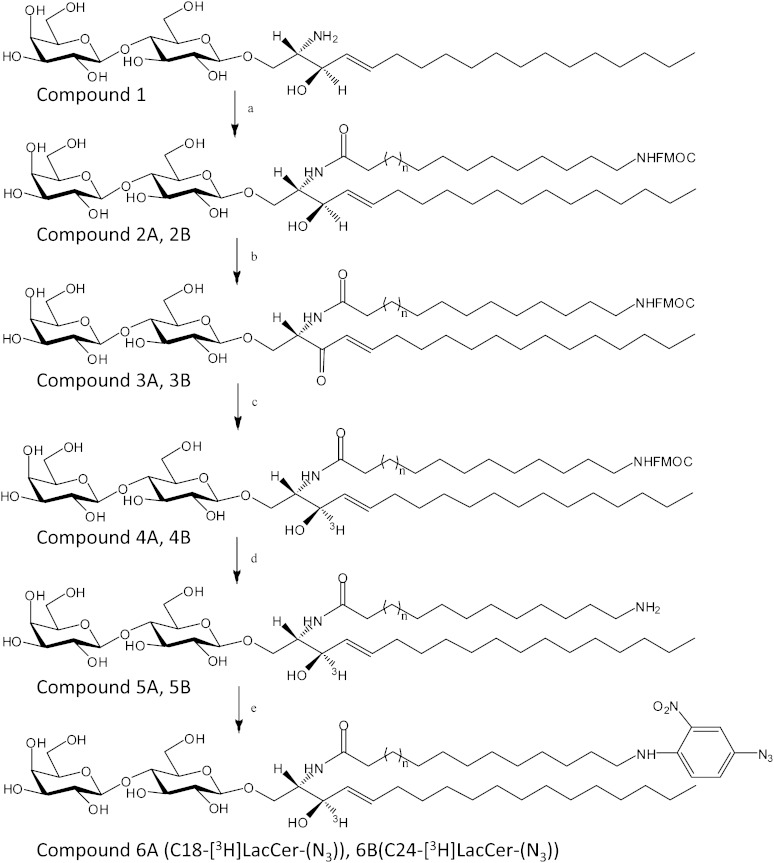
Tritium labeled and photoactivable LacCer containing an azide group at the end of the acyl chain with total lengths corresponding to stearic acid, C18-[3H]LacCer(N3), and lignoceric acid, C24-[3H]LacCer-(N3), were synthesized according to the scheme shown in Fig. 1. C18-[3H]LacCer-(N3) was synthesized from commercially available 12-aminododecanoic acid (11, 12), whereas C24-[3H]LacCer-(N3) was synthesized from 18-amino-octadecanoic acid, compound 16, which had been synthesized according to the scheme shown in Fig. 2.
Fig. 1.
Scheme of the reactions for the synthesis of tritiated and photoactivable LacCer. PentafluorophenolC12NHFMOC or pentafluorophenolC18NHFMOC, hydroxybenzotriazole, Bu3N, DCM/MeOH (a); DDQ, dioxane, 50°C (b); [3H]NaB3H4, MeOH (c); aqueous NH3 (d); Et3N, 4-F-3-NO2-phenilazide, DMF (e). A, n = 1; B, n = 6.
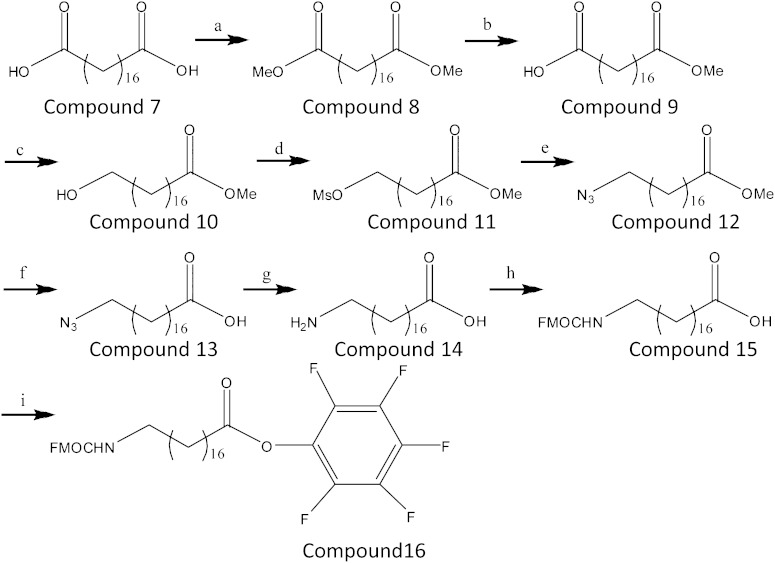
Fig. 2.
Scheme of the reactions for the synthesis of ω-aminooctadecanoic acid. CH3OH, H2SO4 (a), reflux; Ba(OH)2, MeOH, 40°C (b); BH3/THF, −20°C to room temperature (c); MsCl, Et3N, CH2Cl2, 0°C to room temperature (d); NaN3, DMF, 45°C (e); KOH, MeOH (f); Pd/C, 5% CH3COOH in MeOH (g); 9-fluorenylmethyl chloroformate, 10% Na2CO3 (h); 2-chloro-1-methylpyridinium iodide, pentafluorophenol, Bu3N, CH2Cl2, reflux (i).
Synthesis of 18-amino-octadecanoic acid (see Fig. 2).
To 8 ml of a suspension of 0.75 M octadecanedioic acid (compound 7) in CH3OH was added 104 μl of 98% H2SO4, and the reaction mixture was stirred overnight under reflux. The mixture was concentrated under vacuum, diluted with ethyl ether, and washed three times with saturated Na2CO3 solution. The organic phase was dried over Na2SO4 end evaporated under reduced pressure, resulting in a 97% yield of compound 8 (23).
Compound 8 (5.8 mmol) was added to a solution of 0.07 M Ba(OH)2 in dry CH3OH (85 ml) and the mixture was stirred for 24 h at 40°C and filtered under reduced pressure. The precipitate was washed with ethyl ether and dissolved in water (H2O). The solution was acidified with HCl to pH 3, and extracted twice with CH2Cl2. The solvent was evaporated under vacuum and the residue purified by flash chromatography and eluted with an 8:2 mixture (v/v) of petroleum ether:ethyl acetate, resulting in compound 9, with a 52% yield. BH3/THF complex (10 ml, 10 mmol, 1.0 M solution in THF) was added drop wise over 20 min to a solution of compound 9 (3.7 mmol) in anhydrous THF (20 ml) at −20°C. The solution was stirred for 10 min, allowed to warm to room temperature, stirred for 24 h, and quenched with H2O (50 ml) at 0°C. One gram of solid K2CO3 was added after 10 min and the mixture was extracted with ethyl ether (3 × 50 ml). The combined organic layers were dried over Na2SO4 and concentrated under reduced pressure, resulting in compound 10, with a yield of 81% (24).
To a solution of compound 10 (3 mmol) in dry CH2Cl2 (15 ml) was added 850 μl of triethylamine (Et3N), followed by the dropwise addition of methane sulfonyl chloride (MsCl; 4.2 mmol, 326 μl) at 0°C. After allowing it to stand for 30 min, the mixture was stirred for 4 h at room temperature, diluted in 50 ml CH2Cl2, and washed three times with 50 ml H2O. The organic layer was dried over Na2SO4 and evaporated under reduced pressure, resulting in compound 11, with a yield of 93%.
Compound 11 (4.8 mmol) was dissolved in 15 ml anhydrous dimethylformamide (DMF), to which 15 mmol of NaN3 were added. The solution was stirred overnight at 45°C, cooled at room temperature, and concentrated. The crude material was dissolved in 50 ml CH2Cl2 and washed three times with 50 ml H2O. The organic phase was dried over Na2SO4 and evaporated under reduced pressure, resulting in compound 12, with a yield of 58% (25).
Compound 12 (2.8 mmol) was dissolved in 0.5 M KOH in CH3OH (45 ml) and stirred for 48 h. Its pH was adjusted to pH 3 with 1 M HCl, after which it was concentrated and extracted with ethyl ether (3 × 50 ml). The combined organic phases were dried over Na2SO4 and concentrated, resulting in compound 13, at a yield of 71%.
Compound 13 (2 mmol) was dissolved in 5% CH3COOH in CH3OH (5 ml) and a catalytic amount of palladium on charcoal (6 mg) was added in an argon atmosphere. The reaction mixture was hydrogenated at atmospheric pressure for 20 h. The mixture was filtered and the catalyst washed with 5% CH3COOH in CH3OH. The solvent was evaporated, resulting in compound 14, at a yield of 80%.
Protection and activation of 12-aminododecanoic acid and of 18-amino-octadecanoic acid (compound 14).
Protection of (15) and activation of compound 16 of ω-amino acids were as previously described (12).
N-acylation of lactosylsphingosine with activated ω-amino acids, pentafluorophenol-C12NH-FMOC and pentafluorophenol-C18NH-FMOC.
pentafluorophenol-C12NH-FMOC or pentafluorophenol-C18NH-FMOC, (compound 16, 17 μmol), in anhydrous CH2Cl2 (0.5 ml), 1-hydroxybenzotyriazole (19 μmol), and tributylamine (Bu3N; 38 μmol, 9 μl) were added to a solution of lactosylsphingosine, (compound 16, 16 μmol), in anhydrous CH3OH (1 ml). After vigorous stirring for 75 min at room temperature, the reaction mixture was dried and the residue purified by flash chromatography, equilibrated, and eluted with CHCl3-methanol (MeOH)-H2O, 60:25:4 by volume, resulting in compound 2A or 2B, each at a yield of 80%.
Oxidation of 2 at the 3-position of sphingosine.
Compound 2A or 2B (12 μmol) was suspended in 3% 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) in dioxane (10 ml) and incubated at 50°C for 40 h with vigorous stirring in a screw-capped tube. The reaction mixture was evaporated to dryness under vacuum, and excess DDQ was eliminated by flash chromatography with acetone-CH2Cl2-CH3OH, 47:2:1 by volume. Fractions containing compounds 3A or 3B were concentrated and the residue was purified by flash chromatography with CHCl3-MeOH-2-propanol-H2O, 70:5:25:3 by volume, resulting in purifying pure compounds at yields of 80%.
Tritiation with [3H]NaBH4.
Solid [3H]NaBH4 (50 mCi) was added to compound 3A or 3B (1.9 μmol) dissolved in MeOH (1 ml) and incubated at room temperature for 48 h with vigorous stirring. The reaction mixture was dried, and the tritiated compound 4A or 4B with a 2S,3R configuration was purified by flash chromatography with CH2Cl2-MeOH-2-propanol-H2O, 70:10:20:3 by volume.
Deprotection.
Tritiated compound 4A or B (1.7 μmol) was solubilized in 32% aqueous ammonia (2 ml) in a screw-cap flask for 24 h at room temperature with vigorous stirring. The reaction mixture was dried and the residue was purified by column chromatography with CH2Cl2-MeOH-2 N NH3, 60:35:4 by volume, resulting in compound 5A or 5B, at a yield of 80%.
Azide labeling.
This final reaction was conducted in a dark room. Radioactive compound 5A or 5B (10 μmol/ml) was dissolved in anhydrous DMF. An equimolar quantity of Et3N and a 2-fold molar quantity of 4-F-3-NO2-phenylazide, dissolved in ethanol, were added and the mixture was stirred overnight at 80°C. The reaction mixture was concentrated and compound 6A or compound 6B was purified by flash chromatography with CH2Cl2-MeOH-2-propanol-H2O, 70:10:20:3 by volume. Fractions containing the homogeneous tritiated and photoactivable LacCer were immediately solubilized in MeOH and stored at 4°C in a dark glass bottle.
Treatment of cells with [3H]LacCer-(N3)
All procedures before exposure to UV light were performed under red safelight (11–13, 15, 18–20, 26–28). To load D-HL-60 cells with [3H]LacCer-(N3), 10 ml aliquots of D-HL-60 cells (2 × 107 cells/ml) in Dulbecco’s PBS were incubated with 0.25 μg LacCer and 0.25 μg [3H]LacCer-(N3) (final concentrations 0.5 μg/ml) (7, 12) for 30 min at 20°C with gentle shaking (30 strokes/min). [3H]LacCer-(N3) was diluted with LacCer to reduce self-quenching after illumination. After incubation, the cells were washed once with 10 ml PBS containing 0.1% BSA to remove unincorporated LacCer and membrane adherent aggregates of LacCer (7). The cells were washed twice with 10 ml of cold PBS and maintained in 15 ml of cold PBS. Cells were illuminated for 45 min under UV light (λ = 360 nm) on ice.
After photoactivation, the cells were suspended in lysis buffer [1% Triton X-100, 10 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 1 mM diisopropyl fluorophosphate (DFP), 1 mM Na3VO4, 1 mM PMSF, and 1/20 (v/v) cOmplete (Roche Diagnostics, Mannheim, Germany)] at 4°C for 20 min. Cell lysates were gently Dounce homogenized (70 strokes) and centrifuged (1,300 rpm for 5 min) to remove nuclei and cellular debris. The post nuclear supernatant (PNS) was removed and transferred to new tubes.
After loading with [3H]LacCer-(N3), cell viability was assessed by trypan blue exclusion (29, 30). Cell viabilities before and after loading cells were >90%.
Phagocytosis assays
[3H]LacCer-(N3) loaded D-HL-60 cells were incubated with Alexa 647-conjugated non-opsonized zymosan at a concentration of 10 particles per cell for 45 min at 37°C in DMEM/F12. After incubation, the cells were washed with ice-cold PBS and fixed with 2% paraformaldehyde in PBS for 20 min on ice. At least 200 cells in 10 fields per sample were observed using a Leica TCS-SP2 confocal microscope equipped with a Plan-Apochromat ×100 oil differential interference contrast objective (Leica Microsystems, Wetzlar, Germany). Phagocytic index was defined as the percent of cells positive for ingestion multiplied by the average number of phagocytosed particles per cell (7).
Determination of Lyn phosphorylation
All procedures before illumination were performed under red safelight. Culture dishes (10 cm) were coated with 5 ml of 10 μg/ml anti-LacCer mAb T5A7 or normal IgM solutions as described (5). To each dish were added 5 ml of 2.5 × 106 [3H]LacCer-(N3) loaded D-HL-60 cells per ml PBS containing 1 mM MgSO4 and 1 mM CaCl2, and the dishes were incubated for 5 min at 37°C. Reactions were terminated by placing on ice. The cells were illuminated for 45 min under a UV lamp to induce photo-activation. The cells were washed and solubilized, the lysates were cleared by centrifugation and the supernatants were subjected to 8.5% SDS-PAGE and blotted onto polyvinylidene difluoride (PVDF) membranes. To measure Lyn phosphorylation, the blots were incubated with anti-phospho-Lyn IgG and then with HRP-conjugated secondary antibody, followed by detection of phosphorylated Lyn using SuperSignalTM reagent (Pierce Chemical Company, Rockford, IL). The membranes were subsequently stripped of antibody by incubation with stripping buffer [62.5 mM Tris-HCl (pH 6.8), 100 mM β-mercaptoethanol, 2% SDS] for 30 min at 55°C, and then reprobed with anti-Lyn IgG.
Preparation of detergent-resistant membrane fractions by sucrose gradient centrifugation
PNS fractions obtained after illumination of [3H]LacCer-(N3) loaded cells were mixed with an equal volume of 85% of sucrose (w/v) in buffer A [10 mM Tris buffer HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 1 mM Na3VO4], placed at the bottom of a discontinuous sucrose concentration gradient (30–5%) in the same buffer, and centrifuged for 17 h at 200,000 g at 4°C. After ultracentrifugation, 12 fractions were collected, starting at the top of the tube. The light-scattering band, corresponding to detergent-resistant membrane (DRM), was located at the interface between 5% and 30% sucrose and corresponded to fraction 5. The entire procedure was performed at 0–4°C in ice immersion (7, 29, 31). In some experiments, the DRM fractions were diluted with 10 vol of buffer A, and then concentrated at 200,000 g for 1 h at 4°C. The resulting pellets were subjected to SDS-PAGE and Western blotting analysis.
Immunoprecipitation experiments
The lysates, equivalent to 1.2 × 107 cells, or DRM fractions from D-HL-60 cells loaded with C18-[3H]LacCer-(N3) and C24-[3H]LacCer-(N3), were diluted with 10 vol of immunoprecipitation buffer [50 mM HEPES (pH 7.5), 1% Triton X-100, 150 mM NaCl, 2 mM Na3VO4, 10 mM NaF, with 1/20 cOmplete] and precleared by incubation with 30 μl rat anti-mouse IgM/IgG IgG-bound Dynabeads (Invitrogen) for 1 h at 4°C. The supernatants were subsequently incubated overnight at 4°C with 5 μg anti-LacCer IgM Huly-m13 or anti-Lyn IgG, followed by incubation for 4 h at 4°C with 30 μl rat anti-mouse IgM/IgG IgG-bound Dynabeads; as controls, the supernatants were incubated with normal mouse IgM or IgG. The immunoprecipitates were washed three times with immunoprecipitation buffer, denatured under nonreducing conditions, separated on 7.5% polyacrylamide gels, and transferred onto PVDF membranes.
In some experiments, the immunoprecipitated samples were dissolved in 1.0 ml RIPA buffer [30 mM HEPES (pH 7.4), 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, 5 mM EDTA, 1 mM Na3VO4, 50 mM NaF, 1 mM PMSF, 1/20 (v/v) CompleteTM] and immunoprecipitated as above using RIPA buffer. The presence of Lyn protein was assessed by immunoblotting with specific antibody, followed by reaction with secondary HRP-conjugated antibody (7, 12, 13, 32).
Analysis of protein patterns
The concentrated DRM fractions prepared from illuminated C18-[3H]LacCer-(N3) and C24-[3H]LacCer-(N3) loaded cells were denatured under nonreducing conditions, separated on 7.5% polyacrylamide gels, and transferred onto PVDF membranes. Proteins cross-linked to tritium-labeled LacCer derivative were detected by digital autoradiography. The presence of Lyn and Gαi was assessed by immunoblotting with specific antibodies, followed by reaction with secondary HRP-conjugated antibody (11, 12, 15, 18, 19, 27, 30, 33).
Other experimental procedures
1H and 13C NMR spectra were recorded with a Bruker AVANCE-500 spectrometer at a sample temperature of 298 K. NMR spectra were recorded in CDCl3 or CD3OD and calibrated using the TMS signal as internal reference.
Mass spectrometric analyses were performed in positive or negative ESI-MS. MS spectra were recorded on a Hewlett-Packard HP-5988-A or a Thermo Quest Finnigan LCQ™ DECA ion trap mass spectrometer, equipped with a Finnigan ESI interface; data were processed by Finnigan Xcalibur software system.
All reactions were monitored by TLC on silica gel 60 F-254 plates (Merck).
Flash column chromatography was performed on silica gel 60 (230–400 mesh, Merck).
Radioactivity associated with cells and cell fractions was determined by liquid scintillation counting. Digital autoradiography of the PVDF membranes was performed with a Beta-Imager 2000 (Biospace, Paris).
RESULTS
Preparation of C18-[3H]LacCer-(N3) and C24-[3H]LacCer-(N3)
The two LacCer species, C18-[3H]LacCer-(N3) and C24-[3H]LacCer-(N3), were prepared using the strategy previously developed for the synthesis of photoactivable gangliosides, modified to introduce tritium at position 3 of sphingosine. A specific strategy was also developed to synthesize ω-amino-octadecanoic acid. The reaction schemes are shown in Figs. 1 and 2.
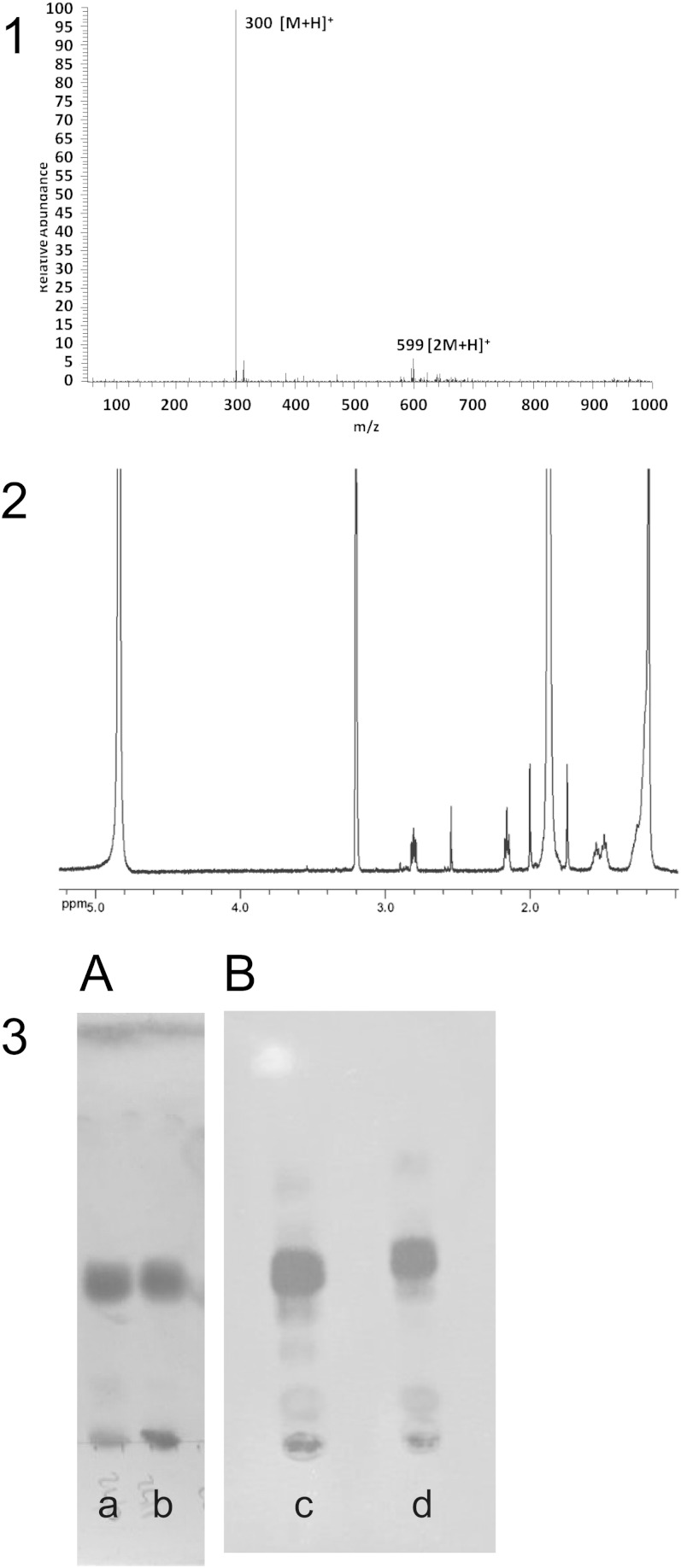
All together, the yield of the GSL synthetic process was around 50%, which was very high considering the final specific radioactivity of 1.5 Ci/mmol. Figure 3 shows the analytical controls as MS and NMR spectra of the synthesized 18-amino-octadecanoic acid (Fig. 2), confirming its structure. Figure 3 also shows the TLC of the two final compounds, C18-[3H]LacCer-(N3) and C24-[3H]LacCer-(N3), showing that both were 98% homogeneous.
Fig. 3.
Analytical controls. 1: ESI-MS (positive-ion mode): m/z = 300 [M+H]+; 599 [2M+H]+ of the ω-amino-octadecanoic acid. 2: 1H NMR (500.13 MHz, MeOD, ppm) 1.18–1.27 [multiplet (m), 26H, alkyl chain]; 1.46–1.59 (m, 4H, C17, C3); 2.17 [triplet (t), 2H; C2]; 2.82 (t, 2H; C18). 3: colorimetric (A) and radioimaging (B) TLC of standard C18-LacCer; standard C24-LacCer (a), C18-[3H]LacCer(N3) (b), and C24-[3H]LacCer(N3) (c). TLCs were developed with the solvent system CH3Cl-CH3OH-2-propanol-H2O, 70:15:15:3 by volume (d).
D-HL-60 cell viability after administration of C18-[3H]LacCer-(N3) and C24-[3H]LacCer-(N3)
Cells were administered the photoactivable species of LacCer together with the natural species, for a total of 0.5 μg/ml of cells. Photoactivable LacCer was diluted with the natural compound to reduce self-quenching at the moment of illumination. Trypan blue assays showed that the viability of untreated cells was 96.33 ± 1.6%; when loaded with C18-[3H]LacCer-(N3) and C24-[3H]LacCer-(N3), they had a viability of 98.33 ± 0.3% and 96.00 ± 1.5%, respectively.
Effects of C18-[3H]LacCer-(N3) and C24-[3H]LacCer-(N3) on the phagocytosis of microorganisms by D-HL-60 cells
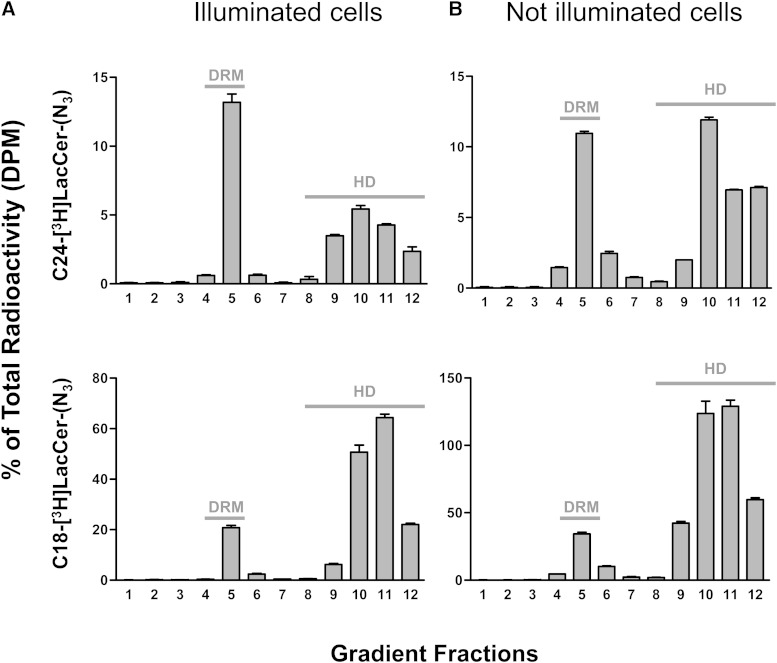
LacCer has been shown to be present in the Triton-X-100 insoluble membrane fractions of neutrophils and D-HL-60 cells (5, 7). After administration of exogenous C18-[3H]LacCer-(N3) and C24-[3H]LacCer-(N3), the cells were illuminated under UV light, harvested, and lysed in lysis buffer containing Triton X-100. The lysates were loaded onto discontinuous sucrose gradients and ultracentrifuged; 12 fractions were collected from the top of each tube and their amounts of radioactivity analyzed. Figure 4 shows that, following loading with both C18-[3H]LacCer-(N3) and C24-[3H]LacCer-(N3), part of the radioactivity was associated with the low density fraction (DRM), corresponding to fraction 5, which is resistant to detergent solubilization. Radioactivity associated with high density fractions 9–12 was due to the photoactivable LacCer molecules peripherally associated with membrane components, but not correctly inserted into the membrane bilayer, or to their catabolites formed after internalization and degradation (12). Thus, this radioactivity does not reflect a physiological interaction.
Fig. 4.
Distribution of radioactivity in sucrose gradient fractions of D-HL-60 cells after incubation with photoactivable and radioactive LacCer derivatives. Cells were incubated in the presence of a 0.25 μg LacCer plus 0.25 μg [3H]LacCer-(N3) as described in the Materials and Methods section. Cells were (A) or were not (B) illuminated for 45 min under UV light to allow cross-linking between LacCer and cellular components. The cells were subsequently subjected to sucrose gradient ultracentrifugation to prepare plasma membrane microdomains (DRM fraction). Twelve fractions were collected from the top of the tube, with fraction 5 corresponding to the DRM fractions and fractions 9–12 corresponding to the high density (HD) fractions. The radioactivity associated with each fraction was determined by liquid scintillation counting. Data are expressed as percentages of total radioactivity. Data are the mean ± SD of three independent experiments.
To determine whether the distribution of radioactivity was dependent on the process of illumination, gradient fractions were also prepared from nonilluminated cells. Figure 4 shows that the illumination process did not significantly alter the distribution of radioactivity.
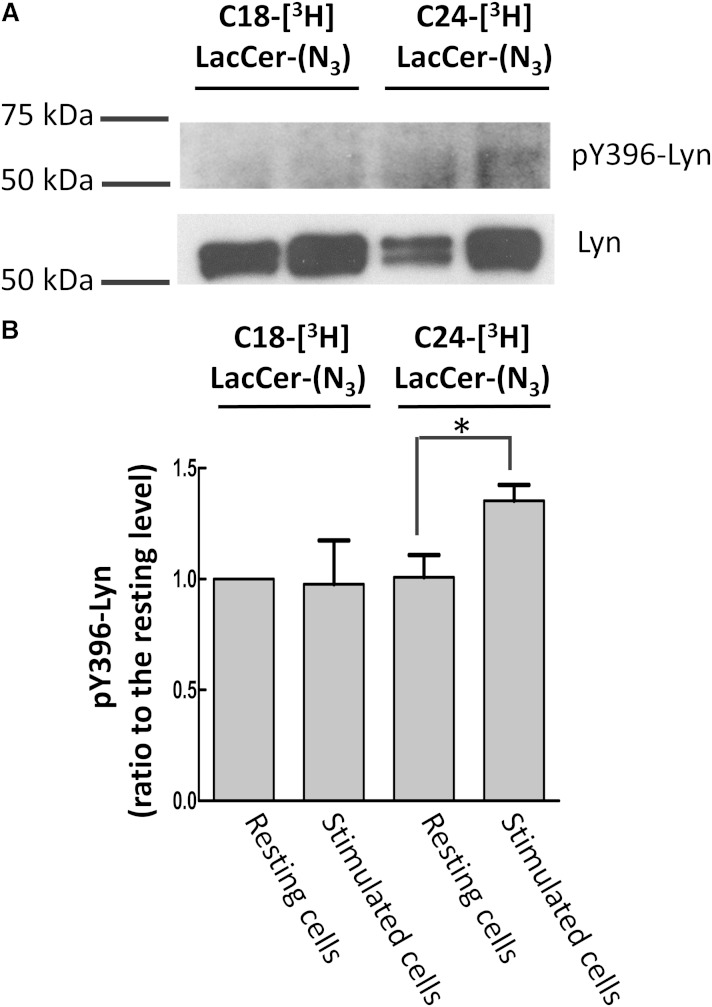
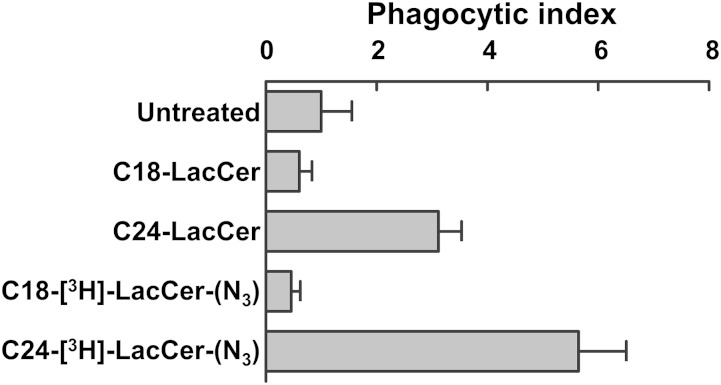
To verify whether the two LacCer analogs maintain the biological properties of the corresponding natural LacCer species, we assessed the phagocytosis activity of D-HL-60 cells loaded with C18-[3H]LacCer-(N3) and C24-[3H]LacCer-(N3). The first step of the signaling process ultimately leading to phagocytosis is represented by Lyn phosphorylation. Thus, D-HL-60 cells loaded with [3H]LacCer-(N3), but not illuminated, were incubated in the presence of anti-LacCer T5A7 (stimulated cells) or normal IgM (resting cells) for 5 min at 37°C. Cell lysates were immunoprecipitated with mouse anti-Lyn IgG or normal mouse IgG, and the immunoprecipitates were separated by SDS-PAGE and blotted onto PVDF membranes, which were probed with rabbit anti-pY369-Lyn IgG (P-Lyn) and rabbit anti-Lyn IgG. Figure 5 clearly shows that Lyn was phosphorylated only in cells loaded with C24-[3H]LacCer-(N3), whereas Fig. 6 shows that D-HL-60 cells loaded with C24-[3H]LacCer-(N3), but not illuminated, maintained the property of cells loaded with natural C24-LacCer and were capable of phagocytosing microorganisms. In contrast, phagocytosis did not occur when the cells were loaded with C18-[3H]LacCer-(N3), similar to findings with natural C19-LacCer (7). These results confirm that LacCer requires a long fatty acyl chain to activate Lyn and induce the phagocytic process and suggest that the photoactivable LacCer derivative is suitable for studying the properties of the natural compound.
Fig. 5.
Phosphorylation of Lyn after [3H]LacCer-(N3) loading of D-HL-60 cells. D-HL-60 cells loaded with 0.25 μg LacCer plus 0.25 μg [3H]LacCer-(N3) for 30 min at 20°C, without illumination, were incubated in the presence of anti-LacCer T5A7 (stimulated cells) or normal IgM (resting cells) for 5 min at 37°C (8). The cell lysates were immunoprecipitated with mouse anti-Lyn IgG or normal mouse IgG. The immunoprecipitates were separated by SDS-PAGE and blotted onto PVDF membranes, which were probed with rabbit anti-pY369-Lyn IgG (p-Lyn). To evaluate the recovery of Lyn, the blotted membrane was probed with rabbit anti-Lyn IgG. A: Blots shown are representative of three independent experiments. B: The ratio of the band intensity of phosphorylated Lyn to total Lyn for each lane was calculated. Data are presented as ratios relative to the phosphorylation rate of the resting cells loaded with C18-[3H]LacCer-(N3) (without anti-LacCer IgM), and are expressed as the mean ± SD of three independent experiments. *P < 0.05.
Fig. 6.
Phagocytosis index after [3H]LacCer-(N3) loading of D-HL-60 cells. D-HL-60 cells were loaded with 0.25 μg LacCer plus 0.25 μg [3H]LacCer-(N3) for 30 min at 20°C, without illumination, and phagocytosis assays were performed. Each bar shows the mean of 20 different fields of two independent experiments.
Coimmunoprecipitation of C24-[3H]LacCer-(N3) and Lyn
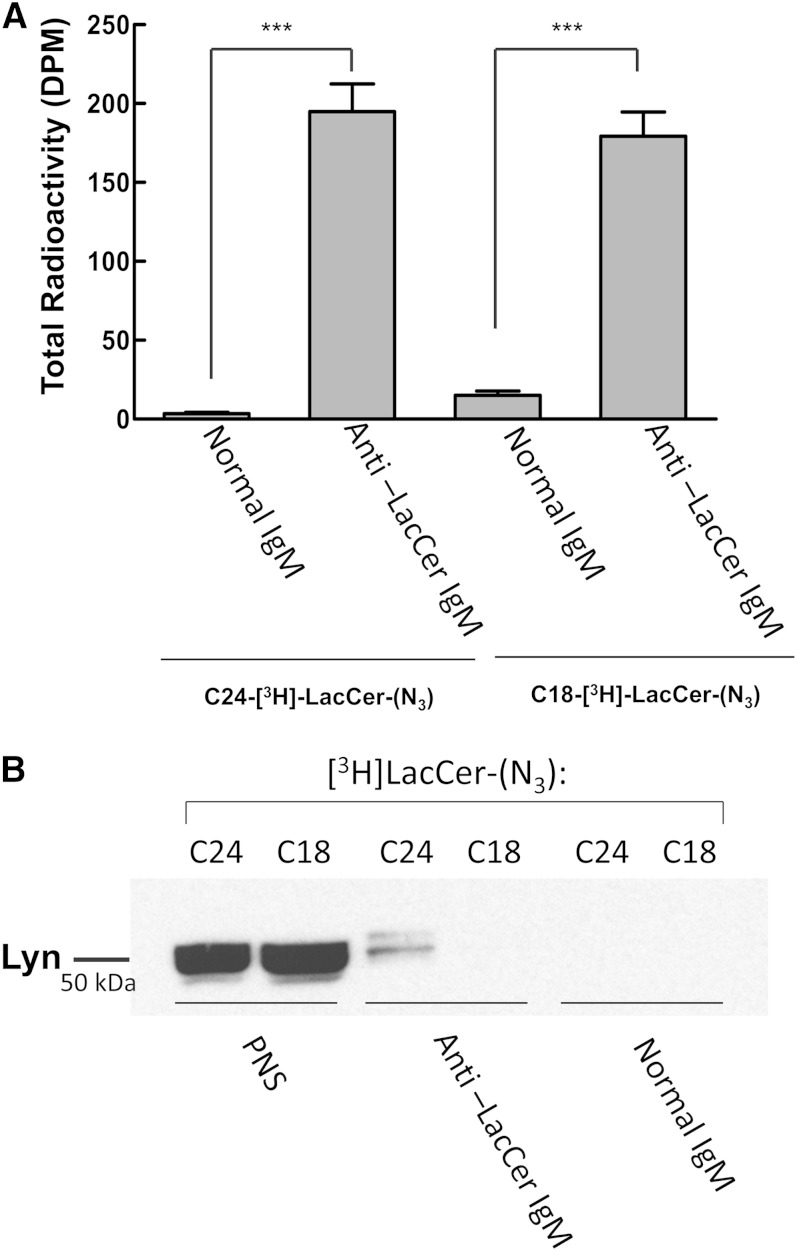
C24-LacCer and Lyn have been shown to belong to the same membrane domain, with both immunoprecipitated by anti-Lyn and anti-LacCer antibodies (5, 7). To determine whether C24-[3H]LacCer-(N3) has the same properties as native C24-LacCer, we attempted immunoprecipitation with anti-LacCer and anti-Lyn antibodies. We first performed immunoprecipitation experiments using anti-LacCer antibody on PNS prepared from D-HL-60 cells loaded with C18-[3H]LacCer-(N3) and C24-[3H]LacCer-(N3), followed by SDS-PAGE of the immunoprecipitates and immunoblotting with anti-Lyn antibody. Figure 7 shows that similar amounts of radioactivity were present in the immunoprecipitates from D-HL-60 cells loaded with the C24 and C18 derivatives (Fig. 7A). However, Lyn was present in immunoprecipitates from cells loaded with C24-[3H]LacCer-(N3), but not from cells loaded with C18-[3H]LacCer-(N3) (Fig. 7B). These results indicate that only LacCer containing very long fatty acids is present in the same membrane domain as Lyn.
Fig. 7.
Anti-LacCer immunoprecipitation from PNS. PNS from D-HL-60 cells loaded with [3H]LacCer-(N3) were immunoprecipitated with mouse anti-LacCer antibody Huly-m13 or normal mouse IgM. A: The radioactivity associated with the immunoprecipitates was determined by liquid scintillation counting. Data are expressed as percentages of total radioactivity. Data are the mean of three different experiments. ***P < 0.0005. B: The immunoprecipitates were analyzed by SDS-PAGE/immunoblotting using rabbit anti-Lyn IgG. The immunoblot shown is representative of three different experiments.
To confirm these findings, we performed immunoprecipitation from DRM fractions with anti-Lyn and under disaggregating conditions. Figure 8 shows that Lyn was immunoprecipitated from DRM fractions prepared from cells loaded with both C18-[3H]LacCer-(N3) and C24-[3H]LacCer-(N3), whereas radioactivity was present only in the latter immunoprecipitate.
Fig. 8.
Anti-Lyn immunoprecipitation from D-HL-60 DRM fractions. DRM fractions from cells loaded with [3H]LacCer-(N3) were immunoprecipitated with mouse anti-Lyn IgG or normal mouse IgG. A: The immunoprecipitates were separated by SDS-PAGE and blotted onto PVDF membranes, which were probed with rabbit anti-Lyn IgG. The immunoblot shown is representative of three different experiments. B: The radioactivity associated with the immunoprecipitates was determined by liquid scintillation counting. Data are expressed as percentages of total radioactivity. Data are the mean of three different experiments. ***P < 0.0005.
Identification of LacCer-protein complexes
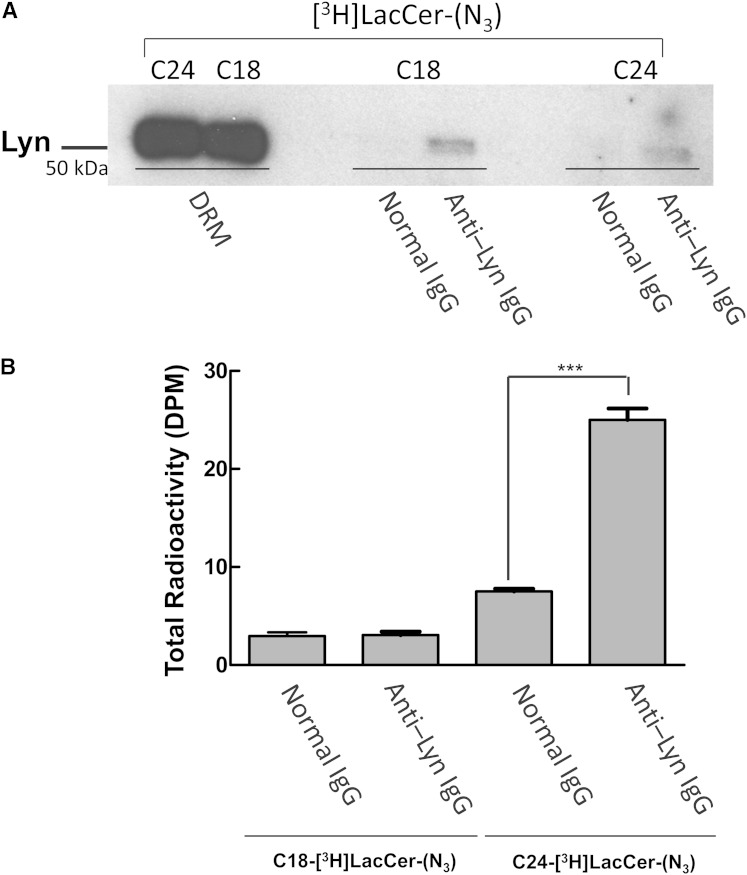
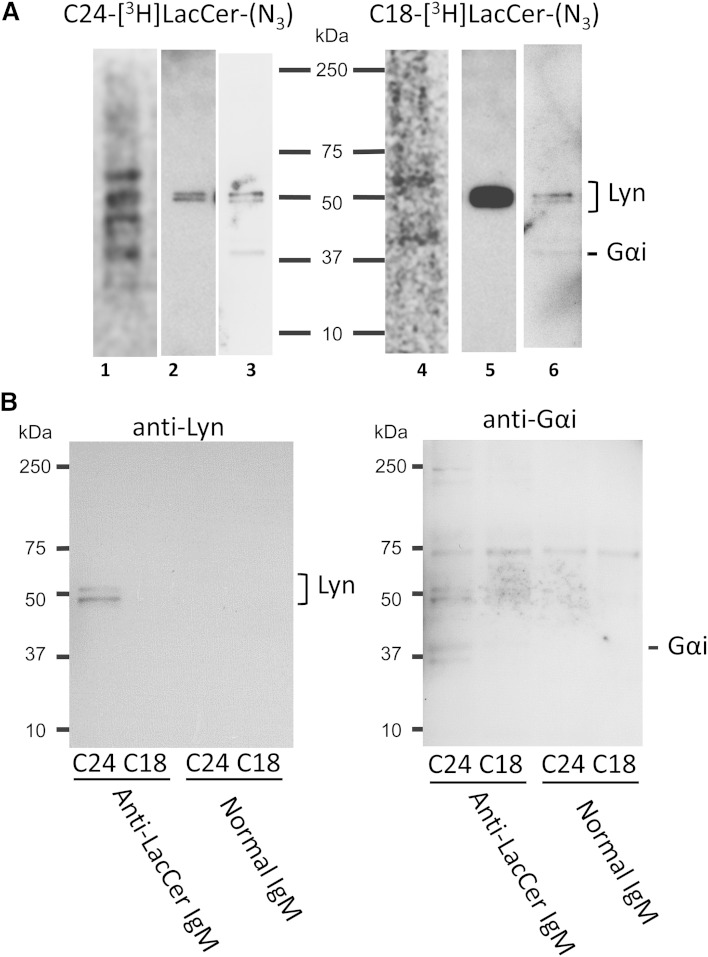
LacCer-enriched lipid raft-mediated neutrophil activities have been shown to involve Src family kinase-, PI-3 kinase-, and heterotrimeric G-protein-mediated signal transduction pathways (5, 8). To better identify the radioactive LacCer-protein complexes, proteins from DRM fractions were concentrated, separated by SDS-PAGE, blotted onto PVDF membranes, and analyzed by digital radioimaging (Fig. 9). Assays of D-HL-60 cells loaded with C24-[3H]LacCer-(N3) showed a few radioactive bands within a wide range of molecular masses, ranging from 20 kDa to 75 kDa, and included bands of 41 kDa and 56 kDa (Fig. 9A), suggesting that specific proteins are present in the membrane environment of LacCer species containing very long fatty acids. After radioimaging, the PVDF membranes were incubated with antibodies to Lyn and Gαi. These two antibodies bound specifically to the bands at 56 kDa and 41 kDa, respectively, suggesting that LacCer may directly interact with Lyn and Gαi. Indeed, anti-LacCer IgM coimmunoprecipitated Lyn and Gαi from DRM of D-HL-60 cells loaded with C24-[3H]LacCer-(N3), but not C18-[3H]LacCer-(N3) (Fig. 9B).
Fig. 9.
Identification of the LacCer-protein complexes. A: Proteins cross-linked with C24-[3H]LacCer-(N3) (lane 1) and C18-[3H]LacCer-(N3) (lane 4) prepared from DRM fractions were concentrated by centrifugation (1 h, 200,000 g, 4°C) and separated by 7.5% SDS-PAGE, blotted onto PVDF membranes, and visualized by digital autoradiography for 96 h. The same PVDF membranes were sequentially visualized by Western blotting using rabbit anti-Lyn (lane 2 and lane 5) and anti-Gαi (lane 3 and lane 6) IgGs. The results shown are representative of three independent experiments. B: DRM fractions from D-HL-60 cells loaded with [3H]LacCer-(N3) were immunoprecipitated with anti-LacCer IgM Huly-m13 or normal mouse IgM. The immunoprecipitants were separated by 7.5% SDS-PAGE, blotted onto PVDF membrane, and sequentially visualized by Western blotting using rabbit anti-Lyn and anti-Gαi IgGs.
DISCUSSION
GSLs are components of the outer layer of plasma membranes, with considerable heterogeneity, due to heterogeneity of both the oligosaccharide and ceramide moieties. They are enriched with respect to glycerophospholipids in limited areas containing a few selected proteins, many of which are associated with cell signaling (28, 34). GSLs have been found to modulate cell signaling processes through specific interactions between their oligosaccharide chains and proteins/glycoproteins in their environment. In contrast, it is unclear whether ceramides play specific roles in such processes, although changes in the structures of both the long-chain base and the fatty acids have been associated with the differentiation and aging processes (35–38). In addition, ceramides resulting from the hydrolysis of GSLs at the cell surface by GSL hydrolases are involved in the regulation of apoptosis (39, 40).
In mammals, ceramides are synthesized by a family of six enzymes, ceramide synthase (CerS)1–6, each of which uses a relatively restricted subset of fatty acyl-CoAs to N-acylate the sphingoid long-chain base (41). The levels of expression of each CerS encoding gene differ among several tissues, suggesting that molecular variations and expression patterns of fatty acid chains of GSLs reflect the functions of these cells. CerS2 is the predominant CerS of C24 ceramides (42, 43). CerS2 knockdown mice exhibited severe hepatopathy, myelin sheath defects, and cerebellar degeneration. The availability of very long-chain fatty acids requires ELOVL enzymes in mammals. ELOVL1 shows high activity toward saturated and monounsaturated C20- and C22-CoAs, and has been shown to be essential for the production of C24 fatty acid containing sphingolipids. ELOVL1 activity is regulated by CerS2. Knockdown of ELOVL1 or CerS2 markedly reduced Lyn activation in HeLa cells (44). These results are consistent with our observation that C24-LacCer molecules are indispensable for Lyn-coupled LacCer-enriched lipid raft-mediated neutrophil functions (7).
LacCer-enriched lipid rafts mediate neutrophil chemotaxis, phagocytosis, and superoxide generation of neutrophils (5, 8, 45). DMSO-differentiated HL-60 cells are commonly used for analyzing neutrophil functions, including migration, phagocytosis of opsonized microorganisms, superoxide generation, and degranulation (5, 8, 46–49). However, those HL-60 cells do not possess LacCer-mediated neutrophil functions, including migration, superoxide generation, or phagocytosis, because the presence of C24-LacCer in plasma membranes is insufficient to form Lyn-coupled LacCer-enriched lipid rafts (7). In contrast, C24-LacCer is present in granules of DMSO-differentiated HL-60 cells, indicating that biosynthesis of C24-LacCer in itself is normal. It seems, therefore, that the delivery system of C24-LacCer from Golgi apparatus to plasma membranes in DMSO-differentiated HL-60 cells is abnormal. Although it remains unclear why C24-LacCer is not selectively delivered to plasma membranes from Golgi apparatus in DMSO-differentiated HL-60 cells, these cells are a good model to analyze the organization and signal transduction mechanisms of LacCer-enriched lipid rafts. LacCer is located on the outer layer of plasma membranes, whereas Lyn is present at their cytosolic face via myristic/palmitic acyl chains. It has been suggested that the long fatty acids of LacCer may interdigitate (50, 51) with the acyl chains of the cytosolic layer, reducing membrane thickness (52, 53). This may allow for direct interactions between LacCer and Lyn, which may be instrumental for Lyn phosphorylation, considered the starting process necessary for the immunological functions of neutrophils (7). To date, however, direct interactions between LacCer and Lyn through the membrane layer have not been observed experimentally.
Photoactivable GSLs have been shown to be good tools to study GSL-protein interactions (13, 14, 16, 17). To verify the direct interaction between C24-LacCer and Lyn through the membrane layers, we synthesized a photoactivable LacCer containing a reactive azide group at the end of the acyl chain. This chain was of a length similar to that of C24 fatty acids, as shown by molecular modeling. The photoactivable C24 fatty acid containing LacCer was labeled with tritium at position 3 of sphingosine, yielding C24-[3H]LacCer-(N3). As a control, we also synthesized a tritium-labeled photoactivable LacCer containing a shorter acyl chain, C18-[3H]LacCer-(N3).
In this study, we showed that C24-[3H]LacCer-(N3) covalently binds to Lyn in LacCer-enriched lipid rafts. D-HL-60 cells were loaded with these two types of [3H]LacCer-(N3), with percentages of each found to be present in the DRM fractions prepared from cell lysates. Lyn phosphorylation and phagocytosis were observed in cells loaded with C24-[3H]LacCer-(N3), but not C18-[3H]LacCer-(N3). These results are similar to those observed when D-HL-60 cells were loaded with natural LacCer molecules containing very long and short/medium fatty acids, suggesting that the photoactivable LacCer derivatives mimic the natural molecules.
After cell loading and illumination, the patterns of the radioactive proteins were analyzed. Illumination rapidly transforms the azide to nitrene, a very reactive group capable of covalently binding to molecules in their nearest environment. Thus any interaction of tritium-labeled LacCer derivative with proteins would yield tritium-labeled LacCer-protein complexes that can be analyzed by PAGE, followed by radioimaging of the blotted material.
The administration of C24-[3H]LacCer-(N3) to the cells followed by illumination resulted in the association of radioactivity with the plasma membrane lipid rafts. Most of the radioactivity was linked to lipids, which was not unexpected, because lipids are the main components of lipid rafts. Some of the radioactivity, however, was linked to proteins, but only a few proteins were labeled, suggesting that their interactions with LacCer are specific. One of the cross-linked proteins was identified as Lyn, whereas a second protein was stained with anti-Gαi. Because the azide moiety is at the end of the LacCer fatty acid, which is inserted into the outer membrane layer, and Lyn is associated with the cytosolic membrane layer by insertion of its acyl chains, the only mechanism by which a LacCer-Lyn complex can form is by the acyl chain of LacCer contacting with the acyl chain(s) of Lyn. In contrast, when C18-[3H]LacCer-(N3) was administered to cells, little radioactivity was associated with the lipid raft proteins, and a LacCer-Lyn complex could not be detected.
These findings confirm that LacCer containing C24 fatty acid is in contact with the acyl chains of Lyn and that this is necessary for the final activation of Lyn, through phosphorylation, followed by the cascade of reactions necessary for transfer of information through the membrane. However, it is difficult to imagine that these interactions are associated with specific hydrophobic moieties. Rather, LacCer containing C24 fatty acids may promote the organization of specific lipid rafts necessary to allow the interaction of several proteins instrumental to the cell signaling processing. Without C24-LacCer molecules, this microenvironment would not be available, Lyn would not be recruited and activated by phosphorylation, and the signaling process would not be induced.
Footnotes
Abbreviations:
- Bu3N
- tributylamine
- CerS
- ceramide synthase
- DDQ
- 2,3-dichloro-5,6-dicyano-1,4-benzoquinone
- DFP
- diisopropyl fluorophosphate
- D-HL-60 cell
- DMSO-treated human promyelocytic leukemia cell
- DMF
- dimethylformamide
- DRM
- detergent-resistant membrane
- Et3N
- triethylamine
- fMLP
- formyl peptide (N-formylmethionine-leucine-phenylalanine)
- GD1b
- Galb1-3GalNAcb1-4(NeuAca2-8NeuAca2-3)Galb1-4Glcb1-1Cer
- GM1
- Galb1-3GalNAcb1-4(NeuAca2-3)Galb1-4Glcb1-1’-Cer
- GM3
- NeuAca2-3Galb1-4Glcb1-1’-Cer
- GSL
- glycosphingolipid
- H2O
- water
- LacCer
- lactosylceramide [β-Gal-(1-4)-β-Glc-(1-1)-Cer]
- MeOH
- methanol
- MsCl
- methane sulfonyl chloride
- PNS
- post nuclear supernatant
- PVDF
- polyvinylidene difluoride
This study was supported in part by a grant-in-aid (S1201013, S1311011) from the Foundation of Strategic Research Projects in Private Universities from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, and by Matsumae Foundation (for K.I.). S.S. was supported by funds obtained through performance in tariff of the Department of Medical Biotechnology and Translational Medicine, University of Milan.
REFERENCES
- 1.Regina Todeschini A., Hakomori S. I. 2008. Functional role of glycosphingolipids and gangliosides in control of cell adhesion, motility, and growth, through glycosynaptic microdomains. Biochim. Biophys. Acta. 1780: 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaga N., Kazuno S., Taka H., Iwabuchi K., Murayama K. 2005. Isolation and mass spectrometry characterization of molecular species of lactosylceramides using liquid chromatography-electrospray ion trap mass spectrometry. Anal. Biochem. 337: 316–324. [DOI] [PubMed] [Google Scholar]
- 3.Yu R. K., Yanagisawa M., Ariga T. 2007. Glycosphingolipid Structures. Elsevier, Oxford, UK. [Google Scholar]
- 4.Fujita A., Cheng J., Fujimoto T. 2009. Segregation of GM1 and GM3 clusters in the cell membrane depends on the intact actin cytoskeleton. Biochim. Biophys. Acta. 1791: 388–396. [DOI] [PubMed] [Google Scholar]
- 5.Iwabuchi K., Nagaoka I. 2002. Lactosylceramide-enriched glycosphingolipid signaling domain mediates superoxide generation from human neutrophils. Blood. 100: 1454–1464. [PubMed] [Google Scholar]
- 6.Kusumi A., Shirai Y. M., Koyama-Honda I., Suzuki K. G., Fujiwara T. K. 2010. Hierarchical organization of the plasma membrane: investigations by single-molecule tracking vs. fluorescence correlation spectroscopy. FEBS Lett. 584: 1814–1823. [DOI] [PubMed] [Google Scholar]
- 7.Iwabuchi K., Prinetti A., Sonnino S., Mauri L., Kobayashi T., Ishii K., Kaga N., Murayama K., Kurihara H., Nakayama H., et al. 2008. Involvement of very long fatty acid-containing lactosylceramide in lactosylceramide-mediated superoxide generation and migration in neutrophils. Glycoconj. J. 25: 357–374. [DOI] [PubMed] [Google Scholar]
- 8.Sato T., Iwabuchi K., Nagaoka I., Adachi Y., Ohno N., Tamura H., Seyama K., Fukuchi Y., Nakayama H., Yoshizaki F., et al. 2006. Induction of human neutrophil chemotaxis by Candida albicans-derived beta-1,6-long glycoside side-chain-branched beta-glucan. J. Leukoc. Biol. 80: 204–211. [DOI] [PubMed] [Google Scholar]
- 9.Ekyalongo R. C., Nakayama H., Kina K., Kaga N., Iwabuchi K. 2014. Organization and functions of glycolipid-enriched microdomains in phagocytes. Biochim. Biophys. Acta. In press. [DOI] [PubMed] [Google Scholar]
- 10.Bisson R., Montecucco C. 1981. Photolabelling of membrane proteins with photoactive phospholipids. Biochem. J. 193: 757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sonnino S., Chigorno V., Acquotti D., Pitto M., Kirschner G., Tettamanti G. 1989. A photoreactive derivative of radiolabeled GM1 ganglioside: preparation and use to establish the involvement of specific proteins in GM1 uptake by human fibroblasts in culture. Biochemistry. 28: 77–84. [DOI] [PubMed] [Google Scholar]
- 12.Mauri L., Prioni S., Loberto N., Chigorno V., Prinetti A., Sonnino S. 2004. Synthesis of radioactive and photoactivable ganglioside derivatives for the study of ganglioside-protein interactions. Glycoconj. J. 20: 11–23. [DOI] [PubMed] [Google Scholar]
- 13.Kabayama K., Sato T., Saito K., Loberto N., Prinetti A., Sonnino S., Kinjo M., Igarashi Y., Inokuchi J. 2007. Dissociation of the insulin receptor and caveolin-1 complex by ganglioside GM3 in the state of insulin resistance. Proc. Natl. Acad. Sci. USA. 104: 13678–13683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ono M., Handa K., Sonnino S., Withers D. A., Nagai H., Hakomori S. 2001. GM3 ganglioside inhibits CD9-facilitated haptotactic cell motility: coexpression of GM3 and CD9 is essential in the downregulation of tumor cell motility and malignancy. Biochemistry. 40: 6414–6421. [DOI] [PubMed] [Google Scholar]
- 15.Chigorno V., Valsecchi M., Acquotti D., Sonnino S., Tettamanti G. 1990. Formation of a cytosolic ganglioside-protein complex following administration of photoreactive ganglioside GM1 to human fibroblasts in culture. FEBS Lett. 263: 329–331. [DOI] [PubMed] [Google Scholar]
- 16.Fra A. M., Masserini M., Palestini P., Sonnino S., Simons K. 1995. A photo-reactive derivative of ganglioside GM1 specifically cross-links VIP21-caveolin on the cell surface. FEBS Lett. 375: 11–14. [DOI] [PubMed] [Google Scholar]
- 17.Palestini P., Pitto M., Tedeschi G., Ferraretto A., Parenti M., Brunner J., Masserini M. 2000. Tubulin anchoring to glycolipid-enriched, detergent-resistant domains of the neuronal plasma membrane. J. Biol. Chem. 275: 9978–9985. [DOI] [PubMed] [Google Scholar]
- 18.Sonnino S., Chigorno V., Valsecchi M., Pitto M., Tettamanti G. 1992. Specific ganglioside-cell protein interactions: a study performed with GM1 ganglioside derivative containing photoactivable azide and rat cerebellar granule cells in culture. Neurochem. Int. 20: 315–321. [DOI] [PubMed] [Google Scholar]
- 19.Loberto N., Prioni S., Prinetti A., Ottico E., Chigorno V., Karagogeos D., Sonnino S. 2003. The adhesion protein TAG-1 has a ganglioside environment in the sphingolipid-enriched membrane domains of neuronal cells in culture. J. Neurochem. 85: 224–233. [DOI] [PubMed] [Google Scholar]
- 20.Prioni S., Mauri L., Loberto N., Casellato R., Chigorno V., Karagogeos D., Prinetti A., Sonnino S. 2004. Interactions between gangliosides and proteins in the exoplasmic leaflet of neuronal plasma membranes: a study performed with a tritium-labeled GM1 derivative containing a photoactivable group linked to the oligosaccharide chain. Glycoconj. J. 21: 461–470. [DOI] [PubMed] [Google Scholar]
- 21.Seino K., Iwabuchi K., Kayagaki N., Miyata R., Nagaoka I., Matsuzawa A., Fukao K., Yagita H., Okumura K. 1998. Chemotactic activity of soluble Fas ligand against phagocytes. J. Immunol. 161: 4484–4488. [PubMed] [Google Scholar]
- 22.Yanagida M., Nakayama H., Yoshizaki F., Fujimura T., Takamori K., Ogawa H., Iwabuchi K. 2007. Proteomic analysis of plasma membrane lipid rafts of HL-60 cells. Proteomics. 7: 2398–2409. [DOI] [PubMed] [Google Scholar]
- 23.Freitas J. M., Abrantes L. M. 2005. Synthesis of long-chain 3-alkylpyrroles bearing terminal carboxy or amino groups. Helv. Chim. Acta. 88: 2470–2478. [Google Scholar]
- 24.Gorczynski M. J., Huang J., King S. B. 2006. Regio- and stereospecific syntheses and nitric oxide donor properties of (E)-9- and (E)-10-nitrooctadec-9-enoic acids. Org. Lett. 8: 2305–2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hang H. C., Geutjes E. J., Grotenbreg G., Pollington A. M., Bijlmakers M. J., Ploegh H. L. 2007. Chemical probes for the rapid detection of fatty-acylated proteins in mammalian cells. J. Am. Chem. Soc. 129: 2744–2745. [DOI] [PubMed] [Google Scholar]
- 26.Aureli M., Prioni S., Mauri L., Loberto N., Casellato R., Ciampa M. G., Chigorno V., Prinetti A., Sonnino S. 2010. Photoactivable sphingosine as a tool to study membrane microenvironments in cultured cells. J. Lipid Res. 51: 798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chigorno V., Palestini P., Sciannamblo M., Dolo V., Pavan A., Tettamanti G., Sonnino S. 2000. Evidence that ganglioside enriched domains are distinct from caveolae in MDCK II and human fibroblast cells in culture. Eur. J. Biochem. 267: 4187–4197. [DOI] [PubMed] [Google Scholar]
- 28.Prinetti A., Chigorno V., Tettamanti G., Sonnino S. 2000. Sphingolipid-enriched membrane domains from rat cerebellar granule cells differentiated in culture. A compositional study. J. Biol. Chem. 275: 11658–11665. [DOI] [PubMed] [Google Scholar]
- 29.Aureli M., Loberto N., Lanteri P., Chigorno V., Prinetti A., Sonnino S. 2011. Cell surface sphingolipid glycohydrolases in neuronal differentiation and aging in culture. J. Neurochem. 116: 891–899. [DOI] [PubMed] [Google Scholar]
- 30.Mehlen P., Rabizadeh S., Snipas S. J., Assa-Munt N., Salvesen G. S., Bredesen D. E. 1998. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature. 395: 801–804. [DOI] [PubMed] [Google Scholar]
- 31.Iwabuchi K., Yamamura S., Prinetti A., Handa K., Hakomori S. 1998. GM3-enriched microdomain involved in cell adhesion and signal transduction through carbohydrate-carbohydrate interaction in mouse melanoma B16 cells. J. Biol. Chem. 273: 9130–9138. [DOI] [PubMed] [Google Scholar]
- 32.Iwabuchi K., Handa K., Hakomori S. 1998. Separation of “glycosphingolipid signaling domain” from caveolin-containing membrane fraction in mouse melanoma B16 cells and its role in cell adhesion coupled with signaling. J. Biol. Chem. 273: 33766–33773. [DOI] [PubMed] [Google Scholar]
- 33.Prinetti A., Marano N., Prioni S., Chigorno V., Mauri L., Casellato R., Tettamanti G., Sonnino S. 2000. Association of Src-family protein tyrosine kinases with sphingolipids in rat cerebellar granule cells differentiated in culture. Glycoconj. J. 17: 223–232. [DOI] [PubMed] [Google Scholar]
- 34.Prinetti A., Chigorno V., Prioni S., Loberto N., Marano N., Tettamanti G., Sonnino S. 2001. Changes in the lipid turnover, composition, and organization, as sphingolipid-enriched membrane domains, in rat cerebellar granule cells developing in vitro. J. Biol. Chem. 276: 21136–21145. [DOI] [PubMed] [Google Scholar]
- 35.Haga Y., Hatanaka K., Hakomori S. I. 2008. Effect of lipid mimetics of GM3 and lyso-GM3 dimer on EGF receptor tyrosine kinase and EGF-induced signal transduction. Biochim. Biophys. Acta. 1780: 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palestini P., Masserini M., Sonnino S., Giuliani A., Tettamanti G. 1990. Changes in the ceramide composition of rat forebrain gangliosides with age. J. Neurochem. 54: 230–235. [DOI] [PubMed] [Google Scholar]
- 37.Riboni L., Acquotti D., Casellato R., Ghidoni R., Montagnolo G., Benevento A., Zecca L., Rubino F., Sonnino S. 1992. Changes of the human liver GM3 ganglioside molecular species during aging. Eur. J. Biochem. 203: 107–113. [DOI] [PubMed] [Google Scholar]
- 38.Suzaki K. 1965. The pattern of mammalian brain gangliosides. 3. Regional and developmental differences. J. Neurochem. 12: 969–979. [DOI] [PubMed] [Google Scholar]
- 39.Aureli M., Bassi R., Prinetti A., Chiricozzi E., Pappalardi B., Chigorno V., Di Muzio N., Loberto N., Sonnino S. 2012. Ionizing radiations increase the activity of the cell surface glycohydrolases and the plasma membrane ceramide content. Glycoconj. J. 29: 585–597. [DOI] [PubMed] [Google Scholar]
- 40.Valaperta R., Chigorno V., Basso L., Prinetti A., Bresciani R., Preti A., Miyagi T., Sonnino S. 2006. Plasma membrane production of ceramide from ganglioside GM3 in human fibroblasts. FASEB J. 20: 1227–1229. [DOI] [PubMed] [Google Scholar]
- 41.Levy M., Futerman A. H. 2010. Mammalian ceramide synthases. IUBMB Life. 62: 347–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imgrund S., Hartmann D., Farwanah H., Eckhardt M., Sandhoff R., Degen J., Gieselmann V., Sandhoff K., Willecke K. 2009. Adult ceramide synthase 2 (CERS2)-deficient mice exhibit myelin sheath defects, cerebellar degeneration, and hepatocarcinomas. J. Biol. Chem. 284: 33549–33560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pewzner-Jung Y., Brenner O., Braun S., Laviad E. L., Ben-Dor S., Feldmesser E., Horn-Saban S., Amann-Zalcenstein D., Raanan C., Berkutzki T., et al. 2010. A critical role for ceramide synthase 2 in liver homeostasis: II. Insights into molecular changes leading to hepatopathy. J. Biol. Chem. 285: 10911–10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohno Y., Suto S., Yamanaka M., Mizutani Y., Mitsutake S., Igarashi Y., Sassa T., Kihara A. 2010. ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proc. Natl. Acad. Sci. USA. 107: 18439–18444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nakayama H., Yoshizaki F., Prinetti A., Sonnino S., Mauri L., Takamori K., Ogawa H., Iwabuchi K. 2008. Lyn-coupled LacCer-enriched lipid rafts are required for CD11b/CD18-mediated neutrophil phagocytosis of nonopsonized microorganisms. J. Leukoc. Biol. 83: 728–741. [DOI] [PubMed] [Google Scholar]
- 46.Newburger P. E., Chovaniec M. E., Greenberger J. S., Cohen H. J. 1979. Functional changes in human leukemic cell line HL-60. A model for myeloid differentiation. J. Cell Biol. 82: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meagher L. C., Cotter T. G. 1988. The degranulation response in differentiated HL-60 cells. Clin. Exp. Immunol. 74: 483–488. [PMC free article] [PubMed] [Google Scholar]
- 48.Wu H., Scher B. M., Chu C. L., Leonard M., Olmedo R., Scher G. S., Stecker S., Scher W., Waxman S. 1991. Reduction in lactate accumulation correlates with differentiation-induced terminal cell division of leukemia cells. Differentiation. 48: 51–58. [DOI] [PubMed] [Google Scholar]
- 49.Xu R. H., Pelicano H., Zhou Y., Carew J. S., Feng L., Bhalla K. N., Keating M. J., Huang P. 2005. Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Res. 65: 613–621. [PubMed] [Google Scholar]
- 50.Allende D., Vidal A., McIntosh T. J. 2004. Jumping to rafts: gatekeeper role of bilayer elasticity. Trends Biochem. Sci. 29: 325–330. [DOI] [PubMed] [Google Scholar]
- 51.Grant C. W., Mehlhorn I. E., Florio E., Barber K. R. 1987. A long chain spin label for glycosphingolipid studies: transbilayer fatty acid interdigitation of lactosyl ceramide. Biochim. Biophys. Acta. 902: 169–177. [DOI] [PubMed] [Google Scholar]
- 52.Ruettinger A., Kiselev M. A., Hauss T., Dante S., Balagurov A. M., Neubert R. H. 2008. Fatty acid interdigitation in stratum corneum model membranes: a neutron diffraction study. Eur. Biophys. J. 37: 759–771. [DOI] [PubMed] [Google Scholar]
- 53.Sonnino S., Prinetti A., Nakayama H., Yangida M., Ogawa H., Iwabuchi K. 2009. Role of very long fatty acid-containing glycosphingolipids in membrane organization and cell signaling: the model of lactosylceramide in neutrophils. Glycoconj. J. 26: 615–621. [DOI] [PubMed] [Google Scholar]