Abstract
A mouse lacking CD28, a T-cell costimulatory molecule, and STAT6, a transcription factor that mediates interleukin-4 (IL-4) signaling, was developed from parental CD28- and STAT6-deficient mice. STAT6/CD28−/− BALB/c mice that were 8 weeks old had a normal phenotype, and IL-4 production was induced following infection with nematode parasites. Unexpectedly, when they were between 4 and 8 months old, all mice examined spontaneously developed severe chronic dermatitis associated with pronounced numbers of Demodex ectoparasites. In addition, pronounced CD4 and CD8 T-cell infiltrates in the dermis and subcutaneous fat, increased serum immunoglobulin G2a levels, and lymphadenopathy associated with increased gamma interferon and IL-12 expression were observed. Single-knockout siblings lacking either CD28 or STAT6 had a phenotype similar to that of BALB/c wild-type controls. To distinguish whether the ectoparasite Demodex or the Th1 immunity was the proximal cause of the inflammatory skin disease, STAT6/CD28−/− mice were treated with a miticide that eliminated the ectoparasites. This treatment markedly reduced the severity of the dermatitis and the associated lymphoid infiltrates. These findings suggest that ubiquitous ectoparasites, which are generally considered to be commensal, may contribute to disease when specific molecules required for an effective Th2 response are blocked.
CD4 T cells differentiate into effector cells following signaling through both the T-cell receptor and costimulatory molecules. T helper effector cells can differentially express specific cytokines and during infectious disease can produce Th1 cytokines, particularly gamma interferon (IFN-γ), or Th2 cytokines, including interleukin-4 (IL-4), IL-5, and IL-13. Conserved microbial structures associated with many viruses and bacteria can bind Toll-like receptors on dendritic cells, triggering IL-12 production and development of the Th1 response. Helminthic parasites and allergens can trigger a Th2 response, although the mechanism through which T helper cells differentiate to produce Th2 cytokines is less clear. Considerable evidence suggests that signaling through the costimulatory molecule CD28 is important in driving the Th2 response (21). Other studies have demonstrated that STAT6 is important for effective signaling through IL-4R (19). However, recent studies have shown that during infectious disease IL-4-producing Th2 cells can develop in the absence of either CD28 (4, 10) or STAT6 (7, 15). Furthermore, CD28−/− T cells, which are refractory to anti-CD3 stimulation in vitro, are activated by addition of IL-4 to develop into Th2 cells (23), suggesting that IL-4 signaling may substitute for CD28 signaling to promote the development of Th2 cells.
The mechanisms by which specific T helper cells might control or contribute to chronic dermatitis are not well understood. The role of the immune response in the development of dermatitis remains unclear partially because of the paucity of suitable murine models. Most models involve an enhanced Th2 response that results in atopic dermatitis associated with increased serum immunoglobulin E (IgE) levels and eosinophilia. These characteristics may develop spontaneously in the case of NC mice, in which they may be triggered by an inappropriate Th2 response to bacteria (12), or in experimental models after introduction of Th2 cytokines (5).
To examine the role of CD28 and STAT6 in the development of the Th2 immune response, we developed a STAT6/CD28−/− mouse. Our studies with this mouse showed that a Th2 response characterized by increases in IL-4 levels could still develop in response to nematode parasite infection. Surprisingly, however, with increased age, STAT6/CD28−/− mice spontaneously developed a severe chronic dermatitis associated with pruritus, erythema, alopecia, blepharitis, undetectable serum IgE levels, and dense lymphoid infiltrates in the dermis and subcutaneous fat without eosinophils. Furthermore, our studies indicated that the inflammatory skin disease observed in the STAT6/CD28−/− mice was caused by spontaneous infestation of the hair follicles with a normally commensal and ubiquitous ectoparasite, Demodex musculi, which does not cause dermatitis in other immunodeficient mouse strains (14), including STAT6−/− and CD28−/− mice. Our studies, which were performed in a specific-pathogen-free (SPF) facility, indicated that the immune response, which was impaired by simultaneous blockade of the STAT6 and CD28 functions, resulted in markedly increased susceptibility to harmful effects of this parasite, which is normally present at low levels on most mice, even in barrier facilities.
MATERIALS AND METHODS
Mice.
CD28−/− BALB/c mice (23) and STAT6−/− BALB/c (19) mice were obtained and interbred in the SPF barrier facility at the Uniformed Services University Laboratory of Animal Medicine. Homozygous STAT6/CD28−/− mice were identified by PCR analysis by using specific primers that individually recognized the original CD28 and STAT6 targeting constructs. BALB/c mice (National Cancer Institute, Frederick, Md.) were used for all studies of wild-type (WT) mice and as controls for the CD28−/− BALB/c, STAT6−/− BALB/c, and STAT6/CD28−/− BALB/c mice. SCID mice with a BALB/c background were obtained from Jackson Laboratories (Bar Harbor, Maine). The experiments were conducted according to the principles set forth in the Guide for the Care and Use of Laboratory Animals of the Institute of Animal Resources, National Research Council, Department of Health, Education and Welfare (publication NIH 78-23) (21a).
Parasite infection.
Mice were inoculated subcutaneously with 500 infective third-stage Nippostrongylus brasiliensis larvae (28). The numbers of parasite eggs and adult worms were determined as described previously (20).
Histology.
Peripheral lymph nodes and skin samples from the dorsal region, ears, and eyelids were collected from killed mice, fixed in 10% formalin in phosphate-buffered saline (PBS) for 1 to 2 days, embedded in paraffin, sectioned, and stained with hematoxylin and eosin, Giemsa stain, and Brown and Hops Gram stain by using standard methods. Slide specimens were analyzed by using a Zeiss Axioskop 2 and were digitally photographed (Zeiss, Thornwood, N.Y.). The frequency of Demodex ectoparasites was determined by counting the number of parasites in each high-power field (magnification, ×200) for five randomly selected fields per skin section.
Immunohistochemistry.
Tissue samples were imbedded in Tissue-Tek O.C.T. compound (Sakura, Torrance, Calif.) and were frozen in the vapor phase of liquid nitrogen. Frozen sections were thawed on microscope slides, allowed to dry at room temperature, and fixed in acetone for 10 min. Nonspecific staining was reduced by incubation with anti-Fc receptor antibody (2.4G2; BD Pharmingin, San Diego, Calif.) and normal rat serum. After slides were washed in PBS, they were simultaneously stained with anti-CD4-phosphatidylethanolamine (PE) (Gk1.5; BD Pharmingen) and biotin-anti-CD8 (Ly-2; BD Pharmingen) followed by streptavidin Alexa 488 (Molecular Probes) at room temperature for 45 min, washed with PBS, and mounted with Fluormount (Southern Biotechnologies, Birmingham, Ala.). The slides were examined by using a Zeiss Axiophot immunofluorescence microscope and Intelligent Imaging software (Intelligent Imaging, Denver, Colo.).
Cytokine gene expression as determined by reverse transcription (RT)-PCR.
Total RNA was prepared from tissues and reverse transcribed as previously described (25). Real-time PCR commercial kits (PE Applied Biosystems, Foster City, Calif.) specific for different cytokines or rRNA were utilized, and all data were normalized to constitutive rRNA values. An Applied Biosystems 7700 sequence detector (PE Applied Biosystems) was used for amplification of target mRNA, and differences between treatment groups were quantified by following the manufacturer's instructions.
Proliferation and cytokine production by cultured lymphocytes.
Lymphocytes were collected from peripheral lymph nodes of different mice and cultured in 96-well round-bottom plates (2 × 105 cells/well) coated with various doses of anti-mouse CD3 monoclonal antibody (2C11; BD/Pharmingin). After 68 h, the supernatants were collected and stored at −70°C. Twenty microliters of CellTiter 96 AQueous One solution (Promega Corp., Madison, Wis.) and 100 μl of RPMI 1640 containing 10% fetal calf serum were added to each well, and after incubation for 4 h, the absorbance at 490 nm was recorded with a 96-well enzyme-linked immunosorbent assay (ELISA) reader. The levels of IL-4, IL-13, IFN-γ, and IL-12 in supernatants were determined by using commercial ELISA kits according to the manufacturer's instructions (R&D Systems, Minneapolis, Minn.).
Treatment of STAT6/CD28−/− mice for ectoparasites.
Ophthalmic ointment was placed in the eyes prior to treatment with an amitraz (Mitaban; Pharmacia and Upjohn, Kalamazoo, Mich.) solution. The amitraz was mixed according to the manufacturer's instructions to obtain 250 ppm of active drug in water. Mice were topically treated with the amitraz solution by thoroughly wetting them by gently pouring the mixture over the entire body and allowing the animals to air dry. A new solution was used for each animal. Mice were treated every 14 days, and four consecutive treatments were administered over a course of 8 weeks. Control mice were treated similarly with water. To monitor pruritus, mice were put into a no-disturbance room and individually videotaped for 5 min, and then the tapes were analyzed for itching behavior, as previously defined (30).
Quantitation of serum immunoglobulin.
Serum IgG1, IgG2a, and IgE levels were quantitated by ELISA, as previously described (11).
Cell labeling and analysis.
Mesenteric lymph node cells were washed and then simultaneously stained with appropriate dilutions of Cy-chrome-anti-CD3 (2C11) FITC-anti-CD8 (Ly2), FITC-anti-CD4 (GK1.5), PE-anti-CD69 (H1F2.3), and FITC-anti-B220 (6B2) (Pharmingen). Two- and three-color flow cytometric analyses were performed with an Epics ELITE flow cytometer (Coulter Electronics, Hialeah, Fla.).
CCCA.
In vivo production of IL-4 was determined by a Cincinnati cytokine capture assay (CCCA) (7). Briefly, to capture secreted IL-4, mice were injected intravenously with 10 μg of biotin-BVD4-1D11 and bled 2 h later. Serum levels of IL-4-biotin-anti-IL-4 complexes were determined by ELISA by using microtiter plates coated with BVD6-24G2.3.
Statistics.
Statistical differences (significance level, P ≤ 0.05) between groups were assessed by using analysis of variance and Tukey's t test for pairwise comparisons. The software program SigmaStat (Jandel Scientific Software, San Rafael, Calif.) was used for all statistical analyses.
RESULTS
STAT6/CD28−/− mice develop a Th2 response following immunization with nematode parasites.
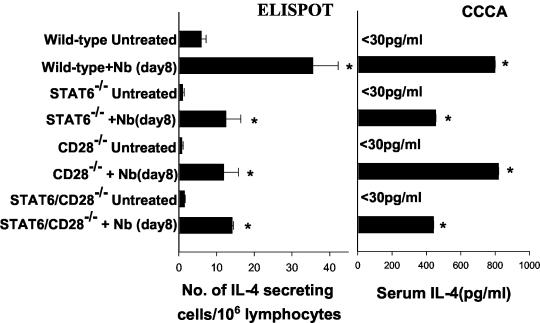
The immune response to the intestinal nematode parasite N. brasiliensis has previously been shown to elicit a strong Th2 immune response in mice, and CD4+ T cells are the primary source of increases in IL-4 levels (8, 9). Furthermore, previous studies have shown that an IL-4 response can develop in the absence of either CD28 (4, 10) or STAT6 (7, 15) signaling. To examine whether blockade of both CD28 and STAT6 interactions inhibited development of the Th2 immune response, 6- to 8-week-old WT, CD28−/−, STAT6−/−, and STAT6/CD28−/− mice (all with a BALB/c background) were immunized subcutaneously with 500 third-stage N. brasiliensis larvae. On day 7, the mice were killed, and IL-4-secreting lymphocytes from the mesenteric lymph nodes were assayed by the ELISPOT procedure, while serum IL-4 levels were measured by using the CCCA. As shown in Fig. 1, pronounced increases in IL-4 levels were observed in N. brasiliensis-infected WT, STAT6−/−, CD28−/−, and STAT6/CD28−/− mice compared to the levels in untreated controls of the same strains (P ≤ 0.01). As determined by the ELISPOT assay, the increases in IL-4 levels in N. brasiliensis-inoculated WT mice were significantly greater (P < 0.05) than the increases in the levels in mice deficient in STAT6, CD28, or both STAT6 and CD28. As expected from previous studies of worm expulsion with STAT6−/− mice (28), worm fecundity remained high in N. brasiliensis-inoculated STAT6−/− and STAT6/CD28−/− mice (data not shown). Worms were successfully expulsed in CD28−/− and WT mice (data not shown), which is consistent with previous studies which showed that B7 blockade does not inhibit expulsion of N. brasiliensis (13). Taken together, the results obtained with the STAT6/CD28−/− mice extended previous findings by indicating that a Th2 response can develop in the simultaneous absence of STAT6 and CD28 and that this response is comparable to the response observed in either STAT6−/− or CD28−/− mice.
FIG. 1.
Increases in the levels of IL-4-producing cells in the mesenteric lymph nodes and the serum IL-4 levels are pronounced in STAT6/CD28−/− BALB/c mice following N. brasiliensis (Nb) inoculation. For the ELISPOT assay, mesenteric lymph node tissues were collected 8 days after inoculation, and the number of IL-4-secreting cells per 106 mesenteric lymph node cells was determined without restimulation. For the CCCA, mice were injected intravenously with 10 μg of biotin-labeled anti-IL-4 monoclonal antibody and bled 2 h after injection. The serum levels of the IL-4-biotin-anti-IL-4 monoclonal antibody complex were determined by ELISA. ELISPOT and CCCA analyses were performed with five mice per treatment group, and the means and standard errors are shown. An asterisk indicates that the P value is ≤0.01.
STAT6/CD28−/− mice develop chronic inflammatory skin disease.
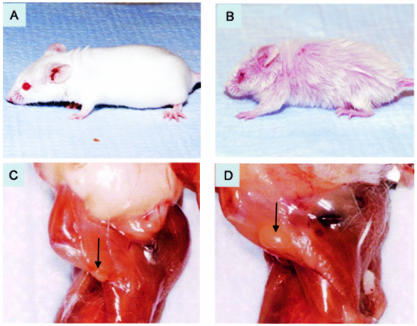
Although mice deficient in either CD28 or STAT6 initially had a phenotype similar to that of WT BALB/c age-matched controls that were between 4 and 8 months old, all STAT6/CD28−/− mice spontaneously developed pruritus associated with crusted and bleeding superficial ulcerations and initially patchy, nonscarring alopecia in the head and neck regions, which became generalized and associated with scarring of the superficial dermis by 8 months (Fig. 2B). In contrast, single-knockout mice deficient in either STAT6 (Fig. 2A) or CD28 (data not shown) had skin and hair that appeared to be normal and was similar to the skin and hair of WT BALB/c mice. Lymph node hyperplasia was also apparent, and the size of the peripheral lymph nodes was markedly increased in STAT6/CD28−/− mice (Fig. 2D) compared to the size in the BALB/c WT controls (Fig. 2C). Splenomegaly was also observed in older mice (age, 8 months); however, the kidneys, liver, and lungs appeared to be normal (data not shown). It should be noted that BALB/c mice deficient in STAT6 or CD28 and mice deficient in both STAT6 and CD28 were housed in adjacent cages in an SPF barrier facility. Even when single-knockout STAT6 or CD28 mice were housed in the same cages as the mice deficient in both CD28 and STAT6, only the double-knockout mice developed dermatitis.
FIG. 2.
Spontaneous inflammatory skin disease and lymph node hyperplasia in STAT6/CD28−/− BALB/c mice. Neither CD28−/− mice (data not shown) nor STAT6−/− mice (A) exhibited dermatitis. In contrast, STAT6/CD28−/− mice exhibited extensive dermatitis associated with alopecia, erythema, blepharitis, and pruritus (B). (C and D) Axillary lymph nodes from a WT BALB/c mouse (C) and a STAT6/CD28−/− mouse (D). The sizes of the peripheral lymph nodes were markedly increased in a STAT6/CD28−/− mouse.
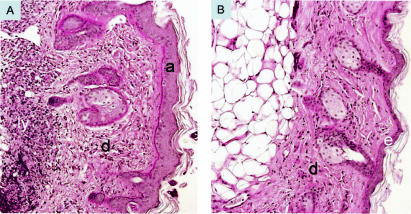
Histological analysis of skin samples revealed severe dermatitis associated with acanthosis, lymphoid infiltrates, an absence of eosinophils, and Demodex infestation.
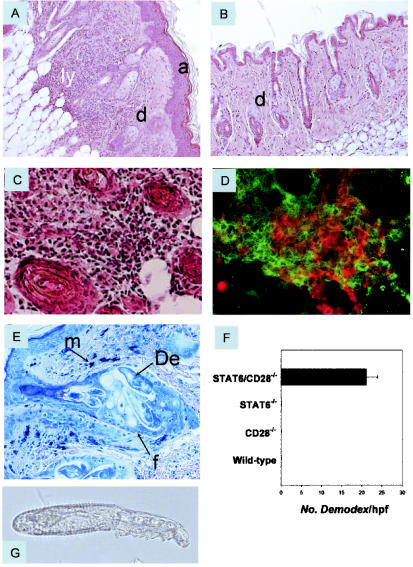
Histological analysis of skin samples from STAT6/CD28−/− mice revealed a dense superficial and deep dermal infiltrate of lymphocytes with scarce eosinophils, as well as changes in the epidermis, including acanthosis and mild or no spongiosis (Fig. 3A). The eyelids and ears were also thickened by lymphoid infiltrates. Single-knockout STAT6−/− (Fig. 3B) and CD28−/− siblings (data not shown) had a normal cutaneous phenotype. In STAT6/CD28−/− mice, the epithelium was characterized by acanthosis and hyperkeratosis with focal areas of parakeratosis. Fibrosis of the dermis was also observed in more severely affected mice with ulcerations, as were pronounced lymphoid infiltrates in the subcutaneous fat that in some cases essentially replaced the adipose tissue. However, there was no evidence of vasculitis. Histological analysis of the deep dermis and subcutaneous fat of STAT6/CD28−/− mice revealed few neutrophils or eosinophils but substantial numbers of lymphocytes (Fig. 3C). Immunofluorescence analysis indicated that the lymphoid infiltrates were comprised primarily of CD4 and CD8 T cells (Fig. 3D). Further flow cytometric analyses showed that there were pronounced increases in CD4 and CD8 T cells in the skin of STAT6/CD28−/− mice, while increases in these T-cell subpopulations were not detected in STAT6−/− or CD28−/− mice.
FIG. 3.
Dermatitis associated with Demodex infestation in STAT6/CD28−/− mice. (A) Hematoxylin- and eosin-stained skin section (magnification, ×200) of an 8-month-old STAT6/CD28−/− mouse revealed acanthosis and a dense lymphoid infiltrate in the dermis extending into the subcutaneous fat. In contrast, skin sections from age-matched STAT6−/− mice (B), CD28−/− mice (data not shown), and WT mice were comparable, and there was no indication of dermatitis. (C and D) High magnification (×400) of the deep dermis of STAT6/CD28−/− mice revealed extensive lymphoid infiltration (C) composed of both CD4 (red) and CD8 (green) T cells (D). (E) Giemsa staining (magnification, ×400) of hair follicles in the skin of 3-month-old STAT/CD28−/− mice revealed numerous Demodex ectoparasites and increased numbers of mast cells. (F) Frequency of Demodex mites in randomly selected high-power fields (magnification, ×200) of hematoxylin- and eosin-stained skin sections (four mice per treatment group) from age-matched STAT6/CD28−/−, STAT6−/−, CD28−/−, and WT mice. (G) Isolated ectoparasites (magnification, ×400) identified as D. musculi. a, acanthosis; d, dermis; ly, lymphoid infiltrate; m, mast cells; De, Demodex; f, follicle; hpf, high-power field.
Analysis of Giemsa-stained preparations showed that there were increases in the numbers of mast cells but not in the frequency of mast cell degranulation (Fig. 3E). The lack of eosinophilia in the skin sections of the STAT6/CD28−/− mice suggested a nonatopic dermatitis, which is consistent with the absence of STAT6, an important pathway for IL-4 signaling. Thus, although an IL-4 response could develop, the absence of STAT6 prevented effective IL-4 signaling, thereby blocking a functional Th2 response. Further histological analysis revealed that large numbers of a specific ectoparasite, Demodex musculi, which normally is an uncommon commensal mite in mice, were present in the hair follicles of the STAT6/CD28−/− mice (Fig. 3E). Demodex was observed either on the skin surface or inside the hair follicles of STAT6/CD28−/− mice in all tissues examined, and there were as many as five mites per hair follicle in a single section. The follicles were enlarged and showed perifollicular inflammation, but there were few or no lymphocytes invading the follicular epithelium. High numbers of Demodex were observed in STAT6/CD28−/− mice as young as 3 months old, although at this time lymphoid infiltrates were not observed (Fig. 3E). Demodex is normally ubiquitous in mice, but it is present at extremely low levels in mouse follicles and there are no associated symptoms. In some immunodeficient strains, increased levels of Demodex are observed, but the increases are not associated with dermatitis (14). The frequency of mites was determined by counting the number of mites in high-power fields in individual skin sections (five mice per treatment group) from age-matched (6- to 8-month-old) STAT6/CD28−/−, STAT6−/−, CD28−/−, and WT mice. As shown in Fig. 3F, significant numbers of mites were detected only in the STAT6/CD28−/− mice. Demodex ectoparasites were isolated and identified as D. musculi (Fig. 3G) based on morphological criteria.
Lymphadenapathy associated with a pronounced Th1 immune response occurs in STAT6/CD28−/− mice.
To identify the expanded lymphocyte populations in the enlarged lymph nodes of the STAT6/CD28−/− mice and to determine whether specific lymphoid populations were preferentially increased, the numbers of T and B cells in the submaxillary lymph nodes of STAT6−/−, CD28−/−, and STAT/CD28−/− mice were compared (Table 1). Marked increases (>10-fold) in the numbers of both T and B cells were observed in STAT6/CD28−/− mice compared to the numbers of these cells in either WT or single-knockout controls (P ≤ 0.01). The number of CD8 T cells increased preferentially, which resulted in a lower CD4/CD8 ratio.
TABLE 1.
Total numbers and distribution of lymphocytes in submaxillary lymph nodes of STAT6−/−, CD28−/−, and STAT6/CD28−/− mice
| Animalsa | No. of cells/lymph node (106)
|
CD4/CD8 T-cell ratio in lymph nodes | No. of cells/lymph node (106)
|
|||
|---|---|---|---|---|---|---|
| Lymphocytes | T cells | B cells | CD4 T cells | CD8 T cells | ||
| WT | 2.10 ± 0.54b | 1.14 ± 0.24 | 0.59 ± 0.12 | 2.67 ± 0.11 | 0.80 ± 0.20 | 0.29 ± 0.06 |
| STAT6−/− | 1.84 ± 0.25 | 1.0 ± 0.15 | 0.51 ± 0.04 | 3.19 ± 0.26 | 0.78 ± 0.11 | 0.25 ± 0.05 |
| CD28−/− | 1.29 ± 0.51 | 0.72 ± 0.30 | 0.43 ± 0.15 | 2.27 ± 0.32 | 0.46 ± 0.19 | 0.21 ± 0.08 |
| STAT6/CD28−/− (4 mo) | 49.43 ± 0.71c | 22.06 ± 0.02c | 14.36 ± 0.28c | 1.94 ± 0.06 | 15.67 ± 0.89c | 7.99 ± 0.24c |
| STAT6/CD28−/− (8 mo) | 28.52 ± 0.60c | 14.49 ± 0.40c | 10.55 ± 0.50c | 1.63 ± 0.05 | 8.37 ± 0.81c | 5.13 ± 0.38c |
WT, STAT6−/−, CD28−/−, and STAT6/CD28−/− BALB/c mice that were 4 and 8 months old (three mice in each group) were killed, and submaxillary lymph nodes were collected. Single-cell suspensions of the lymph nodes were stained to determine the numbers of B cells (B220), T cells (CD3), CD4 T cells (GK1.5), and CD8 T cells (Ly2).
Mean ± standard error for three mice.
P ≤ 0.01.
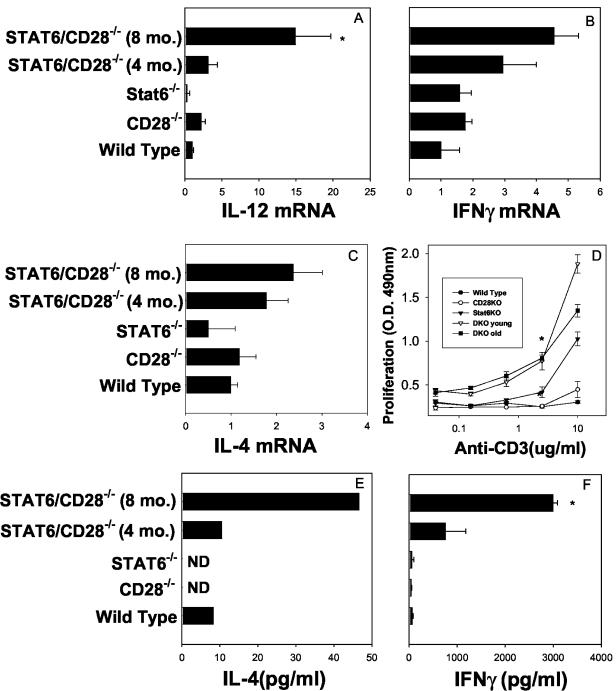
Analysis of cytokine gene expression by real-time RT-PCR showed that there were significant increases in the levels of IL-12 (P ≤ 0.01) (Fig. 4A) and IFN-γ mRNA (Fig. 4B) and smaller increases in the levels of IL-4 (Fig. 4C) in peripheral lymph nodes of older STAT6/CD28−/− mice than in the peripheral lymph nodes of WT, STAT6−/−, or CD28−/− mice. Cell suspensions from the lymph nodes were cultured with anti-CD3 to examine whether T cells were primed compared to the cells in WT or single-knockout controls. As shown in Fig. 4D, lymphocytes from older STAT6/CD28−/− mice showed markedly increased proliferation compared to lymphocytes from WT, CD28−/−, or STAT6−/− mice (at a concentration of 2.5 μg/ml; P ≤ 0.01). Supernatants from lymphocytes cultured with anti-CD3 (2.5 μg/ml) were analyzed to determine their cytokine contents by ELISA, and significant increases in the levels of IL-4 (Fig. 2E) and IFN-γ protein (Fig. 2F) were detected in STAT6/CD28−/− mice compared to the levels in the WT or single-knockout controls (P ≤ 0.01). These experiments were repeated, and similar results were obtained. The data suggest that T cells in the enlarged lymph nodes were activated and primed to produce both Th1 and Th2 cytokines. At 4 and 8 months of age, CD28−/− and STAT6−/− mouse lymph nodes showed similar levels of lymph node cytokine expression.
FIG. 4.
Lymphadenapathy in STAT6/CD28−/− mice is associated with increases in the levels of IL-12 and IFN-γ mRNA and pronounced T-cell activation. (A to C) IL-12 (A), IFN-γ (B), and IL-4 (C) mRNA levels were compared by real-time RT-PCR analysis in submaxillary lymph nodes of WT, CD28−/−, STAT6−/−, and STAT6/CD28−/− mice that were 4 and 8 months old. (D) Lymph node cells from STAT6/CD28−/− mice and single-knockout and WT controls were cultured for 3 days with anti-CD3 antibody, and proliferation was assessed by using a modified 3(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. (E and F) Supernatants from cultured cells were assessed to determine the levels of IL-4 and IFN-γ protein by using ELISA. Lymph nodes were obtained from four mice per treatment group, and the means and standard errors are shown. An asterisk indicates that the P value is ≤0.01. O.D. 490nm, optical density at 490 nm; CD28KO, CD28 knockout; Stat6KO, Stat6 knockout; DKO, double knockout.
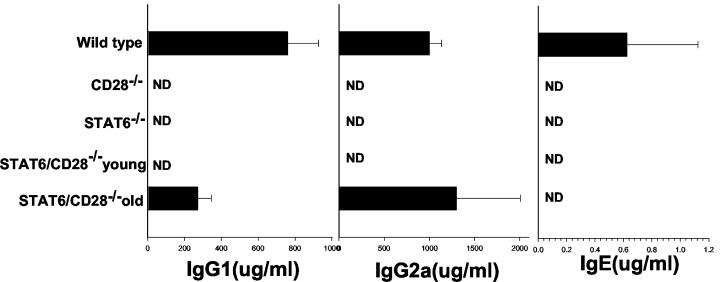
The pronounced increases in the levels of IFN-γ in the draining lymph nodes and the increases in the numbers of CD8 T cells suggested that a Th1 response spontaneously developed in the STAT6/CD28−/− mice. To examine whether this was reflected in the humoral response, serum immunoglobulin isotypes were determined by ELISA. Serum IgE was detectable in WT BALB/c controls but was not detectable in STAT6−/−, CD28−/−, and STAT6/CD28−/− BALB/c mice. In contrast, the IgG1 and IgG2a levels were elevated in older STAT6/CD28−/− mice compared to the levels in either the CD28−/− or STAT6−/− controls (Fig. 5). The increased levels of IgG2a and the absence of IgE are consistent with a Th1 response and the absence of a functional Th2 response. The elevated serum IgG1 levels suggest that there is T-cell help but do not necessarily implicate specific cytokines since previous studies have demonstrated that T-cell-dependent increases in serum IgG1 levels can be IL-4 independent (16, 27).
FIG. 5.
Serum IgG2a levels in STAT6/CD28−/− mice are greater than serum IgG2a levels in STAT6−/− or CD28−/− mice. Serum IgE, IgG1, and IgG2a levels were quantitated by an ELISA. Sera were obtained from four mice per treatment group, and the means and standard errors are shown. ND, not detected.
Administration of an effective miticide markedly reduces acanthosis and dermal lymphoid infiltrates.
Our findings showed that a pronounced immune response and chronic dermatitis developed in STAT6/CD28−/− mice. Infestation by the ectoparasite Demodex was associated with this skin disorder, suggesting that this mite, which normally is ubiquitous in mice but is not known to elicit disease, may cause severe dermatitis in STAT6/CD28−/− mice. Alternatively, the Th1 inflammatory response rather than the ectoparasite may have been the proximal cause of the dermatitis. To examine this possibility, the miticide amitraz or a similar volume of water was topically applied biweekly to separate groups of STAT6/CD28−/− mice (five mice per treatment group). The mice were treated for 8 weeks, and the mites were monitored by using skin scrapings. By 3 weeks, mites were present at almost undetectable levels in amitraz-treated STAT6/CD28−/− mice compared to the levels in the control STAT6/CD28−/− mice that were treated with water (data not shown). The amitraz-treated mice showed increased hair growth on the dorsal region, and the pruritus was markedly decreased compared to that in the control water-treated group (data not shown). Eight weeks after the initial amitraz administration, mice were sacrificed, and dermatitis and immune activation were analyzed. Amitraz treatment resulted in elimination of lymphoid infiltrates in the dermis and subcutaneous fat and markedly reduced hyperkeratosis and acanthosis (Fig. 6B). Increased numbers of anagen hair follicles were also observed in the amitraz-treated mice (Fig. 6B), which is consistent with the observation that there was increased hair growth. These effects were observed in all animals in the amitraz-treated group, whereas skin samples from the control mice treated with water continued to exhibit severe dermatitis (Fig. 6A). These studies indicated that the Demodex ectoparasites caused the dermatitis and associated lymphoid infiltrates. Although induction of dermatitis by transfer of Demodex parasites from infected to uninfected STAT6/CD28−/− mice would further confirm our findings, such experiments are not currently feasible because of the ubiquitous presence of normally small numbers of Demodex ectoparasites on mice in our barrier facilities, as in other barrier facilities.
FIG. 6.
Elimination of Demodex ectoparasites, present at high levels in STAT6/CD28−/− mice, effectively reduced dermatitis: representative hematoxylin- and eosin-stained skin sections (magnification, ×200) from control STAT6/CD28−/− mice (five mice per treatment group) treated with water (A) and STAT6/CD28−/− mice (five mice per treatment group) treated with amitraz (B). a, acanthosis; d, dermis; e, epidermis.
To further examine the possibility that the lymphocytes from Demodex-infested STAT6/CD28−/− mice could induce inflammatory dermatitis, CD4 or CD8 lymphocytes that were obtained from peripheral lymph nodes of STAT6/CD28−/− mice with dermatitis were transferred to SCID mice. Although in reconstituted SCID mice there were pronounced increases in the numbers of donor CD4 and CD8 lymphocytes, no evidence of dermatitis was detected by 12 weeks after transfer (data not shown).
Elimination of Demodex is associated with reduced peripheral T-cell activation.
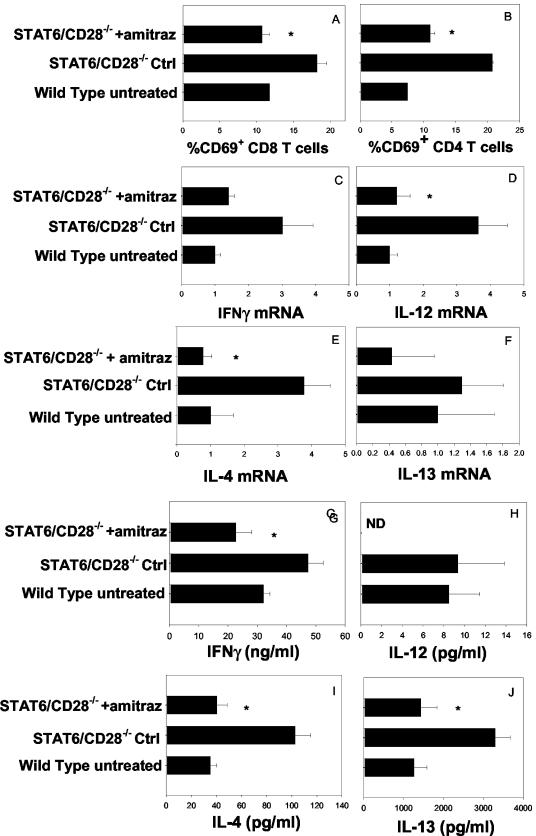
The reduction in lymphoid infiltration of the dermal and subdermal regions of the skin suggested that elimination of Demodex might also reduce lymphocyte activation in the draining lymph nodes. The submaxillary lymph nodes from STAT6/CD28−/− mice treated with amitraz or water were compared to the submaxillary lymph nodes of untreated WT mice (five mice per treatment group). The levels of CD69 expression on CD4 and CD8 T cells in the draining lymph nodes of STAT6/CD28−/− mice were significantly reduced (P ≤ 0.01) compared to the levels in STAT6/CD28−/− mice treated with water and were comparable to the levels observed in WT untreated controls (Fig. 7A and B). Quantitative real-time RT-PCR analysis was used to assess Th1 and Th2 cytokine gene expression from lymph nodes of amitraz-treated and water-treated control STAT6/CD28−/− mice. Increases in the levels of IFN-γ (Fig. 7C), IL-12 (P ≤ 0.05) (Fig. 7D), IL-4 (P ≤ 0.05) (Fig. 7E), and IL-13 (Fig. 7F) observed in STAT6/CD28−/− mice were blocked following administration of amitraz. Lymph node cells from amitraz-treated and water-treated control mice were also stimulated in vitro with anti-CD3 antibody to assess T-cell cytokine production. Decreases in the levels of IFN-γ (P ≤ 0.05) (Fig. 7G), IL-4 (P ≤ 0.05) (Fig. 7I), and IL-13 (P ≤ 0.05) (Fig. 7J) were detected in supernatants of anti-CD3 antibody-stimulated lymph node cells from STAT6/CD28−/− mice treated with amitraz compared to the levels in STAT6/CD28−/− mice treated with water. IL-12 was not detectable in mice treated with amitraz (Fig. 7H). In most cases the increased cytokine concentrations in anti-CD3 antibody-stimulated lymph node cell suspensions from amitraz-treated STAT6/CD28−/− mice were comparable to the levels in WT untreated controls. These studies indicate that the T-cell activation and increases in cytokine expression in the peripheral lymph nodes of STAT6/CD28−/− mice were predominantly caused by localized Demodex infestations in the skin hair follicles.
FIG. 7.
Increases in the numbers of activated T cells and cytokine expression are inhibited in STAT6/CD28−/− mice treated with amitraz. (A and B) STAT6/CD28−/− mice (five mice per treatment group) were treated with amitraz or water biweekly for 8 weeks. Submaxillary lymph nodes were then collected from individual mice, and cell suspensions were stained with FITC-anti-CD8 and PE-anti-CD69 (A) or with FITC-anti-CD4 and PE-anti-CD69 (B). (C to F) Cytokine gene expression in lymphoid tissue as determined by using real-time RT-PCR was measured for IFN-γ (C), IL-12 (D), IL-4 (E), and IL-13 (F). (G to J) Supernatants from lymph node cells cultured with anti-CD3 (2.5 μg/ml) for 3 days were assayed by ELISA to determine the levels of IFN-γ (G), IL-12 (H), IL-4 (I), and IL-13 (J). All data are expressed as means and standard errors for five mice per treatment group. An asterisk indicates that the P value is ≤0.05. Ctrl, control; ND, not determined.
DISCUSSION
Our studies indicate that novel STAT6/CD28−/− mice housed under SPF conditions spontaneously develop a severe chronic inflammatory skin disease associated with pronounced lymphoid infiltrates composed primarily of CD4 and CD8 T cells. The sizes of lymph nodes draining the affected skin region are markedly increased; both the T cells and B cells increased in numbers, and there are marked increases in both IFN-γ and IL-12 levels. In contrast, STAT6−/− mice and CD28−/− mice have a normal skin phenotype when they are housed under similar conditions. Furthermore, large numbers of the ectoparasite Demodex were detected in the hair follicles and on the skin surfaces of STAT6/CD28−/− mice but not in single-knockout mice, and treatment with an effective miticide greatly reduced the severity of the dermatitis. These results suggest that Demodex, which is usually considered a saprophytic commensal in mice, can contribute to severe skin pathogenesis when specific immune cell signaling pathways associated with Th2 cell activation and function are inhibited.
Both STAT6 and CD28 signaling molecules are important in immune cell function during infectious disease. STAT6 mediates IL-4 receptor signaling in both hematopoietic and nonhematopoietic tissues and is required for the development of host protective Th2 immune responses against nematode parasites (7, 28), although IL-4-producing T cells can still develop in the absence of STAT6 (7, 15). CD28 is a T-cell costimulatory molecule, which is important in the development of a number of Th2 immune responses, but it is frequently not required for the development of Th1 responses associated with increases in IFN-γ levels (6, 26). In vitro studies of CD28−/− T cells have suggested that, although unresponsive to anti-CD3, the combination of anti-CD3 and IL-4 promotes activation of these cells and their differentiation to Th2 cells (23). We hypothesized that a deficiency in both CD28 and STAT6 might inhibit the development of CD28-independent in vivo Th2 responses. However, our studies showed that a Th2 response characterized as STAT6 independent can still occur when CD28 signaling pathways, as well as STAT6 signaling pathways, are inhibited. It should be noted that our studies of the N. brasiliensis-induced Th2 response were performed with young mice prior to the onset of observable Demodex-induced inflammatory skin disease.
The unexpected finding that a deficiency in both CD28 and STAT6 triggers the development of Demodex-induced inflammatory skin disease suggests that these signaling molecules support an immune response that normally controls a ubiquitous ectoparasite. Demodex is present, but uncommon, in most mice, even in clean barrier facilities. In some immunodeficient strains of mice, including a T-cell-deficient mouse strain transgenic for human CD3epsilon (29), increased numbers of Demodex have been detected, but associated dermatitis or other pathological effects were not observed (14). Interestingly, neither CD28−/− nor STAT6−/− mice had increased numbers of Demodex, even when they were housed in the same cages, indicating that control of Demodex infestation is lost only when the functions of both molecules are inhibited. The increased numbers of Demodex in the STAT6/CD28−/− mice indicate that these specific molecules support a response that may play a primary role in controlling infestation by ectoparasites. The development of skin inflammatory disease caused by this ectoparasite in STAT6/CD28−/− mice has not previously been reported for immunodeficient strains with known genetic deficiencies. The lymphoid hyperplasia in these mice may result from the development of an ineffective immune response that is continuously stimulated by Demodex infection. It is also possible that the combined deficiency in STAT6 and CD28 promotes a dysregulated immune response that is triggered and sustained by Demodex. The recent finding that CD28−/− NOD mice lack a CD4+ CD25+ regulatory T-cell population that controls the Th1 autoimmune response (24) suggests the possibility that the absence of this population may contribute to the inflammatory skin disease in the STAT6/CD28−/− mice. However, our finding that transfer of STAT6/CD28− /− lymphocytes to SCID mice did not induce dermatitis or Demodex infestation suggests that other cell types, perhaps responding to STAT6-mediated IL-4 signaling, are important in resistance to Demodex. It should also be considered that the viability and/or fecundity of certain parasites is directly enhanced by specific cytokines associated with the inflammatory response, including tumor necrosis factor alpha (1); it is possible that Demodex may be similarly influenced by a factor(s) associated with the inflammatory response.
STAT/CD28−/− mice should provide a useful model for identifying the mechanisms that control the development of dermatitis during ectoparasite infections. Although these mice are capable of developing a Th2 response to intestinal nematode parasites, which typically promote strongly polarized Th2 responses, the absence of STAT6 signaling prevents worm expulsion. The combined blockade of STAT6 and CD28 apparently inhibits an immune response that normally controls the Demodex ectoparasite, suggesting that a redundant protective mechanism that is dependent on CD28 signaling is present in the absence of STAT6. The observed dermatitis is of particular interest because it is nonatopic. Chronic dermatitis is rare in mice, and one well-described mouse strain, the NC mouse strain, spontaneously develops chronic dermatitis. Although the genetic basis for dermatitis in NC mice is unknown, the disease is associated with a pronounced Th2 immune response, including increased serum IgE levels and eosinophil infiltration in the skin. This is a markedly different phenotype than that observed in the STAT6/CD28−/− mice, in which IgE was not detectable and, although massive lymphoid infiltrates occurred, eosinophils remained scarce. Although the number of Demodex mites is increased in NC mice (30), recent studies suggest that bacteria cause the atopic dermatitis by triggering an inappropriate Th2 immune response that mediates allergic inflammation (12). It is unlikely that bacteria play a major role in the etiology of dermatitis in STAT6/CD28−/− mice because (i) Gram stains did not reveal increased numbers of bacteria; (ii) a miticide effectively cured the dermatitis; and (iii) the dermatitis developed in a continuously monitored SPF barrier facility. In an experimental model of atopic dermatitis, a transgenic mouse that overexpressed IL-4 in the epidermis was recently developed (5). Elevated IgE levels and eosinophil infiltration in the skin were observed, similar to the atopic dermatitis observed in the NC mice.
The ectoparasite Demodex is generally considered to be a ubiquitous commensal saprophyte in humans, as well as in mice. In humans, Demodex is found in all types of rosacea and is also associated with blepharitis (also observed in the STAT6/CD28−/− mice), but whether this ectoparasite plays a significant role in the pathogenesis is controversial; in some recent studies the workers have concluded that it is merely a commensal (3, 18). Demodex-associated rosacea has also been observed in human immunodeficiency virus-infected patients (17) and other immunocompromised patients (2, 22). In temperate regions, the Th2 response is generally considered more harmful than beneficial, since it causes immediate hypersensitivity, whereas in tropical regions it may mediate protection against endemic helminthic parasites. The findings reported here suggest the possibility that an intact functional Th2 response is important in controlling ectoparasites, which are ubiquitous in temperate regions, and provide evidence for a potential complication in new allergy treatments that target blockade of the Th2 immune response.
Acknowledgments
We thank Clifford Desch (University of Massachusetts) for identifying the Demodex species and for providing helpful advice concerning quantitating of Demodex organisms.
This work was supported by National Institutes of Health grant AI31678.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Amiri, P., R. M. Locksley, T. G. Parslow, M. Sadick, E. Rector, D. Ritter, and J. H. McKerrow. 1992. Tumour necrosis factor alpha restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice. Nature 356:604-607. [DOI] [PubMed] [Google Scholar]
- 2.Aydinggoz, I. E., T. Mansur, and B. Dervent. 1997. Demodex folliculorum in renal transplant patients. Dermatology 195:232-234. [DOI] [PubMed] [Google Scholar]
- 3.Bonnar, E., P. Eustace, and F. C. Powell. 1991. Demodex mite in normal skin. Lancet 337:1168. [DOI] [PubMed] [Google Scholar]
- 4.Brown, D. R., J. M. Green, N. H. Moskowitz, M. Davis, C. B. Thompson, and S. L. Reiner. 1996. Limited role of CD28-mediated signals in T helper subset differentiation. J. Exp. Med. 184:803-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan, L. S., N. Robinson, and L. Xu. 2001. Expression of interleukin-4 in the epidermis of transgenic mice results in a pruritic inflammatory skin disease: an experimental animal model to study atopic dermatitis. J. Investig. Dermatol. 117:977-983. [DOI] [PubMed] [Google Scholar]
- 6.Corry, D. B., S. L. Reiner, P. S. Linsley, and R. M. Locksley. 1994. Differential effects of blockade of CD28-B7 on the development of Th1 or Th2 effector cells in experimental leishmaniasis. J. Immunol. 153:4142-4148. [PubMed] [Google Scholar]
- 7.Finkelman, F. D., S. C. Morris, T. Orekhova, M. Mori, D. Donaldson, S. L. Reiner, N. L. Reilly, L. Schopf, and J. F. Urban, Jr. 2000. Stat6 regulation of in vivo IL-4 responses. J. Immunol. 164:2303-2310. [DOI] [PubMed] [Google Scholar]
- 8.Finkelman, F. D., T. Shea-Donohue, J. Goldhill, C. A. Sullivan, S. C. Morris, K. B. Madden, W. C. Gause, and J. F. Urban, Jr. 1997. Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu. Rev. Immunol. 15:505-533 [DOI] [PubMed] [Google Scholar]
- 9.Fowell, D. J., J. Magram, C. W. Turck, N. Killeen, and R. M. Locksley. 1997. Impaired Th2 subset development in the absence of CD4. Immunity 6:559-569. [DOI] [PubMed] [Google Scholar]
- 10.Gause, W. C., R. Greenwald, M. J. Halvorson, P. Lu, X.-D. Zhou, S. J. Chen, S. C. Morris, K. P. Lee, C. H. June, F. D. Finkelman, J. F. Urban, and R. Abe. 1997. CD28-dependence of T cell differentiation to IL-4 production varies with the particular type 2 immune response. J. Immunol. 158:4082-4087. [PubMed] [Google Scholar]
- 11.Greenwald, R. J., J. F. Urban, M. J. Ekkens, S. Chen, D. Nguyen, H. Fang, F. D. Finkelman, A. H. Sharpe, and W. C. Gause. 1999. B7-2 is required for the progression but not the initiation of the type 2 immune response to a gastrointestinal nematode parasite. J. Immunol. 162:4133-4139. [PubMed] [Google Scholar]
- 12.Habu, Y., S. Seki, E. Takayama, T. Ohkawa, Y. Koike, K. Ami, T. Majima, and H. Hiraide. 2001. The mechanism of a defective IFN-gamma response to bacterial toxins in an atopic dermatitis model, NC/Nga mice, and the therapeutic effect of IFN-gamma, IL-12, or IL-18 on dermatitis. J. Immunol. 166:5439-5447. [DOI] [PubMed] [Google Scholar]
- 13.Harris, N. L., R. J. Peach, and F. Ronchese. 1999. CTLA4-Ig inhibits optimal T helper 2 cell development but not protective immunity or memory response to Nippostrongylus brasiliensis. Eur. J. Immunol. 29:311-316. [DOI] [PubMed] [Google Scholar]
- 14.Hill, L. R., P. S. Kille, D. A. Weiss, T. M. Craig, and L. G. Coghlan. 2001. Demodex musculi in the skin of transgenic mice. Contemp. Top. Lab. Med. 38:13-18. [PubMed] [Google Scholar]
- 15.Jankovic, D., M. C. Kullberg, N. Noben-Trauth, P. Caspar, W. E. Paul, and A. Sher. 2000. Single cell analysis reveals that IL-4 receptor/Stat6 signaling is not required for the in vivo or in vitro development of CD4+ lymphocytes with a Th2 cytokine profile. J. Immunol. 164:3047-3055. [DOI] [PubMed] [Google Scholar]
- 16.Jankovic, D., M. C. Kullberg, N. Noben-Trauth, P. Caspar, J. M. Ward, A. W. Cheever, W. E. Paul, and A. Sher. 1999. Schistosome-infected IL-4 receptor knockout (KO) mice, in contrast to IL-4 KO mice, fail to develop granulomatous pathology while maintaining the same lymphokine expression profile. J. Immunol. 163:337-342. [PubMed] [Google Scholar]
- 17. Jansen, T., U. Kastner, A. Kreuter, and P. Altmeyer. 2001. Rosacea-like demodicidosis associated with acquired immunodeficiency syndrome. Br. J. Dermatol. 144:139-142. [DOI] [PubMed] [Google Scholar]
- 18.Kamoun, B., M. Fourati, J. Feki, M. Mlik, F. Karray, A. Trigui, S. Ellouze, B. Hammami, M. Chaabouni, and A. Ayadi. 1999. Blepharitis due to Demodex: myth or reality?. J. Fr. Ophtalmol. 22:525-527. [PubMed] [Google Scholar]
- 19.Kaplan, M. H., U. Schindler, S. T. Smiley, and M. J. Grusby. 1996. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity 4:313-319. [DOI] [PubMed] [Google Scholar]
- 20.Katona, I. M., J. F. Urban, Jr., I. Scher, C. Kanelopoulos-Langevin, and F. D. Finkelman. 1983. Induction of an IgE response in mice by Nippostrongylus brasiliensis: characterization of lymphoid cells with intracytoplasmic or surface IgE. J. Immunol. 130:350. [PubMed] [Google Scholar]
- 21.Lenschow, D. J., T. L. Walunas, and J. A. Bluestone. 1996. CD28/B7 system of T cell costimulation. Annu. Rev. Immunol. 14:233-259. [DOI] [PubMed] [Google Scholar]
- 21a.National Institutes of Health. 1985. Guide for the care and use of laboratory animals. National Institutes of Health publication no. 85-23. National Institutes of Health, Bethesda, Md.
- 22.Roihu, T., and A. L. Kariniemi. 1998. Demodex mites in acne rosacea. J. Cutan. Pathol. 25:550-552. [DOI] [PubMed] [Google Scholar]
- 23.Rulifson, I. C., A. I. Sperling, P. E. Fields, F. W. Fitch, and J. A. Bluestone. 1997. CD28 costimulation promotes the production of Th2 cytokines. J. Immunol. 158:658-665. [PubMed] [Google Scholar]
- 24.Salomon, B., D. J. Lenschow, L. Rhee, N. Ashourian, B. Singh, A. Sharpe, and J. A. Bluestone. 2000. B7/CD28 costimulation is essential for the homeostasis of the CD4+ CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 12:431-440. [DOI] [PubMed] [Google Scholar]
- 25.Svetic, A., F. D. Finkelman, Y. C. Jian, C. W. Diffenbach, D. E. Scott, K. F. McCarthy, A. D. Steinberg, and W. C. Gause. 1991. Cytokine gene expression after in vivo primary immunization with goat antibody to mouse IgD antibody. J. Immunol. 147:2391-2397. [PubMed] [Google Scholar]
- 26.Urban, J., H. Fang, Q. Liu, M. J. Ekkens, S. J. Chen, D. Nguyen, V. Mitro, D. D. Donaldson, C. Byrd, R. Peach, S. C. Morris, F. D. Finkelman, L. Schopf, and W. C. Gause. 2000. IL-13-mediated worm expulsion is B7 independent and IFN-gamma sensitive. J. Immunol. 164:4250-4256. [DOI] [PubMed] [Google Scholar]
- 27.Urban, J. F., I. M. Katona, W. E. Paul, and F. D. Finkelman. 1992. Interleukin 4 is important in protective immunity to a gastrointestinal nematode infection in mice. Proc. Natl. Acad. Sci. USA 88:5513.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Urban, J. F., Jr., N. Noben-Trauth, D. D. Donaldson, K. B. Madden, S. C. Morris, M. Collins, and F. D. Finkelman. 1998. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity 8:255-264. [DOI] [PubMed] [Google Scholar]
- 29.Wang, B., C. Biron, J. She, K. Higgins, M. J. Sunshine, E. Lacy, N. Lonberg, and C. Terhorst. 1994. A block in both early T lymphocyte and natural killer cell development in transgenic mice with high-copy numbers of the human CD3E gene. Proc. Natl. Acad. Sci. USA 91:9402-9406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamaguchi, T., T. Maekawa, Y. Nishikawa, H. Nojima, M. Kaneko, T. Kawakita, T. Miyamoto, and Y. Kuraishi. 2001. Characterization of itch-associated responses of NC mice with mite-induced chronic dermatitis. J. Dermatol. Sci. 25:20-28. [DOI] [PubMed] [Google Scholar]