Abstract
Vibrio cholerae is a noninvasive enteric bacterium that causes the severe diarrheal disease cholera. Candidate cholera vaccines have been engineered by deleting genes encoding known virulence factors in V. cholerae; however, many of these attenuated strains were still reactogenic in human volunteers. In this study, DNA arrays were utilized to monitor the transcriptional responses of human intestinal epithelial cells (T84) to eight strains of V. cholerae, including attenuated, toxigenic, and environmental isolates. cDNA probes generated from host RNA samples were hybridized against low- and high-density gene arrays. V. cholerae induced the transcription of a variety of host genes and repressed the expression of a lower number of genes. Expression patterns were confirmed for certain genes by reverse transcriptase PCR and enzyme-linked immunosorbent assays. A core subset of genes was found to be differentially regulated in all experiments. These genes included genes involved in innate mucosal immunity, intracellular signaling, and cellular proliferation. Reactogenic vaccine strains induced greater expression of genes for certain proinflammatory cytokines than nonreactogenic strains. Wild-type and attenuated derivatives induced and repressed many genes in common, although there were differences in the transcription profiles. These results indicate that the types of host genes modulated by attenuated V. cholerae, and the extent of their induction, may mediate the symptoms seen with reactogenic cholera vaccine strains.
The human diarrheal disease cholera results from infection with pathogenic strains of the gram-negative enteric bacterium Vibrio cholerae. Many serogroups of V. cholerae have been identified, but only two of these (O1 and O139) are important for epidemic disease. The O1 serogroup is further subdivided into the classical and El Tor biotypes, as well as the Inaba, Ogawa, and Hikojima serotypes. After ingestion, pathogenic V. cholerae bacteria colonize the small intestine and produce a number of virulence factors, notably cholera toxin (CT). This toxin targets and activates the adenylate cyclase within host epithelial cells, provoking the net secretion of chloride ions and water into the intestinal lumen, resulting in the extensive diarrhea that is characteristic of this disease (25). As such, the pathology of cholera has traditionally been considered noninflammatory (5). However, several reports provide evidence for an inflammatory response in cholera disease. Lymphocytes and mononuclear cells have been observed in the intestinal lamina propria in biopsy specimens from cholera patients (16, 40), and increased levels of lactoferrin, myeloperoxidase, and prostaglandins have been measured in stool samples from infected humans (43, 50). Leukocytes and erythrocytes were also detected in the stools (48), and elevated concentrations of nitric oxide metabolites were observed in urine and serum samples (22, 43, 44) from cholera patients. Finally, and of most relevance for this study, V. cholerae vaccine strains caused symptoms consistent with inflammation in human volunteers (31).
When the first recombinant live oral cholera vaccines were originally being planned and constructed, it was assumed that simple deletion of the genes encoding CT (ctx) would be sufficient to render these strains safe and totally avirulent (23, 24, 34). However, although these initial Δctx strains no longer caused the severe diarrhea characteristic of cholera, they still produced a variety of symptoms in several human volunteers, including mild diarrhea, nausea, vomiting, fever, and abdominal cramps (19, 25, 31). Consequently, these strains were not further tested and developed. One subsequent vaccine candidate that is well tolerated is CVD103-HgR (30). This strain has been extensively evaluated for safety and protection in thousands of individuals (37, 46, 51). Although the efficacy of CVD103-HgR in a trial in an environment where cholera is endemic has yet to be established (46), a randomized, double-blind, placebo-controlled trial in North Americans showed that a single dose of this strain engendered 91% protective efficacy against moderate or severe diarrhea due to toxigenic El Tor V. cholerae (51).
The underlying mechanism(s) responsible for the reactogenicity of V. cholerae Δctx strains is unknown. A number of additional toxins have been identified in V. cholerae. These toxins include the zonula occludens toxin (Zot) (14), accessory cholera enterotoxin (Ace) (54), hemagglutinin/protease (Hap) (3), and RtxA (repeats-in-toxin) (32). However, the importance of these factors in the reactogenicity of cholera vaccines in human volunteers has not been established conclusively. Beyond an extensive understanding of the mechanism of CT, the knowledge of the responses of host cells to infection with the cholera pathogen is limited. With the development of new technologies, such as DNA microarrays, it is now possible to monitor the global transcriptional profiles of cells infected with microbes.
In this study, gene arrays were utilized to screen the effects of eight different strains of V. cholerae on gene expression in a human intestinal epithelial cell line. The findings of this investigation indicate that infection with V. cholerae modulates the expression of diverse genes, but predominantly those involved in defense, intracellular signaling, transcription, and cell proliferation. Reactogenic vaccine strains induced more marked expression of these factors than less reactogenic strains. Although related strains regulated a majority of genes in common, each strain provokes a unique transcriptional profile.
MATERIALS AND METHODS
Bacterial strains and human cell line.
The strains of V. cholerae used in this study are listed in Table 1 and are from collections at the Center for Vaccine Development, University of Maryland. Stocks of strains were maintained at −80°C in Luria-Bertani (LB) broth with 50% glycerol and transferred to LB agar plates, as required. Cells of the human intestinal epithelial line T84 were maintained in Dulbecco modified Eagle medium with F-12 nutrient mixture (DMEM/F-12) (Gibco BRL Life Technologies) supplemented with 10% heat-inactivated fetal bovine serum (FBS) and antibiotic-antimycotic solution (Gibco BRL Life Technologies) at 37°C in an atmosphere of 5% CO2.
TABLE 1.
V. cholerae strains used in this study
| Strain | Characteristic(s) | Reference |
|---|---|---|
| 395 | O1 classical, Ogawa; wild type | 27 |
| CVD101 | 395 ΔctxA | 23 |
| CVD103-HgR | O1 classical, Inaba; ΔctxA hlyA::mer | 30 |
| N16961 | O1 El Tor, Inaba; wild-type | 29 |
| JBK70 | N16961 ΔctxAB | 24 |
| CVD110 | O1 El Tor, Ogawa; ΔctxA Δzot Δace hlyA::mer | 36 |
| CVD112 | O139; ΔctxA Δzot Δace hlyA::mer | 52 |
| 1074-78 | O1 El Tor; nontoxigenic environmental isolate | 28 |
Infection protocol: RNA isolation and adhesion assays.
T84 cells were seeded into six-well tissue culture plates (BD Falcon) in DMEM/F-12 with 10% FBS plus antibiotics and grown at 37°C with 5% CO2 to confluency. Prior to infection, the monolayers were washed twice with phosphate-buffered saline (PBS). Fresh DMEM/F-12 supplemented with 10% FBS but without antibiotics was added to the monolayers, and the plates were incubated for 1 h at 37°C with 5% CO2. Cultures of V. cholerae were grown from single colonies in LB broth for approximately 16 h at 37°C on a rotary shaker (250 rpm). Samples of the bacterial cultures equivalent to 108 CFU (multiplicity of infection of approximately 50) were added directly to the wells of plates containing T84 monolayers, and the plates were incubated for 3 h at 37°C and 5% CO2. The T84 cells were washed three times with fresh DMEM/F-12 to remove nonadherent bacteria.
To isolate RNA, the washed cells were lysed through the addition of Trizol (Gibco BRL Life Technologies), and total RNA was isolated according to the manufacturer's instructions. The concentration and purity of the RNA samples were determined spectrophotometrically, and the integrity of the RNA samples was assessed by electrophoresis through a nondenaturing 1.2% agarose gel. Purified RNA was stored at −80°C until required.
Adhesion assays were performed using a modified version of a previously published protocol (4). Briefly, PBS containing 1% Triton X-100 was added to the cells, and the plates were agitated for 30 min at room temperature. The suspension was mixed thoroughly by repeat pipetting, and samples were removed for serial dilutions and plating on LB agar for the determination of cell counts. For enzyme-linked immunosorbent assays (ELISAs), 3 ml of DMEM/F-12 supplemented with 10% FBS and 100 μg of gentamicin per ml were added to each well of the washed monolayers. The cells were then incubated for 18 h at 37°C and 5% CO2, and samples of culture supernatants were removed, centrifuged to remove cell debris, and frozen at −80°C until required.
DNA microarray analysis.
For the low-density gene arrays, radiolabeled cDNAs were prepared from 2-μg samples of total RNA through the incorporation of [α-33P]dCTP (Amersham Biosciences) using the Panorama human cytokine cDNA labeling kit (Sigma Genosys), following the manufacturer's instructions. Unincorporated nucleotides were removed with Microspin G-25 columns (Amersham Biosciences). Samples of the labeled cDNA were hybridized against the Panorama human cytokine gene array (Sigma Genosys) membranes overnight at 65°C. The membranes were washed multiple times, dried, and exposed against a phosphor screen (Molecular Dynamics). Images were scanned (Molecular Dynamics Storm 840) and analyzed using Phoretix Array software (Nonlinear Dynamics).
For high-density arrays, microarrays were printed with 8,064 clones from the Research Genetics cDNA library (Research Genetics, Huntsville, Ala.) enriched for the named genes and printed at 200-μm spacing using a GeneMachines OmniGrid microarray printer with 16 Majer Precision pins (Majer Precision, Tempe, Ariz.). The sequence of each clone used in printing was independently verified in the University of Maryland Greenebaum Cancer Center Genomics Shared Service laboratory prior to printing. Fluorescently labeled cDNA probes were prepared from approximately 20-μg samples of total RNA using CyScribe first-strand labeling kit (Amersham Biosciences), according to the manufacturer's instructions. Probes were purified and concentrated using Microcon YM-30 centrifuge filter devices (Amicon). Fifteen-microliter samples of probes were combined with 5 μl of 20× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 4.25 μl of 10× Denhardt solution, and 0.75 μl of 10% sodium dodecyl sulfate and boiled for 5 min. Probes were applied to high-density DNA microarray slides, which were provided by the University of Maryland Cancer Center Genomics Core Facility, and hybridized overnight at 65°C. Slides were washed in decreasing concentrations of SSC solution, dried, and scanned with a GenePix 4000B microarray scanner (Axon). Data were gathered using GenePix 3.0 software (Axon).
Reverse transcriptase PCR (RT-PCR).
cDNA was prepared from 1-μg samples of total RNA using Superscript first-strand synthesis system (Invitrogen) with the oligo(dT)12-18 primer, according to the manufacturer's instructions. RNA samples were first treated with DNase I (Invitrogen) for 15 min at room temperature. Two-microliter samples of cDNA were used as the template in PCRs with the following primer pairs (5′ → 3′): IL8S (ATGACTTCCAAGCTGGCCGTGGCT) and IL8A (TCTCAGCCCTCTTCAAAACTTCTC) for interleukin-8 (IL-8), MIPS (ATGTGCTGTACCAAGAGTTTGC) and MIPA (CTAAACCCTCCATGATGTGCAAG) for macrophage inflammatory protein 3 alpha (MIP-3α), EGFS (CCACACCAAACAAGGAGGAG) and EGFA (ATGAGAAGCCCCACGATGAC) for diphtheria toxin receptor (Dtr), EGRS (TCACCTATACTGGCCGCTTT) and EGRA (TGAGTGGCAAAGGCCTTAAT) for early growth response factor 1 (Egr-1), and GAPS (ACCACAGTCCATGCCATCAC) and GAPA (TCCACCACCCTGTTGCTGTA) for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Reaction conditions consisted of an initial denaturation step (2 min at 94°C), followed by cycles of denaturation (1 min at 94°C), annealing (1 min at 60°C), and extension (1 min at 72°C). Preliminary experiments were performed with each primer pair to determine the number of reaction cycles that provided a good correlation between template concentration and signal intensity. Ten-microliter samples of the PCR products were resolved through 1% agarose, stained with ethidium bromide, and visualized.
ELISA.
The levels of IL-8 protein in tissue culture supernatants were determined by sandwich ELISA using reagents purchased from BD Pharmingen. The wells on Immuno Maxisorp plates (96-well plates; Nunc) were coated with 0.25-μg amounts of anti-human IL-8 monoclonal antibody per well overnight at 4°C, washed with PBS containing 0.1% Tween 20, and blocked with 5% nonfat dry milk in PBS for 1 h. Plates were washed, and 100-μl aliquots of samples or recombinant human IL-8 standard were then added for 3 h. Plates were washed again, and biotinylated anti-human IL-8 monoclonal detection antibody was added at 0.25 μg/ml for 1 h. After the plates were thoroughly washed, binding was detected by using avidin-horseradish peroxidase (100 μl/well) and hydrogen peroxide tetramethylbenzidine substrate (100 μl/well). Optical density at 405 nm (OD405) was measured with a Labsystems Multiskan Plus reader. Levels of cholera toxin B subunit (CTB) in tissue culture supernatants were also determined by sandwich ELISA using reagents purchased from BD Pharmingen.
Motility assays.
Cultures of V. cholerae were grown from single colonies in LB broth for approximately 16 h at 37°C on a rotary shaker (250 rpm). The cultures were normalized to equivalent OD600 values, and samples were transferred to plates containing LB plus 0.3% agar by deep inoculation. The plates were incubated for 12 h at 37°C, and the swarm zone sizes were recorded.
RESULTS
Effects of V. cholerae infection on global gene expression patterns in host cells.
Human intestinal epithelial cells (T84) were infected for 3 h with strains of V. cholerae, and total RNA samples were purified and used to prepare fluorescently labeled cDNA probes. These probes were hybridized against a high-density gene array containing 8,064 genes to identify changes in host gene transcription. Eight bacterial strains were tested, which are described in Table 1. This group includes toxigenic clinical isolates (395 and N16961), several attenuated candidate cholera vaccine strains (CVD101, CVD103-HgR, JBK70, CVD110, and CVD112), as well as a nontoxigenic environmental strain (1074-78). All of the strains displayed comparable growth characteristics under the experimental conditions (data not shown). Three independent DNA microarray experiments were performed for each strain. The median values from the three data sets for each strain were combined. Flagged entries were removed, and the values were normalized. The data were then arranged by average gene expression in descending order. The full data sets are available as supplementary material via the Gene Expression Omnibus website (http://www.ncbi.nlm.nih.gov/geo/).
Each strain induced a unique transcriptional expression profile. However, in each case, the majority of genes were unchanged for expression after infection. The numbers of genes exhibiting an average increase in expression of twofold or greater in all the strains were comparable, ranging from 89 (strain 1074-78) to 154 (strain CVD110). These genes represent approximately 1.1 to 1.9% of the total number of genes on the array. The numbers of genes exhibiting an average decrease in expression of twofold or greater in the strains were more variable. These numbers ranged from 16 (strain CVD112) to 141 (strain 395), which represents 0.2 to 1.7% of the total number of genes on the array. There was no quantitative association between the numbers of genes with increased expression and genes with decreased expression. However, the number of genes induced exceeded the number repressed for all strains except for strain 395.
Host responses were also assessed for three of the attenuated strains, CVD101, CVD103-HgR, and JBK70, using Panorama human cytokine gene arrays, which contain 375 genes for cytokines, receptors, growth factors, and related factors. The results from these experiments are displayed in Table 2. Using this system, only a limited number of genes were identified as activated, whereas no genes were found to be consistently down-regulated. The majority of the genes with increased expression in this array were also identified as having increased expression in the high-density microarray analysis.
TABLE 2.
Summary of data from cytokine gene array experiments
| Strain | Genea | Expressionb |
|---|---|---|
| CVD101 | Gro-β | +5.8 |
| IL-8 | +4.9 | |
| Gro-α | +4.8 | |
| MIP-3α | +4.1 | |
| Gro-γ | +3.5 | |
| TNF-α | +2.9 | |
| CVD103-HgR | Gro-β | +6.6 |
| Gro-α | +6.0 | |
| Gro-γ | +5.7 | |
| MIP-3α | +3.5 | |
| TNF-α | +3.4 | |
| Dtr (HB-EGF) | +3.3 | |
| LIF | +3.1 | |
| MIC-1 | +3.0 | |
| FGF-5 | +2.8 | |
| IL-5 | +2.7 | |
| Urokinase receptor | +2.6 | |
| Ephrin A1 | +2.4 | |
| IL-8 | +2.3 | |
| EphA2 | +2.1 | |
| JBK70 | Gro-β | +5.6 |
| Gro-α | +5.5 | |
| Gro-γ | +4.6 | |
| IL-8 | +4.3 | |
| MIP-3α | +3.9 | |
| MIC-1 | +3.1 |
Genes also identified as differentially regulated by high-density microarray analysis are shown in bold type. Abbreviations: LIF, leukemia inhibitory factor; MIC-1, macrophage inhibitory cytokine 1; FGF-5, fibroblast growth factor 5.
Average fold change in gene expression in cells infected with the indicated strain of V. cholerae relative to uninfected cells in at least two independent experiments. Only those genes that were up-regulated twofold or greater are included.
Core cellular transcriptional response to V. cholerae infection.
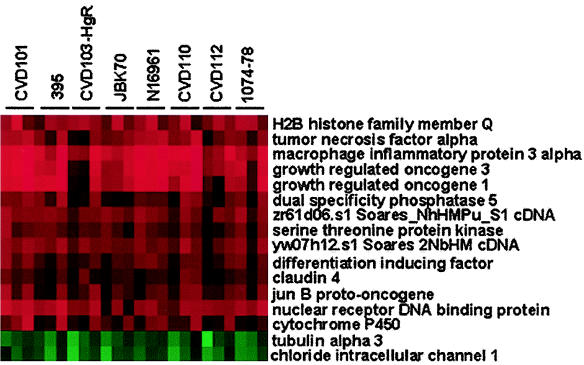
To identify consistently differentially regulated genes, the data from the high-density microarray experiments for all eight strains were combined, transformed, and analyzed by hierarchical clustering (13). This analysis revealed a small, core subset of 16 independent genes that were modulated in all 24 experiments (Fig. 1). Fourteen genes showed increased expression, while only two showed decreased expression after infection with any of the strains of V. cholerae tested, irrespective of serogroup, biotype, or toxigenicity. Prominent among the induced genes are the genes encoding four cytokines that are involved in the inflammatory response (tumor necrosis factor alpha [TNF-α], growth-regulated oncogenes 1 and 3 [GRO1/Gro-α and GRO3/Gro-γ], and MIP-3α/LARC). These were commonly among the most highly induced genes by all strains. In addition, proinflammatory cytokine genes were also identified as up-regulated using the cytokine gene array (Table 2). Also evident were genes encoding three enzymes (dual-specificity phosphatase 5 [DUSP5], serine threonine kinase, cytochrome P450 [CYP1A1]), two DNA-binding proteins (nuclear receptor DNA-binding protein [NAK1] and H2B histone family member Q [H2BFQ]), a gene involved in cell cycle progression (differentiation-inducing factor [DIF-2]), a transcriptional activator of AP-1 (jun-B proto-oncogene [JUNB]), a tight junction protein (claudin 4 [CLDN4]), and two unidentified clones. The genes that were consistently down-regulated encode an intracellular chloride channel (CLIC1) and a subunit of tubulin (TUBA3).
FIG. 1.
Host genes consistently differentially regulated by V. cholerae infection. Cluster analysis was performed on the combined data sets from the high-density microarray analysis for the eight strains of V. cholerae listed at the top of the figure. The genes displaying twofold or greater increases (red) or decreases (green) in expression were identified and are listed to the right of the figure. Each strain was tested in triplicate.
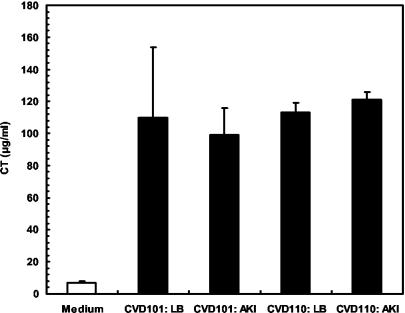
To verify expression of virulence factors under the experimental conditions used, the expression of CTB by two strains, CVD101 and CVD110, was measured by ELISA (Fig. 2). CTB levels were negligible in the control experiment with medium alone added. In contrast, CTB was readily detected in tissue culture medium inoculated with either strain, irrespective of whether the strain had previously been grown in LB medium or AKI medium, which is known to stimulate CT expression. These data indicate that V. cholerae virulence factors are expressed under the experimental conditions used in this investigation (i.e., 3-h incubation with T84 cells), and these factors may elicit some of the observed host cell responses.
FIG. 2.
Expression of CT in tissue culture medium. V. cholerae strains CVD101 (classical) and CVD110 (El Tor) were grown at 37°C in LB medium or AKI medium. The strains were grown for 4 h (statically) and then for 16 h with shaking. Fifty-microliter samples of cultures grown overnight were subcultured into 1-ml portions of DMEM/F-12 and incubated at 37°C for 3 h. Samples of the cultures or uninoculated medium (control) were removed, and concentrations of CT (CTB) were measured by ELISA. The values are the mean CTB levels ± standard deviations (error bars) produced by the two strains that had first been grown in LB or AKI medium and then grown in DMEM/F-12.
A reactogenic vaccine strain induces greater chemokine gene expression than a nonreactogenic vaccine strain.
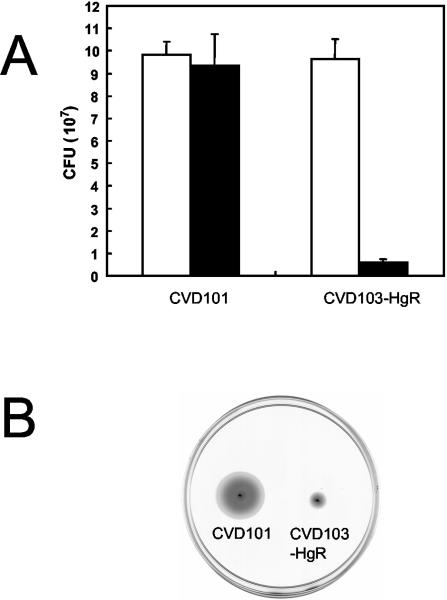
Data from the high-density microarray studies indicated that the attenuated classical vaccine strain, CVD101, induced expression of genes for certain proinflammatory cytokines, or chemokines, more than the classical vaccine strain CVD103-HgR (Fig. 1). This pattern was also observed using the low-density cytokine gene arrays. For example, CVD101 activated greater expression of the genes for IL-8 and MIP-3α than CVD103-HgR (Table 2). These results are consistent with previous studies that have shown CVD103-HgR to be nonreactogenic and well tolerated in human volunteers (30) and also to induce less IL-8 expression in the intestinal epithelial cell line HT29-18N2 (47). The nonreactogenic nature of CVD103-HgR may be due to it being derived from a strain (569B) with reduced ability to colonize the intestine (30). In an adhesion assay, adhesion of CVD103-HgR to T84 cells was approximately 10-fold less than that of CVD101 (Fig. 3A). As several studies have established associations between motility, adhesion, and virulence in V. cholerae (1, 17, 18, 42), the motility of these two strains was compared. As shown in Fig. 3, CVD103-HgR was considerably less motile than CVD101.
FIG. 3.
Adhesion and motility of classical V. cholerae vaccine strains. (A) T84 cells were infected with inocula (white bars) of strains CVD101 and CVD103-HgR for 3 h, and the numbers of adherent vibrios (black bars) were determined. (B) LB agar (0.3%) was inoculated with equivalent samples of cultures of CVD101 and CVD103-HgR grown overnight and incubated at 37°C for 12 h.
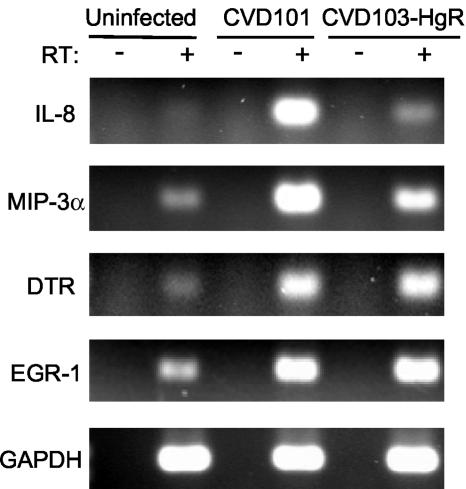
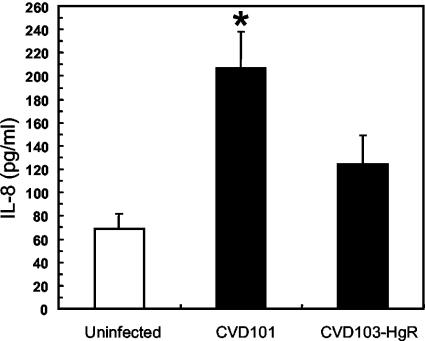
RT-PCR analysis of gene expression was performed to confirm the results of the microarray experiments for selected genes. These data are presented in Fig. 4. In uninfected T84 cells, IL-8 transcripts were not detected, but they were readily measured in cells infected with strain CVD101. In contrast, IL-8 expression was barely detected in cells infected with strain CVD103-HgR. The abundance of MIP-3α transcripts was also greater in cells infected with CVD101 than in cells infected with CVD103-HgR. For all treatments, the levels of expression of the housekeeping GAPDH gene were comparable. Also, no signals were detected in samples that were not treated with RT, indicating that the observed signals were not the result of amplification of genomic DNA or other contaminants. The pattern of synthesis of the IL-8 protein was similar to the transcription pattern of the gene for this chemokine. ELISA showed that infection of T84 cells with CVD101 provoked a statistically significant increase in IL-8 protein secretion over the levels secreted by uninfected cells (Fig. 5). Infection with CVD103-HgR did not significantly increase IL-8 secretion.
FIG. 4.
RT-PCR analysis of gene expression in host cells. T84 cells were infected with V. cholerae strains CVD101 and CVD103-HgR or left uninfected for 3 h. RNA was isolated and used to prepare cDNA using RT. Samples without (−) RT were included to control for genomic DNA contamination. Transcripts were amplified by PCR using primers against IL-8, MIP-3α, Dtr, Egr-1, and the housekeeping gene GAPDH.
FIG. 5.
Levels of IL-8 secreted by infected and uninfected host cells. T84 cells were infected with strains CVD101 and CVD103-HgR or not infected. The concentrations of IL-8 in the culture supernatants were analyzed by ELISA. The values are averages ± standard errors of the means (error bars) for three experiments. Infection with CVD101 induced a statistically significant increase in IL-8 synthesis over that in uninfected cells (P = 0.01) (indicated by the asterisk).
High-density microarray studies also revealed that infection with strains CVD101 and CVD103-HgR induced expression of other, noncytokine genes to similar degrees. Two genes that exhibited this pattern were those encoding Dtr, also known as heparin-binding epidermal growth factor-like growth factor (HB-EGF), and Egr-1. The average expression of these genes was increased 2.3- and 2.5-fold (dtr) and 7.6- and 7.3-fold (egr-1) by CVD101 and CVD103-HgR, respectively. RT-PCR analysis confirmed this pattern, as the abundance of transcripts of both genes was increased to similar extents after infection by CVD101 and CVD103-HgR (Fig. 4).
Comparative transcriptional profiles modulated by toxigenic, attenuated, and nonpathogenic V. cholerae El Tor.
A comparison of the total numbers of genes regulated by infection with three O1 El Tor strains (JBK70, N16961, and 1074-78) was performed to allow a comparison of the transcriptional profiles modulated by these strains. The results from these analyses are displayed diagrammatically in Fig. 6. Although these strains regulated a core of common genes, each strain provoked a distinct transcriptional response.
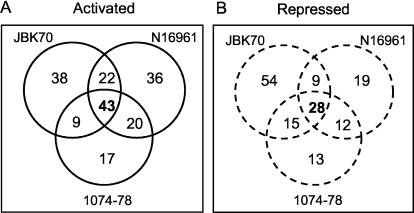
FIG. 6.
Comparative host gene transcription profiles modulated by El Tor strains of V. cholerae. The number of genes induced (A) or repressed (B) in T84 cells infected with attenuated (JBK70), wild-type (N16961), and environmental strains of V. cholerae were compared and illustrated by Venn diagrams. The numbers of common genes modulated by all three strains are indicated in bold type.
A total of 43 genes were activated by all three strains, which is greater than the number of genes induced by any strain individually or by any combination of two strains (Fig. 6A). Notable among the genes specifically induced by the wild-type strain were mitogen-activated protein kinase 6 (MAPK6), the growth factor epiregulin, and an inhibitor of apoptosis (MIHC). Strain 1074-78 specifically activated only 17 genes, which code for diverse functions. Interestingly, however, this environmental isolate induced the gene for Dtr/HB-EGF, which was also activated by infection with the classical strains CVD101 and CVD103-HgR.
The expression of 28 genes was repressed by infection with all three strains. The 28 genes included two genes (TUBA1 and TUBA2) encoding additional subunits of tubulin. Two of the most down-regulated genes by all three strains were those encoding components of the major histocompatibility complex I (HLA-F and HLA-G). Also evident were three genes encoding components related to the structural integrity of the host cells, including two for keratin (KRT14 and KRT19) and a cytoskeleton-associated protein 4 (CKAP4). Interestingly, JBK70 down-regulated considerably more genes, a total of 54 genes, than either of the other two strains, including a number of metabolic enzymes, such as malate dehydrogenase (MDH2) and NADH dehydrogenase (NDFUA9). Again, strain 1074-78 specifically repressed only a limited number of genes.
The attenuated Δctx strain of the O1 serogroup provokes stronger host transcriptional response than a Δctx strain of the O139 serogroup.
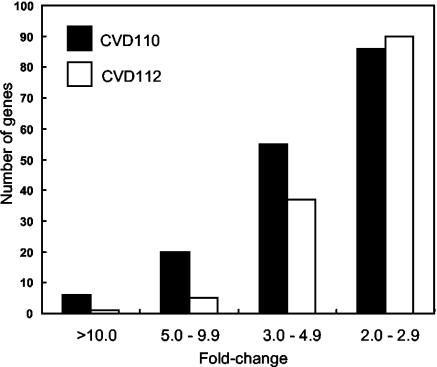
The attenuated Δctx strain vaccine strains CVD110 and CVD112 were engineered by similar methods but are derived from different serogroups. CVD110 was constructed from the parental strain E7946 of the O1 serogroup, while CVD112 was constructed from the parental strain AI-1837 of the O139 serogroup. Although the O1 and O139 serogroups are considered genetically very similar (11), CVD110 was somewhat more reactogenic in human volunteers (7 of 10 subjects had diarrhea with a mean stool volume of 861 ml [range, 377 to 1,687 ml]) (53) than CVD112 (3 of 6 subjects with a mean stool volume of 648 ml [range, 519 to 747 ml]) (52). Data from the high-density microarrays indicated that CVD110 induced greater expression of the core genes than CVD112 in T84 cells (Fig. 1). To determine whether this was a general trend for all the genes activated by these two strains, the entire data sets of these two strains were compared, and the analysis confirmed the pattern seen for the core genes. Among the most highly activated genes, i.e., those induced with average expression ratios of greater than 10-fold, CVD110 induced six genes, while CVD112 induced only one gene (Fig. 7). Among genes activated 5- to 10-fold, CVD110 induced four times as many such genes as CVD112, and among genes activated between three- and fivefold, CVD110 induced more than CVD112 (55 versus 37 genes). Only for modestly induced genes (two- to threefold) was the number of genes activated comparable (86 genes for CVD110 and 90 genes for CVD112). Comparing specific genes confirms this trend. CVD110 activated MIP-3α 37.7-fold compared to only 10.8-fold by CVD112. CVD110 activated Gro-α 29.3-fold compared to only 4.9-fold by CVD112. CVD110 activated interferon regulatory factor 1 (IRF-1) 8.3-fold versus only 2.9-fold by CVD112. Similarly, CVD110 down-regulated the expression of 45 genes more than twofold, compared to CVD112, which repressed only 16 genes.
FIG. 7.
CVD110 elicits a greater host cell transcriptional response than CVD112. The number of host genes induced by infection with strain CVD110 and CVD112 was classified according to the average fold change in expression of three high-density microarray experiments.
DISCUSSION
DNA microarrays represent a powerful tool to study host-pathogen interactions during the process of infection. This technology has been successfully applied to understand further the response of host cells to a range of bacterial, viral, and fungal pathogens (2, 7, 9, 10, 12, 20, 21, 33, 38, 45). This study represents the first report of applying this technology to elucidate the responses of human cells to infection with the causative agent of cholera. More specifically, this study screened host responses to eight strains of V. cholerae, including a number of attenuated live oral cholera vaccine candidates. The findings of this study provide further insight into the dynamics of the V. cholerae-host cell interaction and how this response varies between different wild-type and attenuated strains.
The microarray studies reported here revealed that genes encoding chemokines were markedly induced after infection. Chemokines are important biochemical mediators of the inflammatory response of the intestinal mucosa (56). As such, they function to attract neutrophils, leukocytes, and monocytes to the focus of infection to destroy the infecting microbe. Thus, increased transcription of these genes after infection with V. cholerae was anticipated. Previously it has been demonstrated that attenuated strains of V. cholerae induce IL-8 expression in the intestinal epithelial cell line HT29-18N2 (47). The findings of this investigation indicate that this response is also observed with the T84 intestinal epithelial cell line. Another recent study discovered that infection with attenuated strains of V. cholerae increased TNF-α, IL-6, and MIP-2 concentrations in a murine pulmonary cholera model (15). Thus, the induction of proinflammatory cytokines appears to be an important component of the cellular response to V. cholerae. This provides further support to the theory that cholera has an inflammatory component. Elevated expression of cytokine genes has been identified using DNA microarray technology in host cells infected with a range of other pathogenic bacteria, including enteropathogenic Escherichia coli (9), Salmonella (10, 12, 38), Listeria monocytogenes (7, 38), Bordetella pertussis (2), Mycobacterium tuberculosis (38, 45), Staphylococcus aureus (38), and Helicobacter pylori (33).
Interestingly, however, the expression of chemokine genes was not uniformly activated by all of the strains of V. cholerae tested. Rather, these components were differentially affected by the different strains. Strain CVD101 induced greater expression of IL-8 and MIP-3α than CVD103-HgR. This pattern was confirmed by an independent technique, RT-PCR, for both of these genes (Fig. 4). Moreover, these alterations in expression translated into changes in protein levels for IL-8 (Fig. 5). This observation is consistent with the findings of a separate study, which demonstrated that strain CVD103-HgR induced less transcription and synthesis of IL-8 than other attenuated strains of V. cholerae in another intestinal epithelial cell line (47). These findings correlate with volunteer studies with these two classical strains, which found CVD101 to be reactogenic, while CVD103-HgR was nonreactogenic. Therefore, the degree of induction of these inflammatory genes may correlate with the level of reactogenicity in human volunteers. This is further supported by the finding that the El Tor vaccine strains JBK70 and CVD110, which were both very reactogenic in human volunteers (30, 53), induced greater expression of these genes than strain CVD112 (Fig. 1 and 7), which was less reactogenic in human volunteers (52). Therefore, it may be possible to use the analyses described in this study as a predictive in vitro screen to determine the suitability of live cholera vaccine candidates for further characterization in vivo. It is noteworthy that the well-tolerated strain CVD103-HgR did not induce any anti-inflammatory cytokines (e.g., IL-4 and IL-10), under these conditions.
A key goal in the development of a suitable live cholera vaccine is the characterization of the bacterial mechanism(s) that provoke a reactogenic response in humans. These mechanisms are probably complex and multifaceted (35) and may differ between the various serogroups and biotypes of V. cholerae. For example, RtxA, which appears to be an important reactogenicity factor in El Tor strains (15, 32), is not expressed by classical strains. However, the classical vaccine strain CVD101 is one of the most reactogenic vaccine strains developed (32). One characteristic that may be universally important in reactogenicity is motility. Two well-tolerated attenuated vaccine candidates, Peru-15 (26) and Bengal-15 (8), have been developed from O1 El Tor and O139 strains, respectively. Both of these strains are nonmotile. As indicated in this investigation, the well-tolerated classical vaccine strain CVD103-HgR also exhibits reduced motility (Fig. 3B). Thus, motility may be a universally important reactogenicity factor.
In addition to inducing an inflammatory response, infection with V. cholerae also affects several other cellular processes. Notably, it perturbs intracellular cell signaling, as evidenced by the increased transcription of a dual-specificity phosphatase (DUSP5) and a serine threonine kinase. Dual-specificity phosphatases dephosphorylate residues within mitogen-activated protein kinases and thereby negatively regulate their activity (6). Alterations in intracellular signaling in response to cellular stress lead to the activation of a number of effector transcription factors, which in turn regulate expression of many genes. One of these factors is JunB, a member of the activator protein 1 (AP-1) family. This gene was consistently up-regulated (Fig. 1). Also, many of the strains, including both CVD101 and CVD103-HgR (Fig. 4), induced expression of the transcription factor Egr-1. This gene was up-regulated in epithelial cells after infection with enteropathogenic E. coli (9). Also, many of the strains induced activating transcription factor 3 (ATF3), a gene which was also shown recently to be up-regulated in gastric cells cocultured with H. pylori (49). V. cholerae also appears to inhibit host cell development and proliferation, as infection consistently up-regulated the gene DIF-2 and down-regulated the gene for CLIC1 (Fig. 1). DIF-2 factor is normally down-regulated during differentiation of monocytes (41), while CLIC1 is active in dividing cells (55). Moreover, JunB has been described as a negative regulator of cell proliferation (39).
Comparative analyses of the genes modulated by closely related strains of V. cholerae, e.g., JBK70 (CT negative) and N16961 (CT positive), indicated that these strains modulate a core of common genes (Fig. 6). However, each strain induces and represses a considerable number of specific genes, presumably due to the presence or absence of CT. The nonpathogenic El Tor environmental isolate 1074-78, which did not colonize volunteers (28), regulated fewer genes than the other strains, suggesting that the response it engenders is a subset of the cellular response to the human-adapted JBK70 and N16961 strains that proficiently colonize the human intestine. The proportion of common genes modulated by these various strains was lower than has been recorded in studies with other pathogens. A recent report investigating the responses of macrophages to diverse bacterial pathogens identified 132 genes induced and 59 repressed by all the species studied (38). A separate study using dendritic cells found 166 common genes to be highly regulated by infection with E. coli, Candida albicans, and the influenza virus (20). The different conclusions of those studies compared with this one may reflect technical differences, such as the microarrays used and the criteria employed for selecting regulated genes. Alternatively, differences may relate to the type of host cell used, i.e., epithelial cell versus macrophage, or the bacterial species. Although strain JBK70 is derived from strain N16961, it lacks the ctx genes encoding CT, as does the noncolonizing environmental isolate 1074-78. It would be of interest to determine the cellular transcriptional reaction to purified CT.
In conclusion, this study provides the first report of the global cellular response to V. cholerae infection. This analysis has identified a number of highly regulated factors, the expression of which may mediate the persistent reactogenicity of certain cholera vaccine strains. The results suggest that reactogenicity is more likely determined by the quantitative differences in expression of these factors, rather than any associations between specific factors and certain strains. The preliminary findings of this investigation suggest a number of further lines of investigation using experimental strategies similar to those detailed here. It will be of interest to determine whether the patterns of gene transcription described here are duplicated in other human cell types. Also, as more comprehensive gene arrays become available, it will be important to identify additional factors modulated by infection. Additional studies should also address the temporal patterns of host gene expression during infection. A key question concerns dissecting components of the pathogen to determine whether the host cell responses are modulated by secreted factors, cell surface components, or the adhesion process itself. This study has demonstrated the utility of gene arrays for elucidating the molecular aspects of cholera pathogenesis. It should not be regarded as a definitive, comprehensive study but rather as an initial study that has led to the generation of a number of hypotheses concerning epithelial cell responses and bacterial strain differences that can be tested in subsequent studies. The further application of this technology will provide insights that will be important for the development of superior live cholera vaccines.
Acknowledgments
We thank Diana Gomez for assistance with cell culture and Kristen Kanack, Jing Yin, Yan Xu, and Florin Selaru for advice on preparation and analysis of the microarrays.
This work was supported in part by grants from the National Institutes of Allergy and Infectious Diseases (AI19716) and National Cancer Institute (CA77057, CA85069, CA95323, CA98450, and CA01808)
Editor: V. J. DiRita
REFERENCES
- 1.Attridge, S. R., and D. Rowley. 1983. The role of the flagellum in the adherence of Vibrio cholerae. J. Infect. Dis. 147:864-872. [DOI] [PubMed] [Google Scholar]
- 2.Belcher, C. E., J. Drenkow, B. Kehoe, T. R. Gingeras, N. McNamara, H. Lemjabbar, C. Basbaum, and D. A. Relman. 2000. The transcriptional responses of respiratory epithelial cells to Bordetella pertussis reveal host defensive and pathogen counter-defensive strategies. Proc. Natl. Acad. Sci. USA 97:13847-13852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benitez, J. A., L. Garcia, A. Silva, H. Garcia, R. Fando, B. Cedre, A. Perez, J. Campos, B. L. Rodriguez, J. L. Perez, T. Valmaseda, O. Perez, A. Perez, M. Ramirez, T. Ledon, M. D. Jidy, M. Lastre, L. Bravo, and G. Sierra. 1999. Preliminary assessment of the safety and immunogenicity of a new CTXφ-negative, hemagglutinin/protease-defective El Tor strain as a cholera vaccine candidate. Infect. Immun. 67:539-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benitez, J. A., R. G. Spelbrink, A. Silva, T. E. Phillips, C. M. Stanley, M. Boesman-Finkelstein, and R. A. Finkelstein. 1997. Adherence of Vibrio cholerae to cultured differentiated human intestinal cells: an in vitro colonization model. Infect. Immun. 65:3474-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennish, M. L. 1994. Cholera: pathophysiology, clinical features, and treatment, p. 229-255. In I. K. Wachsmuth, P. A. Blake, and O. Olsvik (ed.), Vibrio cholerae and cholera: molecular to global perspectives. ASM Press, Washington, D.C.
- 6.Camps, M., A. Nichols, and S. Arkinstall. 2000. Dual specificity phosphatases: a gene family for control of MAP kinase function. FASEB J. 14:6-16. [PubMed] [Google Scholar]
- 7.Cohen, P., M. Bouaboula, M. Bellis, V. Baron, O. Jbilo, C. Poinot-Chazel, S. Galiegue, E. H. Hadibi, and P. Casellas. 2000. Monitoring cellular responses to Listeria monocytogenes with oligonucleotide arrays. J. Biol. Chem. 275:11181-11190. [DOI] [PubMed] [Google Scholar]
- 8.Coster, T. S., K. P. Killeen, M. K. Waldor, D. T. Beattie, D. R. Spriggs, J. R. Kenner, A. Trofa, J. C. Sadoff, J. J. Mekalanos, and D. N. Taylor. 1995. Safety, immunogenicity, and efficacy of live attenuated Vibrio cholerae O139 vaccine prototype. Lancet 345:949-952. [DOI] [PubMed] [Google Scholar]
- 9.de Grado, M., C. M. Rosenberger, A. Gauthier, B. A. Vallance, and B. B. Finlay. 2001. Enteropathogenic Escherichia coli infection induces expression of the early growth response factor by activating mitogen-activated protein kinase cascades in epithelial cells. Infect. Immun. 69:6217-6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Detweiler, C. S., D. B. Cunanan, and S. Falkow. 2001. Host microarray analysis reveals a role for the Salmonella response regulator phoP in human macrophage cell death. Proc. Natl. Acad. Sci. USA 98:5850-5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dziejman, M., E. Balon, D. Boyd, C. M. Fraser, J. F. Heidelberg, and J. J. Mekalanos. 2002. Comparative genomic analysis of Vibrio cholerae: genes that correlate with cholera endemic and pandemic disease. Proc. Natl. Acad. Sci. USA 99:1556-1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckmann, L., J. R. Smith, M. P. Housley, M. B. Dwinell, and M. F. Kagnoff. 2000. Analysis by high density cDNA arrays of altered gene expression in human intestinal epithelial cells in response to infection with the invasive enteric bacteria Salmonella. J. Biol. Chem. 275:14084-14094. [DOI] [PubMed] [Google Scholar]
- 13.Eisen, M. B., P. T. Spellman, P. O. Brown, and D. Botstein. 1998. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 95:14863-14868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fasano, A., B. Baudry, D. W. Pumplin, S. S. Wasserman, B. D. Tall, J. M. Ketley, and J. B. Kaper. 1991. Vibrio cholerae produces a second enterotoxin, which affects intestinal tight junctions. Proc. Natl. Acad. Sci. USA 88:5242-5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fullner, K. J., J. C. Boucher, M. A. Hanes, G. K. Haines III, B. M. Meehan, C. Walchle, P. J. Sansonetti, and J. J. Mekalanos. 2002. The contribution of accessory toxins of Vibrio cholerae O1 El Tor to the proinflammatory response in a murine pulmonary cholera model. J. Exp. Med. 195:1455-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gangarosa, E. J., W. R. Beisel, C. M. Benyajati, H. Sprinz, and P. Piyaratn. 1960. The nature of the gastrointestinal lesion in Asiatic cholera and its relation to pathogenesis: a biopsy study. J. Trop. Med. Hyg. 9:125-135. [DOI] [PubMed] [Google Scholar]
- 17.Gardel, C. L., and J. J. Mekalanos. 1996. Alterations in Vibrio cholerae motility phenotypes correlate with changes in virulence factor expression. Infect. Immun. 64:2246-2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guentzel, M. N., and L. J. Berry. 1975. Motility as a virulence factor for Vibrio cholerae. Infect. Immun. 11:890-897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrington, D. A., R. H. Hall, G. Losonsky, J. J. Mekalanos, R. K. Taylor, and M. M. Levine. 1988. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J. Exp. Med. 168:1487-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang, Q., D. Liu, P. Majewski, L. C. Schulte, J. M. Korn, R. A. Young, E. S. Lander, and N. Hacohen. 2001. The plasticity of dendritic cell responses to pathogens and their components. Science 294:870-875. [DOI] [PubMed] [Google Scholar]
- 21.Ichikawa, J. K., A. Norris, M. G. Bangera, G. K. Geiss, A. B. van't Wout, R. E. Bumgarner, and S. Lory. 2000. Interaction of Pseudomonas aeruginosa with epithelial cells: identification of differentially regulated genes by expression microarray analysis of human cDNAs. Proc. Natl. Acad. Sci. USA 97:9659-9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janoff, E. N., H. Hayakawa, D. N. Taylor, C. E. Fasching, J. R. Kenner, E. Jaimes, and L. Raij. 1997. Nitric oxide production during Vibrio cholerae infection. Am. J. Physiol. 273:G1160-G1167. [DOI] [PubMed] [Google Scholar]
- 23.Kaper, J. B., H. Lockman, M. M. Baldini, and M. M. Levine. 1984. A recombinant live oral cholera vaccine. Bio/Technology 2:345-359. [Google Scholar]
- 24.Kaper, J. B., H. Lockman, M. M. Baldini, and M. M. Levine. 1984. Recombinant nontoxinogenic Vibrio cholerae strains as attenuated cholera vaccine candidates. Nature 308:655-658. [DOI] [PubMed] [Google Scholar]
- 25.Kaper, J. B., J. G. Morris, Jr., and M. M. Levine. 1995. Cholera. Clin. Microbiol. Rev. 8:48-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kenner, J. R., T. S. Coster, D. N. Taylor, A. F. Trofa, M. Barrera-Oro, T. Hyman, J. M. Adams, D. T. Beattie, K. P. Killeen, D. R. Spriggs, J. J. Mekalanos, and J. C. Sadoff. 1995. Peru-15, an improved live attenuated oral vaccine candidate for Vibrio cholerae O1. J. Infect. Dis. 172:1126-1129. [DOI] [PubMed] [Google Scholar]
- 27.Levine, M. M., R. E. Black, M. L. Clements, L. Cisneros, D. R. Nalin, and C. R. Young. 1981. Duration of infection-derived immunity to cholera. J. Infect. Dis. 143:818-820. [DOI] [PubMed] [Google Scholar]
- 28.Levine, M. M., R. E. Black, M. L. Clements, L. Cisneros, A. Saah, D. R. Nalin, D. M. Gill, J. P. Craig, C. R. Young, and P. Ristaino. 1982. The pathogenicity of nonenterotoxigenic Vibrio cholerae serogroup O1 biotype El Tor isolated from sewage water in Brazil. J. Infect. Dis. 145:296-299. [DOI] [PubMed] [Google Scholar]
- 29.Levine, M. M., R. E. Black, M. L. Clements, D. R. Nalin, L. Cisneros, and R. A. Finkelstein. 1981. Volunteer studies in development of vaccines against cholera and enterotoxigenic Escherichia coli: a review, p. 443-459. In T. Holme, J. Holmgren, M. H. Merson, and R. Mollby (ed.), Acute enteric infections in children. New prospects for treatment and prevention. Elsevier/North-Holland Biomedical Press, Amsterdam, The Netherlands.
- 30.Levine, M. M., J. B. Kaper, D. Herrington, J. Ketley, G. Losonsky, C. O. Tacket, B. Tall, and S. Cryz. 1988. Safety, immunogenicity, and efficacy of recombinant live oral cholera vaccines, CVD 103 and CVD 103-HgR. Lancet 2:467-470. [DOI] [PubMed] [Google Scholar]
- 31.Levine, M. M., J. B. Kaper, D. Herrington, G. Losonsky, J. G. Morris, M. L. Clements, R. E. Black, B. Tall, and R. Hall. 1988. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect. Immun. 56:161-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin, W., K. J. Fullner, R. Clayton, J. A. Sexton, M. B. Rogers, K. E. Calia, S. B. Calderwood, C. Fraser, and J. J. Mekalanos. 1999. Identification of a Vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc. Natl. Acad. Sci. USA 96:1071-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maeda, S., M. Otsuka, Y. Hirata, Y. Mitsuno, H. Yoshida, Y. Shiratori, Y. Masuho, M. Muramatsu, N. Seki, and M. Omata. 2001. cDNA microarray analysis of Helicobacter pylori-mediated alteration of gene expression in gastric cancer cells. Biochem. Biophys. Res. Commun. 284:443-449. [DOI] [PubMed] [Google Scholar]
- 34.Mekalanos, J. J., D. J. Swartz, G. D. Pearson, N. Harford, F. Groyne, and M. de Wilde. 1983. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature 306:551-557. [DOI] [PubMed] [Google Scholar]
- 35.Mel, S. F., K. J. Fullner, S. Wimer-Mackin, W. I. Lencer, and J. J. Mekalanos. 2000. Association of protease activity in Vibrio cholerae vaccine strains with decreases in transcellular epithelial resistance of polarized T84 intestinal epithelial cells. Infect. Immun. 68:6487-6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michalski, J., J. E. Galen, A. Fasano, and J. B. Kaper. 1993. CVD110, an attenuated Vibrio cholerae O1 El Tor live oral vaccine strain. Infect. Immun. 61:4462-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Migasena, S., P. Pitisuttitham, B. Prayurahong, P. Suntharasami, W. Suparanond, V. Desakorn, U. Vongsthongsri, B. Tall, J. Ketley, G. Losonsky, S. Cryz, J. B. Kaper, and M. M. Levine. 1989. Preliminary assessment of the safety and immunogenicity of live oral cholera vaccine strain CVD103-HgR in healthy Thai adults. Infect. Immun. 57:3261-3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nau, G. J., J. F. Richmond, A. Schlesinger, E. G. Jennings, E. S. Lander, and R. A. Young. 2002. Human macrophage activation programs induced by bacterial pathogens. Proc. Natl. Acad. Sci. USA 99:1503-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Passegue, E., and E. F. Wagner. 2000. JunB suppresses cell proliferation by transcriptional activation of p16INK4a expression. EMBO J. 19:2969-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pastore, G., G. Schiraldi, G. Fera, E. Sforza, and O. Schiraldi. 1976. A bioptic study of gastrointestinal mucosa in cholera patients during an epidemic in southern Italy. Am. J. Dig. Dis. 21:613-617. [DOI] [PubMed] [Google Scholar]
- 41.Pietzsch, A., C. Buchler, C. Aslanidis, and G. Schmitz. 1997. Identification and characterization of a novel monocyte/macrophage differentiation-dependent gene that is responsive to lipopolysaccharide, ceramide, and lysophosphatidylcholine. Biochem. Biophys. Res. Commun. 235:4-9. [DOI] [PubMed] [Google Scholar]
- 42.Postnova, T., O. G. Gomez-Duarte, and K. Richardson. 1996. Motility mutants of Vibrio cholerae O1 have reduced adherence in vitro to human small intestinal epithelial cells as demonstrated by ELISA. Microbiology 142:2767-2776. [DOI] [PubMed] [Google Scholar]
- 43.Qadri, F., R. Raqib, F. Ahmed, T. Rahman, C. Wenneras, S. K. Das, N. H. Alam, M. M. Mathan, and A. M. Svennerholm. 2002. Increased levels of inflammatory mediators in children and adults infected with Vibrio cholerae O1 and O139. Clin. Diagn. Lab. Immunol. 9:221-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rabbani, G. H., S. Islam, A. K. Chowdhury, A. K. Mitra, M. J. Miller, and G. Fuchs. 2001. Increased nitrite and nitrate concentrations in sera and urine of patients with cholera or shigellosis. Am. J. Gastroenterol. 96:467-472. [DOI] [PubMed] [Google Scholar]
- 45.Ragno, S., M. Romano, S. Howell, D. J. Pappin, P. J. Jenner, and M. J. Colston. 2001. Changes in gene expression in macrophages infected with Mycobacterium tuberculosis: a combined transcriptomic and proteomic approach. Immunology 104:99-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richie, E. E., N. H. Punjabi, Y. Y. Sidharta, K. K. Peetosutan, M. M. Sukandar, S. S. Wasserman, M. M. Lesmana, F. F. Wangsasaputra, S. S. Pandam, M. M. Levine, P. P. O'Hanley, S. J. Cryz, and C. H. Simanjuntak. 2000. Efficacy trial of single-dose live oral cholera vaccine CVD 103-HgR in North Jakarta, Indonesia, a cholera-endemic area. Vaccine 18:2399-2410. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez, B. L., A. Rojas, J. Campos, T. Ledon, E. Valle, W. Toledo, and R. Fando. 2001. Differential interleukin-8 response of intestinal epithelial cell line to reactogenic and nonreactogenic candidate vaccine strains of Vibrio cholerae. Infect. Immun. 69:613-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saha, D. R., S. K. Niyogi, G. B. Nair, B. Manna, and S. K. Bhattacharya. 2000. Detection of faecal leucocytes and erythrocytes from stools of cholera patients suggesting an evidence of an inflammatory response in cholera. Indian J. Med. Res. 112:5-8. [PubMed] [Google Scholar]
- 49.Sepulveda, A. R., H. Tao, E. Carloni, J. Sepulveda, D. Y. Graham, and L. E. Peterson. 2002. Screening of gene expression profiles in gastric epithelial cells induced by Helicobacter pylori using microarray analysis. Aliment. Pharmacol. Ther. 16(Suppl. 2):145-157. [DOI] [PubMed] [Google Scholar]
- 50.Silva, T. M., M. A. Schleupner, C. O. Tacket, T. S. Steiner, J. B. Kaper, R. Edelman, and R. Guerrant. 1996. New evidence for an inflammatory component in diarrhea caused by selected new, live attenuated cholera vaccines and by El Tor and O139 Vibrio cholerae. Infect. Immun. 64:2362-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tacket, C. O., M. B. Cohen, S. S. Wasserman, G. Losonsky, S. Livio, K. Kotloff, R. Edelman, J. B. Kaper, S. J. Cryz, R. A. Giannella, G. Schiff, and M. M. Levine. 1999. Randomized, double-blind, placebo-controlled, multicentered trial of the efficacy of a single dose of live oral cholera vaccine CVD 103-HgR in preventing cholera following challenge with Vibrio cholerae O1 El Tor Inaba three months after vaccination. Infect. Immun. 67:6341-6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tacket, C. O., G. Losonsky, J. P. Nataro, L. Comstock, J. Michalski, R. Edelman, J. B. Kaper, and M. M. Levine. 1995. Initial clinical studies of CVD 112 Vibrio cholerae O139 live oral vaccine: safety and efficacy against experimental challenge. J. Infect. Dis. 172:883-886. [DOI] [PubMed] [Google Scholar]
- 53.Tacket, C. O., G. Losonsky, J. P. Nataro, S. J. Cryz, R. Edelman, A. Fasano, J. Michalski, J. B. Kaper, and M. M. Levine. 1993. Safety and immunogenicity of live oral cholera vaccine candidate CVD 110, a ΔctxA Δzot Δace derivative of El Tor Ogawa Vibrio cholerae. J. Infect. Dis. 168:1536-1540. [DOI] [PubMed] [Google Scholar]
- 54.Trucksis, M., J. E. Galen, J. Michalski, A. Fasano, and J. B. Kaper. 1993. Accessory cholera enterotoxin (Ace), the third toxin of a Vibrio cholerae virulence cassette. Proc. Natl. Acad. Sci. USA 90:5267-5271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Warton, K., R. Tonini, W. D. Fairlie, J. M. Matthews, S. M. Valenzuela, M. R. Qiu, W. M. Wu, S. Pankhurst, A. R. Bauskin, S. J. Harrop, T. J. Campbell, P. M. Curmi, S. N. Breit, and M. Mazzanti. 2002. Recombinant CLIC1 (NCC27) assembles in lipid bilayers via a pH-dependent two-state process to form chloride ion channels with identical characteristics to those observed in Chinese hamster ovary cells expressing CLIC1. J. Biol. Chem. 277:26003-26011. [DOI] [PubMed] [Google Scholar]
- 56.Yang, S. K., L. Eckmann, A. Panja, and M. F. Kagnoff. 1997. Differential and regulated expression of C-X-C, C-C, and C-chemokines by human colon epithelial cells. Gastroenterology 113:1214-1223. [DOI] [PubMed] [Google Scholar]